Fabrication of Ultra-High Aspect Ratio (>420:1) Al2O3 Nanotube Arraysby Sidewall TransferMetal Assistant Chemical Etching
Abstract
1. Introduction
2. Materials and Methods
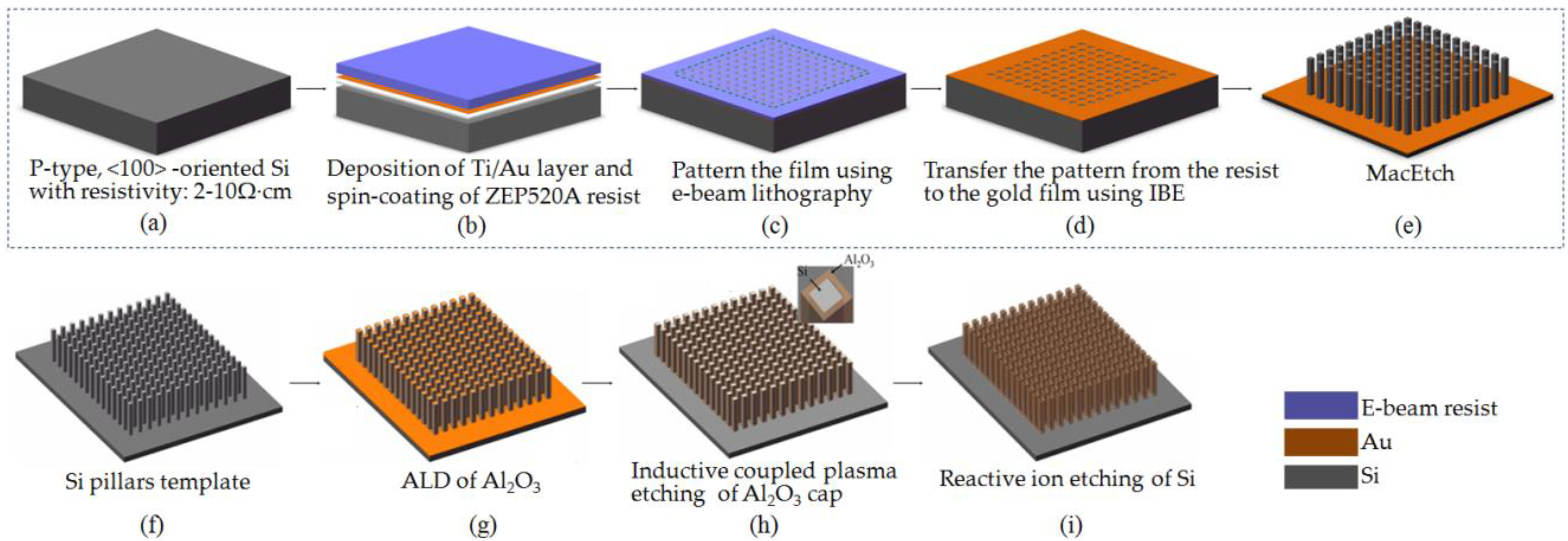
2.1. Fabrication ofTi/Au Nanostructures with Low Aspect Ratio
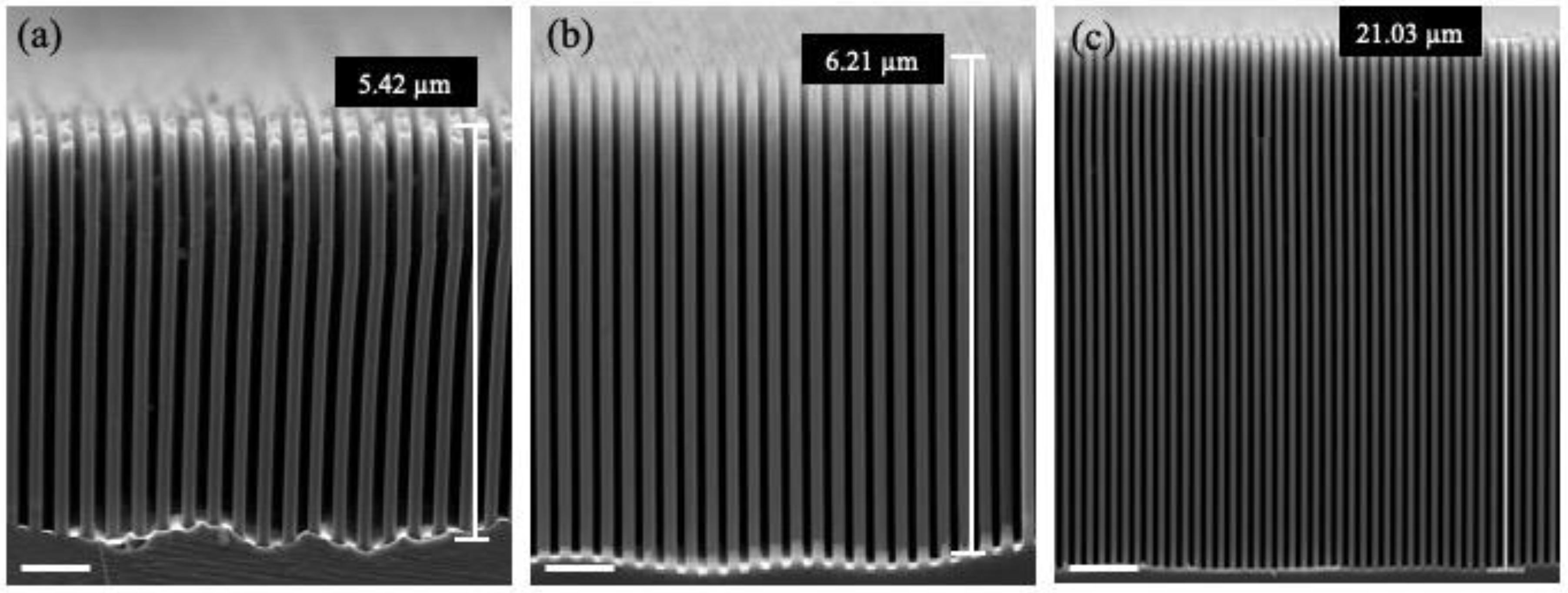
2.2. Fabrication of Silicon Nanostructures with High Aspect Ratio
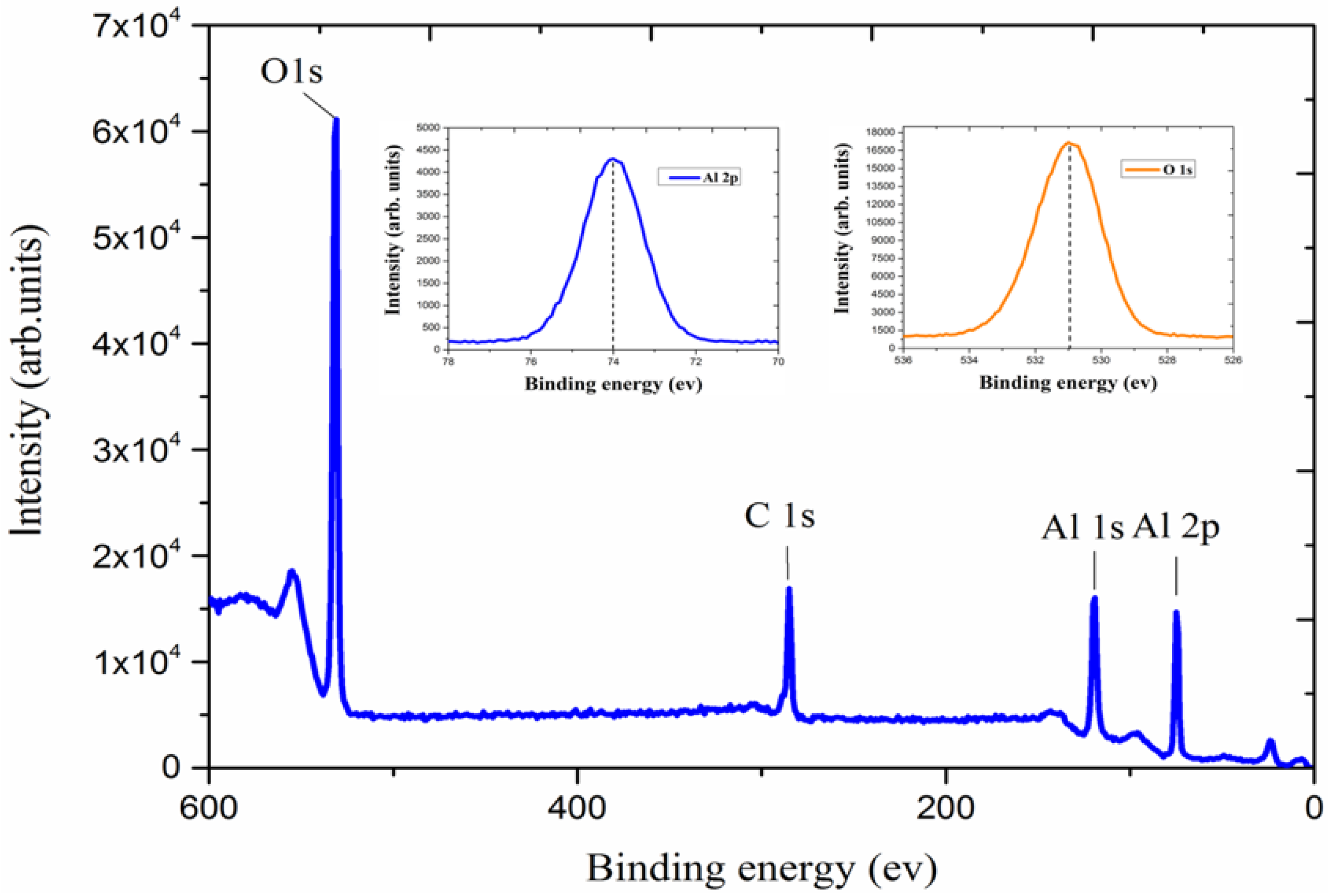
2.3. Al2O3 Film Deposition by ALD
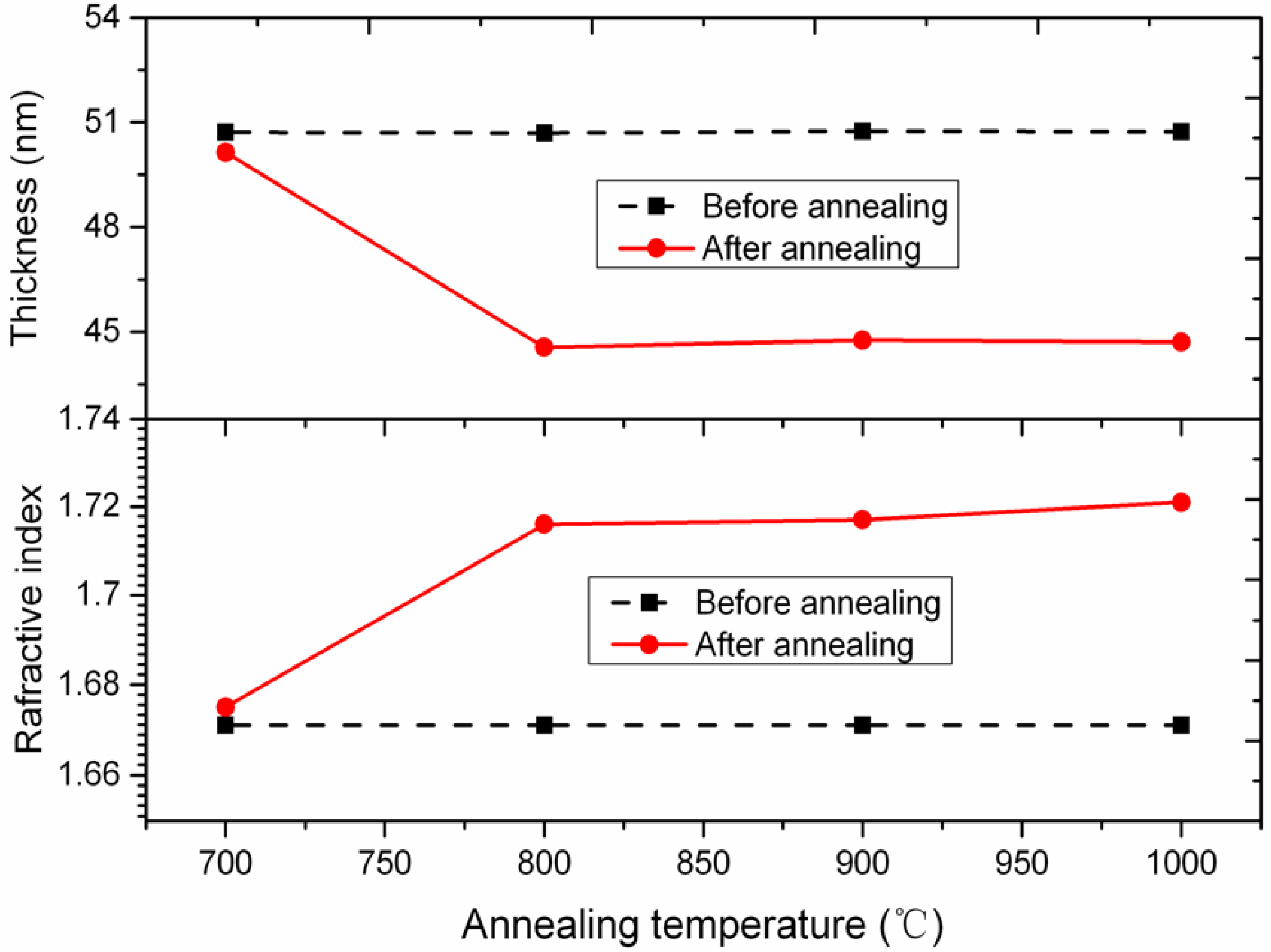
2.4. Annealing Processand Characterization of the Al2O3 Film
2.5. Formation of Al2O3 Nanotube Arrays with an Ultra-High Aspect Ratio
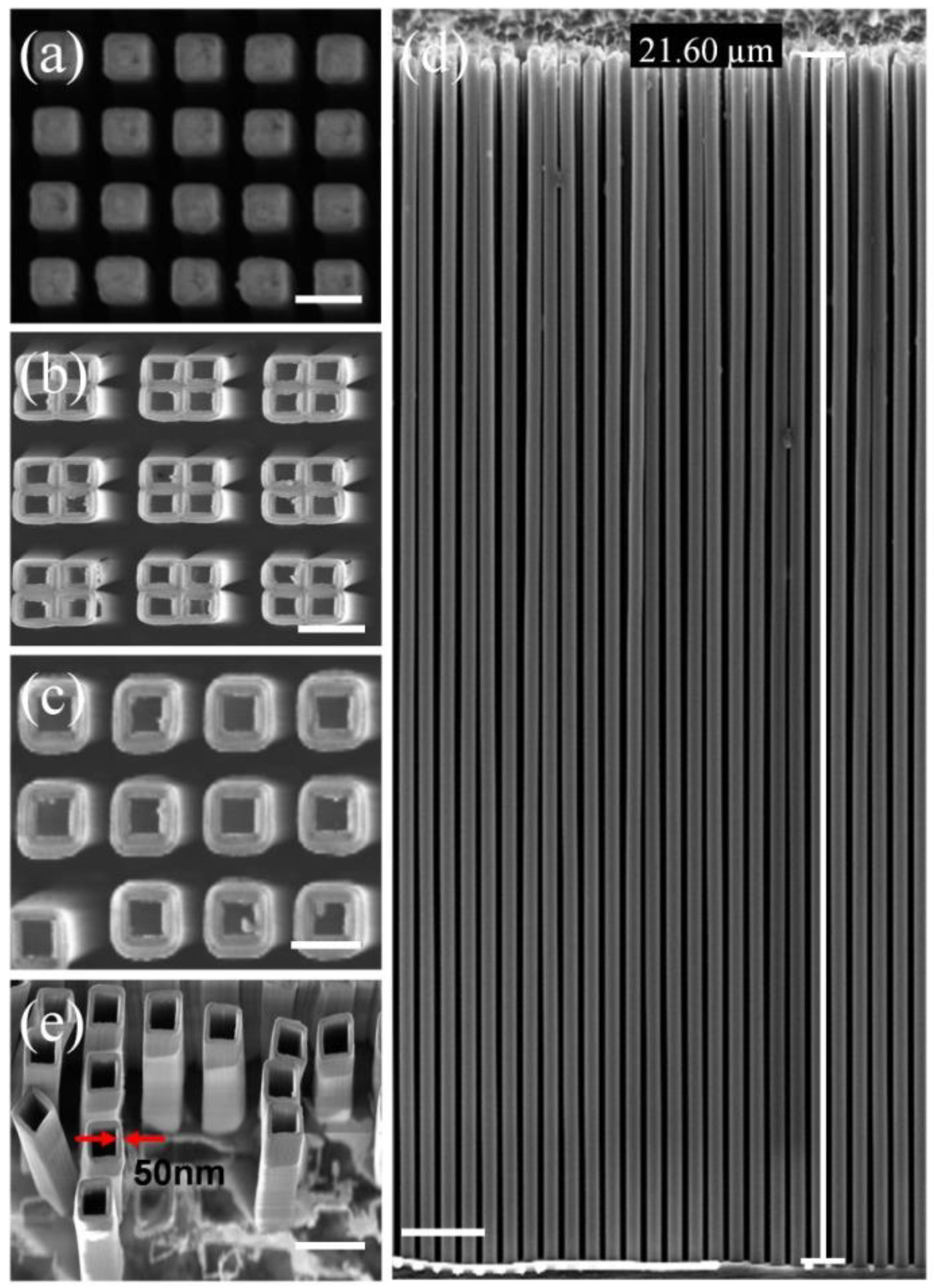
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shanmugham, S.; Hendrick, M.; Richards, N.; Oljaca, M. Candidate oxidation resistant coatings via combustion chemical vapor deposition. J. Mater. Sci. 2004, 39, 377–381. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Lloret, F.; Pham, T.T.; Cañas, J.; Reyes, D.F.D.L.; Eon, D.; Pernot, J.; Araujo, D. Control of the Alumina Microstructure to Reduce Gate Leaks in Diamond MOSFETs. Nanomater 2018, 8, 584. [Google Scholar] [CrossRef] [PubMed]
- Thamaraiselvi, T.V.; Rajewari, S. Biological evaluation of bioceramic materials—Areview. Trends Biomater. Artif. Organs 2004, 81, 9–17. [Google Scholar]
- Xifré-Pérez, E.; Ferre-Borull, J.; Pallarès, J.; Marsal, L.F. Mesoporous alumina as a biomaterial for biomedical applications. Open Mater. Sci. 2015, 2, 13–32. [Google Scholar] [CrossRef]
- De Wit, H.; Crevecoeur, C. The dielectric breakdown of anodic aluminum oxide. Phys. Lett. A 1974, 50, 365–366. [Google Scholar] [CrossRef]
- Mei, Y.; Wu, X.; Shao, X.F.; Siu, G.G.; Bao, X.M. Formation of an array of isolated alumina nanotubes. EPL Europhys. Lett. 2003, 62, 595–599. [Google Scholar] [CrossRef]
- Lee, H.C.; Kim, H.J.; Chung, S.H.; Lee, K.H.; Lee, H.C.; Lee, J.S. Synthesis of unidirectional alumina nanostructures without added organics olvents. J. Am. Chem. Soc. 2003, 125, 2882–2883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; He, R.; Zhang, Q.; Zhang, X.; Zhu, J. Synthesis of alumina nanotubes using carbon nanotubes as templates. Chem. Phys. Lett. 2002, 360, 579–584. [Google Scholar] [CrossRef]
- Mei, Y.; Wu, X.; Shao, X.; Huang, G.; Siu, G. Formation mechanism of alumina nanotube array. Phys. Lett. A 2003, 309, 109–113. [Google Scholar] [CrossRef]
- Pu, L.; Bao, X.M.; Zou, J.P.; Feng, D. Individual alumina nanotubes. Angew. Chem. Int. Ed. 2001, 113, 1538. [Google Scholar] [CrossRef]
- Xiao, Z.-L.; Han, C.; Welp, U.; Wang, H.H.; Vlasko-Vlasov, V.; Kwok, W.K.; Miller, D.J.; Hiller, J.; Cook, R.E.; Willing, G.A.; et al. Nickel antidot arrays on anodic alumina substrates. Appl. Phys. Lett. 2002, 81, 2869–2871. [Google Scholar] [CrossRef]
- Huang, G.S.; Wu, X.L.; Xie, Y.; Shao, X.F.; Wang, S.H. Light emission from silicon-based porous anodic alumina form edin 0.5 M oxalicacid. J. Appl. Phys. 2003, 94, 2407–2410. [Google Scholar] [CrossRef]
- Jensen, D.S.; Kanyal, S.S.; Madaan, N.; Miles, A.J.; Davis, R.C.; Vanfleet, R.; Vail, M.A.; Dadson, A.E.; Linford, M.R. Ozone priming of patterned carbon nanotube forests for subsequent atomic layer deposition-like deposition of SiO2 for the preparation of microfabricated thin layer chromatography plates. J. Vac. Sci. Technol. B 2013, 31, 31803. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, M.; Ren, W.; Ye, Z.-G. Well-ordered ZnO nanotube arrays and networks grown by atomic layer deposition. Appl. Surf. Sci. 2015, 340, 120–125. [Google Scholar] [CrossRef]
- Elam, J.W.; Routkevitch, D.; Mardilovich, P.P.; George, S.M. Conformal coating on ultra high-aspect-ratio nanopores of anodic alumina by atomic layer deposition. Chem. Mater. 2003, 15, 3507–3517. [Google Scholar] [CrossRef]
- Zazpe, R.; Knaut, M.; Sopha, H.; Hromadko, L.; Albert, M.; Prikryl, J.; Gärtnerová, V.; Bartha, J.W.; Macak, J.M. Atomic Layer Deposition for Coating of High Aspect Ratio TiO2 Nanotube Layers. Langmuir 2016, 32, 10551–10558. [Google Scholar] [CrossRef]
- Shin, H.; Jeong, D.-K.; Lee, J.; Sung, M.M.; Kim, J. Formation of TiO2 and ZrO2 Nanotubes Using Atomic Layer Deposition with Ultraprecise Control of the Wall Thickness. Adv. Mater. 2004, 16, 1197–1200. [Google Scholar] [CrossRef]
- Li, X.; Bohn, P.W. Metal-assisted chemical etching in HF/H2O2 produces porous silicon. Appl. Phys. Lett. 2000, 77, 2572–2574. [Google Scholar] [CrossRef]
- Bai, S.; Du, Y.; Wang, C.; Wu, J.; Sugioka, K. Reusable Surface-Enhanced Raman Spectroscopy Substrates Made of Silicon Nanowire Array Coated with Silver Nanoparticles Fabricated by Metal-Assisted Chemical Etching and Photonic Reduction. Nanomater 2019, 9, 1531. [Google Scholar] [CrossRef]
- Chang, C.; Sakdinawat, A. Ultra-high aspect ratio high-resolution nanofabrication for hard X-ray diffractive optics. Nat. Commun. 2014, 5, 4243. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Bi, Q.; Sajjad, M.; Wang, X.; Ren, Y.; Zhou, X.; Xu, W.; Liu, Z. One-Dimensional Porous Silicon Nanowires with Large Surface Area for Fast Charge–Discharge Lithium-Ion Batteries. Nanomaterials 2018, 8, 285. [Google Scholar] [CrossRef] [PubMed]
- Hoshian, S.; Gaspar, C.; Vasara, T.; Jahangiri, F.; Jokinen, V.; Franssila, S. Non-Lithographic Silicon Micromachining Using Inkjet and Chemical Etching. Micromachines 2016, 7, 222. [Google Scholar] [CrossRef]
- Rezvani, S.J.; Pinto, N.; Boarino, L. Rapid formation of single crystalline Ge nanowires by anodic metal assisted etching. CrystEngComm 2016, 18, 7843–7848. [Google Scholar] [CrossRef]
- Geng, X.; Duan, B.K.; Grismer, D.A.; Zhao, L.; Bohn, P.W. Monodisperse GaN nanowires prepared by metal-assisted chemical etching with in situ catalyst deposition. Electrochem. Commun. 2012, 19, 39–42. [Google Scholar] [CrossRef]
- Duan, B.K.; Bohn, P.W. High sensitivity hydrogen sensing with Pt-decorated porous gallium nitride prepared by metal-assisted electroless etching. Analyst 2010, 135, 902. [Google Scholar] [CrossRef] [PubMed]
- DeJarld, M.; Shin, J.C.; Chern, W.; Chanda, D.; Balasundaram, K.; Rogers, J.A.; Li, X. Formation of High Aspect Ratio GaAs Nanostructures with Metal-Assisted Chemical Etching. Nano Lett. 2011, 11, 5259–5263. [Google Scholar] [CrossRef]
- Wilhelm, T.S.; Soule, C.W.; Baboli, M.A.; O’Connell, C.J.; Mohseni, P.K. Fabrication of Suspended III–V Nanofoils by Inverse Metal-Assisted Chemical Etching of In0.49Ga0.51P/GaAs Heteroepitaxial Films. ACS Appl. Mater. Interfaces 2018, 10, 2058–2066. [Google Scholar] [CrossRef]
- Lova, P.; Robbiano, V.; Cacialli, F.; Comoretto, D.; Soci, C. Black GaAs by Metal-Assisted Chemical Etching. ACS Appl. Mater. Interfaces 2018, 10, 33434–33440. [Google Scholar] [CrossRef]
- Kim, J.; Oh, J. Formation of GaP Nanocones and Micro-mesas by Metal-assisted Chemical Etching. Phys. Chem. Chem. Phys. 2016, 18, 3402–3408. [Google Scholar] [CrossRef]
- Kim, S.H.; Mohseni, P.K.; Song, Y.; Ishihara, T.; Li, X. Inverse Metal-Assisted Chemical Etching Produces Smooth High Aspect Ratio InP Nanostructures. Nano Lett. 2014, 15, 641–648. [Google Scholar] [CrossRef]
- Wilhelm, T.S.; Wang, Z.-H.; Baboli, M.A.; Yan, J.; Preble, S.F.; Mohseni, P.K. Ordered AlxGa1–xAs Nanopillar Arrays via Inverse Metal-Assisted Chemical Etching. ACS Appl. Mater. Interfaces 2018, 10, 27488–27497. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Song, Y.; Kim, J.D.; Yu, L.; Wasserman, D.; Chim, W.K.; Chiam, S.Y.; Li, X. Damage-Free Smooth-Sidewall InGaAs Nanopillar Array by Metal-Assisted Chemical Etching. ACS Nano 2017, 11, 10193–10205. [Google Scholar] [CrossRef]
- Romano, L.; Vila-Comamala, J.; Jefimovs, K.; Stampanoni, M. Effect of isopropanol on gold assisted chemical etching of silicon microstructures. Microelectron. Eng. 2017, 177, 59–65. [Google Scholar] [CrossRef][Green Version]
- Scuderi, V.; Impellizzeri, G.; Romano, L.; Scuderi, M.; Nicotra, G.; Bergum, K.; Irrera, A.; Svensson, B.; Privitera, V. TiO2-coated nanostructures for dye photo-degradation in water. Nanoscale Res. Lett. 2014, 9, 458. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Boukai, A.; Yang, P. High Density n-Si/n-TiO2Core/Shell Nanowire Arrays with Enhanced Photoactivity. Nano Lett. 2009, 9, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ye, T.; Shi, L.; Xie, C. Fabrication of ultra-high aspect ratio (>160:1) silicon nanostructures by using Au metal assisted chemical etching. J. Micromech. Microeng. 2017, 27, 124002. [Google Scholar] [CrossRef]
- Li, H.; Niu, J.; Wang, G.; Wang, E.; Xie, C. Direct Production of Silicon Nanostructures with Electrochemical Nanoimprinting. ACS Appl. Electron. Mater. 2019, 1, 1070–1075. [Google Scholar] [CrossRef]
- Kim, D.H.; Sung, S.-K.; Sim, J.S.; Kim, K.R.; Lee, J.D.; Park, B.-G.; Choi, B.H.; Hwang, S.; Ahn, D. Single-electron transistor based on a silicon-on-insulator quantum wire fabricated by a side-wall patterning method. Appl. Phys. Lett. 2001, 79, 3812–3814. [Google Scholar] [CrossRef]
- Liu, D.; Syms, R.R.A. NEMS by Sidewall Transfer Lithography. J. Microelectromech. Syst. 2014, 23, 1366–1373. [Google Scholar] [CrossRef]
- Hållstedt, J.; Hellstrom, P.-E.; Radamson, H. Sidewall transfer lithography for reliable fabrication of nanowires and deca-nanometer MOSFETs. Thin Solid Films 2008, 517, 117–120. [Google Scholar] [CrossRef]
- Sainiemi, L.; Franssila, S. Mask material effects in cryogenic deep reactive ion etching. J. Vac. Sci. Technol. B Microelectron. Nanometer. Struct. 2007, 25, 801. [Google Scholar] [CrossRef]
- Moulder, J.F. Hand Book of X-ray Photoelectron Spectroscopy; Perkin Elmer Corporation: Eden Prairie, MN, USA, 1992; pp. 182–187. [Google Scholar]
- Ghiraldelli, E.; Pelosi, C.; Gombia, E.; Chiavarotti, G.; Vanzetti, L. ALD growth, thermal treatments and characterisation of Al2O3 layers. Thin Solid Films 2008, 517, 434–436. [Google Scholar] [CrossRef]
- Böse, O.; Kemnitz, E.; Lippitz, A.; Unger, W.E.S. C 1s and Au 4f7/2 referenced XPS binding energy data obtained with different aluminium oxides,-hydroxides and-fluorides. Fresenius’ J. Anal. Chem. 1997, 358, 175–179. [Google Scholar]
- Gehr, R.J.; Boyd, R.W. Optical Properties of Nanostructured Optical Materials. Chem. Mater. 1996, 8, 1807–1819. [Google Scholar] [CrossRef]
| Process Parameters | Al2O3 Etch | Si Etch |
|---|---|---|
| Cl2 (sccm) | 1.2 | − |
| BCl3 (sccm) | 6.8 | − |
| SF6 (sccm) | − | 90 |
| Pressure (mtorr) | 3 | 4 |
| Coil power (W) | 900 | 400 |
| Platen power (W) | 200 | 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Xie, C. Fabrication of Ultra-High Aspect Ratio (>420:1) Al2O3 Nanotube Arraysby Sidewall TransferMetal Assistant Chemical Etching. Micromachines 2020, 11, 378. https://doi.org/10.3390/mi11040378
Li H, Xie C. Fabrication of Ultra-High Aspect Ratio (>420:1) Al2O3 Nanotube Arraysby Sidewall TransferMetal Assistant Chemical Etching. Micromachines. 2020; 11(4):378. https://doi.org/10.3390/mi11040378
Chicago/Turabian StyleLi, Hailiang, and Changqing Xie. 2020. "Fabrication of Ultra-High Aspect Ratio (>420:1) Al2O3 Nanotube Arraysby Sidewall TransferMetal Assistant Chemical Etching" Micromachines 11, no. 4: 378. https://doi.org/10.3390/mi11040378
APA StyleLi, H., & Xie, C. (2020). Fabrication of Ultra-High Aspect Ratio (>420:1) Al2O3 Nanotube Arraysby Sidewall TransferMetal Assistant Chemical Etching. Micromachines, 11(4), 378. https://doi.org/10.3390/mi11040378




