Electrospun Fibers and Sorbents as a Possible Basis for Effective Composite Wound Dressings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Sorbent Preparation
2.2.2. Electrospinning
2.2.3. Fibers Characterization
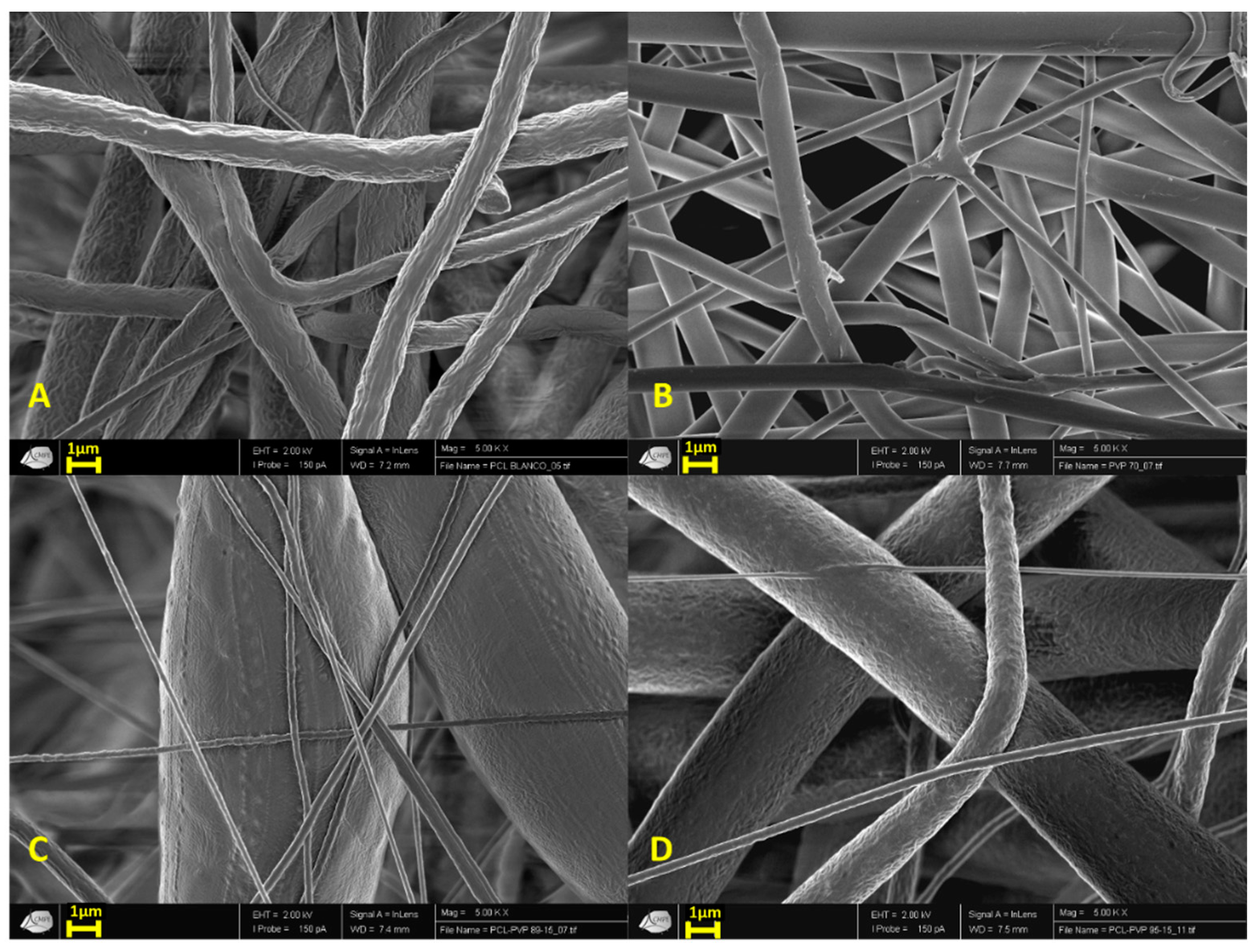
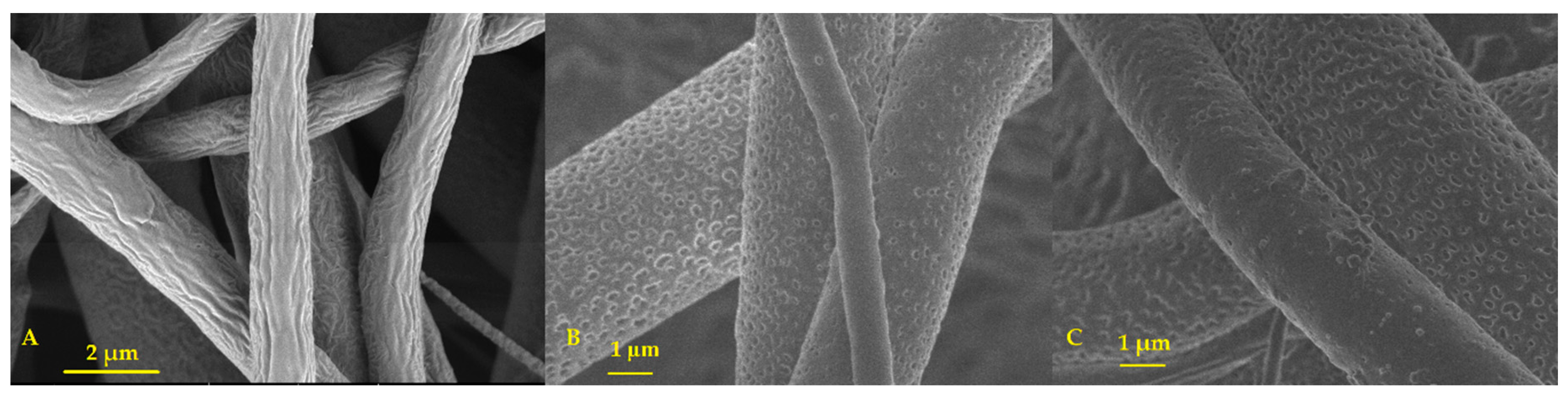
Scanning Electron Microscopy (SEM)
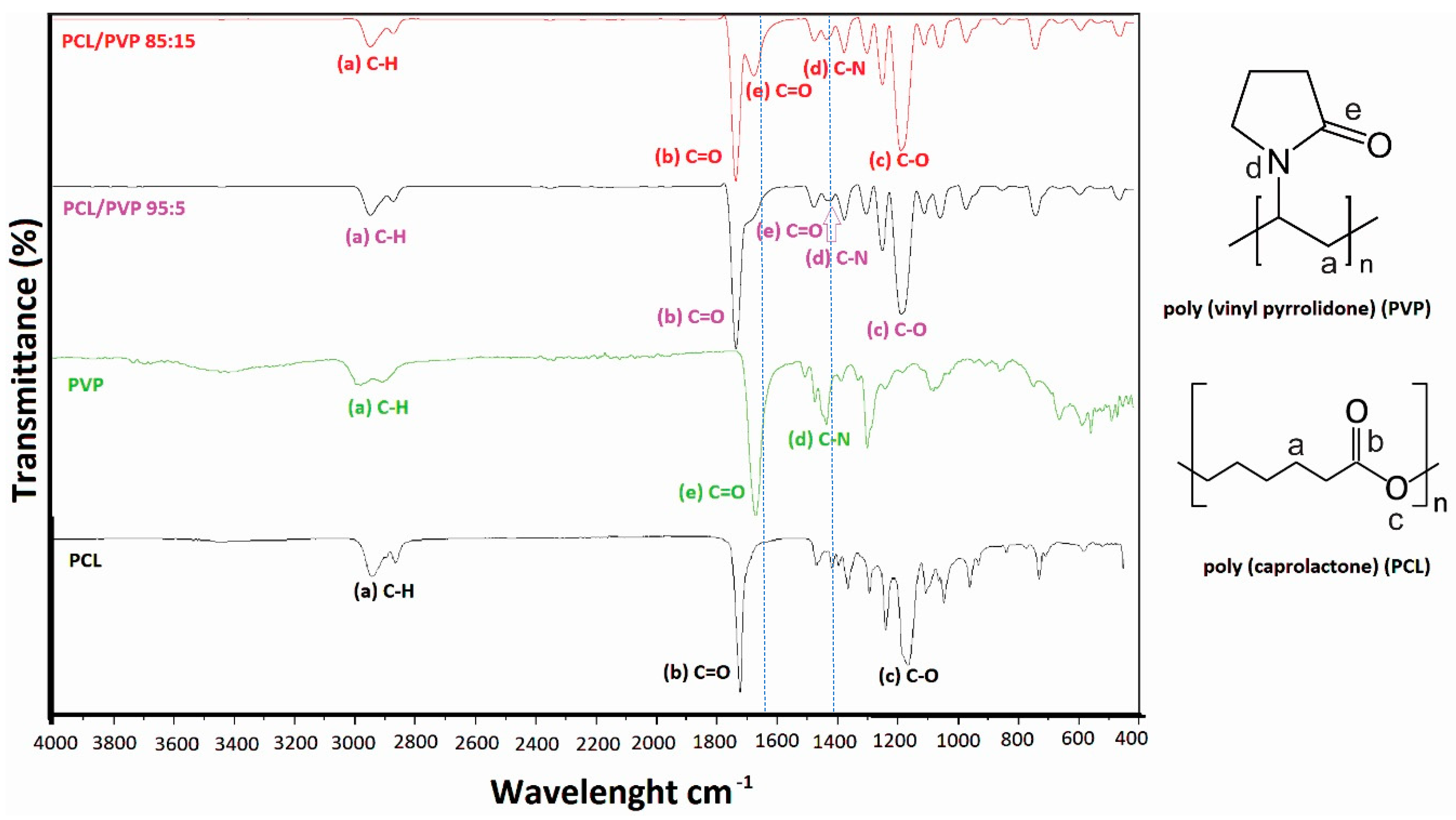
Fourier Transform Infrared Spectroscopy (FTIR)
Differential Scattering Calorimetry (DSC)
PVP Dissolution Test
Macro-Tensile Measurement on PCL/PVP Fiber Scaffolds
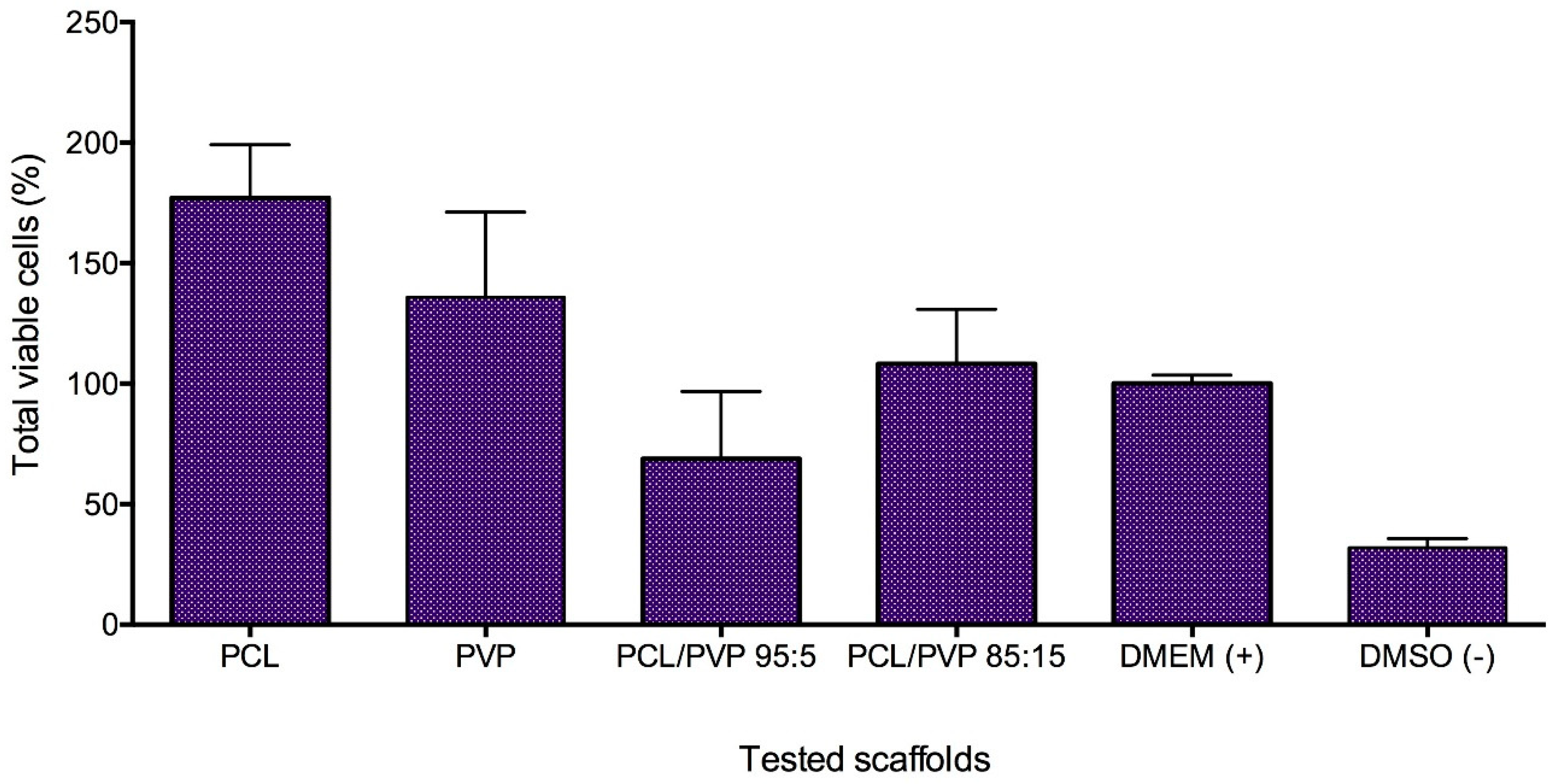
In Vitro HFF-1 Cytotoxicity Test
2.2.4. Sorbents Characterization
Optical Emission Spectroscopy (ICP-OES)
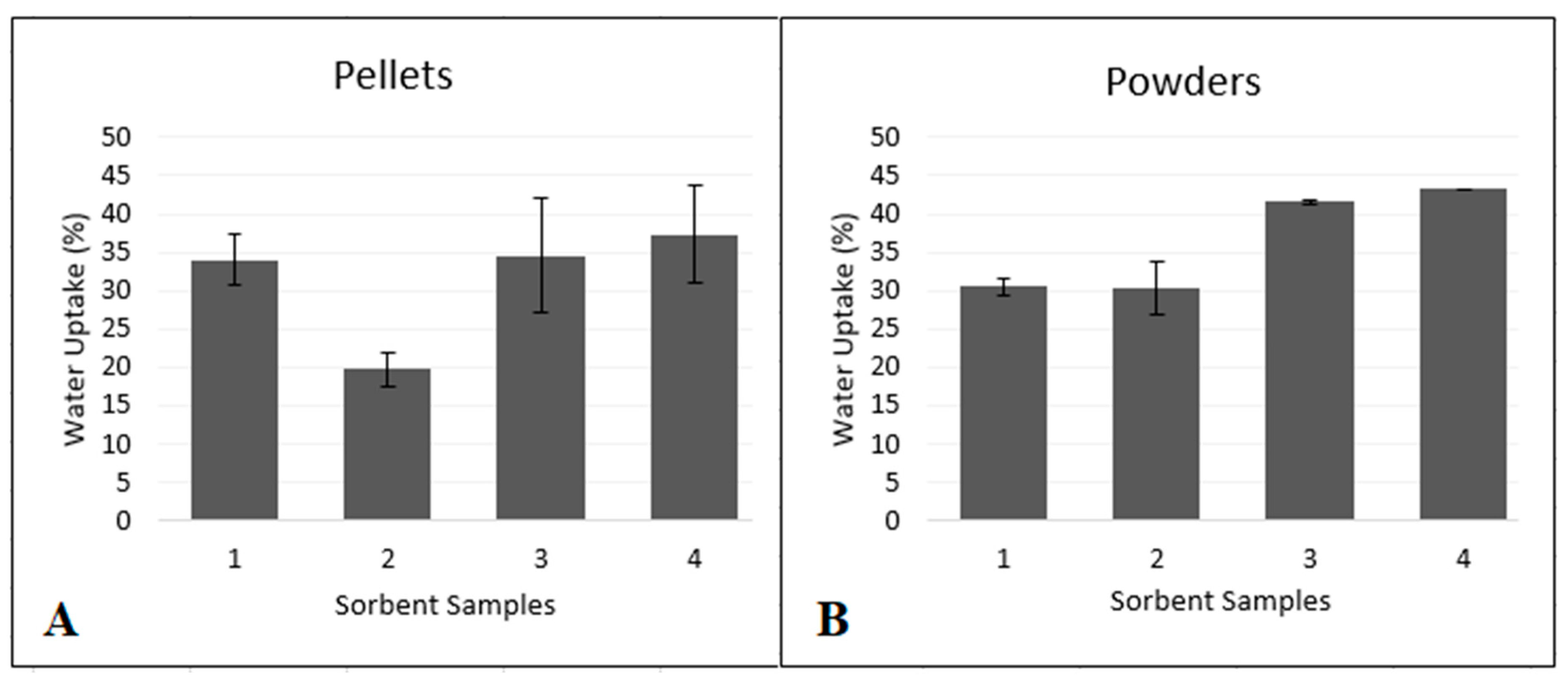
Water Uptake Test
2.2.5. Dressings Assembly
2.2.6. In Vitro Antimicrobial Test
Antibacterial Test
Antifungal Test
Statistical Analysis
3. Results
3.1. Fibers Characterization
3.1.1. Scanning Electron Microscopy (SEM)
3.1.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.1.3. Differential Scattering Calorimetry (DSC)
3.1.4. PVP Dissolution Test
3.1.5. Macro-Tensile Measurement on PCL/PVP Fiber Scaffolds
3.1.6. In Vitro HFF-1 Cytotoxicity Test
3.2. Sorbents Characterization
Water Uptake Test
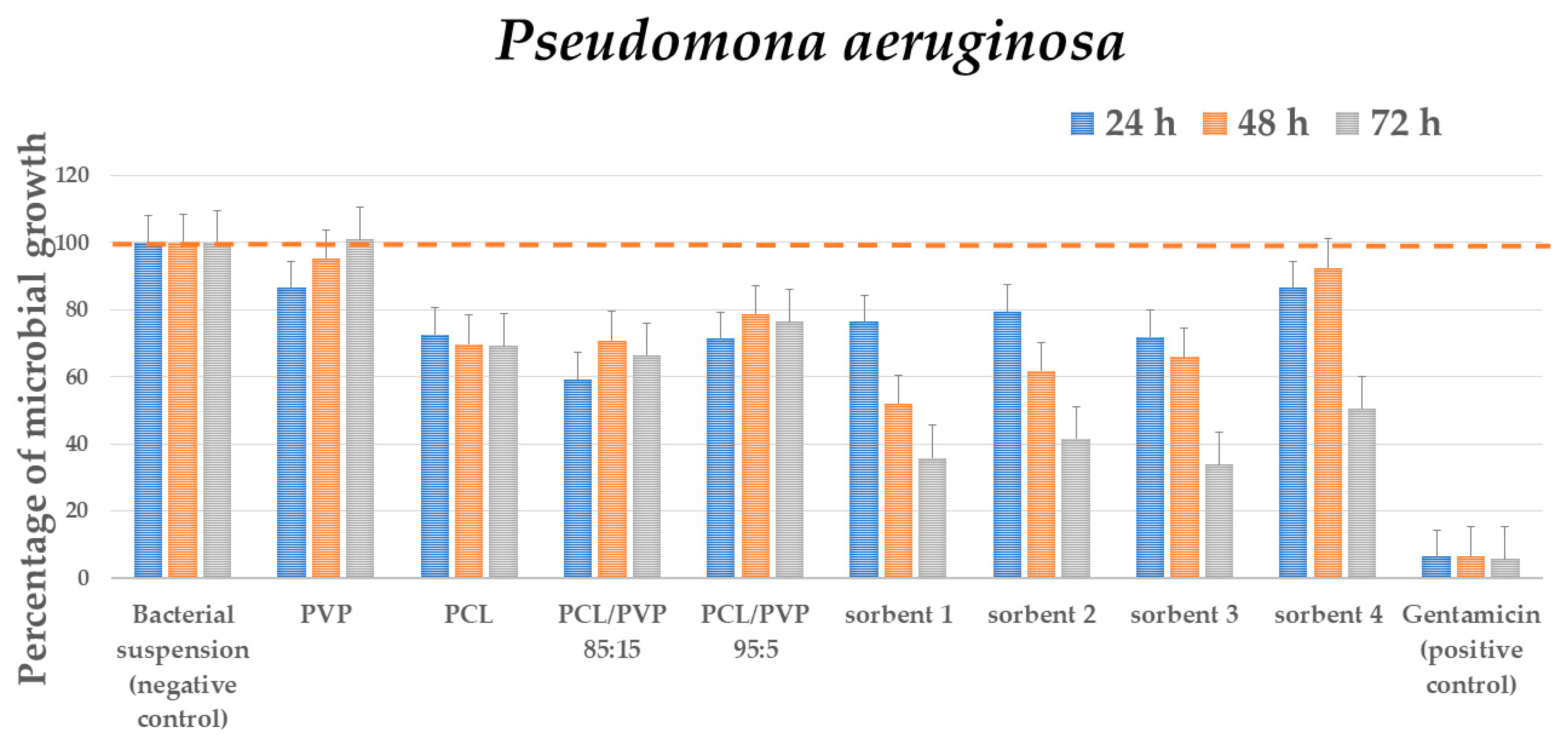
3.3. Antimicrobials Tests
3.3.1. Antibacterial Test
3.3.2. Antifungal Test
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mir, M.; Ali, M.N.; Barakullah, A.; Gulzar, A.; Arshad, M.; Fatima, S.; Asad, M. Synthetic polymeric biomaterials for wound healing: A review. Prog. Biomater. 2018, 7, 1–21. [Google Scholar] [CrossRef]
- Loke, W.-K.; Lau, S.-K.; Yong, L.L.; Khor, E.; Sum, C.K. Wound dressing with sustained anti-microbial capability. J. Biomed. Mater. Res. 2002, 53, 8–17. [Google Scholar] [CrossRef]
- Romanelli, M.; Vowden, K.; Weir, D. Exudate management made easy. Wounds Int. 2010, 1, 1–6. [Google Scholar]
- Expert Working Group; Satellite Expert Working Group. Wound exudate and the role of dressings. A consensus document. Int. Wound J. 2008, 5 (Suppl. 1), 3–12. [Google Scholar] [CrossRef]
- Cutting, K.F. Wound exudate: Composition and functions. Br. J. Community Nurs. 2003, 8 (Suppl. 3), S4–S9. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Harries, R.L.; Bosanquet, D.C.; Harding, K.G. Wound bed preparation: TIME for an update. Int. Wound J. 2016, 13 (Suppl. 3), 8–14. [Google Scholar] [CrossRef]
- Moore, R.A.; Liedl, D.A.; Jenkins, S.; Andrews, K.L. Using a silver-coated polymeric substrate for the management of chronic ulcerations: The initial mayo clinic experience. Adv. Skin Wound Care 2008, 21, 517–520. [Google Scholar] [CrossRef]
- Miguel, S.P.; Figueira, D.R.; Simões, D.; Ribeiro, M.P.; Coutinho, P.; Ferreira, P.; Correia, I.J. Electrospun polymeric nanofibres as wound dressings: A review. Colloids Surf. B 2018, 169, 60–71. [Google Scholar] [CrossRef]
- Narayanan, N.; Kuang, L.; Del Ponte, M.; Chain, C.; Deng, M. Design and fabrication of nanocomposites for musculoskeletal tissue regeneration. Nanocompos. Musculoskelet. Tissue Regen. 2016, 1, 3–29. [Google Scholar] [CrossRef]
- Ragaert, K.; Cardon, L. Bulk mechanical properties of thermoplastic poly-ε-caprolactone. J. Mater. Sci. Mater. Med. 2009, 20, 1255–1262. [Google Scholar]
- Eshraghi, S.; Das, S. Mechanical and microstructural properties of polycaprolactone scaffolds with one-dimensional, two-dimensional, and three-dimensional orthogonally oriented porous architectures produced by selective laser sintering. Acta Biomater. 2010, 6, 2467–2476. [Google Scholar] [CrossRef] [PubMed]
- Christen, M.O.; Vercesi, F. Polycaprolactone: How a well-known and futuristic polymer has become an innovative collagen-stimulator in esthetics. Clin. Cosmet. Investig. Dermatol. 2020, 13, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Wilikinson, L.J.; White, R.J.; Chipman, J.K. Silver, and nanoparticles of silver in wound dressing: A review of efficacy and safety. J. Wound Care 2011, 20, 543–549. [Google Scholar] [CrossRef]
- Norman, G.; Westby, M.J.; Rithalia, A.D.; Stubbs, N.; Soares, M.O.; Dumville, J.C. Dressings and topical agents for treating venous leg ulcers. Cochrane Database Syst. Rev. 2018, 6, CD012583. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, H.; Yang, S.; Xi, Z.; Tang, T.; Yin, R.; Zhang, W. Electrospun PLGA membrane incorporated with andrographolide-loaded mesoporous silica nanoparticles for sustained antibacterial wound dressing. Nanomedicine (Lond.) 2018, 13, 2881–2899. [Google Scholar] [CrossRef]
- Yang, S.; Han, X.; Jia, Y.; Zhang, H.; Tang, T. Hydroxypropyltrimethyl ammonium chloride chitosan functionalized-PLGA electrospun fibrous membranes as antibacterial wound dressing: In vitro and in vivo evaluation. Polymers (Basel) 2017, 9, 697. [Google Scholar] [CrossRef]
- Hadrup, N.; Sharma, A.K.; Loeschner, K. Toxicity of silver ions, metallic silver and silver nanoparticles materials after in vivo dermal and mucosal surface exposure: A review. Regul. Toxicol. Pharmacol. 2018, 98, 257–267. [Google Scholar] [CrossRef]
- Najabat Ali, M.; Ansari, U.; Sami, J.; Qayyum, F.; Mir, M. To develop a biocompatible and biodegradable polymer-metal composite with good; mechanical and drug release properties. J. Mater. Sci. Eng. 2016, 5, 274. [Google Scholar] [CrossRef]
- Podkopaev, D.O.; Shaburova, L.N.; Balandin, G.V.; Kraineva, O.V.; Labutina, N.V.; Suvorov, O.A.; Sidorenko, Y.I. Comparative evaluation of antimicrobial activity of silver nanoparticles. Nanotechnol. Russ. 2014, 9, 93–97. [Google Scholar] [CrossRef]
- Vazquez-Muñoz, R.; Avalos-Borja, M.; Castro-Longoria, E. Ultrastructural analysis of Candida albicans when exposed to silver nanoparticles. PLoS ONE 2014, 9, e108876. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ruiz, B.; Arellano-García, M.E.; Radilla-Chávez, P.; Salas-Vargas, D.S.; Toledano-Magaña, Y.; Casillas-Figueroa, F.; Vazquez-Gomez, R.L.; Pestryakov, A.; García-Ramos, J.C.; Bogdanchikova, N. The cytokinesis-block micronucleus assay using human lymphocytes as sensitive tool for AgNPs cytotoxicity/genotoxicity evaluation. ACS Omega 2020, in press. [Google Scholar]
- Semenov, F.V.; Fidarova, K.M. The treatment of the patients presenting with chronic inflammation of the trepanation cavity with a preparation containing silver nanoparticles following sanitation surgery of the open type. Vestn. Otorinolaringol. 2012, 6, 117–119. [Google Scholar]
- Burmistrov, V.A.; Pestryakov, A.N.; Odegova, G.V.; Burmistrov, I.V.; Burmistrov, A.V.; Bogdanchikova, N.E. Complex Drug to Prevent and Treat Intestinal Infections. RU Patent 2,519,659, 27 September 2014. [Google Scholar]
- Burmistrov, V.A.; Pestryakov, A.N.; Odegova, G.V.; Burmistrov, I.V.; Burmistrov, A.V.; Bogdanchikova, N.E. Method of Prevention and Treatment of Gastritis, Stomach and Duodenal Ulcer. RU Patent 2,549,975, 23 June 2015. [Google Scholar]
- Blagitko, E.M.; Rodionov, P.P.; Bugaichewnko, N.V.; Shorina, G.N.; Ilina, V.N.; Minina, A.V.; Mikhailov, Y.I.; Burmistrov, V.A.; Odegova, G.V.; Polunina, O.A.; et al. Mean Argovit for Treating the Infected Wounds. RU Patent 2,245,151, 27 January 2005. [Google Scholar]
- Voronzova, N.A.; Rodionov, P.P.; Burmistrov, V.A.; Blagitko, E.M.; Mikhailov, Y.I.; Odegova, G.V.; Bogdanchikova, N.E.; Avalos, M.B.; Polunina, O.A.; Mikhailov, K.Y.; et al. Method of Treatment of Ear, Throat and Nose Diseases. RU Patent 2,307,657, 10 October 2007. [Google Scholar]
- Velasco Barraza, R.D.; Álvarez Suarez, A.S.; Villarreal Gómez, L.J.; Paz González, J.A.; Iglesias, A.L.; Vera Graziano, R. Designing a low-cost electrospinning device for practical learning in a Bioengineering Biomaterials course. Rev. Mex. Ing. Biomed. 2016, 37, 27–36. [Google Scholar] [CrossRef]
- Velasco-Barraza, R.D.; Vera-Graziano, R.; López-Maldonado, E.A.; Oropeza, M.; Dastager, S.; Álvarez-Andrade, A.; Iglesias, A.I.; Villarreal-Gómez, L.J. Study of nanofiber scaffolds of PAA, PAA/CS, and PAA/ALG for its potential use in biotechnological applications. Int. J. Polym. Mater. 2017. [Google Scholar] [CrossRef]
- Villarreal-Gómez, L.J.; Vera-Graziano, R.; Vega-Ríos, M.R.; Pineda-Camacho, J.L.; Almanza, H.; Mier-Maldonado, P.A.; Cornejo-Bravo, J.M. Biocompatibility evaluation of electrospun scaffolds of poly (L-Lactide) with pure and grafted hydroxyapatite. J. Mex. Chem. Soc. 2014, 58, 435–443. [Google Scholar]
- Villarreal-Gómez, L.J.; Vera-Graziano, R.; Vega-Ríos, M.R.; Pineda-Camacho, J.L.; Mier-Maldonado, P.A.; Almanza-Reyes, H.; Bravo, J.M.C. In vivo biocompatibility of dental scaffolds for tissue regeneration. Adv. Mater. Res. 2014, 976, 191–195. [Google Scholar] [CrossRef]
- Villarreal-Gómez, L.J.; Cornejo-Bravo, J.M.; Vera-Graziano, R.; Grande, D. Electrospinning as a powerful technique for biomedical applications: A critically selected survey. J. Biomater. Sci. Polym. Ed. 2016, 27, 157–176. [Google Scholar] [CrossRef]
- Torres-Martinez, E.J.; Cornejo-Bravo, J.M.; Serrano-Medina, A.; Villarreal-Gomez, L.J. A Summary of electrospun nanofibers as drug delivery system: Drugs loaded and biopolymers used as matrices. Curr. Drug. Deliv. 2018, 15, 1–16. [Google Scholar] [CrossRef]
- Cornejo-Bravo, J.M.; Villarreal-Gómez, L.J.; Serrano-Medina, A. Electrospinning for drug delivery systems: Drug incorporation techniques. In Techniques, and Biomedical Applications; Chapter 7; Haider, S., Ed.; InTech: Rijeka, Croatia, 2016. [Google Scholar] [CrossRef]
- Villarreal-Gómez, L.J.; Serrano-Medina, A.; Torres-Martínez, E.J.; Pérez-González, G.L.; Cornejo-Bravo, J.M. Polymeric advanced delivery systems for antineoplastic drugs: Doxorubicin and 5-fluorouracil. e-Polymers 2018, 18, 359–372. [Google Scholar] [CrossRef]
- Li, X.; Kanjwal, M.A.; Lin, L.; Chronakis, I.S. Electrospun poly vinyl-alcohol nanofibers as oral fast-dissolving delivery system of caffeine and riboflavin. Colloids Surf. B Biointerfaces 2013, 103, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Uyar, T. Fast dissolving oral drug delivery system based on electrospun nano fi brous webs of cyclodextrin/ibuprofen inclusion complex nanofibers. Mol. Pharm. 2019, 16, 4387–4398. [Google Scholar] [CrossRef]
- ASTM D882-10. Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar] [CrossRef]
- Ong, S.-Y.; Wu, J.; Moochhala, S.M.; Tan, M.-H.; Lu, J. Development of a chitosan-based wound dressing with improved haemostatic and antimicrobial properties. Biomaterials 2008, 29, 4323–4332. [Google Scholar] [CrossRef] [PubMed]
- Mizuma, T.; Kiyokawa, Y.; Wakai, Y. Water absorption characteristics and structural properties of rice for sake brewing. J. Biosci. Bioeng. 2008, 106, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Rachkovskaya, L.N.; Popova, T.V.; Letyagin, A.; Tolstikova, T.G.; Korolyov, M.A.; Bogdanchikova, N.; Pestryakov, A.; Kotlyarova, A.; Burmistrov, V.; Konenkov, V. Silver containing sorbents: Physicochemical and biological properties. Resour. Technol. 2016, 2, 43–49. [Google Scholar] [CrossRef]
- Dai, M.; Jin, S.; Nugen, S.R. Water-soluble electrospun nanofibers as a method for on-chip reagent storage. Biosensors (Basel) 2012, 2, 388–395. [Google Scholar] [CrossRef]
- Chadha, R.; Kapoor, V.K.; Kumar, A. Analytical techniques used to characterize drug-polyvinylpyrrolidone systems in solid and liquid states—An overview. J. Sci. Ind. Res. 2006, 65, 459–469. [Google Scholar]
- D’Amelia, R.P.; Gentile, S.; Nirode, W.F.; Huang, L. Quantitative analysis of copolymers and blends of Polyvinyl Acetate (PVAc) using Fourier Transform Infrared Spectroscopy (FTIR) and Elemental Analysis (EA). World J. Chem. Educ. 2016, 4, 25–31. [Google Scholar] [CrossRef]
- Yuan, Y.; Lee, T.R. Contact angle, and wetting properties. In Surface Science Techniques; Springer Series in Surface Sciences; Bracco, G., Holst, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 51. [Google Scholar] [CrossRef]
- Franca, D.C.; Bezerra, E.B.; de Morais, D.D.S.; Araujo, E.M.; Wellen, R.M.R. Hydrolytic and thermal degradation of PCL and PCL/bentonite compounds. Mater. Res. 2016, 19, 618–627. [Google Scholar] [CrossRef]
- Peh, K.; Khan, T.; Ch’ng, H. Mechanical, bioadhesive strength and biological evaluations of chitosan films for wound dressing. J. Pharm. Pharm. Sci. 2000, 3, 303–311. [Google Scholar]
- Beachley, V.; Wen, X. Effect of electrospinning parameters on the nanofiber diameter and length. Mater. Sci. Eng. C Mater. Biol. Appl. 2009, 29, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Rho, J.; Shin, J.Y.; Lee, D.Y.; Hwang, T.; Kim, K.J. Mechanical properties and cytotoxicity of PLA/PCL films. Biomed. Eng. Lett. 2018, 8, 267–272. [Google Scholar] [CrossRef] [PubMed]
- de Espinosa, L.M.; Meesorn, W.; Moatsou, D.; Weder, C. Bioinspired polymer systems with stimuli-responsive mechanical properties. Chem. Rev. 2017, 117, 12851–12892. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.K.; Gangwar, M.; Mondal, S.C.; Jana, S. Protective effects of tetrahydrocurcumin (THC) on fibroblast and melanoma cell lines in vitro: It’s implication for wound healing. J. Food Sci. Technol. 2017, 54, 1137–1145. [Google Scholar] [CrossRef]
- Rahman, M.S.; Islam, R.; Rana, M.M.; Spitzhorn, L.-S.; Rahman, M.S.; Adjaye, J.; Asaduzzaman, S.M. Characterization of burn wound healing gel prepared from human amniotic membrane and Aloe vera extract. BMC Complement. Altern. Med. 2019, 19, 115. [Google Scholar] [CrossRef]
- Ankawi, G.; Fan, W.; Pomarè-Montin, D.; Lorenzin, A.; Neri, M.; Caprara, C.; de Cal, M.; Ronco, C. A new series of sorbent devices for multiple clinical purposes: Current evidence and future directions. Blood Purif. 2019, 47, 94–100. [Google Scholar] [CrossRef]
- Pomarè-Montin, D.; Ankawi, G.; Lorenzin, A.; Neri, M.; Caprara, C.; Ronco, C. Biocompatibility and cytotoxic evaluation of new sorbent cartridges for blood hemoperfusion. Blood Purif. 2018, 46, 187–195. [Google Scholar] [CrossRef]
- Cipoletti, J.J. Resin technology in medicine. In Sorbents and Their Clinical Applications; Cipoletti, J.J., Kunin, R., Meyer, F., Eds.; Academic Press: New York, NY, USA, 1980; pp. 221–248. [Google Scholar]
- Maltsev, V.; Smagin, A.; Rachkovskaya, L.; Rachkovsky, E.; Nimaev, V.; Yastrebova, E.; Volodin, A.; Korolev, M.; Vedyagin, A.; Shurlygina, A.; et al. Carbon-mineral carriers for active pharmaceutical substance. In Proceedings of the 2019 International Multi-Conference on Engineering, Computer and Information Sciences (SIBIRCON), Novosibirsk, Russia, 21–22 October 2019. [Google Scholar]
- Lykov, A.; Rachkovskaya, L.; Poveshchenko, O.; Surovtseva, A.; Kim, I.; Bondarenko, N.; Yankaite, E.; Rachkovsky, E.; Volodin, A.; Konenkov, V. Single wall carbon nanotubes functionalized with composition of gamma-aluminum oxide and polydimethylsiloxane properties. In Proceedings of the 2019 International Multi-Conference on Engineering, Computer and Information Sciences (SIBIRCON), Novosibirsk, Russia, 21–22 October 2019. [Google Scholar]
- Madonov, P.; Rachkovskaya, L.; Michurina, S.; Robinson, M.; Rachkovsky, E.; Ishchenko, I.; Lykov, A.; Shurlygina, A.; Poveshchenko, O.; Popova, T. Aluminum and silica containing porous carrier for active pharmaceutical ingredients. In Proceedings of the 2019 International Multi-Conference on Engineering, Computer and Information Sciences (SIBIRCON), Novosibirsk, Russia, 21–22 October 2019. [Google Scholar]
- Sheianov, S.D.; Ul’chenko, V.; Shashkov, B.V. The treatment of soft-tissue wounds by using sorbent bandages. Voen. Med. Zh. 1993, 8, 31–35; 79–80. [Google Scholar]
- Zykova, A.K.; Pantyukhov, P.V.; Kolesnikova, N.N.; Popov, A.A.; Olkhov, A.A. Influence of particle size on water absorption capacity and mechanical properties of polyethylene-wood flour composites. AIP Conf. Proc. 2015, 1683, 20242. [Google Scholar]
- Greg, S.; Singh, K. Adsorption, Specific Surface, Porosity; Mir: Moscow, Russia, 1984; p. 306. [Google Scholar]
- Tiwari, U.; Cummins, E. Functional and Physicochemical Properties of Legume Fibers; Tiwari, B.K., Gowen, A., McKenna, B.B.T., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 121–156. [Google Scholar]
- Patrício, T.; Domingos, M.; Gloria, A.; Bártolo, P. Characterisation of PCL and PCL/PLA scaffolds for tissue engineering. Procedia CIRP 2013, 5, 110–114. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.H.; Cho, Y.S. Analysis of degradation rate for dimensionless surface area of well-interconnected PCL scaffold via in-vitro accelerated degradation experiment. Tissue Eng. Regen. Med. 2014, 11, 446–452. [Google Scholar] [CrossRef]
- Díaz, E.; Sandonis, I.; Valle, M.B. In vitro degradation of Poly (caprolactone)/nHA composites. J. Nanomater. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Mim, L.M.; Sultana, N. Comparison on in vitro degradation of polycaprolactone and polycaprolactone/gelatin nanofibrous scaffold. Malays. J. Anal. Sci. 2017, 21, 627–632. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-M.; Le, K.H.T.; Giannitelli, S.M.; Lee, Y.J.; Rainer, A.; Trombetta, M. Electrospinning of PCL/PVP blends for tissue engineering scaffolds. J. Mater. Sci. Mater. Med. 2013, 24, 1425–1442. [Google Scholar] [CrossRef]
- Dini, V.; Salvo, P.; Janowska, A.; Di Francesco, F.; Barbini, A.; Romanelli, M. Correlation between wound temperatures obtained with an infrared camera and clinical wound bed score in venous leg ulcers. Wounds 2015, 27, 274–278. [Google Scholar]
- Salgado, C.L.; Sánchez, E.M.; Zavaglia, C.A.; Granja, P.L. Biocompatibility and biodegradation of polycaprolactone-sebacic acid blended gels. J. Biomed. Mater. Res. A 2012, 100, 243–251. [Google Scholar] [CrossRef]
- Walker, M.; Jones, S.; Parsons, D.; Booth, R.; Cochrane, C.; Bowler, P. Evaluation of low-adherent antimicrobial dressings. Wounds 2011, 7, 32–45. [Google Scholar]
- Renner, R.; Rogalski, C.; Friedlein, H.; Simon, J.C. Vacuum therapy in dermatology: A review. J. Dtsch. Dermatol. Ges. 2006, 4, 468–475. [Google Scholar] [CrossRef]
- Yannas, I.V. Tissue regeneration by use of collagen-glycosaminoglycan copolymers. Clin. Mater. 1992, 9, 179–187. [Google Scholar] [CrossRef]
- Jansen, J.A.; von Recum, A.F. Textured and porous materials. In Biomaterials Science: An Introduction to Materials and Medicine; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Elsevier Academic Press: London, UK, 2016; pp. 218–225. [Google Scholar] [CrossRef]
- Doneva, T.A.; Yina, H.B.; Stephens, P.; Bowena, W.R.; Thomas, D.W. Development and AFM study of porous scaffolds for wound healing applications. Spectroscopy 2004, 18, 587–596. [Google Scholar] [CrossRef][Green Version]
- Rnjak-Kovacina, J.; Wise, S.G.; Li, Z.; Maitz, P.K.M.; Young, C.J.; Wang, Y.; Weiss, A.S. Tailoring the porosity and pore size of electrospun synthetic human elastin scaffolds for dermal tissue engineering. Biomaterials 2011, 32, 6729–6736. [Google Scholar] [CrossRef] [PubMed]
- Rnjak-Kovacina, J.; Weiss, A.S. Increasing the pore size of electrospun scaffolds. Tissue Eng. Part B Rev. 2011, 17, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Croisier, F.; Duwez, A.S.; Jérôme, C.; Léonard, A.F.; van der Werf, K.O.; Dijkstra, P.J.; Bennink, M.L. Mechanical testing of electrospun PCL fibers. Acta Biomater. 2012, 8, 218–224. [Google Scholar] [CrossRef]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.-O.; Jafari, S.-H.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2010, 21, 77–95. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Zarghami, N.; Melat Yar, H.; Akbarzadeh, A. Nanofiber: Synthesis and biomedical applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Averous, L.; Moro, L.; Dole, P.; Fringant, C. Properties of thermoplastic blends: Starch-polycaprolactone. Polymer 2000, 41, 4157–4167. [Google Scholar] [CrossRef]
- Huang, S.; Zhou, L.; Li, M.C.; Wu, Q.; Kojima, Y.; Zhou, D. Preparation and properties of electrospun poly (vinyl pyrrolidone)/cellulose nanocrystal/silver nanoparticle composite fibers. Materials (Basel) 2016, 9, 523. [Google Scholar] [CrossRef]
- Pawlaczyk, M.; Lelonkiewicz, M.; Wieczorowski, M. Age-dependent biomechanical properties of the skin. Postepy Dermatol. Alergol. 2013, 30, 302–306. [Google Scholar] [CrossRef]
- Mohd Azam, N.A.N.; Amin, K.A.M. The physical and mechanical properties of gellan gum films incorporated manuka honey as wound dressing materials. IOP Conf. Ser. Mater. Sci. Eng. 2017, 209, 012027. [Google Scholar] [CrossRef]
- Mo, X.; Sun, B.; Wu, T.; Li, D. Electrospun nanofibers for tissue engineering. In Electrospinning: Nanofabrication and Applications (Micro and Nano Technologies); Chapter 24; Ding, B., Wang, X., Yu, J., Andrew, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 719–731. [Google Scholar] [CrossRef]
- Higa, O.Z.; Rogero, S.O.; Machado, L.D.B.; Mathor, M.B.; Lugão, A.B. Biocompatibility study for PVP wound dressing obtained in different conditions. Radiat. Phys. Chem. 1999, 55, 705–707. [Google Scholar] [CrossRef]
- Jimi, S.; Kimura, M.; De Francesco, F.; Riccio, M.; Hara, S.; Ohjimi, H. Acceleration mechanisms of skin wound healing by autologous micrograft in mice. Int. J. Mol. Sci. 2017, 18, 1675. [Google Scholar] [CrossRef] [PubMed]
- Borjigin, M.; Eskridge, C.; Niamat, R.; Strouse, B.; Bialk, P.; Kmiec, E. Electrospun fiber membranes enable proliferation of genetically modified cells. Int. J. Nanomed. 2013, 8, 855–864. [Google Scholar] [CrossRef]
- Sadeghi-Avalshahr, A.R.; Nokhasteh, S.; Molavi, A.M. Tailored PCL scaffolds as skin substitutes using sacrificial PVP fibers and collagen/chitosan blends. Int. J. Mol. Sci. 2020, 21, 2311. [Google Scholar] [CrossRef] [PubMed]
- Safaeijavan, R.; Soleimani, M.; Divsalar, A.; Eidi, A. Biological behavior study of gelatin coated PCL nanofiberous electrospun scaffolds using fibroblasts. J. Paramed. Sci. 2014, 5, 67–73. [Google Scholar] [CrossRef]
- Rogero, S.O.; Malmonge, S.M.; Lugão, A.B.; Ikeda, T.I.; Miyamaru, L.; Cruz, A.S. Biocompatibility study of polymeric biomaterials. Artif. Organs. 2003, 27, 424–427. [Google Scholar] [CrossRef]
- Sousa Dominguez, A.; Perez-Rodríguez, M.T.; Nodar, A.; Martinez-Lamas, L.; Perez-Landeiro, A.; Crespo Casal, M. Successful treatment of MDR Pseudomonas aeruginosa skin and soft-tissue infection with ceftolozane/tazobactam. J. Antimicrob Chemother. 2016, 72, 1262–1263. [Google Scholar] [CrossRef]
- Petkovsek, Z.; Elersic, K.; Gubina, M.; Zgur-Bertok, D.; Starcic Erjavec, M. Virulence potential of Escherichia coli isolates from skin and soft tissue infections. J. Clin. Microbiol. 2009, 47, 1811–1817. [Google Scholar] [CrossRef]
- McCaig, L.F.; McDonald, L.C.; Mandal, S.; Jernigan, D.B. Staphylococcus aureus-associated skin and soft tissue infections in ambulatory care. Emerg. Infect. Dis. 2006, 12, 1715–1723. [Google Scholar] [CrossRef]
- Kühbacher, A.; Burger-Kentischer, A.; Rupp, S. Interaction of Candida Species with the Skin. Microorganisms 2017, 5, 32. [Google Scholar] [CrossRef]
- Salević, A.; Prieto, C.; Cabedo, L.; Nedović, V.; Lagaron, J.M. Physicochemical, antioxidant and antimicrobial properties of electrospun poly(ε-caprolactone) films containing a solid dispersion of sage (Salvia officinalis L.) extract. Nanomaterials (Basel) 2019, 9, 270. [Google Scholar] [CrossRef]
- Kumar, S.; Bose, S.; Chatterjee, K. Amine-functionalized multiwall carbon nanotubes impart osteoinductive and bactericidal properties in poly (ε-caprolactone) composites. RSC Adv. 2014, 4, 19086–19098. [Google Scholar] [CrossRef]
- Sun, P.; Zhao, J.; Luo, Z.; Zhang, P.; Chen, P.; Zhang, X.; Luo, S.; Yang, D.; Tan, J.; Zhou, Y.; et al. Diluted povidone-iodine inhibits tumor growyh through apoptosis induction and suppresion of SOD activity. Oncol. Rep. 2012, 27, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Adomavičiūtė, E.; Stanys, S.; Žilius, M.; Juškaitė, V.; Pavilonis, A.; Briedis, V. formation and biopharmaceutical characterization of electrospun PVP mats with propolis and silver nanoparticles for fast releasing wound dressing. Biomed. Res. Int. 2016, 2016, 4648287. [Google Scholar] [CrossRef] [PubMed]
- Salman, J.A.S.; Al-Kadhemy, M.F.H.; Madhloom, S.A. Preparation of titanium dioxide nanoparticles and polyvinyl pyrrolidone polymer films as antibacterial, antibiofilm against pathogenic bacteria on different surfaces. Malays. J. Sci. 2017, 36, 132–140. [Google Scholar] [CrossRef][Green Version]
- Fiorelli, A.; Pentimalli, F.; D’Urso, V.; Di Marzo, D.; Forte, I.M.; Giordano, A.; Di Domenico, M.; Accardo, M.; Di Serio, U.; Santini, M. Antineoplastic activity of povidone-iodine on different mesothelioma cell lines: Results of in vitro study. Eur. J. Cardiothorac. Surg. 2014, 45, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Cedillo, E.; Ortega-Lara, W.; Rocha-Pizaña, M.R.; Gutierrez-Uribe, J.A.; Elías-Zúñiga, A.; Rodríguez, C. Electrospun polycaprolactone fibrous membranes containing Ag, TiO2 and Na2Ti6O13 particles for potential use in bone regeneration. Membranes (Basel) 2019, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Adeli-sardou, M.; Torkzadeh-Mahani, M.; Yaghoobi, M.M.; Dodel, M. Antibacterial and Anti-biofilm Investigation of Electrospun PCL/gelatin/Lawsone Nano Fiber Scaffolds against Biofilm Producing Bacteria. Biomacromol. J. 2018, 4, 46–57. [Google Scholar]
- Sadeghianmaryan, A.; Yazdanpanah, Z.; Soltani, Y.A.; Sardroud, H.A.; Nasirtabrizi, M.H.; Chen, X. Curcumin-loaded electrospun polycaprolactone/montmorillonite nanocomposite: Wound dressing application with anti-bacterial and low cell toxicity properties. J. Biomater. Sci. Polym. Ed. 2020, 31, 169–187. [Google Scholar] [CrossRef]
- Ruckh, T.T.; Oldinski, R.A.; Carroll, D.A.; Mikhova, K.; Bryers, J.D.; Popat, K.C. Antimicrobial effects of nanofiber poly(caprolactone) tissue scaffolds releasing rifampicin. J. Mater. Sci. Mater. Med. 2012, 23, 1411–1420. [Google Scholar] [CrossRef]
- Tartanson, M.-A.; Soussan, L.; Rivallin, M.; Pecastaings, S.; Chis, C.V.; Penaranda, D.; Roques, C.; Faura, C. Dynamic Mechanisms of the Bactericidal Action of an Al2O3-TiO2-Ag Granular Material on an Escherichia coli Strain. Appl. Environ. Microbiol. 2015, 81, 7135–7142. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębska, A.M.; Kunicki, A.R.; Olszyna, A.R.; Karwowska, E. Al2O3-Ag nanopowders: New method of synthesis, characterisation and biocidal activity. Adv. Appl. Ceram. 2011, 110, 108–113. [Google Scholar] [CrossRef]
- Kędziora, A.; Speruda, M.; Krzyżewska, E.; Rybka, J.; Łukowiak, A.; Bugla-Płoskońska, G. Similarities and differences between silver ions and silver in nanoforms as antibacterial agents. Int. J. Mol. Sci. 2018, 19, 444. [Google Scholar] [CrossRef]
- Almonaci-Hernández, C.A.; Juarez-Moreno, K.; Castañeda-Juarez, M.E.; Almanza-Reyes, H.; Pestryakov, A.; Bogdanchikova, N. Silver nanoparticles for the rapid healing of diabetic foot ulcers. Int. J. Med. Nano Res. 2017, 4, 19. [Google Scholar] [CrossRef]

| Sample. | Thickness (×10−4 m) | Ultimate Tensile Strength (MPa) | Elongation at Break (%) | Young´s Modules (MPa) | Yield Strength (MPa) |
|---|---|---|---|---|---|
| PCL | 2.3 ± 1.2 | 2.5 ± 0.5 | 94.9 ± 15.7 | 0.3 | 1.4 |
| PVP | 3.1 ± 2.4 | 2.3 ± 0.2 | 20.7 ± 7.5 | 0.4 | 2.2 |
| PCL/PVP 95:5 | 3.1 ± 1.3 | 1.2 ± 0.3 | 107.1 ± 3.6 | 0.1 | 0.6 |
| PCL/PVP 85:15 | 3.1 ± 1.2 | 0.8 ± 0.1 | 60.6 ± 19.2 | 0.1 | 0.5 |
| Ag- Si/Al2O3 Sorbents Number | Ag (%) | Particle Size (mm) | Sspec (m2/g) | V∑pores (cm3/g) | P (g/cm3) |
|---|---|---|---|---|---|
| Ag- Si/Al2O3-1 | 0.01 | 0.10 | 100.0 | 0.25 | 0.90 |
| Ag- Si/Al2O3-2 | 0.003 | 0.04 | 96.6 | 0.20 | 1.10 |
| Ag- Si/Al2O3-3 | 0.01 | 1.00 | 245.0 | 0.35 | 0.70 |
| Ag- Si/Al2O3-4 | 0.01 | 0.80 | 250.0 | 0.35 | 0.75 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Suárez, A.S.; Dastager, S.G.; Bogdanchikova, N.; Grande, D.; Pestryakov, A.; García-Ramos, J.C.; Pérez-González, G.L.; Juárez-Moreno, K.; Toledano-Magaña, Y.; Smolentseva, E.; et al. Electrospun Fibers and Sorbents as a Possible Basis for Effective Composite Wound Dressings. Micromachines 2020, 11, 441. https://doi.org/10.3390/mi11040441
Álvarez-Suárez AS, Dastager SG, Bogdanchikova N, Grande D, Pestryakov A, García-Ramos JC, Pérez-González GL, Juárez-Moreno K, Toledano-Magaña Y, Smolentseva E, et al. Electrospun Fibers and Sorbents as a Possible Basis for Effective Composite Wound Dressings. Micromachines. 2020; 11(4):441. https://doi.org/10.3390/mi11040441
Chicago/Turabian StyleÁlvarez-Suárez, Alan Saúl, Syed G. Dastager, Nina Bogdanchikova, Daniel Grande, Alexey Pestryakov, Juan Carlos García-Ramos, Graciela Lizeth Pérez-González, Karla Juárez-Moreno, Yanis Toledano-Magaña, Elena Smolentseva, and et al. 2020. "Electrospun Fibers and Sorbents as a Possible Basis for Effective Composite Wound Dressings" Micromachines 11, no. 4: 441. https://doi.org/10.3390/mi11040441
APA StyleÁlvarez-Suárez, A. S., Dastager, S. G., Bogdanchikova, N., Grande, D., Pestryakov, A., García-Ramos, J. C., Pérez-González, G. L., Juárez-Moreno, K., Toledano-Magaña, Y., Smolentseva, E., Paz-González, J. A., Popova, T., Rachkovskaya, L., Nimaev, V., Kotlyarova, A., Korolev, M., Letyagin, A., & Villarreal-Gómez, L. J. (2020). Electrospun Fibers and Sorbents as a Possible Basis for Effective Composite Wound Dressings. Micromachines, 11(4), 441. https://doi.org/10.3390/mi11040441










