Sperm Cell Driven Microrobots—Emerging Opportunities and Challenges for Biologically Inspired Robotic Design
Abstract
1. Introduction
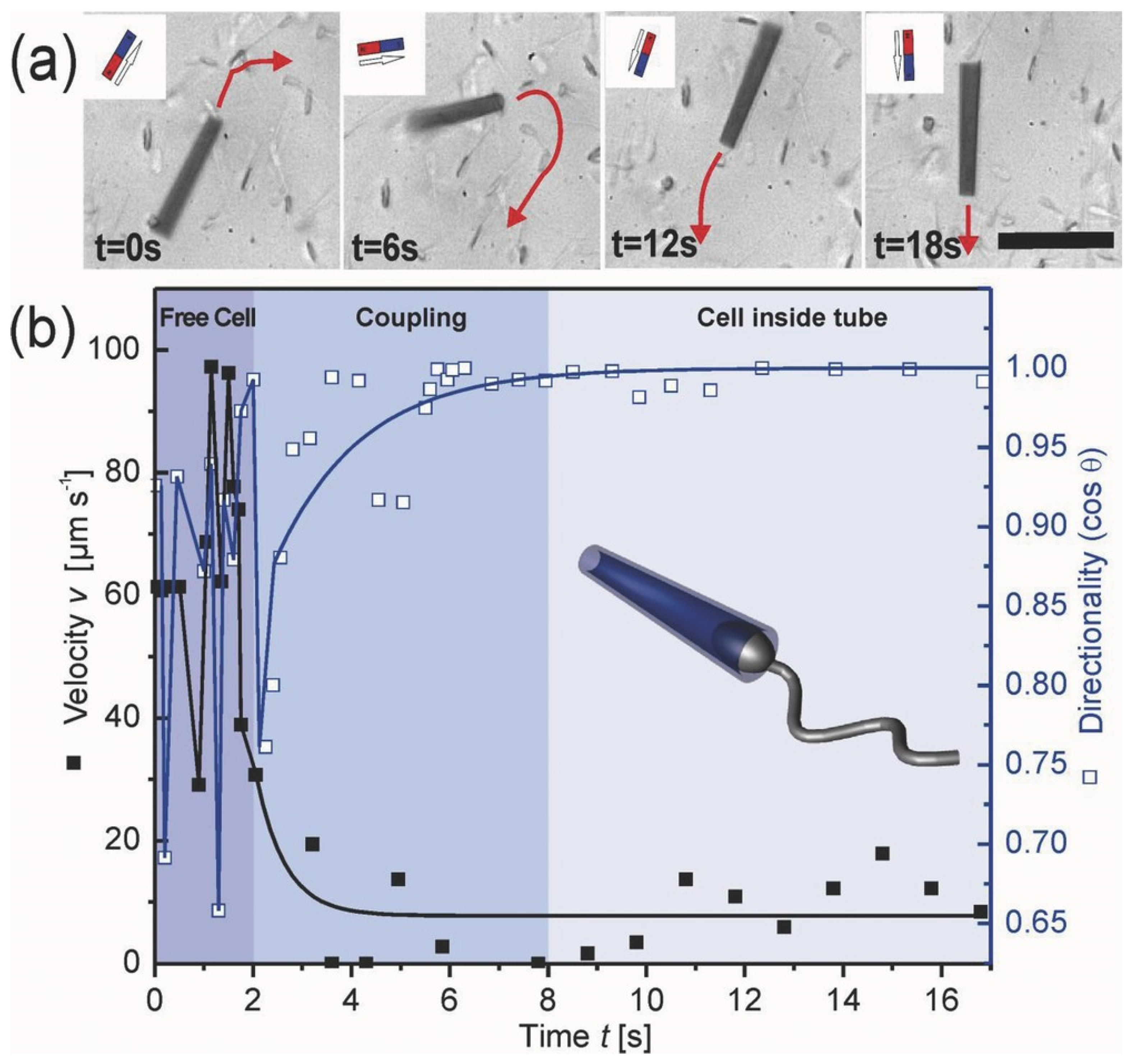
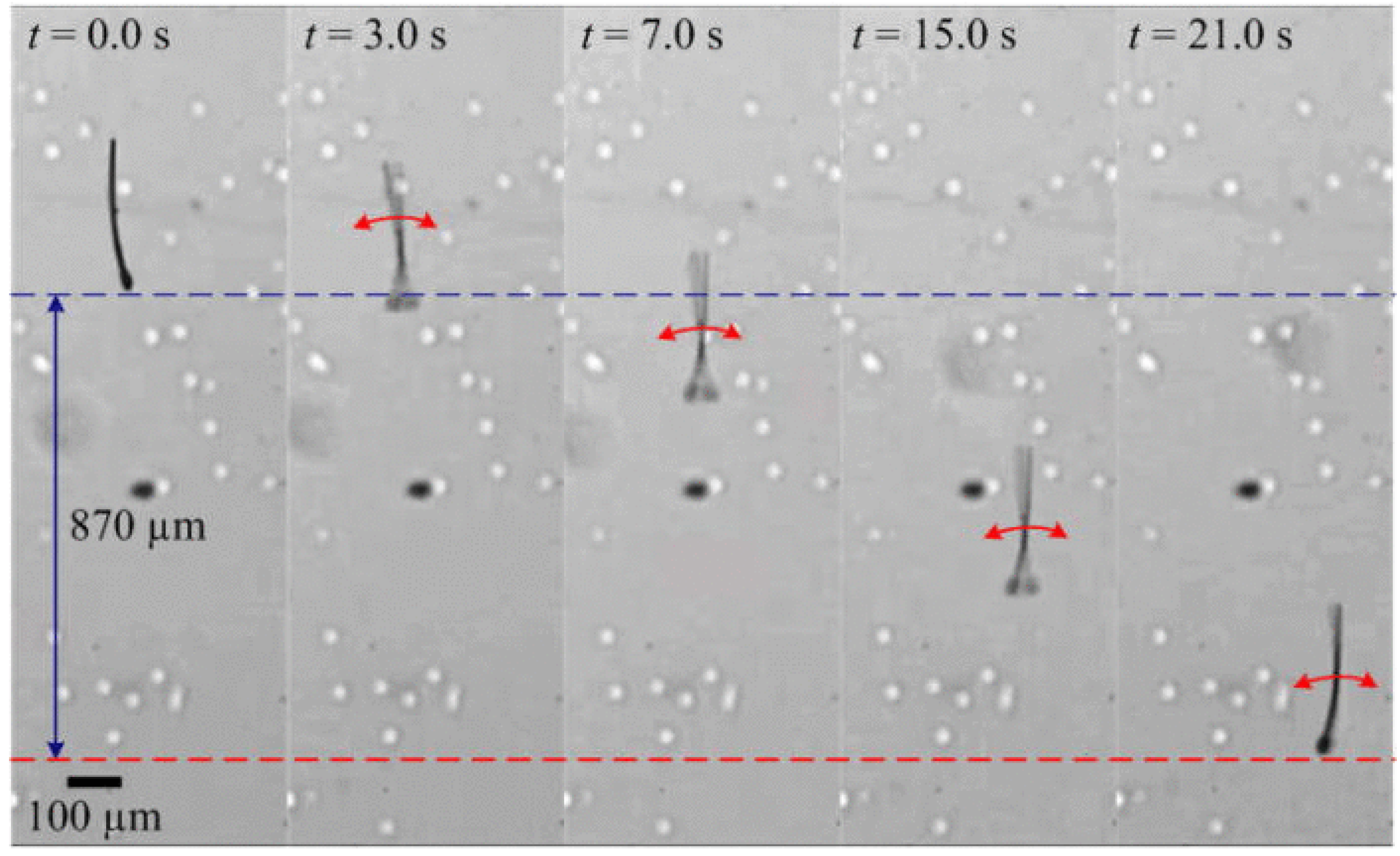
2. Concept of Spermbots with Undulatory Locomotion
3. Creating Biohybrid and Synthetic Spermbots—Technical Challenges and Solutions
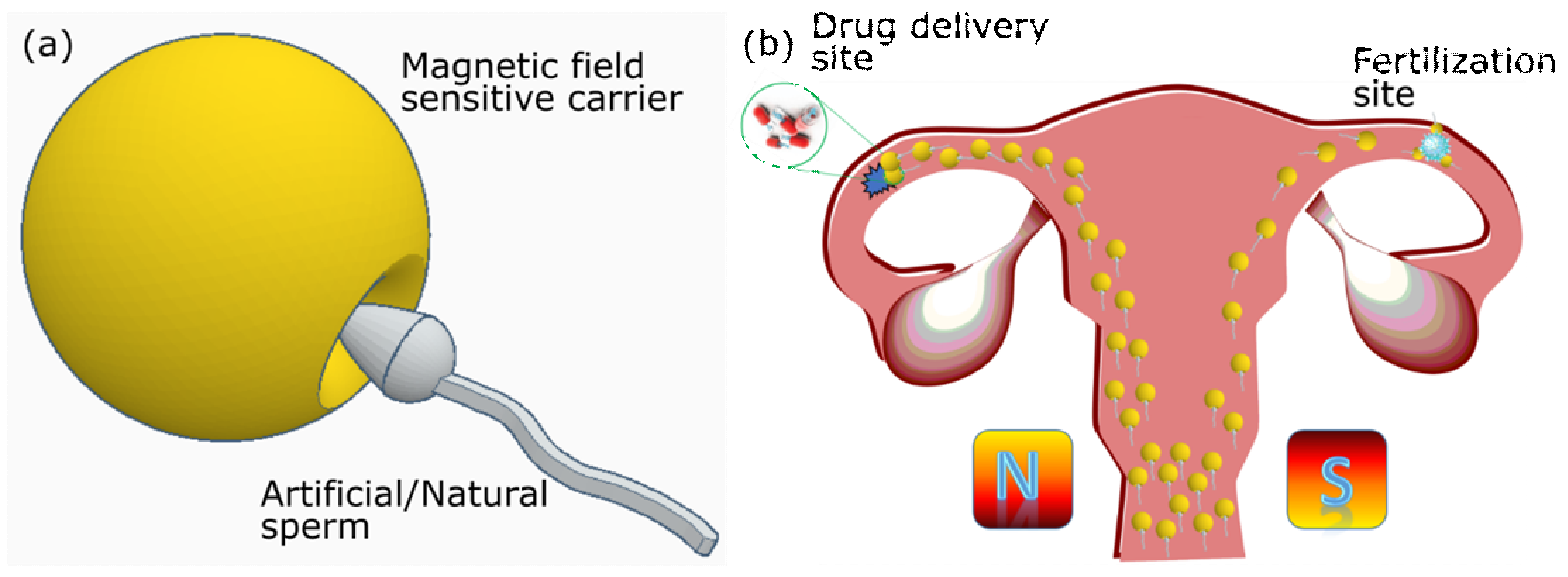
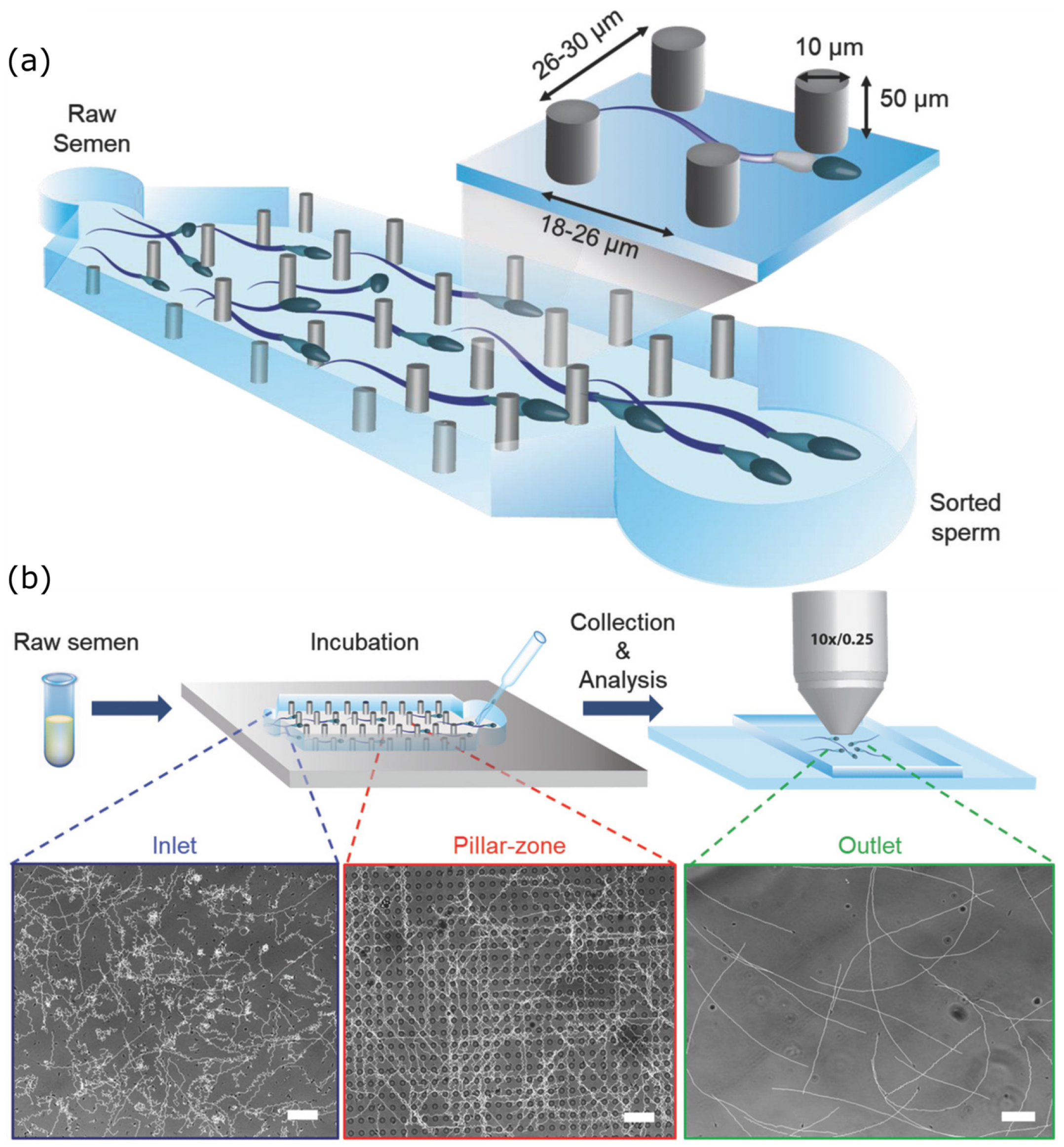
4. Spermbot Assisted Targeted Delivery—Proof of Concept Examples with Assisted Fertilization and Drug Delivery
Artificial Spermbots
5. Challenges
- Sperm cells do not all have the same motility. Their motility varies from individual to individual and from cell to cell even from the same individual. This is a cause for concern because we need sperm cells that are highly motile, to be as efficient as possible for actuation. While there is considerable interest in this particular field [94,95,96,97,98,99,100,101,102,103,104], a standardized method and protocol is highly crucial and is the need of the hour.
- The load/microtubes by itself should not be toxic and should be able to pass through any barriers that it may encounter on the way to the targeted site.
- The attachment of load or microtubes to the sperm cell is random and a very low-yielding process [27]. Therefore, robust methods to increase this coupling efficiency are needed [38,73]. Electrostatic-based self-assembly helps in partial coating of all sperm heads with magnetic nanoparticle aggregates [38].
- While the sperm cell by itself is highly biocompatible, there is a chance that harmful microbes could attach themselves to it and render it useless for medical use. To avoid this, antibacterial agents must be applied to the inside of the microtubes to protect the sperm cells.
- In order to ensure accurate site-targeting, an appropriate tracking technology is necessary for imaging and guiding of the spermbots. Photoacoustic, Ultrasound and magnetic resonance imaging (MRI) techniques are worth mentioning here as each comes with its own advantages and promises [109,110,111,112,113,114,115,116].
- There must be an easy-to-control strategy to help release the drug at the intended site once the spermbots reaches there.
- There may be other application specific challenges. For example, in the case of fertilization with spermbots, we also need to select only the most fertile sperm cells.
6. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, A.V.; Rahman, A.; Sudhir Kumar, N.V.G.; Aditi, A.S.; Galluzzi, M.; Bovio, S.; Barozzi, S.; Montani, E.; Parazzoli, D. Bio-inspired approaches to design smart fabrics. Mater. Des. (1980–2015) 2012, 36, 829–839. [Google Scholar] [CrossRef]
- Singh, A.V.; Dad Ansari, M.H.; Dayan, C.B.; Giltinan, J.; Wang, S.; Yu, Y.; Kishore, V.; Laux, P.; Luch, A.; Sitti, M. Multifunctional magnetic hairbot for untethered osteogenesis, ultrasound contrast imaging and drug delivery. Biomaterials 2019, 219, 119394. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Laschi, C.; Trimmer, B. Soft robotics: A bioinspired evolution in robotics. Trends Biotechnol. 2013, 31, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Albu-Schaffer, A.; Eiberger, O.; Grebenstein, M.; Haddadin, S.; Ott, C.; Wimbock, T.; Wolf, S.; Hirzinger, G. Soft robotics. IEEE Robot. Autom. Mag. 2008, 15, 20–30. [Google Scholar] [CrossRef]
- Cianchetti, M.; Laschi, C.; Menciassi, A.; Dario, P. Biomedical applications of soft robotics. Nat. Rev. Mater. 2018, 3, 143–153. [Google Scholar] [CrossRef]
- Abidi, H.; Gerboni, G.; Brancadoro, M.; Fras, J.; Diodato, A.; Cianchetti, M.; Wurdemann, H.; Althoefer, K.; Menciassi, A. Highly dexterous 2-module soft robot for intra-organ navigation in minimally invasive surgery. Int. J. Med. Robot. Comput. Assist. Surg. 2018, 14, e1875. [Google Scholar] [CrossRef]
- Cianchetti, M.; Ranzani, T.; Gerboni, G.; Falco, I.D.; Laschi, C.; Menciassi, A. STIFF-FLOP surgical manipulator: Mechanical design and experimental characterization of the single module. In Proceedings of the 2013 IEEE/RSJ International Conference on Intelligent Robots and Systems, Tokyo, Japan, 3–7 November 2013; pp. 3576–3581. [Google Scholar]
- Runciman, M.; Darzi, A.; Mylonas, G.P. Soft Robotics in Minimally Invasive Surgery. Soft Robot. 2019, 6, 423–443. [Google Scholar] [CrossRef]
- Singh, A.V.; Hosseinidoust, Z.; Park, B.-W.; Yasa, O.; Sitti, M. Microemulsion-Based Soft Bacteria-Driven Microswimmers for Active Cargo Delivery. ACS Nano 2017, 11, 9759–9769. [Google Scholar] [CrossRef]
- Iacovacci, V.; Blanc, A.; Huang, H.; Ricotti, L.; Schibli, R.; Menciassi, A.; Behe, M.; Pané, S.; Nelson, B.J. High-Resolution SPECT Imaging of Stimuli-Responsive Soft Microrobots. Small 2019, 15, 1900709. [Google Scholar] [CrossRef]
- Palagi, S.; Singh, D.P.; Fischer, P. Light-Controlled Micromotors and Soft Microrobots. Adv. Opt. Mater. 2019, 7, 1900370. [Google Scholar] [CrossRef]
- Hu, C.; Pané, S.; Nelson, B.J. Soft Micro- and Nanorobotics. Annu. Rev. Control Robot. Auton. Syst. 2018, 1, 53–75. [Google Scholar] [CrossRef]
- Carlsen, R.W.; Sitti, M. Bio-Hybrid Cell-Based Actuators for Microsystems. Small 2014, 10, 3831–3851. [Google Scholar] [CrossRef] [PubMed]
- Macnab, R.M. The bacterial flagellum: Reversible rotary propellor and type III export apparatus. J. Bacteriol. 1999, 181, 7149–7153. [Google Scholar] [CrossRef] [PubMed]
- Baccetti, B.; Afzelius, B.A. The Biology of the Sperm Cell; Karger AG: Basel, Switzerland, 1976. [Google Scholar]
- Astbury, W.T.; Saha, N.N. Structure of Algal Flagella. Nature 1953, 171, 280–283. [Google Scholar] [CrossRef]
- Singh, A.V.; Ansari, M.H.D.; Laux, P.; Luch, A. Micro-nanorobots: Important considerations when developing novel drug delivery platforms. Expert Opin. Drug Deliv. 2019, 16, 1259–1275. [Google Scholar] [CrossRef] [PubMed]
- Hosseinidoust, Z.; Mostaghaci, B.; Yasa, O.; Park, B.-W.; Singh, A.V.; Sitti, M. Bioengineered and biohybrid bacteria-based systems for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 27–44. [Google Scholar] [CrossRef]
- Singh, A.V.; Jahnke, T.; Xiao, Y.; Wang, S.; Yu, Y.; David, H.; Richter, G.; Laux, P.; Luch, A.; Srivastava, A.; et al. Peptide-Induced Biomineralization of Tin Oxide (SnO2) Nanoparticles for Antibacterial Applications. J. Nanosci. Nanotechnol. 2019, 19, 5674–5686. [Google Scholar] [CrossRef]
- Zhang, L.; Abbott, J.J.; Dong, L.; Kratochvil, B.E.; Bell, D.; Nelson, B.J. Artificial bacterial flagella: Fabrication and magnetic control. Appl. Phys. Lett. 2009, 94, 064107. [Google Scholar] [CrossRef]
- Behkam, B.; Sitti, M. Bacterial flagella-based propulsion and on/off motion control of microscale objects. Appl. Phys. Lett. 2007, 90, 023902. [Google Scholar] [CrossRef]
- Magdanz, V.; Medina-Sánchez, M.; Schwarz, L.; Xu, H.; Elgeti, J.; Schmidt, O.G. Spermatozoa as Functional Components of Robotic Microswimmers. Adv. Mater. 2017, 29, 1606301. [Google Scholar] [CrossRef]
- Ricotti, L.; Menciassi, A. Bio-hybrid muscle cell-based actuators. Biomed. Microdevices 2012, 14, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.J.; Anand, S.V.; Rajagopalan, J.; Saif, M.T.A. A self-propelled biohybrid swimmer at low Reynolds number. Nat. Commun. 2014, 5, 3081. [Google Scholar] [CrossRef] [PubMed]
- Dreyfus, R.; Baudry, J.; Roper, M.L.; Fermigier, M.; Stone, H.A.; Bibette, J. Microscopic artificial swimmers. Nature 2005, 437, 862–865. [Google Scholar] [CrossRef] [PubMed]
- Weibel, D.B.; Garstecki, P.; Ryan, D.; DiLuzio, W.R.; Mayer, M.; Seto, J.E.; Whitesides, G.M. Microoxen: Microorganisms to move microscale loads. Proc. Natl. Acad. Sci. USA 2005, 102, 11963. [Google Scholar] [CrossRef] [PubMed]
- Magdanz, V.; Sanchez, S.; Schmidt, O.G. Development of a Sperm-Flagella Driven Micro-Bio-Robot. Adv. Mater. 2013, 25, 6581–6588. [Google Scholar] [CrossRef]
- Khalil, I.S.M.; Dijkslag, H.C.; Abelmann, L.; Misra, S. MagnetoSperm: A microrobot that navigates using weak magnetic fields. Appl. Phys. Lett. 2014, 104, 223701. [Google Scholar] [CrossRef]
- Khalil, I.S.M.; Youakim, K.; Sánchez, A.; Misra, S. Magnetic-based motion control of sperm-shaped microrobots using weak oscillating magnetic fields. In Proceedings of the 2014 IEEE/RSJ International Conference on Intelligent Robots and Systems, Chicago, IL, USA, 14–18 September 2014; pp. 4686–4691. [Google Scholar]
- Medina-Sánchez, M.; Schwarz, L.; Meyer, A.K.; Hebenstreit, F.; Schmidt, O.G. Cellular Cargo Delivery: Toward Assisted Fertilization by Sperm-Carrying Micromotors. Nano Lett. 2016, 16, 555–561. [Google Scholar] [CrossRef]
- Xu, H.; Medina-Sánchez, M.; Magdanz, V.; Schwarz, L.; Hebenstreit, F.; Schmidt, O.G. Sperm-Hybrid Micromotor for Targeted Drug Delivery. ACS Nano 2018, 12, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Magdanz, V.; Boryshpolets, S.; Ridzewski, C.; Eckel, B.; Reinhardt, K. The motility-based swim-up technique separates bull sperm based on differences in metabolic rates and tail length. PLoS ONE 2019, 14, e0223576. [Google Scholar] [CrossRef]
- Khalil, I.S.M.; Klingner, A.; Magdanz, V.; Striggow, F.; Medina-Sánchez, M.; Schmidt, O.G.; Misra, S. Modeling of Spermbots in a Viscous Colloidal Suspension. Adv. Theory Simul. 2019, 2, 1900072. [Google Scholar] [CrossRef]
- Magdanz, V.; Gebauer, J.; Sharan, P.; Eltoukhy, S.; Voigt, D.; Simmchen, J. Sperm–Particle Interactions and Their Prospects for Charge Mapping. Adv. Biosyst. 2019, 3, 1900061. [Google Scholar] [CrossRef]
- Khalil, I.S.M.; Klingner, A.; Hamed, Y.; Magdanz, V.; Toubar, M.; Misra, S. Characterization of Flagellar Propulsion of Soft Microrobotic Sperm in a Viscous Heterogeneous Medium. Front. Robot. AI 2019, 6, 65. [Google Scholar] [CrossRef]
- Ridzewski, C.; Li, M.; Dong, B.; Magdanz, V. Gelatin Microcartridges for Onboard Activation and Antioxidant Protection of Sperm. ACS Appl. Bio Mater. 2020. [Google Scholar] [CrossRef]
- Xu, H.; Medina-Sánchez, M.; Maitz, M.F.; Werner, C.; Schmidt, O.G. Sperm-Micromotors for Cargo-Delivery through Flowing Blood. ACS Nano 2020. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.S.M.; Magdanz, V.; Simmchen, J.; Klingner, A.; Misra, S. Resemblance between motile and magnetically actuated sperm cells. Appl. Phys. Lett. 2020, 116, 063702. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Felfoul, O.; Mohammadi, M.; Taherkhani, S.; de Lanauze, D.; Xu, Y.Z.; Loghin, D.; Essa, S.; Jancik, S.; Houle, D.; Lafleur, M.; et al. Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions. Nat. Nanotechnol. 2016, 11, 941–947. [Google Scholar] [CrossRef]
- Park, B.-W.; Zhuang, J.; Yasa, O.; Sitti, M. Multifunctional Bacteria-Driven Microswimmers for Targeted Active Drug Delivery. ACS Nano 2017, 11, 8910–8923. [Google Scholar] [CrossRef]
- Martel, S.; Mathieu, J.-B.; Felfoul, O.; Chanu, A.; Aboussouan, E.; Tamaz, S.; Pouponneau, P.; Yahia, L.H.; Beaudoin, G.; Soulez, G.; et al. Automatic navigation of an untethered device in the artery of a living animal using a conventional clinical magnetic resonance imaging system. Appl. Phys. Lett. 2007, 90, 114105. [Google Scholar] [CrossRef]
- Pouponneau, P.; Leroux, J.-C.; Soulez, G.; Gaboury, L.; Martel, S. Co-encapsulation of magnetic nanoparticles and doxorubicin into biodegradable microcarriers for deep tissue targeting by vascular MRI navigation. Biomaterials 2011, 32, 3481–3486. [Google Scholar] [CrossRef]
- Folio, D.; Ferreira, A. Two-Dimensional Robust Magnetic Resonance Navigation of a Ferromagnetic Microrobot Using Pareto Optimality. IEEE Trans. Robot. 2017, 33, 583–593. [Google Scholar] [CrossRef]
- Felfoul, O.; Becker, A.T.; Fagogenis, G.; Dupont, P.E. Simultaneous steering and imaging of magnetic particles using MRI toward delivery of therapeutics. Sci. Rep. 2016, 6, 33567. [Google Scholar] [CrossRef]
- Egolf, P.W.; Shamsudhin, N.; Pané, S.; Vuarnoz, D.; Pokki, J.; Pawlowski, A.-G.; Tsague, P.; de Marco, B.; Bovy, W.; Tucev, S.; et al. Hyperthermia with rotating magnetic nanowires inducing heat into tumor by fluid friction. J. Appl. Phys. 2016, 120, 064304. [Google Scholar] [CrossRef]
- Iacovacci, V.; Ricotti, L.; Sinibaldi, E.; Signore, G.; Vistoli, F.; Menciassi, A. An Intravascular Magnetic Catheter Enables the Retrieval of Nanoagents from the Bloodstream. Adv. Sci. 2018, 5, 1800807. [Google Scholar] [CrossRef]
- Iacovacci, V.; Ricotti, L.; Signore, G.; Vistoli, F.; Sinibaldi, E.; Menciassi, A. Retrieval of magnetic medical microrobots from the bloodstream. In Proceedings of the 2019 International Conference on Robotics and Automation (ICRA), Montreal, QC, Canada, 20–24 May 2019; pp. 2495–2501. [Google Scholar]
- Levy, M.; Luciani, N.; Alloyeau, D.; Elgrabli, D.; Deveaux, V.; Pechoux, C.; Chat, S.; Wang, G.; Vats, N.; Gendron, F.; et al. Long term in vivo biotransformation of iron oxide nanoparticles. Biomaterials 2011, 32, 3988–3999. [Google Scholar] [CrossRef] [PubMed]
- Mejías, R.; Gutiérrez, L.; Salas, G.; Pérez-Yagüe, S.; Zotes, T.M.; Lázaro, F.J.; Morales, M.P.; Barber, D.F. Long term biotransformation and toxicity of dimercaptosuccinic acid-coated magnetic nanoparticles support their use in biomedical applications. J. Control. Release 2013, 171, 225–233. [Google Scholar] [CrossRef]
- Magdanz, V.; Schmidt, O.G. Spermbots: Potential impact for drug delivery and assisted reproductive technologies. Expert Opin. Drug Deliv. 2014, 11, 1125–1129. [Google Scholar] [CrossRef]
- Magdanz, V.; Gebauer, J.; Mahdy, D.; Simmchen, J.; Khalil, I.S.M. Sperm-templated magnetic microrobots. In Proceedings of the 2019 International Conference on Manipulation, Automation and Robotics at Small Scales (MARSS), Helsinki, Finland, 1–5 July 2019; pp. 1–6. [Google Scholar]
- Jang, D.; Jeong, J.; Song, H.; Chung, S.K. Targeted drug delivery technology using untethered microrobots: A review. J. Micromech. Microeng. 2019, 29, 053002. [Google Scholar] [CrossRef]
- Medina-Sánchez, M.; Xu, H.; Schmidt, O.G. Micro- and nano-motors: The new generation of drug carriers. Ther. Deliv. 2018, 9, 303–316. [Google Scholar] [CrossRef]
- Pan, Y.; Du, X.; Zhao, F.; Xu, B. Magnetic nanoparticles for the manipulation of proteins and cells. Chem. Soc. Rev. 2012, 41, 2912–2942. [Google Scholar] [CrossRef]
- Gao, W.; Sattayasamitsathit, S.; Manesh, K.M.; Weihs, D.; Wang, J. Magnetically Powered Flexible Metal Nanowire Motors. J. Am. Chem. Soc. 2010, 132, 14403–14405. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J. Gene therapy progress and prospects: Magnetic nanoparticle-based gene delivery. Gene Ther. 2006, 13, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Scarberry, K.E.; Dickerson, E.B.; McDonald, J.F.; Zhang, Z.J. Magnetic Nanoparticle−Peptide Conjugates for in Vitro and in Vivo Targeting and Extraction of Cancer Cells. J. Am. Chem. Soc. 2008, 130, 10258–10262. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, N.; Dykman, L. Biodistribution and toxicity of engineered gold nanoparticles: A review of in vitro and in vivo studies. Chem. Soc. Rev. 2011, 40, 1647–1671. [Google Scholar] [CrossRef]
- Ansari, M.H.D.; Lavhale, S.; Kalunke, R.M.; Srivastava, P.L.; Pandit, V.; Gade, S.; Yadav, S.; Laux, P.; Luch, A.; Gemmati, D. Recent Advances in Plant Nanobionics and Nanobiosensors for Toxicology Applications. Curr. Nanosci. 2019, 16, 27–41. [Google Scholar] [CrossRef]
- Schmidt, O.G.; Eberl, K. Thin solid films roll up into nanotubes. Nature 2001, 410, 168. [Google Scholar] [CrossRef]
- Mei, Y.; Huang, G.; Solovev, A.A.; Ureña, E.B.; Mönch, I.; Ding, F.; Reindl, T.; Fu, R.K.Y.; Chu, P.K.; Schmidt, O.G. Versatile Approach for Integrative and Functionalized Tubes by Strain Engineering of Nanomembranes on Polymers. Adv. Mater. 2008, 20, 4085–4090. [Google Scholar] [CrossRef]
- Magdanz, V.; Guix, M.; Schmidt, O.G. Tubular micromotors: From microjets to spermbots. Robot. Biomim. 2014, 1, 11. [Google Scholar] [CrossRef][Green Version]
- Bansal, P.; Gupta, S.K. Binding characteristics of sperm with recombinant human zona pellucida glycoprotein-3 coated beads. Indian J. Med. Res. 2009, 130, 37. [Google Scholar]
- Diaz, E.S.; Kong, M.; Morales, P. Effect of fibronectin on proteasome activity, acrosome reaction, tyrosine phosphorylation and intracellular calcium concentrations of human sperm. Hum. Reprod. 2007, 22, 1420–1430. [Google Scholar] [CrossRef]
- Wennemuth, G.; Schiemann, P.J.; Krause, W.; Gressner, A.M.; AumÜLler, G. Influence of fibronectin on the motility of human spermatozoa. Int. J. Androl. 1997, 20, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Frimat, J.P.; Bronkhorst, M.; de Wagenaar, B.; Bomer, J.G.; van der Heijden, F.; van den Berg, A.; Segerink, L.I. Make it spin: Individual trapping of sperm for analysis and recovery using micro-contact printing. Lab Chip 2014, 14, 2635–2641. [Google Scholar] [CrossRef]
- Huszar, G.; Ozenci, C.C.; Cayli, S.; Zavaczki, Z.; Hansch, E.; Vigue, L. Hyaluronic acid binding by human sperm indicates cellular maturity, viability and unreacted acrosomal status. Fertil. Steril. 2003, 79, 1616–1624. [Google Scholar] [CrossRef]
- Yagci, A.; Murk, W.; Stronk, J.; Huszar, G. Spermatozoa Bound to Solid State Hyaluronic Acid Show Chromatin Structure with High DNA Chain Integrity: An Acridine Orange Fluorescence Study. J. Androl. 2010, 31, 566–572. [Google Scholar] [CrossRef]
- Parmegiani, L.; Cognigni, G.E.; Bernardi, S.; Troilo, E.; Taraborrelli, S.; Arnone, A.; Maccarini, A.M.; Filicori, M. Comparison of two ready-to-use systems designed for sperm–hyaluronic acid binding selection before intracytoplasmic sperm injection: PICSI vs. Sperm Slow: A prospective, randomized trial. Fertil. Steril. 2012, 98, 632–637. [Google Scholar] [CrossRef]
- Campos, L.B.; Peixoto, G.C.X.; da Silva, A.M.; Souza, A.L.P.; de Souza Castelo, T.; Maia, K.M.; Pereira, A.F.; Silva, A.R. Estimating the binding ability of collared peccary (Pecari tajacu Linnaeus, 1758) sperm using heterologous substrates. Theriogenology 2017, 92, 57–62. [Google Scholar] [CrossRef]
- Tecle, E.; Reynoso, H.S.; Wang, R.; Gagneux, P. The female reproductive tract contains multiple innate sialic acid-binding immunoglobulin-like lectins (Siglecs) that facilitate sperm survival. J. Biol. Chem. 2019, 294, 11910–11919. [Google Scholar] [CrossRef]
- Magdanz, V.; Medina-Sánchez, M.; Chen, Y.; Guix, M.; Schmidt, O.G. How to Improve Spermbot Performance. Adv. Funct. Mater. 2015, 25, 2763–2770. [Google Scholar] [CrossRef]
- Singh, A.V.; Patil, R.; Thombre, D.K.; Gade, W.N. Micro-nanopatterning as tool to study the role of physicochemical properties on cell–surface interactions. J. Biomed. Mater. Res. Part A 2013, 101, 3019–3032. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, C.A.; Hippler, M.; Münchinger, A.; Bastmeyer, M.; Barner-Kowollik, C.; Wegener, M.; Blasco, E. 4D Printing at the Microscale. Adv. Funct. Mater. 2019, 1907615. [Google Scholar] [CrossRef]
- Vikram Singh, A.; Hasan Dad Ansari, M.; Wang, S.; Laux, P.; Luch, A.; Kumar, A.; Patil, R.; Nussberger, S. The adoption of three-dimensional additive manufacturing from biomedical material design to 3d organ printing. Appl. Sci. 2019, 9, 811. [Google Scholar] [CrossRef]
- Singh, A.V.; Laux, P.; Luch, A.; Balkrishnan, S.; Dakua, S.P. Bottom-UP assembly of nanorobots: Extending synthetic biology to complex material design. Front. Nanosci. Nanotechnol. 2019, 5, 1–2. [Google Scholar]
- Singh, A. Top-Down Versus Bottom-Up Nanoengineering Routes to Design Advanced Oropharmacological Products. Curr. Pharm. Des. 2016, 22, 1534–1545. [Google Scholar] [CrossRef] [PubMed]
- Martel, S. Bacterial microsystems and microrobots. Biomed. Microdevices 2012, 14, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Soong, R.K.; Bachand, G.D.; Neves, H.P.; Olkhovets, A.G.; Craighead, H.G.; Montemagno, C.D. Powering an Inorganic Nanodevice with a Biomolecular Motor. Science 2000, 290, 1555. [Google Scholar] [CrossRef] [PubMed]
- Mukai, C.; Bergkvist, M.; Nelson, J.L.; Travis, A.J. Sequential Reactions of Surface- Tethered Glycolytic Enzymes. Chem. Biol. 2009, 16, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.S.M.; Magdanz, V.; Sanchez, S.; Schmidt, O.G.; Misra, S. Biocompatible, accurate and fully autonomous: A sperm-driven micro-bio-robot. J. Micro-Bio Robot. 2014, 9, 79–86. [Google Scholar] [CrossRef]
- Raz, O.; Leshansky, A.M. Efficiency of cargo towing by a microswimmer. Phys. Rev. E 2008, 77, 055305. [Google Scholar] [CrossRef]
- Grosjean, G.; Hubert, M.; Vandewalle, N. Magnetocapillary self-assemblies: Locomotion and micromanipulation along a liquid interface. Adv. Colloid Interface Sci. 2018, 255, 84–93. [Google Scholar] [CrossRef]
- Gao, W.; Kagan, D.; Pak, O.S.; Clawson, C.; Campuzano, S.; Chuluun-Erdene, E.; Shipton, E.; Fullerton, E.E.; Zhang, L.; Lauga, E.; et al. Cargo-Towing Fuel-Free Magnetic Nanoswimmers for Targeted Drug Delivery. Small 2012, 8, 460–467. [Google Scholar] [CrossRef]
- Luo, M.; Feng, Y.; Wang, T.; Guan, J. Micro-/Nanorobots at Work in Active Drug Delivery. Adv. Funct. Mater. 2018, 28, 1706100. [Google Scholar] [CrossRef]
- Yan, W. Toward Better Treatment for Women’s Reproductive Health: New Devices, Nanoparticles and Even Robotic Sperm May Hold the Key to Preventing a Range of Health Conditions. IEEE Pulse 2018, 9, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Bendich, A.; Borenfreund, E.; Sternberg, S.S. Penetration of Somatic Mammalian Cells by Sperm. Science 1974, 183, 857. [Google Scholar] [CrossRef]
- Lindsay, T.J.; Vitrikas, K.R. Evaluation and treatment of infertility. Am. Fam. Physician 2015, 91, 308–314. [Google Scholar] [PubMed]
- Khalil, I.S.M.; Fatih Tabak, A.; Klingner, A.; Sitti, M. Magnetic propulsion of robotic sperms at low-Reynolds number. Appl. Phys. Lett. 2016, 109, 033701. [Google Scholar] [CrossRef]
- Khalil, I.S.M.; Tabak, A.F.; Abou Seif, M.; Klingner, A.; Sitti, M. Controllable switching between planar and helical flagellar swimming of a soft robotic sperm. PLoS ONE 2018, 13, e0206456. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.S.M.; Tabak, A.F.; Hamed, Y.; Mitwally, M.E.; Tawakol, M.; Klingner, A.; Sitti, M. Swimming Back and Forth Using Planar Flagellar Propulsion at Low Reynolds Numbers. Adv. Sci. 2018, 5, 1700461. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.S.; Pacey, A.A. Sperm transport in the female reproductive tract. Hum. Reprod. Update 2005, 12, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Schuster, T.G.; Cho, B.; Keller, L.M.; Takayama, S.; Smith, G.D. Isolation of motile spermatozoa from semen samples using microfluidics. Reprod. Biomed. Online 2003, 7, 75–81. [Google Scholar] [CrossRef]
- Cho, B.S.; Schuster, T.G.; Zhu, X.; Chang, D.; Smith, G.D.; Takayama, S. Passively Driven Integrated Microfluidic System for Separation of Motile Sperm. Anal. Chem. 2003, 75, 1671–1675. [Google Scholar] [CrossRef]
- Zhang, B.; Yin, T.L.; Yang, J. A novel microfluidic device for selecting human sperm to increase the proportion of morphologically normal, motile sperm with uncompromised DNA integrity. Anal. Methods 2015, 7, 5981–5988. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, R.-R.; Yin, T.; Zou, W.; Tang, Y.; Ding, J.; Yang, J. Generation of Gradients on a Microfluidic Device: Toward a High-Throughput Investigation of Spermatozoa Chemotaxis. PLoS ONE 2015, 10, e0142555. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, R.; Vollmer, M.; Eamer, L.; San Gabriel, M.C.; Zeidan, K.; Zini, A.; Sinton, D. Rapid selection of sperm with high DNA integrity. Lab Chip 2014, 14, 1142–1150. [Google Scholar] [CrossRef]
- Eamer, L.; Vollmer, M.; Nosrati, R.; San Gabriel, M.C.; Zeidan, K.; Zini, A.; Sinton, D. Turning the corner in fertility: High DNA integrity of boundary-following sperm. Lab Chip 2016, 16, 2418–2422. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, R.; Driouchi, A.; Yip, C.M.; Sinton, D. Two-dimensional slither swimming of sperm within a micrometre of a surface. Nat. Commun. 2015, 6, 8703. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, R.; Graham, P.J.; Liu, Q.; Sinton, D. Predominance of sperm motion in corners. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Tasoglu, S.; Safaee, H.; Zhang, X.; Kingsley, J.L.; Catalano, P.N.; Gurkan, U.A.; Nureddin, A.; Kayaalp, E.; Anchan, R.M.; Maas, R.L.; et al. Exhaustion of Racing Sperm in Nature-Mimicking Microfluidic Channels During Sorting. Small 2013, 9, 3374–3384. [Google Scholar] [CrossRef]
- Asghar, W.; Velasco, V.; Kingsley, J.L.; Shoukat, M.S.; Shafiee, H.; Anchan, R.M.; Mutter, G.L.; Tüzel, E.; Demirci, U. Selection of Functional Human Sperm with Higher DNA Integrity and Fewer Reactive Oxygen Species. Adv. Healthc. Mater. 2014, 3, 1671–1679. [Google Scholar] [CrossRef]
- Chinnasamy, T.; Kingsley, J.L.; Inci, F.; Turek, P.J.; Rosen, M.P.; Behr, B.; Tüzel, E.; Demirci, U. Guidance and Self-Sorting of Active Swimmers: 3D Periodic Arrays Increase Persistence Length of Human Sperm Selecting for the Fittest. Adv. Sci. 2018, 5, 1700531. [Google Scholar] [CrossRef]
- Kantsler, V.; Dunkel, J.; Goldstein, R.E. Surface Interactions in Suspensions of Swimming Cells. Biophys. J. 2014, 106, 210a. [Google Scholar] [CrossRef]
- Kantsler, V.; Dunkel, J.; Blayney, M.; Goldstein, R.E. Rheotaxis facilitates upstream navigation of mammalian sperm cells. eLife 2014, 3, e02403. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Meriano, J.; Ru, C.; Xie, S.; Luo, J.; Sun, Y. Human sperm rheotaxis: A passive physical process. Sci. Rep. 2016, 6, 23553. [Google Scholar] [CrossRef] [PubMed]
- Woolley, D.M. Motility of spermatozoa at surfaces. Reproduction 2003, 126, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Kruger, R.A. Photoacoustic ultrasound. Med. Phys. 1994, 21, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.; Schwarz, M.; Soliman, D.; Symvoulidis, P.; Ntziachristos, V. Pushing the Optical Imaging Limits of Cancer with Multi-Frequency-Band Raster-Scan Optoacoustic Mesoscopy (RSOM). Neoplasia 2015, 17, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Neuschmelting, V.; Lockau, H.; Ntziachristos, V.; Grimm, J.; Kircher, M.F. Lymph Node Micrometastases and In-Transit Metastases from Melanoma: In Vivo Detection with Multispectral Optoacoustic Imaging in a Mouse Model. Radiology 2016, 280, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.S.M.; Ferreira, P.; Eleutério, R.; Korte, C.L.d.; Misra, S. Magnetic-based closed-loop control of paramagnetic microparticles using ultrasound feedback. In Proceedings of the 2014 IEEE International Conference on Robotics and Automation (ICRA), Hong Kong, China, 31 May–7 June 2014; pp. 3807–3812. [Google Scholar]
- Sánchez, A.; Magdanz, V.; Schmidt, O.G.; Misra, S. Magnetic control of self-propelled microjets under ultrasound image guidance. In Proceedings of the 5th IEEE RAS/EMBS International Conference on Biomedical Robotics and Biomechatronics, Sao Paulo, Brazil, 12–15 August 2014; pp. 169–174. [Google Scholar]
- Weissleder, R.; Moore, A.; Mahmood, U.; Bhorade, R.; Benveniste, H.; Chiocca, E.A.; Basilion, J.P. In vivo magnetic resonance imaging of transgene expression. Nat. Med. 2000, 6, 351–354. [Google Scholar] [CrossRef]
- Lee, S.-C.; Kim, K.; Kim, J.; Lee, S.; Han Yi, J.; Woo Kim, S.; Ha, K.-S.; Cheong, C. One Micrometer Resolution NMR Microscopy. J. Magn. Reson. 2001, 150, 207–213. [Google Scholar] [CrossRef]
- Uecker, M.; Zhang, S.; Voit, D.; Karaus, A.; Merboldt, K.-D.; Frahm, J. Real-time MRI at a resolution of 20 ms. NMR Biomed. 2010, 23, 986–994. [Google Scholar] [CrossRef]
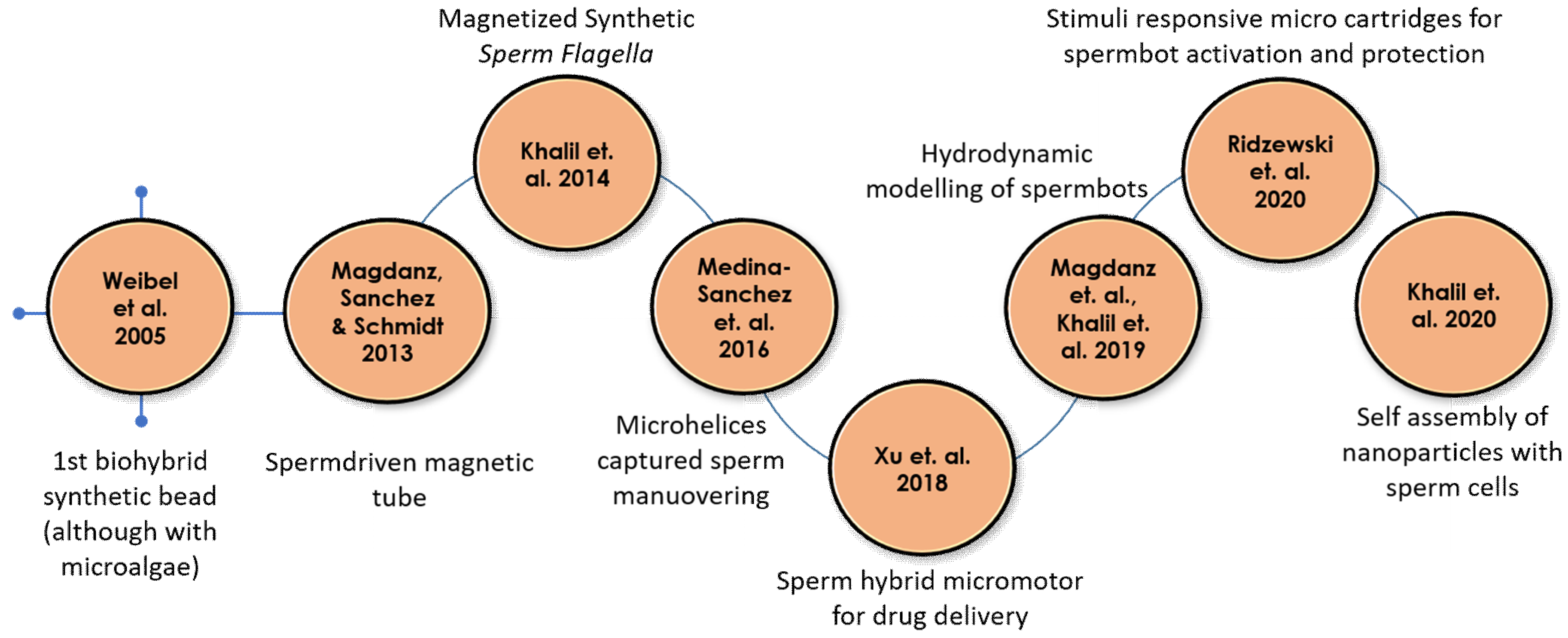


| Year | Authors | Motor Type | Load Type | Reference |
|---|---|---|---|---|
| 2005 | Dreyfus et al. | Artificial flagella | Red blood cells | [25] |
| 2005 | Weibel et al. | Algae | Polystyrene beads | [26] |
| 2013 | Magdanz, Sanchez & Schmidt | Spermatozoa | Magnetic microtubes | [27] |
| 2014 | Khalil et al. | Sperm shaped synthetic magnetic microbot | - | [28,29] |
| 2016 | Medina-Sanchez et al. | Magnetic microhelices | Low motility spermatozoa | [30] |
| 2018 | Xu et al. | Spermatozoa | Doxorubicin hydrochloride (Anti-cancer drug) | [31] |
| 2019 | Magdanz et al. Khalil et al. | Spermatozoa | - | [32,33,34,35] |
| 2020 | Ridzewski et al. | Spermatozoa | Gelatin microtubes | [36] |
| 2020 | Xu et al. | Spermatozoa with a streamlined-horned cap | Heparin-loaded liposomes (Anticoagulant) | [37] |
| 2020 | Khalil et al. | Spermatozoa | Magnetic nanoparticles | [38] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.V.; Ansari, M.H.D.; Mahajan, M.; Srivastava, S.; Kashyap, S.; Dwivedi, P.; Pandit, V.; Katha, U. Sperm Cell Driven Microrobots—Emerging Opportunities and Challenges for Biologically Inspired Robotic Design. Micromachines 2020, 11, 448. https://doi.org/10.3390/mi11040448
Singh AV, Ansari MHD, Mahajan M, Srivastava S, Kashyap S, Dwivedi P, Pandit V, Katha U. Sperm Cell Driven Microrobots—Emerging Opportunities and Challenges for Biologically Inspired Robotic Design. Micromachines. 2020; 11(4):448. https://doi.org/10.3390/mi11040448
Chicago/Turabian StyleSingh, Ajay Vikram, Mohammad Hasan Dad Ansari, Mihir Mahajan, Shubhangi Srivastava, Shubham Kashyap, Prajjwal Dwivedi, Vaibhav Pandit, and Uma Katha. 2020. "Sperm Cell Driven Microrobots—Emerging Opportunities and Challenges for Biologically Inspired Robotic Design" Micromachines 11, no. 4: 448. https://doi.org/10.3390/mi11040448
APA StyleSingh, A. V., Ansari, M. H. D., Mahajan, M., Srivastava, S., Kashyap, S., Dwivedi, P., Pandit, V., & Katha, U. (2020). Sperm Cell Driven Microrobots—Emerging Opportunities and Challenges for Biologically Inspired Robotic Design. Micromachines, 11(4), 448. https://doi.org/10.3390/mi11040448







