Light Gradient-Based Screening of Arabidopsis thaliana on a 384-Well Type Plant Array Chip
Abstract
:1. Introduction
2. Materials and Methods
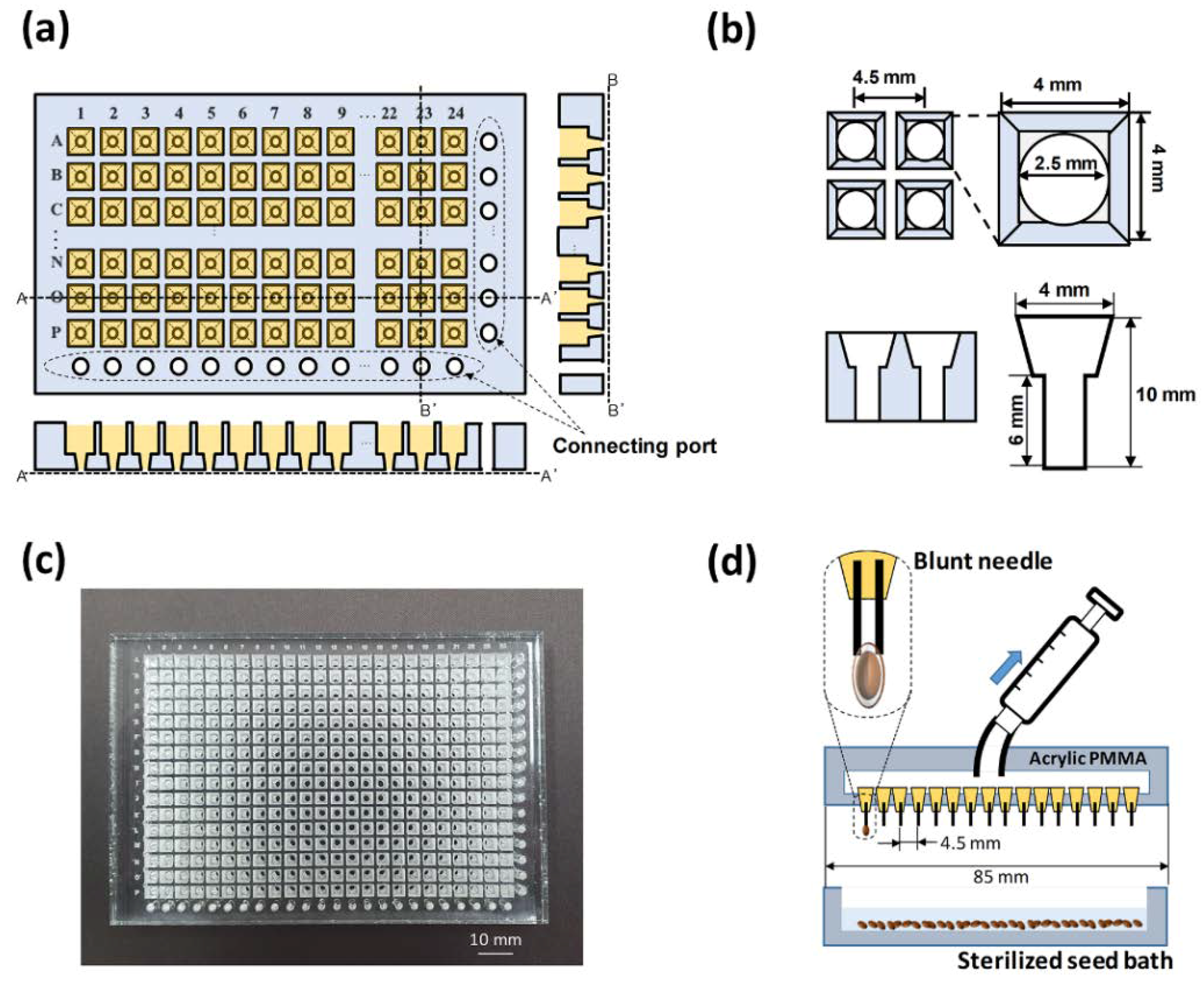
2.1. Design and Concept
2.2. Fabrication of a 384-Well Type Plant Array Chip
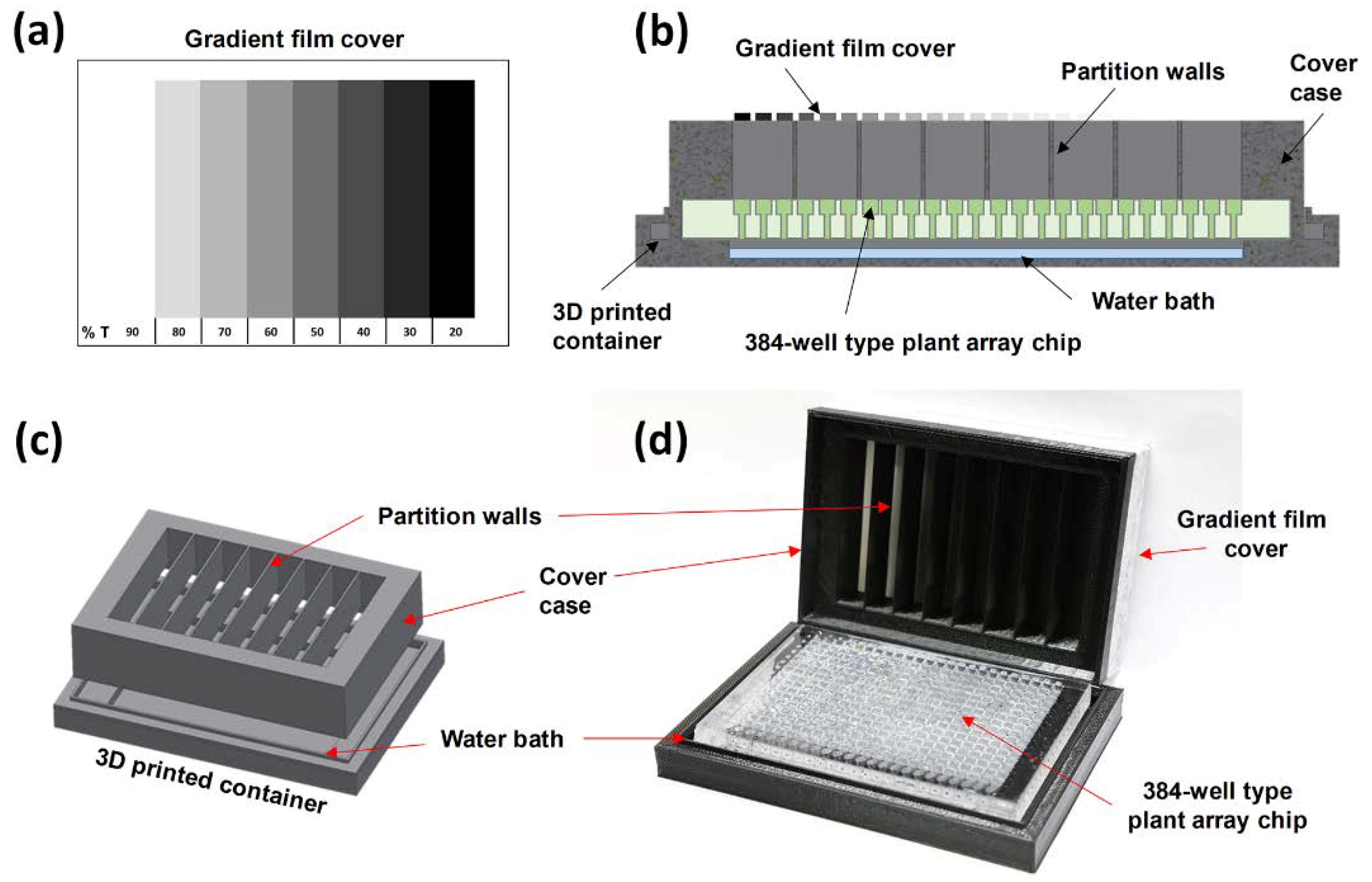
2.3. Integration of a Light Gradient Module
2.4. Incubation of Arabidopsis in a 384-Well Type Plant Array Chip
3. Results and Discussion
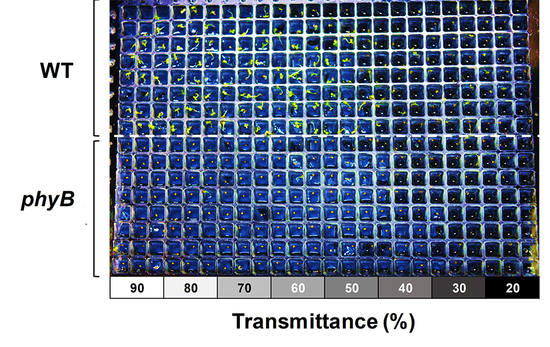
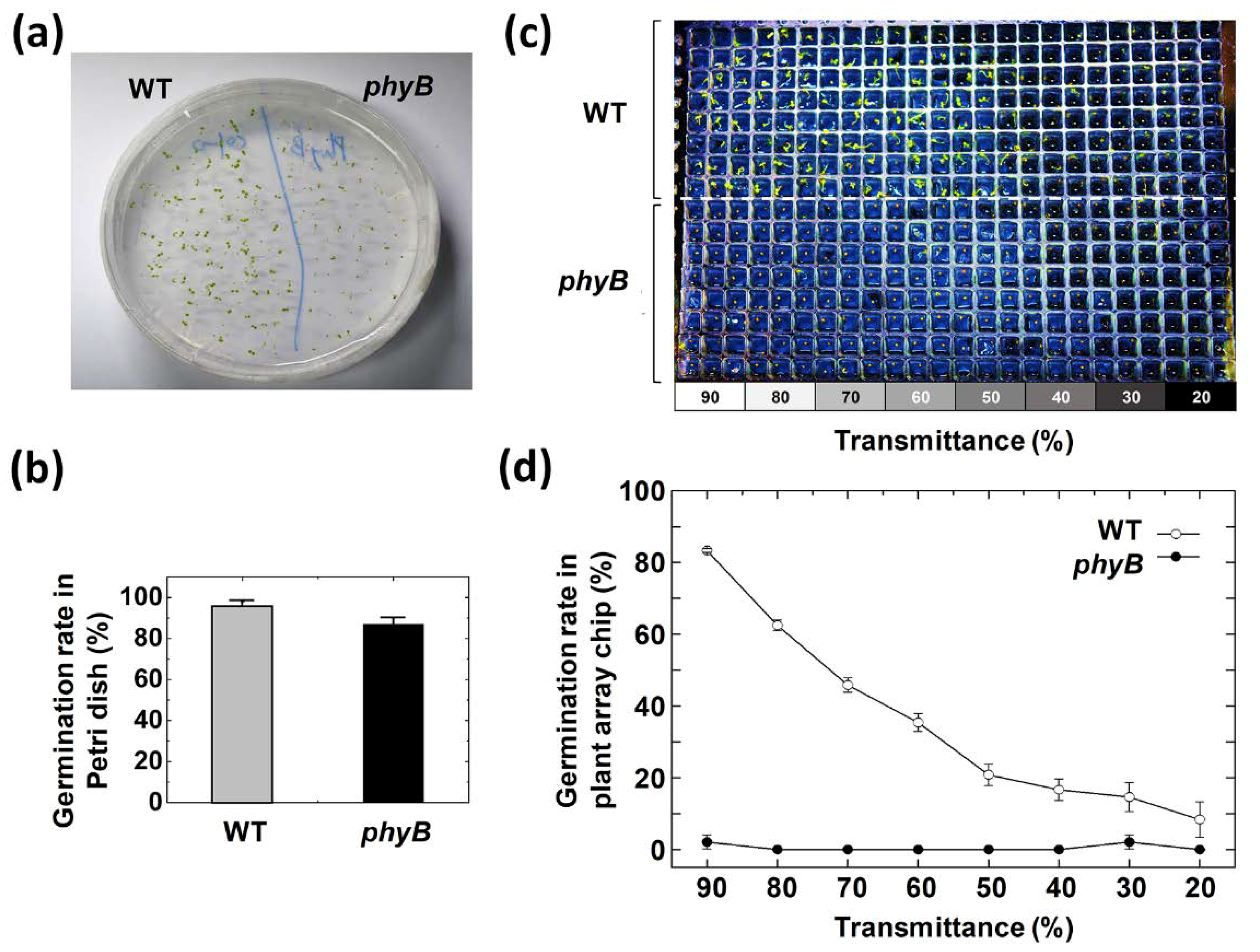
3.1. Germination and Growth Assay on a 384-Well Type Plant Array Chip
3.2. Effect of White Light Intensity on Arabidopsis Growth
3.3. Effect of Red Light Intensity on Germination Rate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Houle, D.; Govindaraju, D.R.; Omholt, S. Phenomics: The next challenge. Nat. Rev. Genet. 2010, 11, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J. Arabidopsis: An Atlas of Morphology and Development; Springer: New York, NY, USA, 1994. [Google Scholar]
- Iyer-Pascuzzi, A.S.; Symonova, O.; Mileyko, Y.; Hao, Y.; Belcher, H.; Harer, J.; Weitz, J.S.; Benfey, P.N. Imaging and analysis platform for automatic phenotyping and trait ranking of plant root systems. Plant Physiol. 2010, 152, 1148–1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stavang, J.A.; Gallego-Bartolomé, J.; Gómez, M.D.; Yoshida, S.; Asami, T.; Olsen, J.E.; García-Martínez, J.L.; Alabadí, D.; Blázquez, M.A. Hormonal regulation of temperature-induced growth in Arabidopsis. Plant J. 2009, 60, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qin, T.; Ma, Q.; Sun, J.; Liu, Z.; Yuan, M.; Mao, T. Light-regulated hypocotyl elongation involves proteasome-dependent degradation of the microtubule regulatory protein WDL3 in Arabidopsis. Plant Cell 2013, 25, 1740–1755. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Kendy, E.; Qiang, Y.; Changming, L.; Yanjun, S.; Hongyong, S. Effect of soil water deficit on evapotranspiration, crop yield, and water use efficiency in the North China Plain. Agric. Water Manag. 2004, 64, 107–122. [Google Scholar] [CrossRef]
- Koziol, M.J.; Whatley, F.R. Gaseous Air Pollutants and Plant Metabolism; Butterworth-Heinemann: London, UK, 1984. [Google Scholar] [CrossRef]
- Chailakhyan, M.K. Internal factors of plant flowering. Ann. Rev. Plant Physiol. 1968, 19, 1–37. [Google Scholar] [CrossRef]
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Chapter two-light-regulated plant growth and development. Curr. Top. Dev. Biol. 2010, 91, 29–66. [Google Scholar] [CrossRef] [Green Version]
- Kasahara, M.; Swartz, T.E.; Olney, M.A.; Onodera, A.; Mochizuki, N.; Fukuzawa, H.; Asamizu, E.; Tabata, S.; Kanegae, H.; Takano, M.; et al. Photochemical properties of the flavin mononucleotide-binding domains of the phototropins from Arabidopsis, Rice, and Chlamydomonas reinhardtii. Plant Physiol. 2002, 129, 762–773. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, G.; Wang, H.; Deng, X.W. Phytochrome signaling mechanisms. Arab. B 2011, 9, e0148. [Google Scholar] [CrossRef] [Green Version]
- Shinomura, T.; Nagatanit, A.; Hanzawa, H.; Kubotat, M.; Watanabet, M.; Furuya, M. Action spectra for phytochrome A- and B-specific photoinduction of seed germination in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1996, 93, 8129–8133. [Google Scholar] [CrossRef] [Green Version]
- Tatic-Lucic, S. Applications of bioMEMS in cell-related research. Semi. Device Research Symp. 2007, 12–14. [Google Scholar] [CrossRef]
- Takayama, S.; Ostuni, E.; LeDuc, P.; Naruse, K.; Ingber, D.E.; Whitesides, G.M. Subcellular positioning of small molecules. Nature 2001, 411, 1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horade, M.; Yanagisawa, N.; Mizuta, Y.; Higashiyama, T.; Arata, H. Growth assay of individual pollen tubes arrayed by microchannel device. Microelec. Eng. 2014, 118, 25–28. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, X.; Aluru, M.R.; Dong, L. Plant miniature greenhouse. Sens. Actuator A Phys. 2019, 111572. [Google Scholar] [CrossRef]
- Jiang, H.; Xu, Z.; Aluru, M.R.; Dong, L. Plant chip for high-throughput phenotyping of Arabidopsis. Lab Chip 2014, 14, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, C.G.; Nezhad, A.S.; Ghanbari, M.; Naghavi, M.; Packirisamy, M.; Geitmann, A. TipChip: A modular, MEMS-based platform for experimentation and phenotyping of tip-growing cells. Plant J. 2013, 73, 1057–1068. [Google Scholar] [CrossRef]
- Grossmann, G.; Guo, W.; Ehrhardt, D.W.; Frommer, W.B.; Sit, R.V.; Quake, S.R.; Meier, M. The RootChip: An integrated microfluidic chip for plant science. Plant Cell 2011, 23, 4234–4240. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Jiao, Y.; Maneesha, M.R.; Dong, L. Electrospun nanofibrous membranes for temperature regulation of microfluidic seed growth chips. J. Nanosci. Nanotechnol. 2012, 12, 6333–6339. [Google Scholar] [CrossRef]
- Meier, M.; Lucchetta, E.M.; Ismagilov, R.F. Chemical stimulation of the Arabidopsis thaliana root using multi-laminar flow on a microfluidic chip. Lab Chip 2010, 10, 2147–2153. [Google Scholar] [CrossRef] [Green Version]
- Busch, W.; Brad, T.M.; Bradley, M.; Mace, D.L.; Twigg, R.W.; Jung, J.; Pruteanu-Malinici, I.; Kennedy, J.S.; Fricke, G.K.; Clark, R.L.; et al. A microfluidic device and computational platform for high-throughput live imaging of gene expression. Nat. Methods 2012, 9, 1101–1106. [Google Scholar] [CrossRef] [Green Version]
- Sanati Nezhad, A. Microfluidic platforms for plant cells studies. Lab Chip 2014, 14, 3262–3274. [Google Scholar] [CrossRef] [PubMed]
- Parashar, A.; Pandey, S. Plant-in-chip: Microfluidic system for studying root growth and pathogenic interactions in Arabidopsis. Appl. Phys. Lett. 2011, 98, 263703. [Google Scholar] [CrossRef]
- Park, Y.-H.; Lee, N.; Choi, G.; Park, J.-K. Plant array chip for the germination and growth screening of Arabidopsis thaliana. Lab Chip 2017, 17, 3071–3077. [Google Scholar] [CrossRef] [PubMed]
- Chory, J.; Peto, C.A.; Ashbaugh, M.; Saganich, R.; Pratt, L.; Ausubel, F. Different roles for phytochrome in etiolated and green plants deduced from characterization of Arabidopsis thaliana mutants. Plant Cell 1989, 1, 867–880. [Google Scholar] [CrossRef] [Green Version]
- Jankowska-blaszczuk, M.; Daws, M.I. Impact of red: Far red ratios on germination of temperate forest herbs in relation to shade tolerance, seed mass and persistence in the soil. Funct. Ecol. 2007, 21, 1055–1062. [Google Scholar] [CrossRef]
- Halliday, K.J.; Koornneef, M.; Whitelam, G.C. Phytochromes B, D, and E act redundantly to control multiple physiological responses in Arabidopsis. Plant Physiol. 1994, 104, 1311–1315. [Google Scholar] [CrossRef] [Green Version]
- Jensen, P.J.; Hangarter, R.P.; Estelle, M. Auxin transport is required for hypocotyl elongation in light-grown but not dark-grown Arabidopsis. Plant Physiol. 1998, 116, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Beligni, M.V.; Lamattina, L. Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta 2000, 210, 215–221. [Google Scholar] [CrossRef]
- Reed, J.W.; Nagpal, P.; Poole, D.S.; Furuya, M.; Chory, J. Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 1993, 5, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Leyser, O.; Day, S. Mechanisms in Plant Development; Wiley-Blackwell: Hoboken, NJ, USA, 2009. [Google Scholar]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.-H.; Park, J.-K. Light Gradient-Based Screening of Arabidopsis thaliana on a 384-Well Type Plant Array Chip. Micromachines 2020, 11, 191. https://doi.org/10.3390/mi11020191
Park Y-H, Park J-K. Light Gradient-Based Screening of Arabidopsis thaliana on a 384-Well Type Plant Array Chip. Micromachines. 2020; 11(2):191. https://doi.org/10.3390/mi11020191
Chicago/Turabian StylePark, Youn-Hee, and Je-Kyun Park. 2020. "Light Gradient-Based Screening of Arabidopsis thaliana on a 384-Well Type Plant Array Chip" Micromachines 11, no. 2: 191. https://doi.org/10.3390/mi11020191
APA StylePark, Y.-H., & Park, J.-K. (2020). Light Gradient-Based Screening of Arabidopsis thaliana on a 384-Well Type Plant Array Chip. Micromachines, 11(2), 191. https://doi.org/10.3390/mi11020191