Dripping, Jetting and Regime Transition of Droplet Formation in a Buoyancy-Assisted Microfluidic Device
Abstract
:1. Introduction
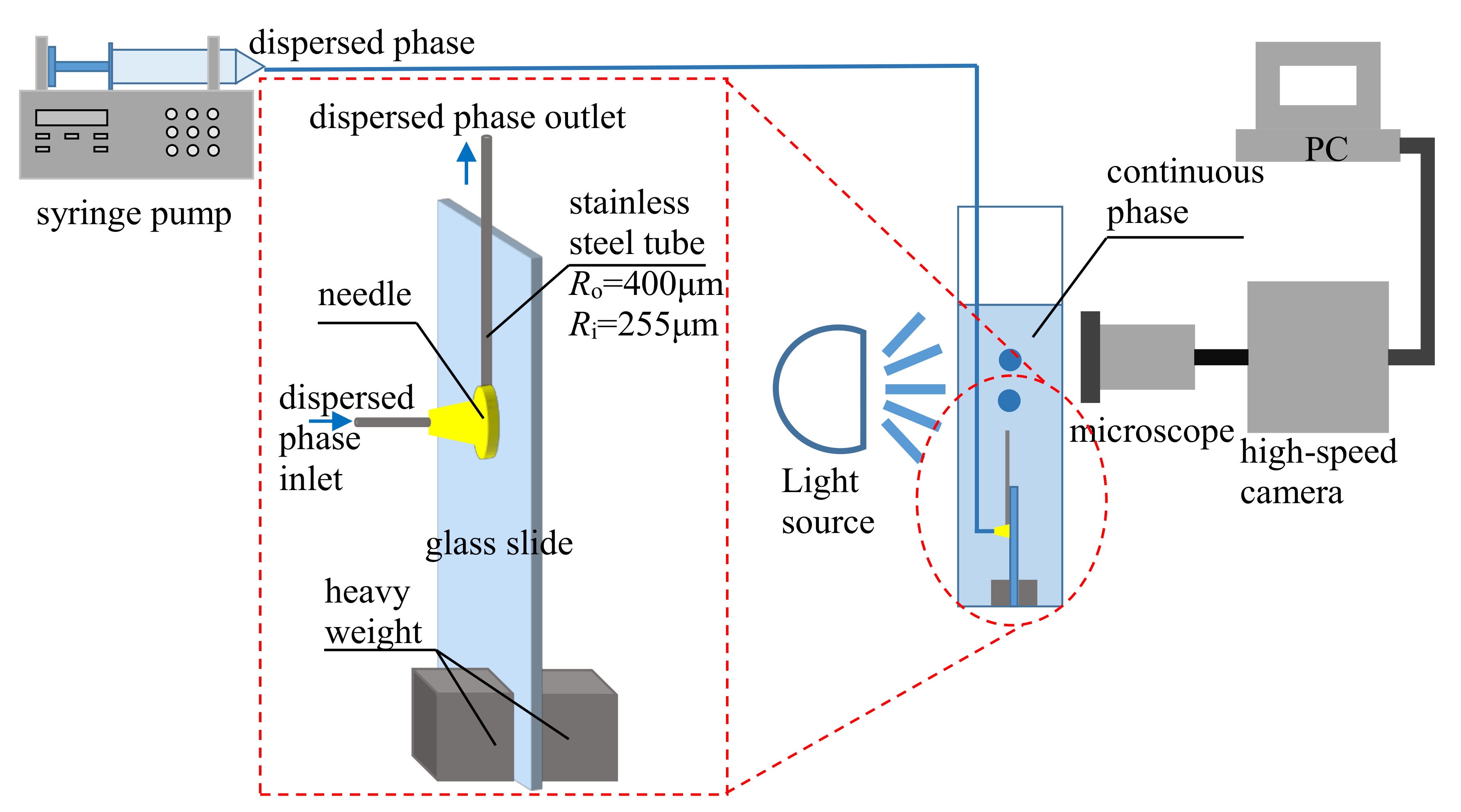
2. Experimental Setup
3. Mathematical Model
3.1. Governing Equations
3.2. Numerical Solution
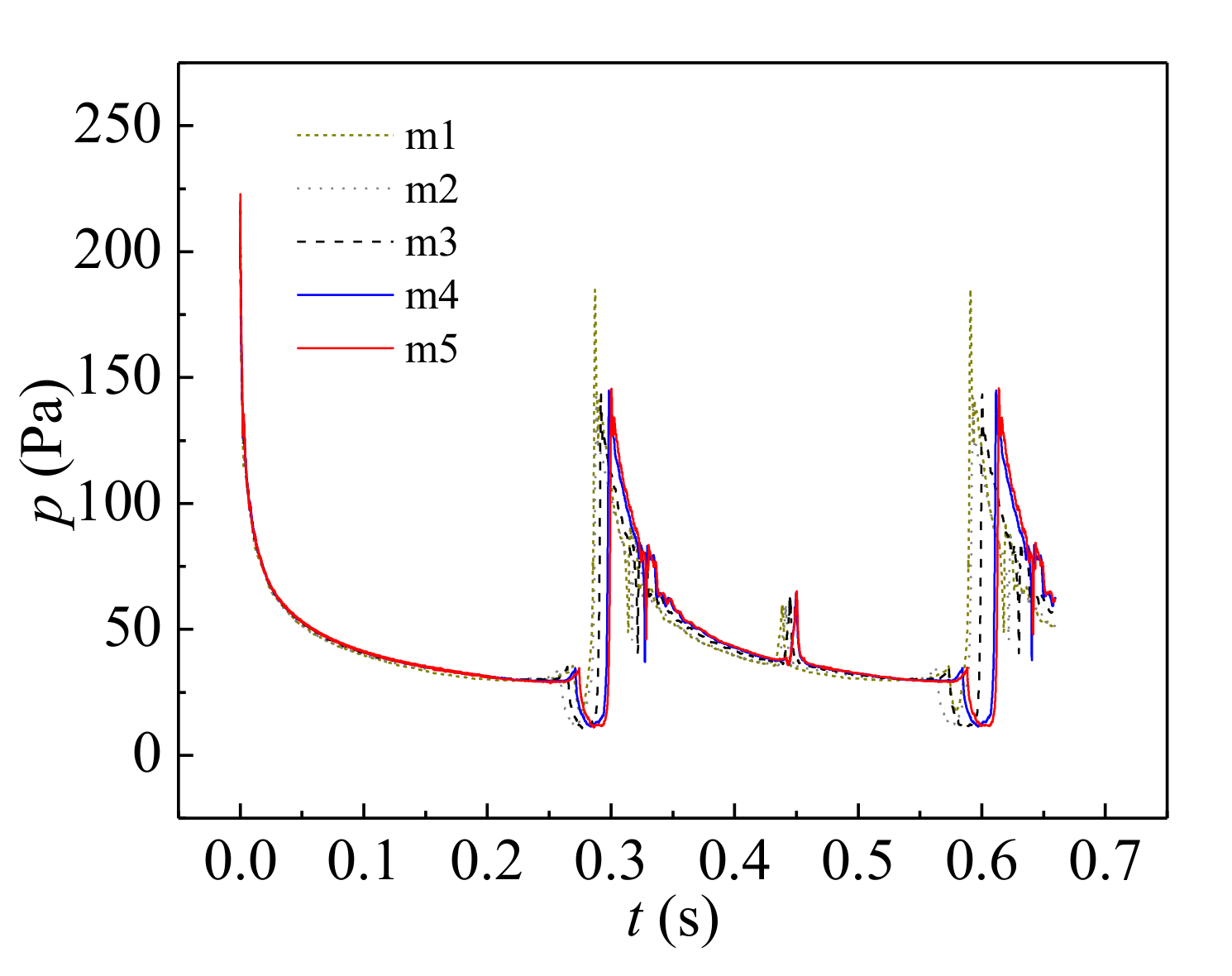
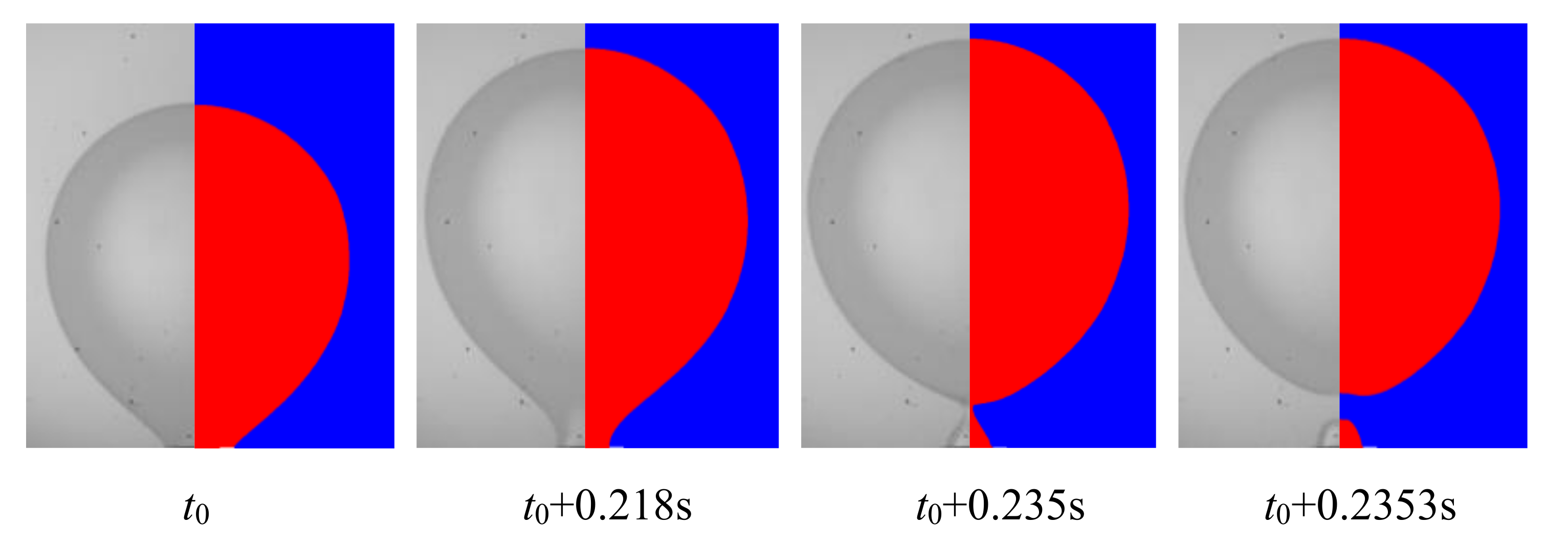
3.3. Case Validation
4. Results and Discussion
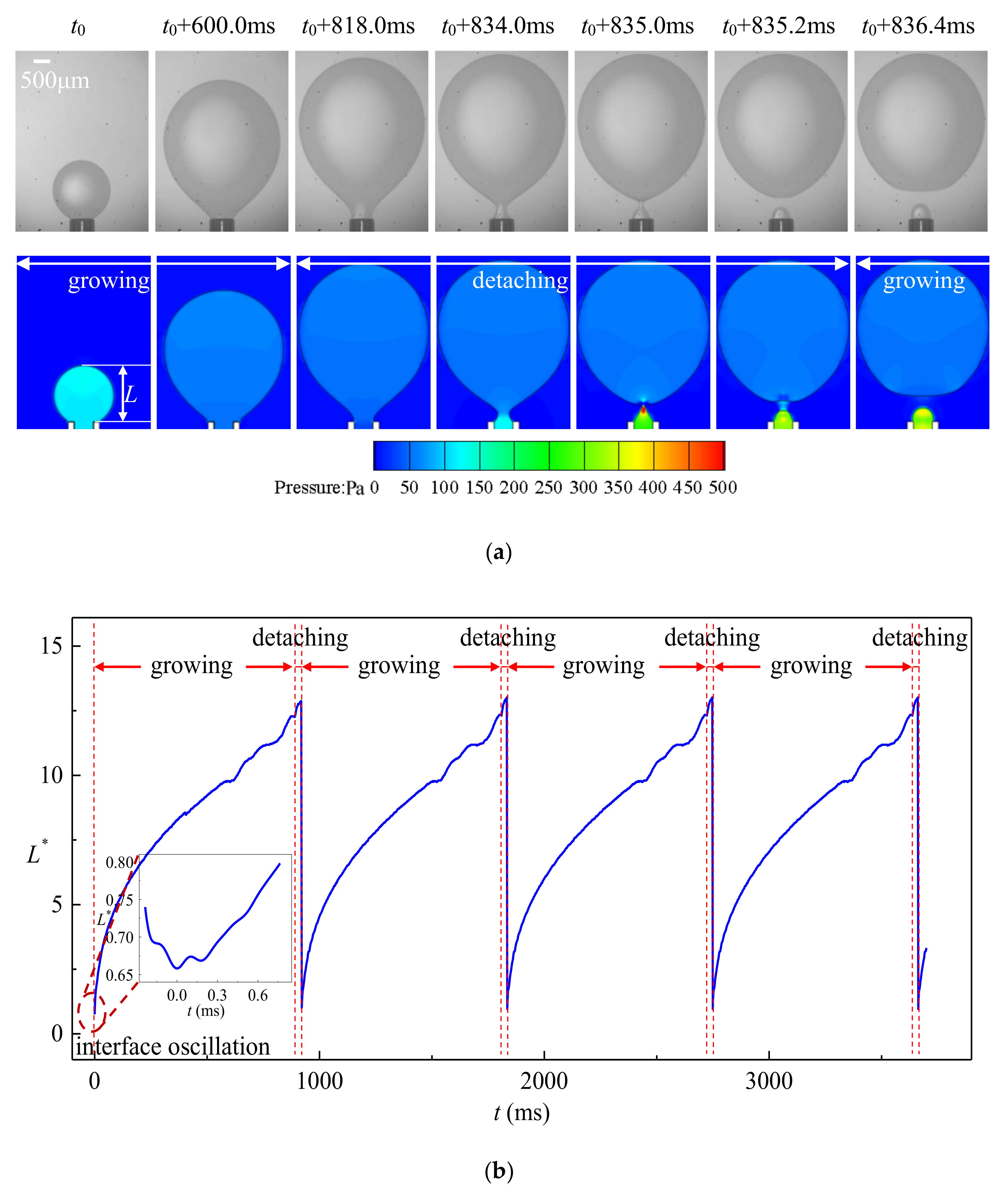
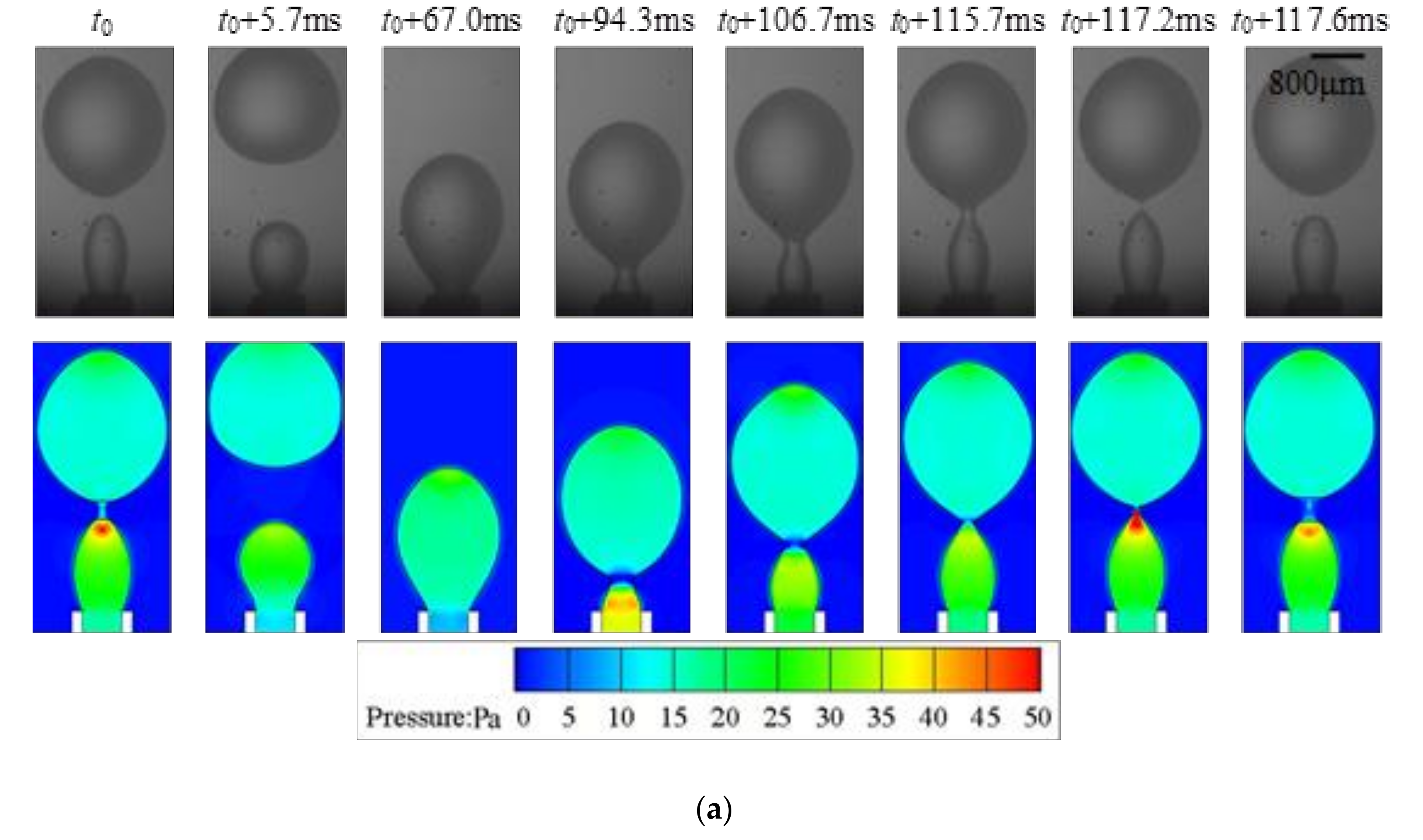
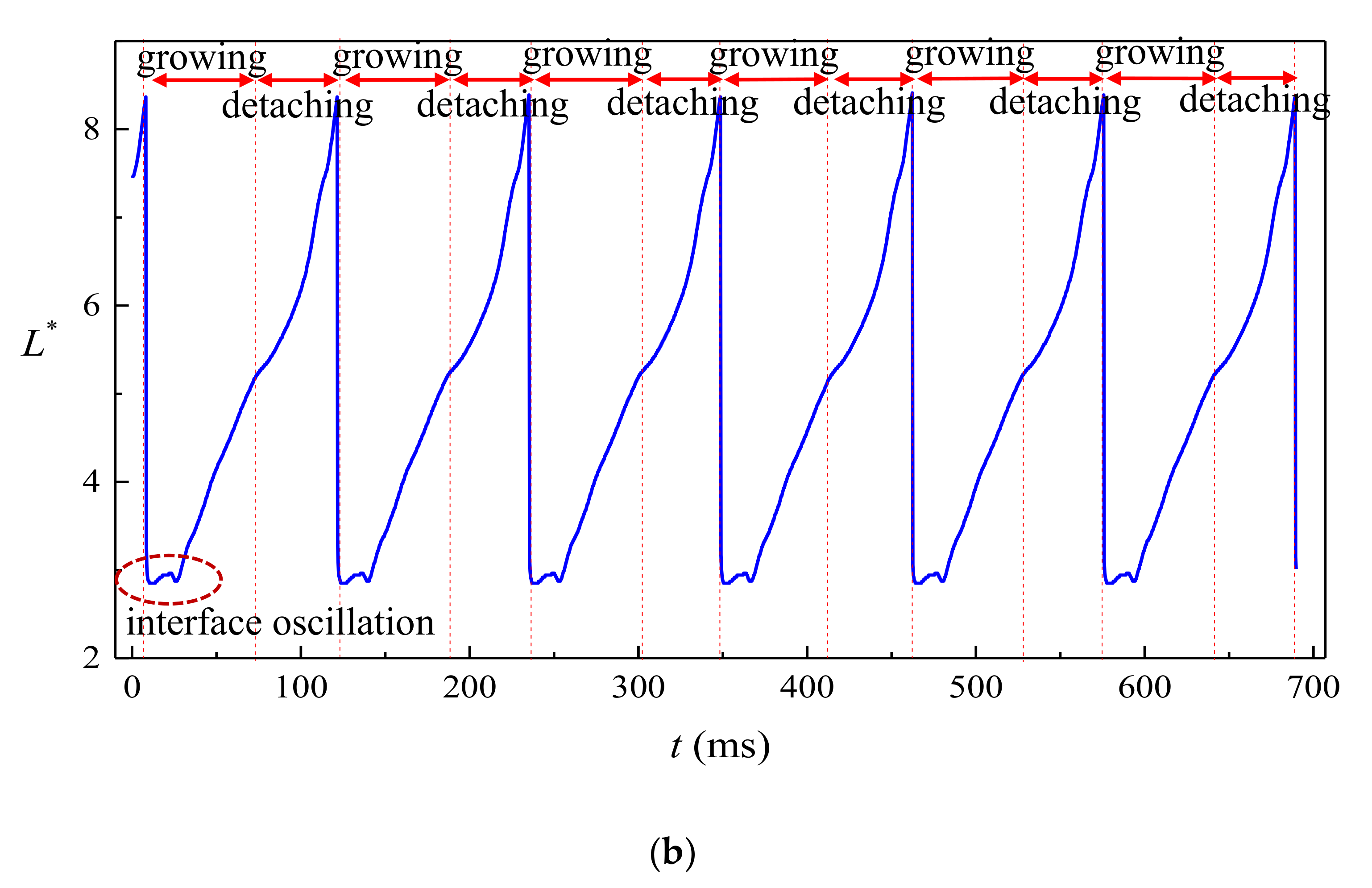
4.1. Dripping
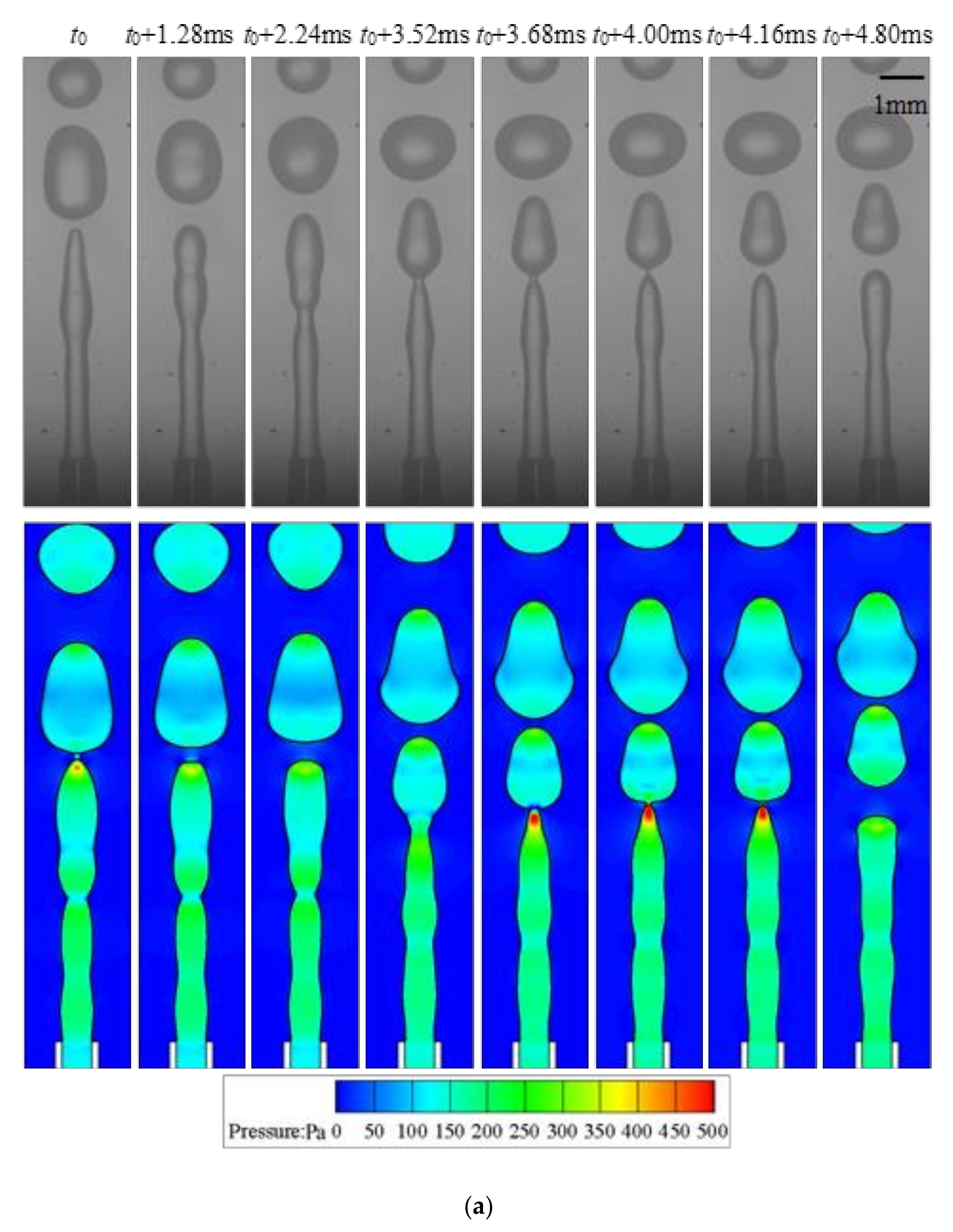
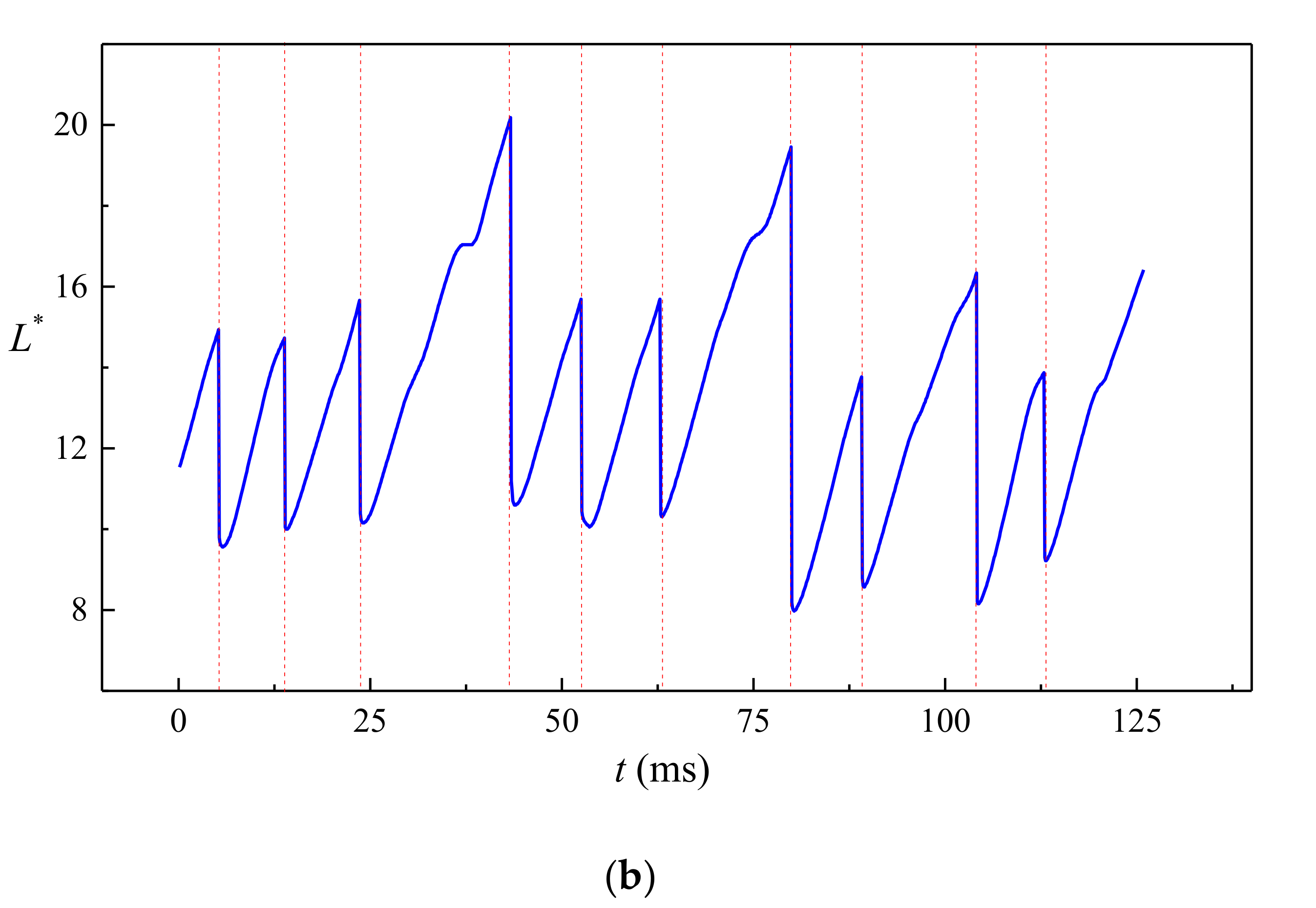
4.2. Jetting
4.3. Dripping-to-Jetting Transition
4.4. Effect of Interfacial Tension Coefficient
4.5. Regime Diagram
5. Conclusions
- (1)
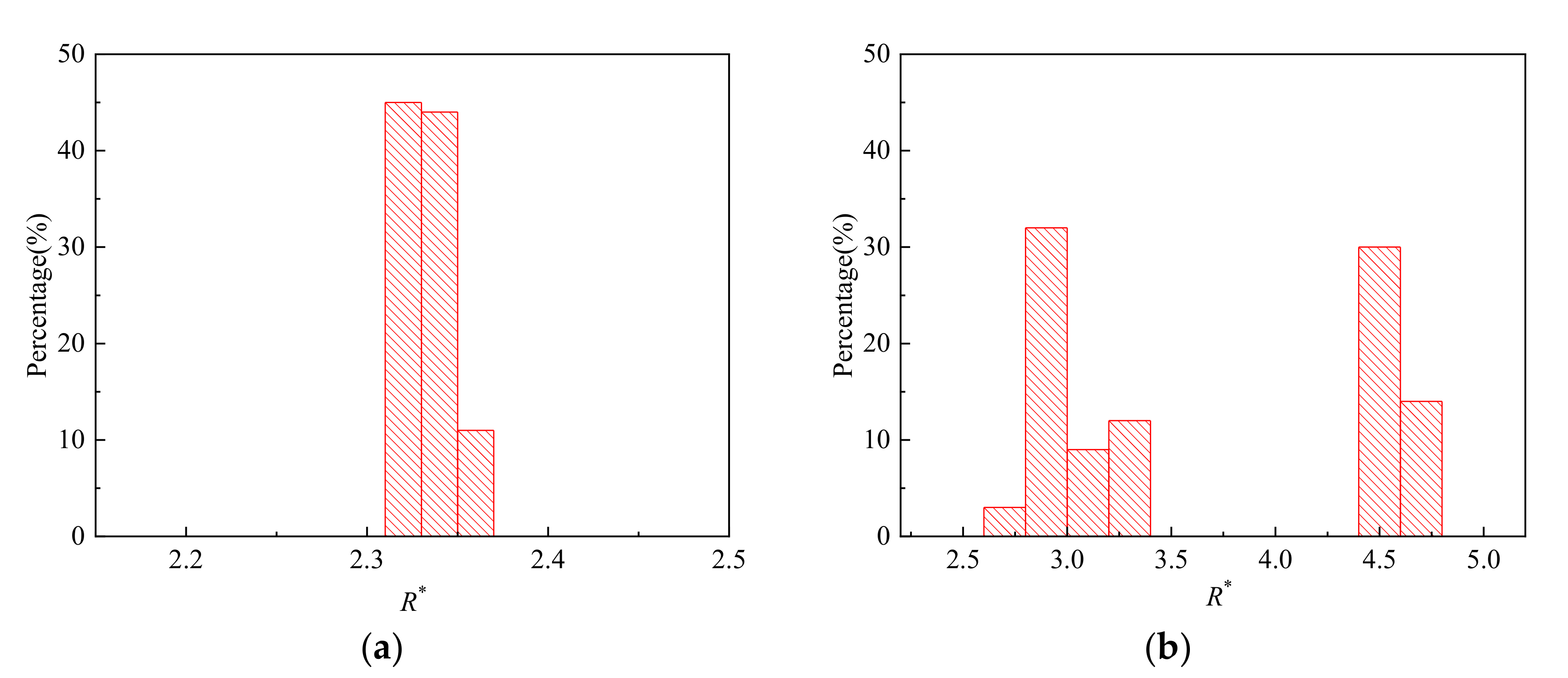
- Strong periodicity is observed in the dripping regime where highly monodispersed droplets can be produced with c.v. in radius below 0.1%. The growing stage occupies over 90% of the time in one droplet formation period. During the detaching stage, axisymmetric vortexes are observed inside the droplet caused by reversed flow of the dispersed phase near the axis. The dispersed phase downstream of the neck is squeezed and accelerated towards the flow direction, resulting in fast draining of the dispersed phase inside the neck.
- (2)
- The droplet length L* varies almost linearly with time in each droplet formation period in the jetting regime. Fluctuations on the interface are observed due to the development of the capillary wave. Droplets produced in the jetting regimes in buoyancy-assisted devices are always larger than the inner radius of the injection tube.
- (3)
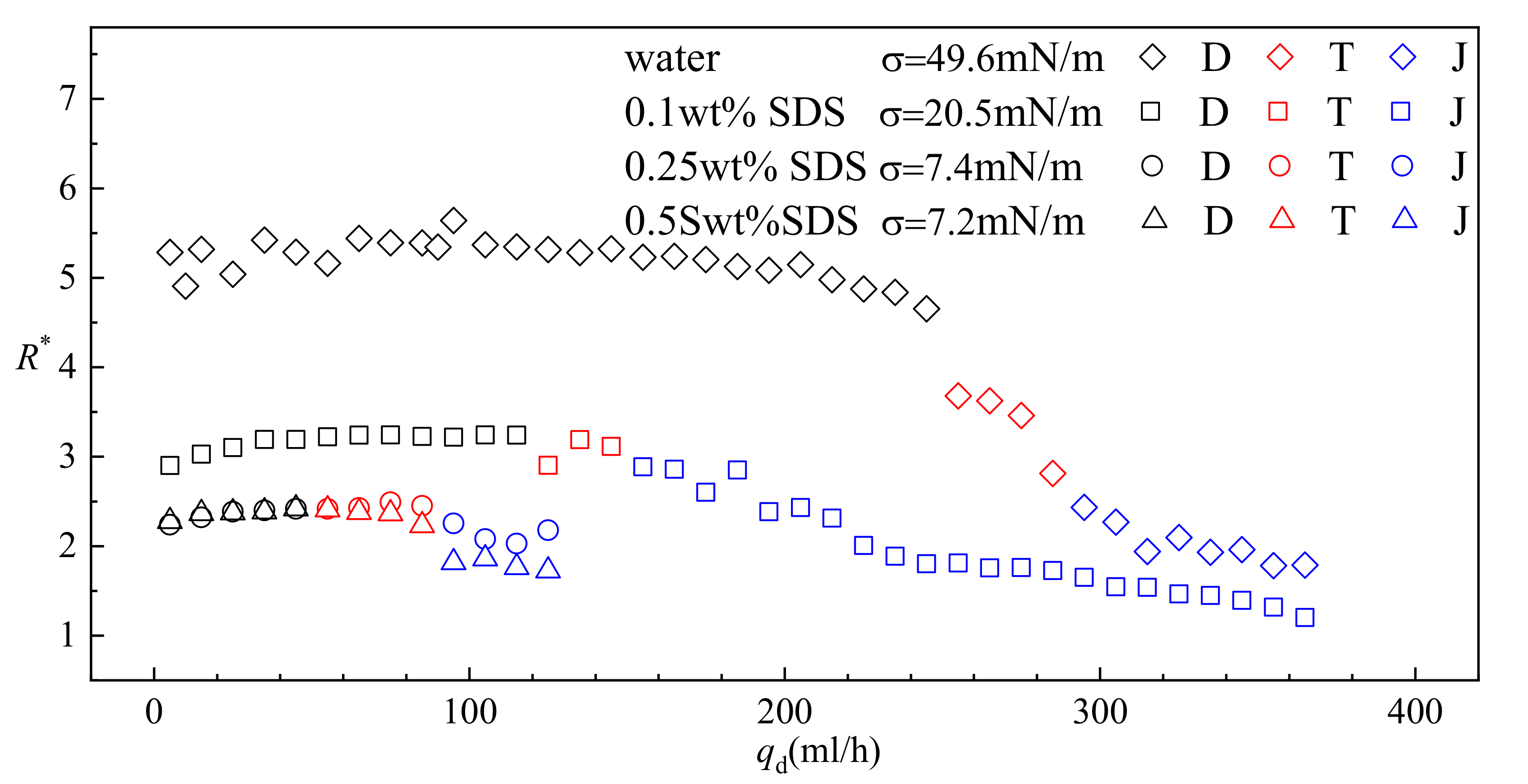
- Transition regimes with the characteristics of both dripping and jetting are observed when Weber number We is increased. The duration of the growing state in a transitional regime is shortened compared to dripping.
- (4)
- The sizes of the droplets are insensitive to the flow rate qd in dripping regimes. Reducing interfacial tension coefficient results in narrower range of qd that is covered by dripping. Moreover, the transition from dripping to jetting can be triggered at lower qd.
- (5)
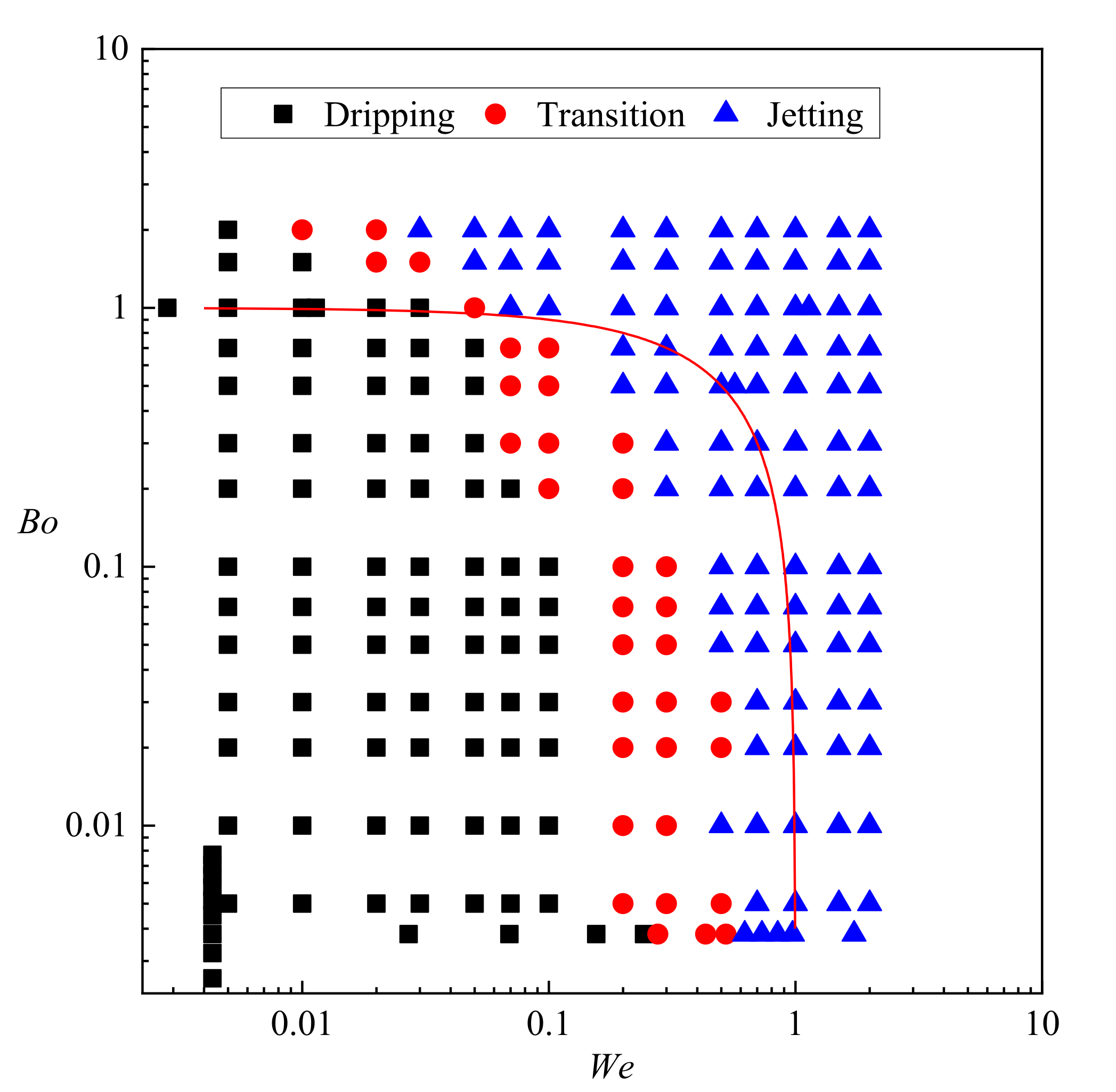
- The regime diagram suggests that most dripping cases occur when We + Bo < 1 while most jetting cases occur when We + Bo > 1. However, only sufficient inertial force from the dispersed phase is the indispensable condition of triggering the transition behavior from dripping to jetting. Even when buoyancy dominates over interfacial tension (Bo > 1), the droplets are still formed under dripping regime as long as We < 0.01. On the other hand, the formation regime is always jetting when We > 0.5 regardless of Bo.
Author Contributions
Funding
Conflicts of Interest
References
- Lan, K.; Liu, J.; Li, Z.; Xie, X.; Huo, W.; Chen, Y.; Ren, G.; Zheng, C.; Yang, D.; Li, S.; et al. Progress in octahedral spherical hohlraum study. Matter Radiat. Extrem. 2016, 1, 8–27. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Gao, W.; Zhang, C.; Zhao, Y. Three-dimensional splitting microfluidics. Lab Chip 2016, 16, 1332–1339. [Google Scholar] [CrossRef]
- Vladisavljevic, G.T.; Kobayashi, I.; Nakajima, M. Production of uniform droplets using membrane, microchannel and microfluidic emulsification devices. Microfluid. Nanofluid. 2012, 13, 151–178. [Google Scholar] [CrossRef] [Green Version]
- Vladisavljevic, G.T.; Khalid, N.; Neves, M.A.; Kuroiwa, T.; Nakajima, M.; Uemura, K.; Ichikawa, S.; Kobayashi, I. Industrial lab-on-a-chip: Design, applications and scale-up for drug discovery and delivery. Adv. Drug Deliver Rev. 2013, 65, 1626–1663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Z.Y.; Bai, B.F. Dynamics of capsules enclosing viscoelastic fluid in simple shear flow. J. Fluid Mech. 2018, 840, 656–687. [Google Scholar] [CrossRef]
- Shin, S.; Doan, V.S.; Feng, J. Osmotic delivery and release of lipid-encapsulated molecules via sequential solution exchange. Phys. Rev. Appl. 2019, 12, 024014. [Google Scholar] [CrossRef]
- Mary, P.; Chen, A.; Chen, I.; Abate, A.R.; Weitz, D.A. On-chip background noise reduction for cell-based assays in droplets. Lab Chip 2011, 11, 2066–2070. [Google Scholar] [CrossRef]
- Wang, J.; Gao, W.; Zhang, H.; Zou, M.H.; Chen, Y.P.; Zhao, Y.J. Programmable wettability on photocontrolled graphene film. Sci. Adv. 2018, 4, eaat7392. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Liu, Y.; Zhao, Y.; Zhang, L.; Zhang, L.; Mao, H.; Huang, C. Nanotechnology-assisted isolation and analysis of circulating tumor cells on microfluidic devices. Micromachines 2020, 11, 774. [Google Scholar] [CrossRef] [PubMed]
- Ganan-Calvo, A.M. Generation of steady liquid microthreads and micron-sized monodisperse sprays in gas streams. Phys. Rev. Lett. 1998, 80, 285–288. [Google Scholar] [CrossRef]
- Chu, L.Y.; Utada, A.S.; Shah, R.K.; Kim, J.W.; Weitz, D.A. Controllable monodisperse multiple emulsions. Angew. Chem. Int. Ed. 2007, 46, 8970–8974. [Google Scholar] [CrossRef]
- Li, E.Q.; Zhang, J.M.; Thoroddsen, S.T. Simple and inexpensive microfluidic devices for the generation of monodisperse multiple emulsions. J. Micromech. Microeng. 2013, 24, 015019. [Google Scholar] [CrossRef]
- Shah, R.K.; Shum, H.C.; Rowat, A.C.; Lee, D.; Agresti, J.J.; Utada, A.S.; Chu, L.Y.; Kim, J.W.; Fernandez-Nieves, A.; Martinez, C.J.; et al. Designer emulsions using microfluidics. Mater. Today 2008, 11, 18–27. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, L. Passive and active droplet generation with microfluidics: A review. Lab Chip 2017, 17, 34–75. [Google Scholar] [CrossRef] [PubMed]
- Lagus, T.P.; Edd, J.F. A review of the theory, methods and recent applications of high-throughput single-cell droplet microfluidics. J. Phys. D Appl. Phys. 2013, 46, 114005. [Google Scholar] [CrossRef]
- Christopher, G.F.; Anna, S.L. Microfluidic methods for generating continuous droplet streams. J. Phys. D Appl. Phys. 2007, 40, R319–R336. [Google Scholar] [CrossRef]
- Karbstein, H.; Schubert, H. Developments in the continuous mechanical production of oil-in-water macro-emulsions. Chem. Eng. Process. Process Intensif. 1995, 34, 205–211. [Google Scholar] [CrossRef]
- Modarres-Gheisari, S.M.M.; Gavagsaz-Ghoachani, R.; Malaki, M.; Safarpour, P.; Zandi, M. Ultrasonic nano-emulsification—A review. Ultrason. Sonochem. 2019, 52, 88–105. [Google Scholar] [CrossRef]
- Anna, S.L.; Bontoux, N.; Stone, H.A. Formation of dispersions using “flow focusing” in microchannels. Appl. Phys. Lett. 2003, 82, 364–366. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Shi, M. Hydrodynamics of double emulsion droplet in shear flow. Appl. Phys. Lett. 2013, 102, 051609. [Google Scholar] [CrossRef]
- Chong, Z.Z.; Tan, S.H.; Ganan-Calvo, A.M.; Tor, S.B.; Loh, N.H.; Nguyen, N.T. Active droplet generation in microfluidics. Lab Chip 2016, 16, 35–58. [Google Scholar] [CrossRef] [Green Version]
- Fu, F.F.; Shang, L.R.; Chen, Z.Y.; Yu, Y.R.; Zhao, Y.J. Bioinspired living structural color hydrogels. Sci. Robot. 2018, 3, eaar8580. [Google Scholar] [CrossRef] [Green Version]
- Umbanhowar, P.B.; Prasad, V.; Weitz, D.A. Monodisperse emulsion generation via drop break off in a coflowing stream. Langmuir 2000, 16, 347–351. [Google Scholar] [CrossRef]
- Takeuchi, S.; Garstecki, P.; Weibel, D.B.; Whitesides, G.M. An axisymmetric flow-focusing microfluidic device. Adv. Mater. 2005, 17, 1067–1072. [Google Scholar] [CrossRef]
- Fu, T.; Ma, Y.; Funfschilling, D.; Zhu, C.; Li, H.Z. Squeezing-to-dripping transition for bubble formation in a microfluidic t-junction. Chem. Eng. Sci. 2010, 65, 3739–3748. [Google Scholar] [CrossRef]
- Ferraro, D.; Champ, J.; Teste, B.; Serra, M.; Malaquin, L.; Viovy, J.L.; de Cremoux, P.; Descroix, S. Microfluidic platform combining droplets and magnetic tweezers: Application to her2 expression in cancer diagnosis. Sci. Rep. 2016, 6, 25540. [Google Scholar] [CrossRef] [Green Version]
- Mao, Y.; Pan, Y.; Li, X.; Li, B.; Chu, J.; Pan, T. High-precision digital droplet pipetting enabled by a plug-and-play microfluidic pipetting chip. Lab Chip 2018, 18, 2720–2729. [Google Scholar] [CrossRef]
- Ofner, A.; Mattich, I.; Hagander, M.; Dutto, A.; Seybold, H.; Rühs, P.A.; Studart, A.R. Controlled massive encapsulation via tandem step emulsification in glass. Adv. Funct. Mater. 2019, 29, 1806821. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, Z. Hydrodynamics of a droplet passing through a microfluidic t-junction. J. Fluid Mech. 2017, 819, 401–434. [Google Scholar] [CrossRef]
- Vladisavljević, G.T.; Al Nuumani, R.; Nabavi, S.A. Microfluidic production of multiple emulsions. Micromachines 2017, 8, 75. [Google Scholar] [CrossRef]
- Richards, J.R.; Beris, A.N.; Lenhoff, A.M. Drop formation in liquid–liquid systems before and after jetting. Phys. Fluids 1995, 7, 2617–2630. [Google Scholar] [CrossRef]
- Zhang, D.F.; Stone, H.A. Drop formation in viscous flows at a vertical capillary tube. Phys. Fluids 1997, 9, 2234–2242. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Basaran, O.A. An experimental study of dynamics of drop formation. Phys. Fluids 1995, 7, 1184–1203. [Google Scholar] [CrossRef]
- Zhang, X. Dynamics of drop formation in viscous flows. Chem. Eng. Sci. 1999, 54, 1759–1774. [Google Scholar] [CrossRef]
- Garstecki, P.; Gitlin, I.; DiLuzio, W.; Whitesides, G.M.; Kumacheva, E.; Stone, H.A. Formation of monodisperse bubbles in a microfluidic flow-focusing device. Appl. Phys. Lett. 2004, 85, 2649–2651. [Google Scholar] [CrossRef] [Green Version]
- Martin-Banderas, L.; Flores-Mosquera, M.; Riesco-Chueca, P.; Rodriguez-Gil, A.; Cebolla, A.; Chavez, S.; Ganan-Calvo, A.M. Flow focusing: A versatile technology to produce size-controlled and specific-morphology microparticles. Small 2005, 1, 688–692. [Google Scholar] [CrossRef]
- Ganan-Calvo, A.M. Perfectly monodisperse microbubbling by capillary flow focusing: An alternate physical description and universal scaling. Phys. Rev. E 2004, 69, 027301. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, W.; Zhao, Y.; Chen, Y. Microfluidic generation of self-contained multicomponent microcapsules for self-healing materials. Appl. Phys. Lett. 2018, 113, 203702. [Google Scholar] [CrossRef]
- Liu, M.; Su, L.; Li, J.; Chen, S.; Liu, Y.; Li, J.; Li, B.; Chen, Y.; Zhang, Z. Investigation of spherical and concentric mechanism of compound droplets. Matter Radiat. Extrem. 2016, 1, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Chaurasia, A.S.; Josephides, D.N.; Sajjadi, S. Buoyancy-driven drop generation via microchannel revisited. Microfluid. Nanofluid. 2015, 18, 943–953. [Google Scholar] [CrossRef]
- Chaurasia, A.S.; Sajjadi, S. Millimetric core–shell drops via buoyancy assisted non-confined microfluidics. Chem. Eng. Sci. 2015, 129, 260–270. [Google Scholar] [CrossRef]
- Che, Z.; Wong, T.N.; Nguyen, N.T. A simple method for the formation of water-in-oil-in-water (w/o/w) double emulsions. Microfluid. Nanofluid. 2017, 21, 8. [Google Scholar] [CrossRef]
- Tate, T. Xxx. On the magnitude of a drop of liquid formed under different circumstances. Philos. Mag. 1864, 27, 176–180. [Google Scholar] [CrossRef]
- Du Noüy, P.L. A new apparatus for measuring surface tension. J. Gen. Physiol. 1919, 1, 521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Yu, F.; Li, X.; Chen, Y. Gravity–capillary evaporation regimes in microgrooves. AIChE J. 2019, 65, 1119–1125. [Google Scholar] [CrossRef]
- Zhou, C.; Yue, P.; Feng, J.J. Formation of simple and compound drops in microfluidic devices. Phys. Fluids 2006, 18, 092105. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Lu, Y.C.; Xu, J.H.; Luo, G.S. Determination of dynamic interfacial tension and its effect on droplet formation in the t-shaped microdispersion process. Langmuir 2009, 25, 2153–2158. [Google Scholar] [CrossRef]
- Roumpea, E.; Kovalchuk, N.M.; Chinaud, M.; Nowak, E.; Simmons, M.J.H.; Angeli, P. Experimental studies on droplet formation in a flow-focusing microchannel in the presence of surfactants. Chem. Eng. Sci. 2019, 195, 507–518. [Google Scholar] [CrossRef]
- Xu, J.H.; Dong, P.F.; Zhao, H.; Tostado, C.P.; Luo, G.S. The dynamic effects of surfactants on droplet formation in coaxial microfluidic devices. Langmuir 2012, 28, 9250–9258. [Google Scholar] [CrossRef]
- Baret, J.-C. Surfactants in droplet-based microfluidics. Lab Chip 2012, 12, 422–433. [Google Scholar] [CrossRef]
- Ferraro, D.; Serra, M.; Filippi, D.; Zago, L.; Guglielmin, E.; Pierno, M.; Descroix, S.; Viovy, J.L.; Mistura, G. Controlling the distance of highly confined droplets in a capillary by interfacial tension for merging on-demand. Lab Chip 2019, 19, 136–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Utada, A.S.; Fernandez-Nieves, A.; Stone, H.A.; Weitz, D.A. Dripping to jetting transitions in coflowing liquid streams. Phys. Rev. Lett. 2007, 99, 094502. [Google Scholar] [CrossRef] [PubMed]


| Material | Density ρ (kg/m3) | Interfacial Tension Coefficient σ (mN/m) |
|---|---|---|
| n-octane | 702 | |
| DI water | 998.2 | 49.6 |
| 0.1 wt% SDS in DI water | 998.2 | 20.5 |
| 0.25 wt% SDS in DI water | 998.2 | 7.4 |
| 0.5 wt% SDS in DI water | 998.2 | 7.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, C.; Liu, F.; Wu, L.; Yu, C.; Yu, W. Dripping, Jetting and Regime Transition of Droplet Formation in a Buoyancy-Assisted Microfluidic Device. Micromachines 2020, 11, 962. https://doi.org/10.3390/mi11110962
Shen C, Liu F, Wu L, Yu C, Yu W. Dripping, Jetting and Regime Transition of Droplet Formation in a Buoyancy-Assisted Microfluidic Device. Micromachines. 2020; 11(11):962. https://doi.org/10.3390/mi11110962
Chicago/Turabian StyleShen, Chaoqun, Feifan Liu, Liangyu Wu, Cheng Yu, and Wei Yu. 2020. "Dripping, Jetting and Regime Transition of Droplet Formation in a Buoyancy-Assisted Microfluidic Device" Micromachines 11, no. 11: 962. https://doi.org/10.3390/mi11110962
APA StyleShen, C., Liu, F., Wu, L., Yu, C., & Yu, W. (2020). Dripping, Jetting and Regime Transition of Droplet Formation in a Buoyancy-Assisted Microfluidic Device. Micromachines, 11(11), 962. https://doi.org/10.3390/mi11110962





