Microfluidic Chamber Design for Controlled Droplet Expansion and Coalescence
Abstract
:1. Introduction
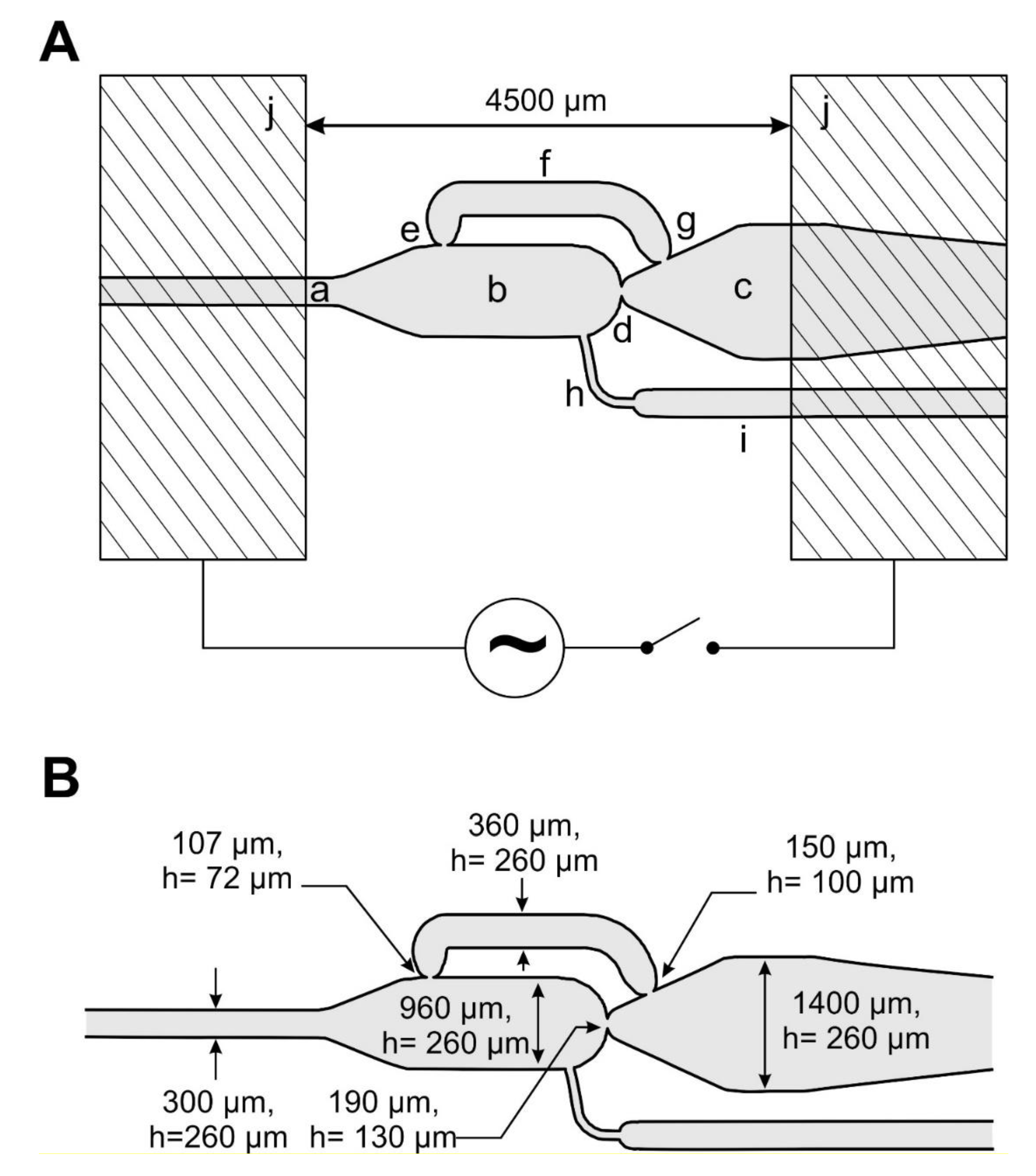
2. Experimental and Setup
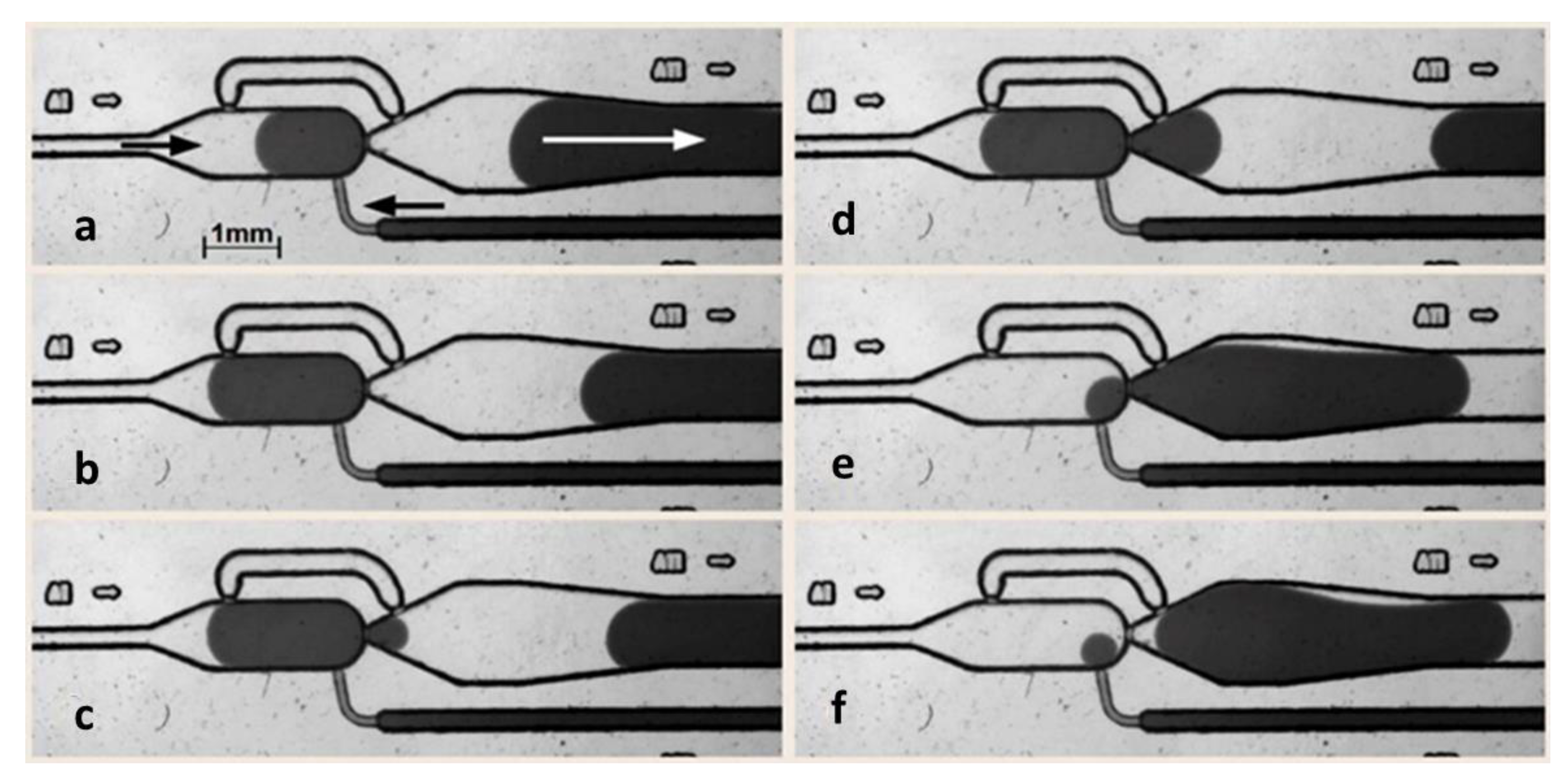
3. Results and Discussion
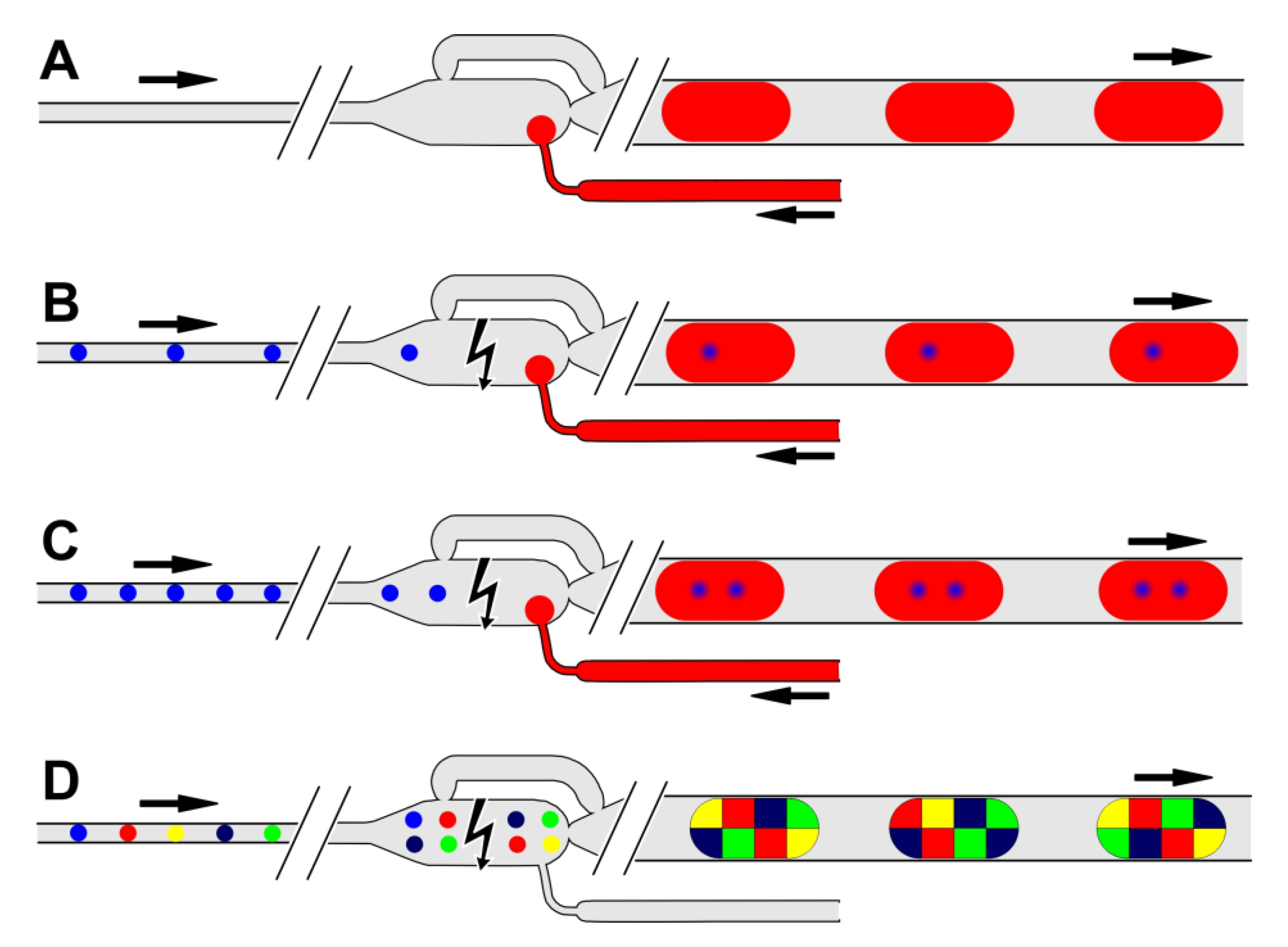
3.1. Self-Controlled Droplet Formation Mode
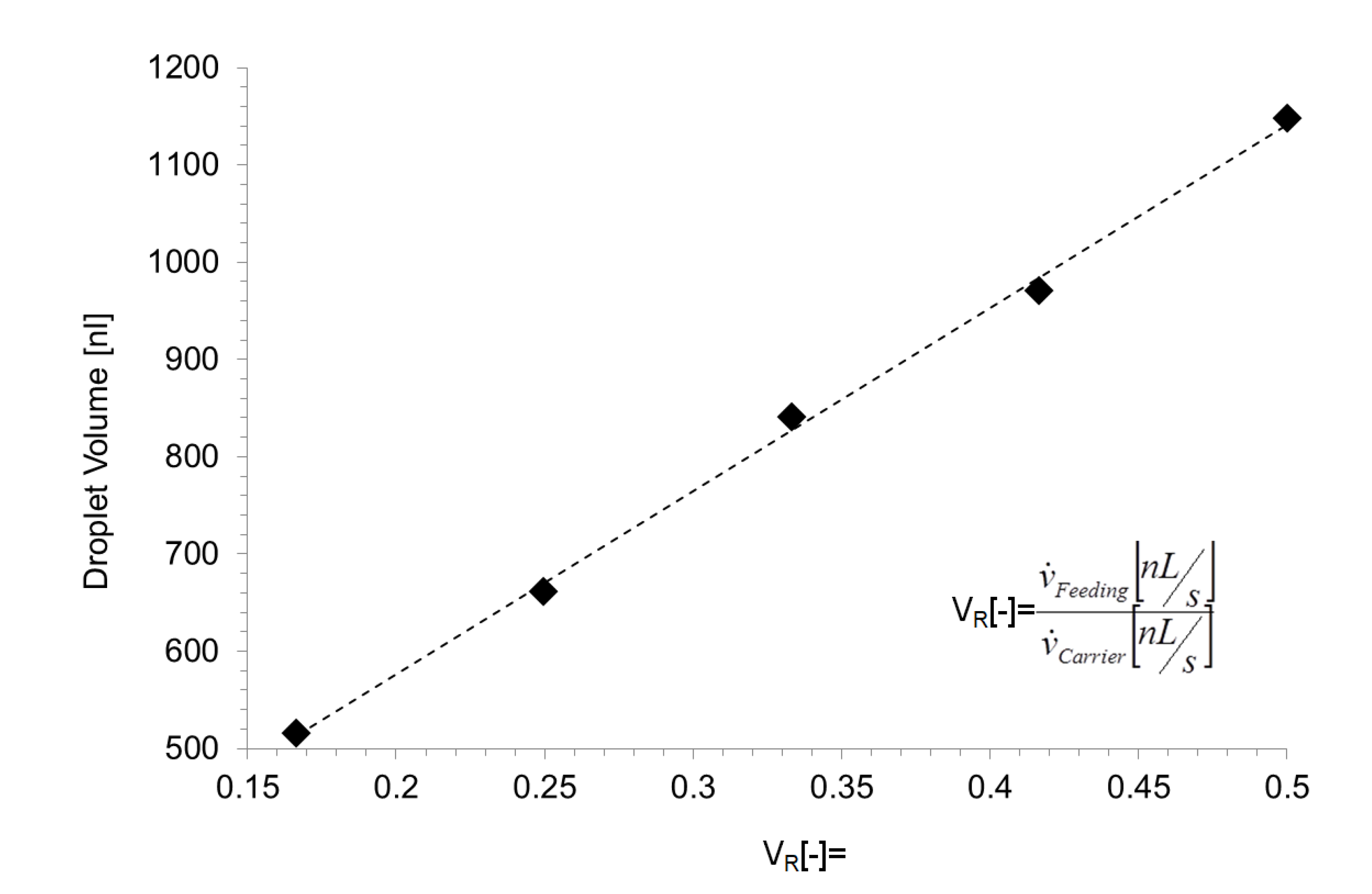
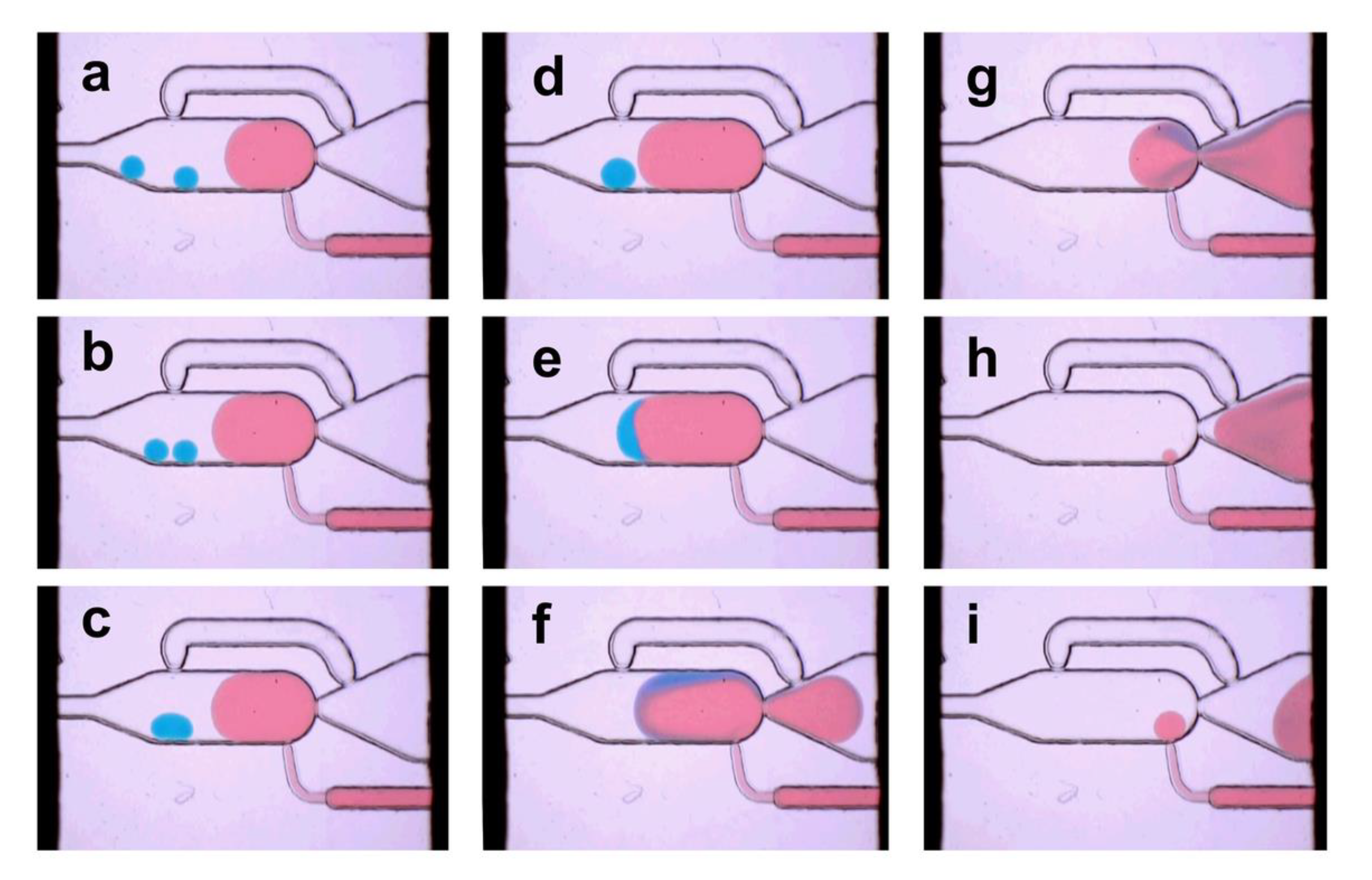
3.2. Single Droplet Dilution Mode
3.3. Multiple Droplet Dilution Mode
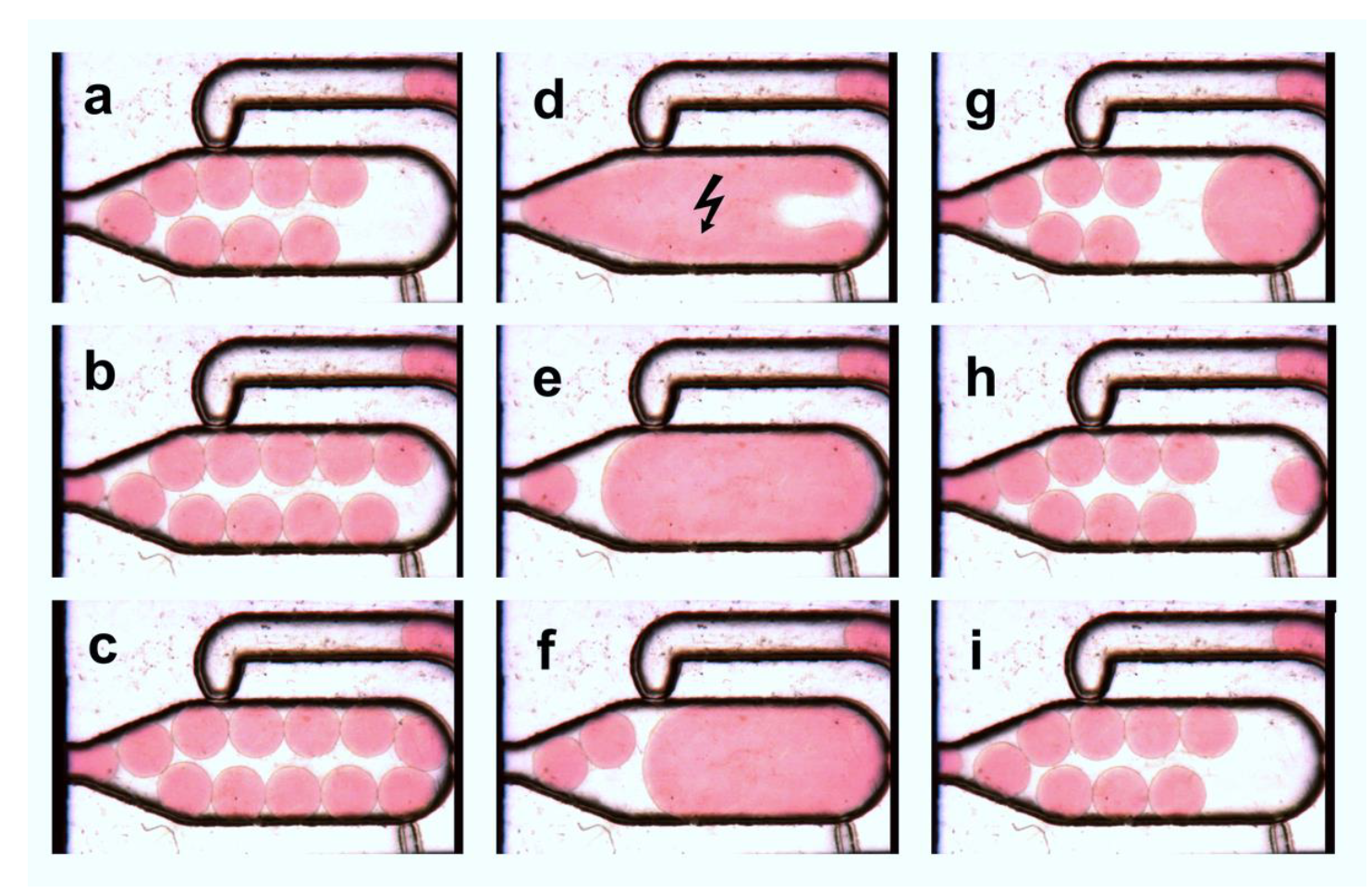
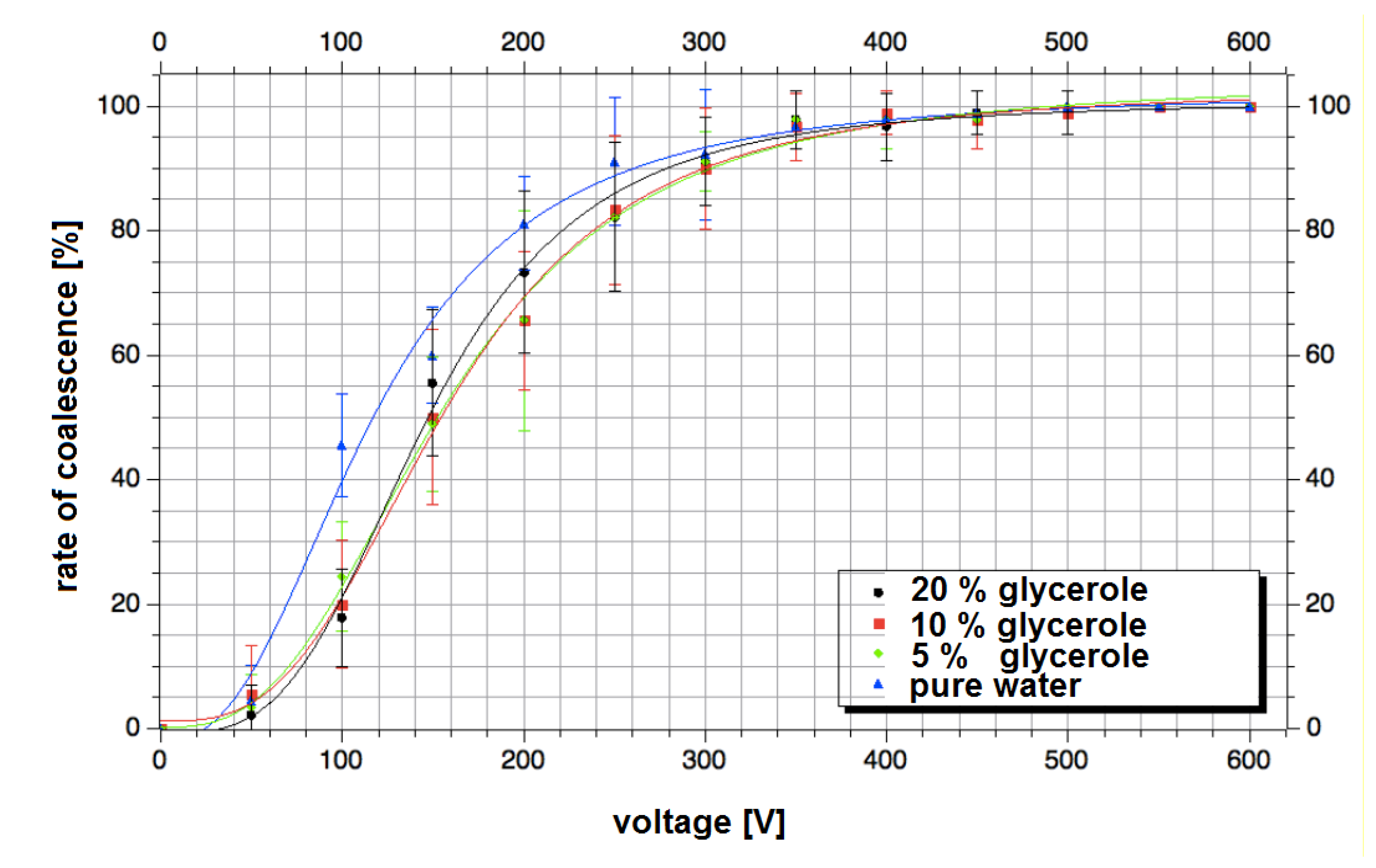
3.4. Multiple Droplet Coalescence Mode (D)
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huebner, A.; Sharma, S.; Srisa-Art, M.; Hollfelder, F.; Edel, J.B.; Demello, A.J. Microdroplets: A sea of applications? Lab Chip 2008, 8, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Günther, A.; Jensen, K.F. Multiphase microfluidics: From flow characteristics to chemical and materials synthesis. Lab Chip 2006, 6, 1487–1503. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Tice, J.D.; Ismagilov, R.F. A microfluidic system for controlling reaction networks in time. Angew. Chem.-Int. Ed. 2003, 42, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Clausell-Tormos, J.; Lieber, D.; Baret, J.C.; El-Harrak, A.; Miller, O.J.; Frenz, L.; Blouwolff, J.; Humphry, K.J.; Koster, S.; Duan, H.; et al. Droplet-based microfluidic platforms for the encapsulation and screening of mammalian cells and multicellular organisms. Chem. Biol. 2008, 15, 427–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodell, J.R.; McMullen, J.P.; Zaborenko, N.; Maloney, J.R.; Ho, C.X.; Jensen, K.F.; Porco, J.A., Jr.; Beeler, A.B. Development of an Automated Microfluidic Reaction Platform for Multidimensional Screening: Reaction Discovery Employing Bicyclo 3.2.1 octanoid Scaffolds. J. Org. Chem. 2009, 74, 6169–6180. [Google Scholar] [CrossRef]
- Seah, Y.F.S.; Hu, H.; Merten, C.A. Microfluidic single-cell technology in immunology and antibody screening. Mol. Asp. Med. 2018, 59, 47–61. [Google Scholar] [CrossRef]
- Kiss, M.M.; Ortoleva-Donnelly, L.; Beer, N.R.; Warner, J.; Bailey, C.G.; Colston, B.W.; Rothberg, J.M.; Link, D.R.; Leamon, J.H. High-Throughput Quantitative Polymerase Chain Reaction in Picoliter Droplets. Anal. Chem. 2008, 80, 8975–8981. [Google Scholar] [CrossRef]
- Lindstrom, S.; Andersson-Svahn, H. Overview of single-cell analyses: Microdevices and applications. Lab Chip 2010, 10, 3363–3372. [Google Scholar] [CrossRef]
- Pekin, D.; Skhiri, Y.; Baret, J.-C.; Le Corre, D.; Mazutis, L.; Ben Salem, C.; Millot, F.; El Harrak, A.; Hutchison, J.B.; Larson, J.W.; et al. Quantitative and sensitive detection of rare mutations using droplet-based microfluidics. Lab Chip 2011, 11, 2156–2166. [Google Scholar] [CrossRef]
- Mazutis, L.; Gilbert, J.; Ung, W.L.; Weitz, D.A.; Griffiths, A.D.; Heyman, J.A. Single-cell analysis and sorting using droplet-based microfluidics. Nat. Protoc. 2013, 8, 870–891. [Google Scholar] [CrossRef]
- Kohler, J.M.; Henkel, T.; Grodrian, A.; Kirner, T.; Roth, M.; Martin, K.; Metze, J. Digital reaction technology by micro segmented flow—Components, concepts and applications. Chem. Eng. J. 2004, 101, 201–216. [Google Scholar] [CrossRef]
- Cao, J.L.; Kürsten, D.; Schneider, S.; Knauser, A.; Günther, P.M.; Köhler, J.M. Uncovering toxicological complexity by multi-dimensional screenings in microsegmented flow: Modulation of antibiotic interference by nanoparticles. Lab Chip 2012, 12, 474–484. [Google Scholar] [CrossRef]
- Kauser, A.; Schneider, S.; Moller, F.; Csaki, A.; Fritzsche, W.; Köhler, J.M. Screening of plasmonic properties of composed metal nanoparticles by combinatorial synthesis in micro-fluid segment sequences. Chem. Eng. J. 2013, 227, 80–89. [Google Scholar] [CrossRef]
- Boedicker, J.Q.; Li, L.; Kline, T.R.; Ismagilov, R.F. Detecting bacteria and determining their susceptibility to antibiotics by stochastic confinement in nanoliter droplets using plug-based microfluidics. Lab Chip 2008, 8, 1265–1272. [Google Scholar] [CrossRef] [Green Version]
- Martin, K.; Henkel, T.; Baier, V.; Grodrian, A.; Schon, T.; Roth, M.; Kohler, J.M.; Metze, J. Generation of larger numbers of separated microbial populations by cultivation in segmented-flow microdevices. Lab Chip 2003, 3, 202–207. [Google Scholar] [CrossRef]
- Cao, J.L.; Köhler, J.M. Droplet-based microfluidics for microtoxicological studies. Eng. Life Sci. 2015, 15, 306–317. [Google Scholar] [CrossRef]
- Kursten, D.; Cao, J.L.; Funfak, A.; Muller, P.; Kohler, J.M. Cultivation of Chlorella vulgaris in microfluid segments and microtoxicological determination of their sensitivity against CuCl2 in the nanoliter range. Eng. Life Sci. 2011, 11, 580–587. [Google Scholar] [CrossRef]
- Kürsten, D.; Moller, F.; Groß, G.A.; Lenk, C.; Visaveliya, N.; Schuler, T.; Köhler, J.M. Identification of response classes from heavy metal-tolerant soil microbial communities by highly resolved concentration-dependent screenings in a microfluidic system. Methods Ecol. Evol. 2015, 6, 600–609. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Kerner, A.; Burns, M.A.; Lin, X.X.N. Microdroplet-Enabled Highly Parallel Co-Cultivation of Microbial Communities. PLoS ONE 2011, 6, e17019. [Google Scholar] [CrossRef] [Green Version]
- Gross, G.A.; Hamann, C.; Guenther, M.; Koehler, J.M. Formation of polymer and nanoparticle doped polymer minirods by use of the microsegmented flow principle. Chem. Eng. Technol. 2007, 30, 341–346. [Google Scholar] [CrossRef]
- Baroud, C.N.; Gallaire, F.; Dangla, R. Dynamics of microfluidic droplets. Lab Chip 2010, 10, 2032–2045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahler, L.; Tovar, M.; Weber, T.; Brandes, S.; Rudolph, M.M.; Ehgartner, J.; Mayr, T.; Figge, M.T.; Roth, M.; Zang, E. Enhanced and homogeneous oxygen availability during incubation of microfluidic droplets. RSC Adv. 2015, 5, 101871–101878. [Google Scholar] [CrossRef] [Green Version]
- Zang, E.; Brandes, S.; Tovar, M.; Martin, K.; Mech, F.; Horbert, P.; Henkel, T.; Figge, M.T.; Roth, M. Real-time image processing for label-free enrichment of Actinobacteria cultivated in picolitre droplets. Lab Chip 2013, 13, 3707–3713. [Google Scholar] [CrossRef] [Green Version]
- Abbyad, P.; Dangla, R.; Alexandrou, A.; Baroud, C.N. Rails and anchors: Guiding and trapping droplet microreactors in two dimensions. Lab Chip 2011, 11, 813–821. [Google Scholar] [CrossRef] [Green Version]
- Kielpinski, M.; Vasold, T.; Horbert, P.; Martin, K.; Mayer, G.; Henkel, T. Potential of sinusoidal gradients for dose response assays in Droplet-Based microfluidics. In Proceedings of the µTAS, Seattle, WA, USA, 2–6 October 2011. [Google Scholar]
- Kielpinski, M.; Mayer, G.; Albert, J.; Malsch, D.; Felbel, J.; Henkel, T. Self controlled droplet fusion of segmented sample streams. In Proceedings of the IMRET9, Potsdam, Germany, 6–8 September 2006; Volume 114. [Google Scholar]
- Fradet, E.; McDougall, C.; Abbyad, P.; Dangla, R.; McGloin, D.; Baroud, C.N. Combining rails and anchors with laser forcing for selective manipulation within 2D droplet arrays. Lab Chip 2011, 11, 4228–4234. [Google Scholar] [CrossRef] [Green Version]
- Kielpinski, M.; Malsch, D.; Gleichmann, N.; Mayer, G.; Henkel, T. Application of Self-Control in Droplet-based Microfluidics. In Proceedings of the ICNMM, ASME, Darmstadt, Germany, 23–25 June 2008; Volume 114. [Google Scholar]
- Abate, A.R.; Hung, T.; Mary, P.; Agresti, J.J.; Weitz, D.A. High-throughput injection with microfluidics using picoinjectors. Proc. Natl. Acad. Sci. USA 2010, 107, 19163–19166. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, B.; Vanapalli, S.A. Electrocoalescence based serial dilution of microfluidic droplets. Biomicrofluidics 2014, 8, 044111. [Google Scholar] [CrossRef] [Green Version]
- Postek, W.; Kaminski, T.S.; Garstecki, P. A precise and accurate microfluidic droplet dilutor. Analyst 2017, 142, 2901–2911. [Google Scholar] [CrossRef]
- Kaminski, T.S.; Jakiela, S.; Czekalska, M.A.; Postek, W.; Garstecki, P. Automated generation of libraries of nL droplets. Lab Chip 2012, 12, 3995–4002. [Google Scholar] [CrossRef]
- Theberge, A.B.; Mayot, E.; El Harrak, A.; Kleinschmidt, F.; Huck, W.T.S.; Griffiths, A.D. Microfluidic platform for combinatorial synthesis in picolitre droplets. Lab Chip 2012, 12, 1320–1326. [Google Scholar] [CrossRef] [Green Version]
- Um, E.; Rogers, M.E.; Stone, H.A. Combinatorial generation of droplets by controlled assembly and coalescence. Lab Chip 2013, 13, 4674–4680. [Google Scholar] [CrossRef] [PubMed]
- Gielen, F.; van Vliet, L.; Koprowski, B.T.; Devenish, S.R.A.; Fischlechner, M.; Edel, J.B.; Niu, X.Z.; deMello, A.J.; Hollfelder, F. A Fully Unsupervised Compartment-on-Demand Platform for Precise Nanoliter Assays of Time-Dependent Steady-State Enzyme Kinetics and Inhibition. Anal. Chem. 2013, 85, 4761–4769. [Google Scholar] [CrossRef] [PubMed]
- Churski, K.; Korczyk, P.; Garstecki, P. High-throughput automated droplet microfluidic system for screening of reaction conditions. Lab Chip 2010, 10, 816–818. [Google Scholar] [CrossRef]
- Niu, X.Z.; Gielen, F.; Edel, J.B.; deMello, A.J. A microdroplet dilutor for high-throughput screening. Nat. Chem. 2011, 3, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Jousse, F.; Farr, R.; Link, D.R.; Fuerstman, M.J.; Garstecki, P. Bifurcation of droplet flows within capillaries. Phys. Rev. E 2006, 74, 036311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanella, A.; Biral, A. Design and Analysis of a Microfluidic Bus Network with Bypass Channels. In Proceedings of the 2014 IEEE International Conference on Communications (ICC), Sydney, Australia, 10–14 June 2014; pp. 3993–3998. [Google Scholar]
- van Steijn, V.; Korczyk, P.M.; Derzsi, L.; Abate, A.R.; Weitz, D.A.; Garstecki, P. Block-and-break generation of microdroplets with fixed volume. Biomicrofluidics 2013, 7, 024108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postek, W.; Kaminski, T.S.; Garstecki, P. A passive microfluidic system based on step emulsification allows the generation of libraries of nanoliter-sized droplets from microliter droplets of varying and known concentrations of a sample. Lab Chip 2017, 17, 1323–1331. [Google Scholar] [CrossRef] [Green Version]
- Niu, X.; Gulati, S.; Edel, J.B.; deMello, A.J. Pillar-induced droplet merging in microfluidic circuits. Lab Chip 2008, 8, 1837–1841. [Google Scholar] [CrossRef]
- Chen, X.M.; Brukson, A.; Ren, C.L. A simple droplet merger design for controlled reaction volumes. Microfluid. Nanofluid. 2017, 21, 34. [Google Scholar] [CrossRef]
- Hoang, D.A.; Haringa, C.; Portela, L.M.; Kreutzer, M.T.; Kleijn, C.R.; van Steijn, V. Design and characterization of bubble-splitting distributor for scaled-out multiphase microreactors. Chem. Eng. J. 2014, 236, 545–554. [Google Scholar] [CrossRef]
- Sajeesh, P.; Manasi, S.; Doble, M.; Sen, A.K. A microfluidic device with focusing and spacing control for resistance-based sorting of droplets and cells. Lab Chip 2015, 15, 3738–3748. [Google Scholar] [CrossRef]
- Darhuber, A.A.; Valentino, J.P.; Davis, J.M.; Troian, S.M.; Wagner, S. Microfluidic actuation by modulation of surface stresses. Appl. Phys. Lett. 2003, 82, 657–659. [Google Scholar] [CrossRef] [Green Version]
- Maddala, J.; Wang, W.S.; Vanapalli, S.A.; Rengaswamy, R. Traffic of pairs of drops in microfluidic ladder networks with fore-aft structural asymmetry. Microfluid. Nanofluid. 2013, 14, 337–344. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.M.; Ren, C.L. A microfluidic chip integrated with droplet generation, pairing, trapping, merging, mixing and releasing. RSC Adv. 2017, 7, 16738–16750. [Google Scholar] [CrossRef] [Green Version]
- Bithi, S.S.; Wang, W.S.; Sun, M.; Blawzdziewicz, J.; Vanapalli, S.A. Coalescing drops in microfluidic parking networks: A multifunctional platform for drop-based microfluidics. Biomicrofluidics 2014, 8, 034118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korczyk, P.M.; Derzsi, L.; Jakiela, S.; Garstecki, P. Microfluidic traps for hard-wired operations on droplets. Lab Chip 2013, 13, 4096–4102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bithi, S.S.; Vanapalli, S.A. Behavior of a train of droplets in a fluidic network with hydrodynamic traps. Biomicrofluidics 2010, 4, 044110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dangla, R.; Lee, S.; Baroud, C.N. Trapping Microfluidic Drops in Wells of Surface Energy. Phys. Rev. Lett. 2011, 107, 124501. [Google Scholar] [CrossRef] [Green Version]
- Holtze, C.; Rowat, A.C.; Agresti, J.J.; Hutchison, J.B.; Angile, F.E.; Schmitz, C.H.J.; Koster, S.; Duan, H.; Humphry, K.J.; Scanga, R.A.; et al. Biocompatible surfactants for water-in-fluorocarbon emulsions. Lab Chip 2008, 8, 1632–1639. [Google Scholar] [CrossRef]
- Baret, J.-C. Surfactants in droplet-based microfluidics. Lab Chip 2012, 12, 422–433. [Google Scholar] [CrossRef]
- Ahn, K.; Agresti, J.; Chong, H.; Marquez, M.; Weitz, D.A. Electrocoalescence of drops synchronized by size-dependent flow in microfluidic channels. Appl. Phys. Lett. 2006, 88, 264105. [Google Scholar] [CrossRef] [Green Version]
- Priest, C.; Herminghaus, S.; Seemann, R. Controlled electrocoalescence in microfluidics: Targeting a single lamella. Appl. Phys. Lett. 2006, 89, 134101. [Google Scholar] [CrossRef]
- Niu, X.; Gielen, F.; deMello, A.J.; Edel, J.B. Electro-Coalescence of Digitally Controlled Droplets. Anal. Chem. 2009, 81, 7321–7325. [Google Scholar] [CrossRef]
- Zagnoni, M.; Cooper, J.M. On-chip electrocoalescence of microdroplets as a function of voltage, frequency and droplet size. Lab Chip 2009, 9, 2652–2658. [Google Scholar] [CrossRef]
- Zagnoni, M.; Le Lain, G.; Cooper, J.M. Electrocoalescence Mechanisms of Microdroplets Using Localized Electric Fields in Microfluidic Channels. Langmuir 2010, 26, 14443–14449. [Google Scholar] [CrossRef]
- Gu, H.; Murade, C.U.; Duits, M.H.G.; Mugele, F. A microfluidic platform for on-demand formation and merging of microdroplets using electric control. Biomicrofluidics 2011, 5, 011101. [Google Scholar] [CrossRef] [Green Version]
- Mazutis, L.; Griffiths, A.D. Selective droplet coalescence using microfluidic systems. Lab Chip 2012, 12, 1800–1806. [Google Scholar] [CrossRef]
- Xu, L.; Lee, H.; Panchapakesan, R.; Oh, K.W. Fusion and sorting of two parallel trains of droplets using a railroad-like channel network and guiding tracks. Lab Chip 2012, 12, 3936–3942. [Google Scholar] [CrossRef]
- Pico-Surf-User-Guide. Available online: https://www.spherefluidics.com/wp-content/uploads/2016/11/Pico-Surf-User-Guide-2016-12-02-V1.pdf (accessed on 1 April 2020).
- neMESYS Syringe Pumps. Available online: https://www.cetoni.com/products/pumps/ (accessed on 1 April 2020).

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kielpinski, M.; Walther, O.; Cao, J.; Henkel, T.; Köhler, J.M.; Groß, G.A. Microfluidic Chamber Design for Controlled Droplet Expansion and Coalescence. Micromachines 2020, 11, 394. https://doi.org/10.3390/mi11040394
Kielpinski M, Walther O, Cao J, Henkel T, Köhler JM, Groß GA. Microfluidic Chamber Design for Controlled Droplet Expansion and Coalescence. Micromachines. 2020; 11(4):394. https://doi.org/10.3390/mi11040394
Chicago/Turabian StyleKielpinski, Mark, Oliver Walther, Jialan Cao, Thomas Henkel, J. Michael Köhler, and G. Alexander Groß. 2020. "Microfluidic Chamber Design for Controlled Droplet Expansion and Coalescence" Micromachines 11, no. 4: 394. https://doi.org/10.3390/mi11040394
APA StyleKielpinski, M., Walther, O., Cao, J., Henkel, T., Köhler, J. M., & Groß, G. A. (2020). Microfluidic Chamber Design for Controlled Droplet Expansion and Coalescence. Micromachines, 11(4), 394. https://doi.org/10.3390/mi11040394







