Acoustic Microfluidic Separation Techniques and Bioapplications: A Review
Abstract
1. Introduction
2. Theory and Background
2.1. Acoustic Wave
2.2. Drag Force
2.3. Acoustic Radiation Force
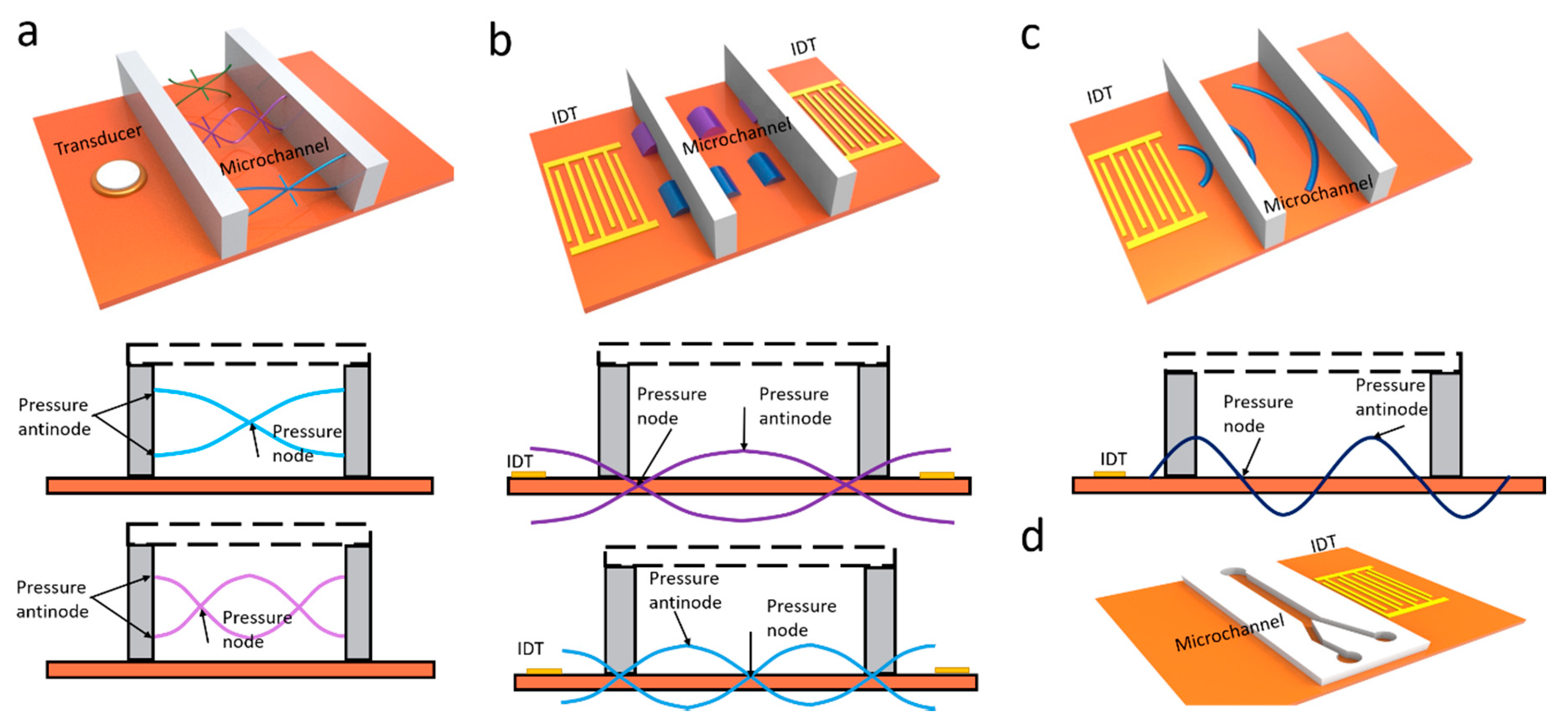
3. BAW-Based Separation
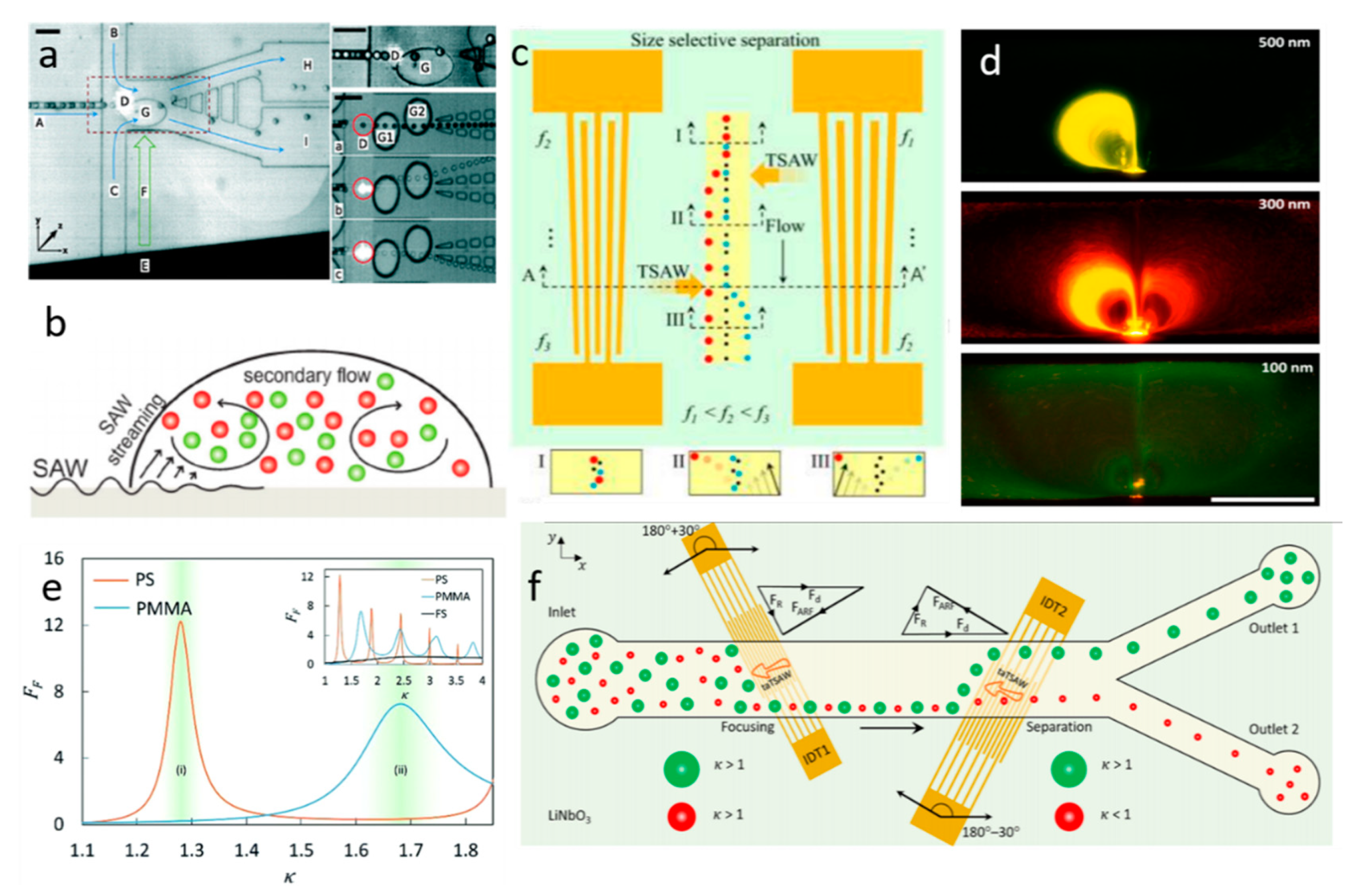
Microbubble-Based Separation
4. SAW-Based Separation
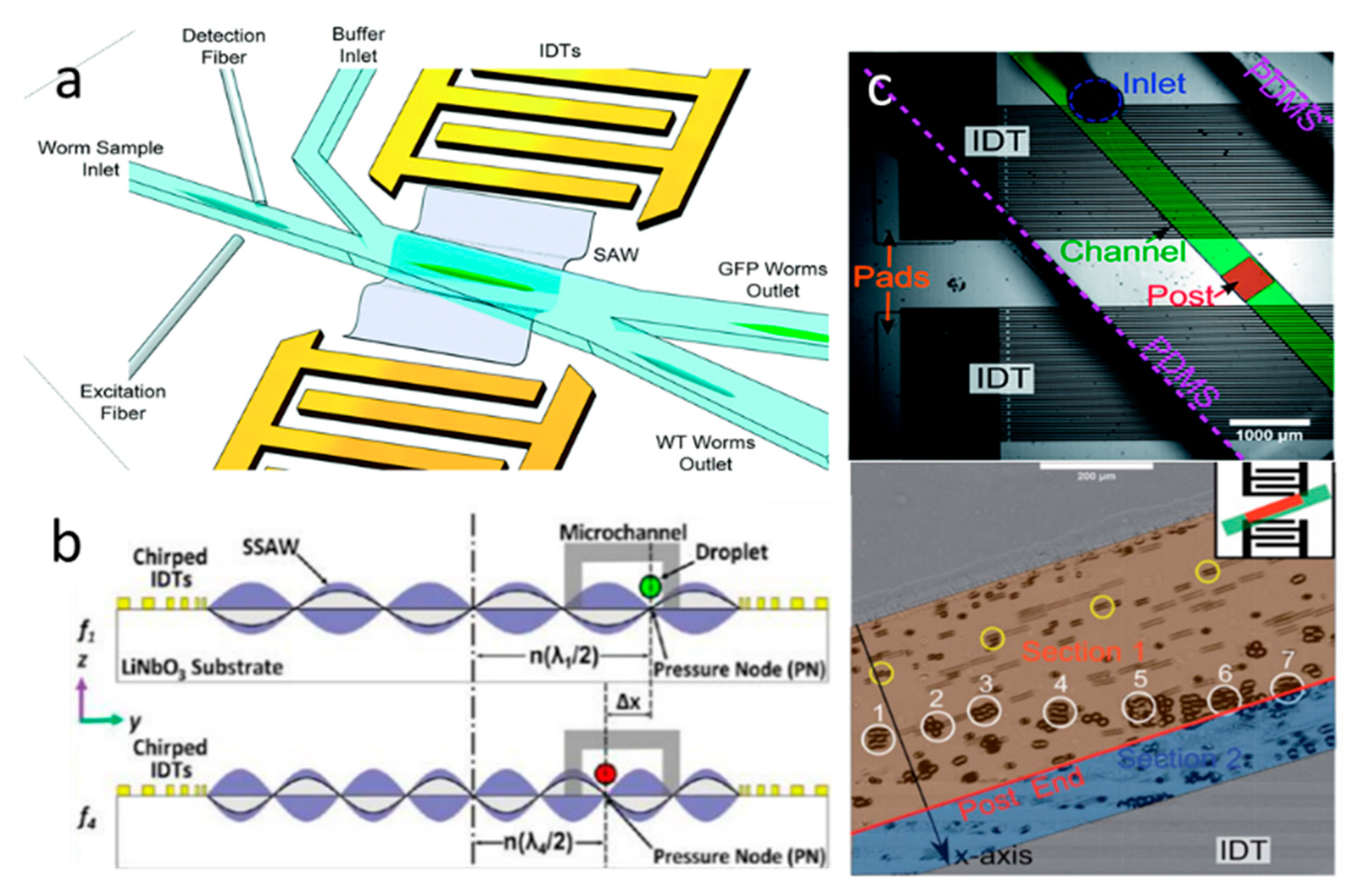
4.1. TSAW-Based Separation
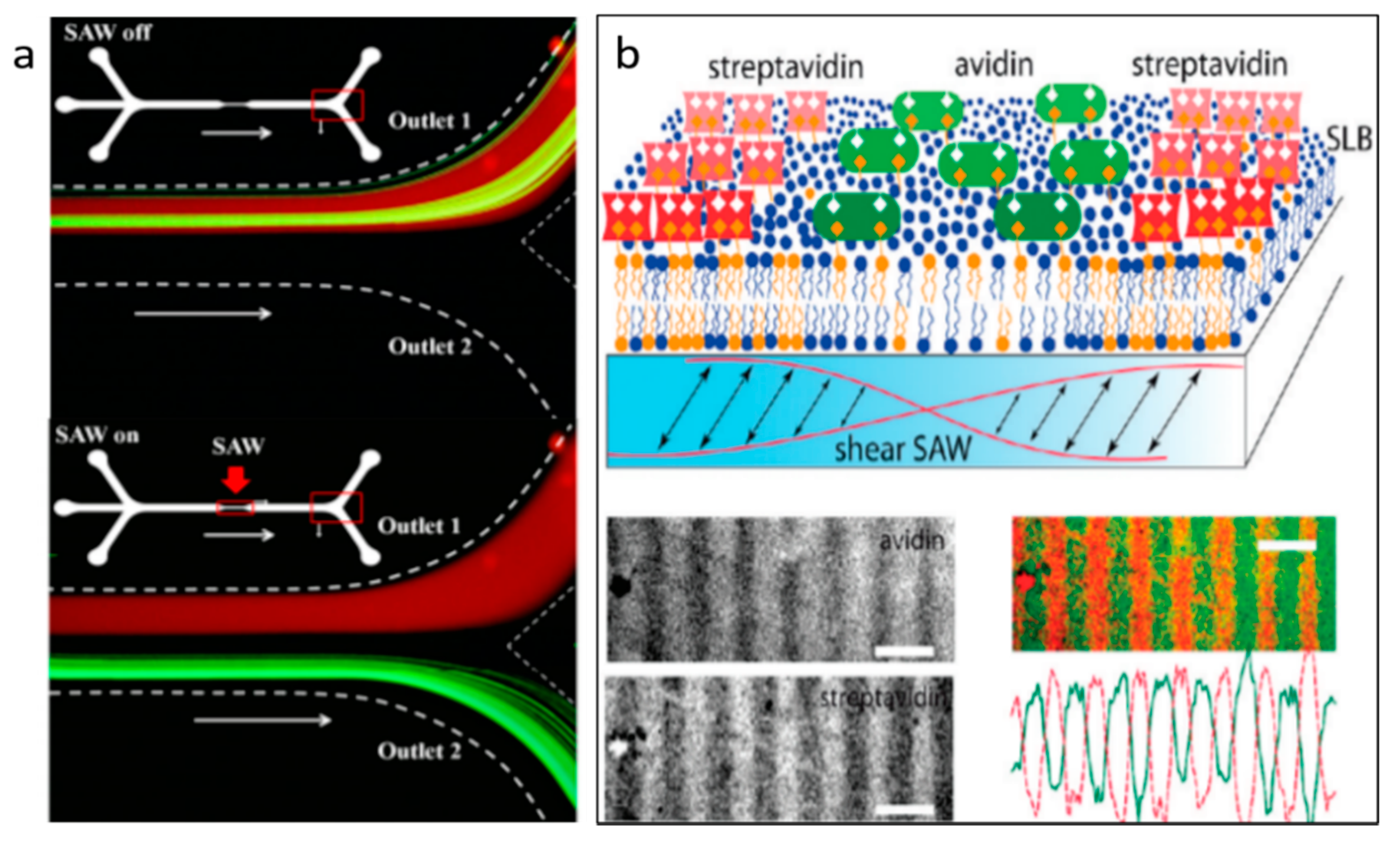
4.2. SSAW-Based Separation
5. Exemplary Applications of Acoustic Micro-Object Separation
5.1. Cell Separation
5.2. Protein Separation
5.3. Virus Separation
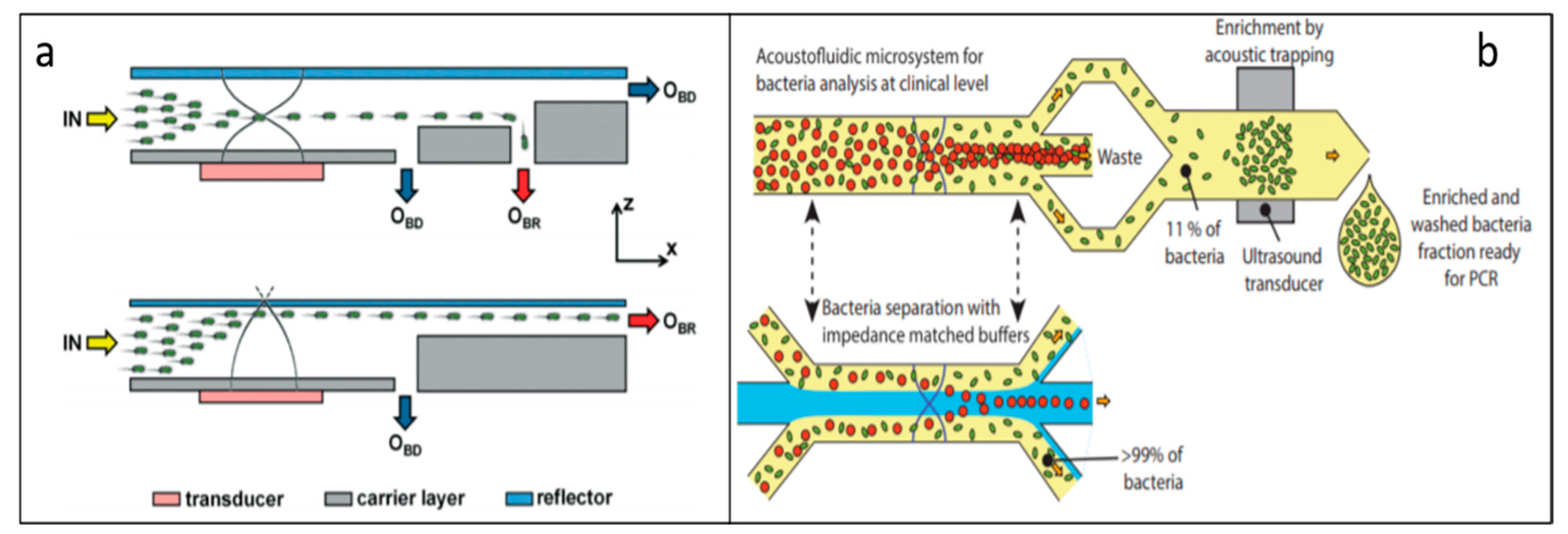
5.4. Bacteria Separation
5.5. DNA/RNA Separation
6. Conclusions and Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- van Voorthuizen, E.; Zwijnenburg, A.; van der Meer, W.; Temmink, H. Biological black water treatment combined with membrane separation. Water Res. 2008, 42, 4334–4340. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.J.; Fluri, K.; Seiler, K.; Fan, Z.; Effenhauser, C.S.; Manz, A. Micromachining a miniaturized capillary electrophoresis-based chemical analysis system on a chip. Science 1993, 261, 895–897. [Google Scholar] [CrossRef] [PubMed]
- Hafez, A.; Khedr, M.; Gadallah, H. Wastewater treatment and water reuse of food processing industries. Part II: Techno-economic study of a membrane separation technique. Desalination 2007, 214, 261–272. [Google Scholar] [CrossRef]
- Marella, C.; Muthukumarappan, K.; Metzger, L. Application of membrane separation technology for developing novel dairy food ingredients. J. Food Process. Technol. 2013, 4, 269. [Google Scholar]
- Dassey, A.J.; Theegala, C.S. Harvesting economics and strategies using centrifugation for cost effective separation of microalgae cells for biodiesel applications. Bioresour. Technol. 2013, 128, 241–245. [Google Scholar] [CrossRef]
- Zhu, Z.; Lu, J.J.; Liu, S. Protein separation by capillary gel electrophoresis: A review. Anal. Chim. Acta 2012, 709, 21–31. [Google Scholar] [CrossRef]
- Jirage, K.B.; Hulteen, J.C.; Martin, C.R. Nanotubule-based molecular-filtration membranes. Science 1997, 278, 655–658. [Google Scholar] [CrossRef]
- Vidal, L.; Riekkola, M.-L.; Canals, A. Ionic liquid-modified materials for solid-phase extraction and separation: A review. Anal. Chim. Acta 2012, 715, 19–41. [Google Scholar] [CrossRef]
- Yao, Y.; Hou, J.; Xu, Z.; Li, G.; Yang, Y. Effects of solvent mixtures on the nanoscale phase separation in polymer solar cells. Adv. Funct. Mater. 2008, 18, 1783–1789. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef]
- Kannel, W.B.; Anderson, K.; Wilson, P.W. White blood cell count and cardiovascular disease: Insights from the Framingham Study. JAMA 1992, 267, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Kragsbjerg, P.; Jones, I.; Vikerfors, T.; Holmberg, H. Diagnostic value of blood cytokine concentrations in acute pneumonia. Thorax 1995, 50, 1253–1257. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Irimia, D.; Dixon, M.; Sekine, K.; Demirci, U.; Zamir, L.; Tompkins, R.G.; Rodriguez, W.; Toner, M. A microfluidic device for practical label-free CD4+ T cell counting of HIV-infected subjects. Lab Chip 2007, 7, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Horsman, K.M.; Barker, S.L.; Ferrance, J.P.; Forrest, K.A.; Koen, K.A.; Landers, J.P. Separation of sperm and epithelial cells in a microfabricated device: Potential application to forensic analysis of sexual assault evidence. Anal. Chem. 2005, 77, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Lewpiriyawong, N.; Yang, C.; Lam, Y.C. Continuous sorting and separation of microparticles by size using AC dielectrophoresis in a PDMS microfluidic device with 3-D conducting PDMS composite electrodes. Electrophoresis 2010, 31, 2622–2631. [Google Scholar] [CrossRef]
- Ng, A.H.; Choi, K.; Luoma, R.P.; Robinson, J.M.; Wheeler, A.R. Digital microfluidic magnetic separation for particle-based immunoassays. Anal. Chem. 2012, 84, 8805–8812. [Google Scholar] [CrossRef]
- Dienerowitz, M.; Mazilu, M.; Dholakia, K. Optical manipulation of nanoparticles: A review. J. Nanophotonics 2008, 2, 021875. [Google Scholar] [CrossRef]
- Wang, G.; Mao, W.; Byler, R.; Patel, K.; Henegar, C.; Alexeev, A.; Sulchek, T. Stiffness dependent separation of cells in a microfluidic device. PLoS ONE 2013, 8, e75901. [Google Scholar] [CrossRef]
- Sajeesh, P.; Sen, A.K. Particle separation and sorting in microfluidic devices: A review. Microfluid. Nanofluid. 2014, 17, 1–52. [Google Scholar] [CrossRef]
- Patel, M.V.; Nanayakkara, I.A.; Simon, M.G.; Lee, A.P. Cavity-induced microstreaming for simultaneous on-chip pumping and size-based separation of cells and particles. Lab Chip 2014, 14, 3860–3872. [Google Scholar] [CrossRef]
- Wu, M.; Ouyang, Y.; Wang, Z.; Zhang, R.; Huang, P.-H.; Chen, C.; Li, H.; Li, P.; Quinn, D.; Dao, M. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc. Natl. Acad. Sci. USA 2017, 114, 10584–10589. [Google Scholar] [CrossRef] [PubMed]
- Wiklund, M. Acoustofluidics 12: Biocompatibility and cell viability in microfluidic acoustic resonators. Lab Chip 2012, 12, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, M.; Lin, Y.; Zhao, W.; Xu, J. Acoustic bubble-based bidirectional micropump. Microfluid. Nanofluid. 2020, 24, 29. [Google Scholar] [CrossRef]
- Wu, M.; Ozcelik, A.; Rufo, J.; Wang, Z.; Fang, R.; Huang, T.J. Acoustofluidic separation of cells and particles. Microsyst. Nanoeng. 2019, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Shields IV, C.W.; Reyes, C.D.; López, G.P. Microfluidic cell sorting: A review of the advances in the separation of cells from debulking to rare cell isolation. Lab Chip 2015, 15, 1230–1249. [Google Scholar] [CrossRef] [PubMed]
- Lenshof, A.; Evander, M.; Laurell, T.; Nilsson, J. Acoustofluidics 5: Building microfluidic acoustic resonators. Lab Chip 2012, 12, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Rayleigh, L. On waves propagated along the plane surface of an elastic solid. Proc. Lond. Math. Soc. 1885, 1, 4–11. [Google Scholar] [CrossRef]
- Destgeer, G.; Sung, H.J. Recent advances in microfluidic actuation and micro-object manipulation via surface acoustic waves. Lab Chip 2015, 15, 2722–2738. [Google Scholar] [CrossRef]
- Gautam, G.P.; Burger, T.; Wilcox, A.; Cumbo, M.J.; Graves, S.W.; Piyasena, M.E. Simple and inexpensive micromachined aluminum microfluidic devices for acoustic focusing of particles and cells. Anal. Bioanal. Chem. 2018, 410, 3385–3394. [Google Scholar] [CrossRef]
- Ding, X.; Li, P.; Lin, S.-C.S.; Stratton, Z.S.; Nama, N.; Guo, F.; Slotcavage, D.; Mao, X.; Shi, J.; Costanzo, F. Surface acoustic wave microfluidics. Lab Chip 2013, 13, 3626–3649. [Google Scholar] [CrossRef]
- Lighthill, J. Acoustic streaming. J. Sound Vib. 1978, 61, 391–418. [Google Scholar] [CrossRef]
- Manasseh, R. Acoustic bubbles, acoustic streaming, and cavitation microstreaming. Handb. Ultrason. Sonochem. 2016, 33–68. [Google Scholar]
- Nyborg, W.L. Acoustic streaming due to attenuated plane waves. J. Acoust. Soc. Am. 1953, 25, 68–75. [Google Scholar] [CrossRef]
- King, L.V. On the acoustic radiation pressure on spheres. Proc. R. Soc. London. Ser. A-Math. Phys. Sci. 1934, 147, 212–240. [Google Scholar]
- Tan, M.K.; Tjeung, R.; Ervin, H.; Yeo, L.Y.; Friend, J. Double aperture focusing transducer for controlling microparticle motions in trapezoidal microchannels with surface acoustic waves. Appl. Phys. Lett. 2009, 95, 134101. [Google Scholar] [CrossRef]
- Yosioka, K.; Kawasima, Y. Acoustic radiation pressure on a compressible sphere. Acta Acust. United Acust. 1955, 5, 167–173. [Google Scholar]
- Woodside, S.M.; Bowen, B.D.; Piret, J.M. Measurement of ultrasonic forces for particle–liquid separations. Aiche J. 1997, 43, 1727–1736. [Google Scholar] [CrossRef]
- Whitworth, G.; Grundy, M.; Coakley, W. Transport and harvesting of suspended particles using modulated ultrasound. Ultrasonics 1991, 29, 439–444. [Google Scholar] [CrossRef]
- Bjerknes, V. Fields of Force: Supplementary Lectures, Applications to Meteorology; A Course of Lectures in Mathematical Physics Delivered December 1 to 23, 1905; The Columbia University Press: New York, NY, USA, 1906. [Google Scholar]
- Saeidi, D.; Saghafian, M.; Haghjooy Javanmard, S.; Wiklund, M. A Quantitative Study of the Secondary Acoustic Radiation Force on Biological Cells during Acoustophoresis. Micromachines 2020, 11, 152. [Google Scholar] [CrossRef]
- Doinikov, A.A. Acoustic radiation forces: Classical theory and recent advances. Recent Res. Dev. Acoust. 2003, 1, 39–67. [Google Scholar]
- Hashmi, A.; Yu, G.; Reilly-Collette, M.; Heiman, G.; Xu, J. Oscillating bubbles: A versatile tool for lab on a chip applications. Lab Chip 2012, 12, 4216–4227. [Google Scholar] [CrossRef] [PubMed]
- Leibacher, I.; Reichert, P.; Dual, J. Microfluidic droplet handling by bulk acoustic wave (BAW) acoustophoresis. Lab Chip 2015, 15, 2896–2905. [Google Scholar] [CrossRef] [PubMed]
- Devendran, C.; Gralinski, I.; Neild, A. Separation of particles using acoustic streaming and radiation forces in an open microfluidic channel. Microfluid. Nanofluid. 2014, 17, 879–890. [Google Scholar] [CrossRef]
- Dauson, E.R.; Gregory, K.B.; Greve, D.W.; Healy, G.P.; Oppenheim, I.J. Mechanically robust microfluidics and bulk wave acoustics to sort microparticles. In Proceedings of the Health Monitoring of Structural and Biological Systems, Las Vegas, NV, USA, 21–24 March 2016; p. 98051I. [Google Scholar]
- Gautam, G.P.; Gurung, R.; Fencl, F.A.; Piyasena, M.E. Separation of sub-micron particles from micron particles using acoustic fluid relocation combined with acoustophoresis. Anal. Bioanal. Chem. 2018, 410, 6561–6571. [Google Scholar] [CrossRef]
- Fornell, A.; Cushing, K.; Nilsson, J.; Tenje, M. Binary particle separation in droplet microfluidics using acoustophoresis. Appl. Phys. Lett. 2018, 112, 063701. [Google Scholar] [CrossRef]
- Lin, Y.; Gao, Y.; Wu, M.; Zhou, R.; Chung, D.; Caraveo, G.; Xu, J. Acoustofluidic stick-and-play micropump built on foil for single-cell trapping. Lab Chip 2019, 19, 3045–3053. [Google Scholar] [CrossRef]
- Lin, Y.; Gao, C.; Gao, Y.; Wu, M.; Yazdi, A.A.; Xu, J. Acoustofluidic micromixer on lab-on-a-foil devices. Sens. Actuators B Chem. 2019, 287, 312–319. [Google Scholar] [CrossRef]
- Ahmed, D.; Mao, X.; Juluri, B.K.; Huang, T.J. A fast microfluidic mixer based on acoustically driven sidewall-trapped microbubbles. Microfluid. Nanofluid. 2009, 7, 727. [Google Scholar] [CrossRef]
- Marmottant, P.; Hilgenfeldt, S. Controlled vesicle deformation and lysis by single oscillating bubbles. Nature 2003, 423, 153–156. [Google Scholar] [CrossRef]
- Rogers, P.; Neild, A. Selective particle trapping using an oscillating microbubble. Lab Chip 2011, 11, 3710–3715. [Google Scholar] [CrossRef]
- Wang, C.; Jalikop, S.V.; Hilgenfeldt, S. Size-sensitive sorting of microparticles through control of flow geometry. Appl. Phys. Lett. 2011, 99, 034101. [Google Scholar] [CrossRef]
- Xu, Y.H.; Hashmi, A.; Yu, G.; Lu, X.N.; Kwon, H.J.; Chen, X.L.; Xu, J. Microbubble array for on-chip worm processing (vol 102, 023702, 2013). Appl. Phys. Lett. 2013, 102, 023702. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, C. Acoustic bubble enhanced pinched flow fractionation for microparticle separation. J. Micromech. Microeng. 2015, 25, 084005. [Google Scholar] [CrossRef]
- Xie, Y.L.; Chindam, C.; Nama, N.; Yang, S.K.; Lu, M.Q.; Zhao, Y.H.; Mai, J.D.; Costanzo, F.; Huang, T.J. Exploring bubble oscillation and mass transfer enhancement in acoustic-assisted liquid-liquid extraction with a microfluidic device. Sci. Rep. 2015, 5, 12572. [Google Scholar] [CrossRef]
- Skowronek, V.; Rambach, R.W.; Schmid, L.; Haase, K.; Franke, T. Particle deflection in a poly (dimethylsiloxane) microchannel using a propagating surface acoustic wave: Size and frequency dependence. Anal. Chem. 2013, 85, 9955–9959. [Google Scholar] [CrossRef]
- Wiklund, M.; Green, R.; Ohlin, M. Acoustofluidics 14: Applications of acoustic streaming in microfluidic devices. Lab Chip 2012, 12, 2438–2451. [Google Scholar] [CrossRef]
- Franke, T.; Braunmuller, S.; Schmid, L.; Wixforth, A.; Weitz, D.A. Surface acoustic wave actuated cell sorting (SAWACS). Lab Chip 2010, 10, 789–794. [Google Scholar] [CrossRef]
- Schmid, L.; Weitz, D.A.; Franke, T. Sorting drops and cells with acoustics: Acoustic microfluidic fluorescence-activated cell sorter. Lab Chip 2014, 14, 3710–3718. [Google Scholar] [CrossRef]
- Bourquin, Y.; Syed, A.; Reboud, J.; Ranford-Cartwright, L.C.; Barrett, M.P.; Cooper, J.M. Rare-Cell Enrichment by a Rapid, Label-Free, Ultrasonic Isopycnic Technique for Medical Diagnostics. Angew. Chem. Int. Ed. 2014, 53, 5587–5590. [Google Scholar] [CrossRef]
- Destgeer, G.; Ha, B.H.; Jung, J.H.; Sung, H.J. Submicron separation of microspheres via travelling surface acoustic waves. Lab Chip 2014, 14, 4665–4672. [Google Scholar] [CrossRef]
- Destgeer, G.; Ha, B.H.; Park, J.; Jung, J.H.; Alazzam, A.; Sung, H.J. Microchannel Anechoic Corner for Size-Selective Separation and Medium Exchange via Traveling Surface Acoustic Waves. Anal. Chem. 2015, 87, 4627–4632. [Google Scholar] [CrossRef] [PubMed]
- Destgeer, G.; Jung, J.H.; Park, J.; Ahmed, H.; Park, K.; Ahmad, R.; Sung, H.J. Acoustic impedance-based manipulation of elastic microspheres using travelling surface acoustic waves. Rsc Adv. 2017, 7, 22524–22530. [Google Scholar] [CrossRef]
- Collins, D.J.; Ma, Z.C.; Ai, Y. Highly Localized Acoustic Streaming and Size-Selective Submicrometer Particle Concentration Using High Frequency Microscale Focused Acoustic Fields. Anal. Chem. 2016, 88, 5513–5522. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Destgeer, G.; Park, J.; Afzal, M.; Sung, H.J. Sheathless Focusing and Separation of Microparticles Using Tilted-Angle Traveling Surface Acoustic Waves. Anal. Chem. 2018, 90, 8546–8552. [Google Scholar] [CrossRef]
- Laurell, T.; Petersson, F.; Nilsson, A. Chip integrated strategies for acoustic separation and manipulation of cells and particles. Chem. Soc. Rev. 2007, 36, 492–506. [Google Scholar] [CrossRef]
- Guldiken, R.; Jo, M.C.; Gallant, N.D.; Demirci, U.; Zhe, J. Sheathless Size-Based Acoustic Particle Separation. Sensors 2012, 12, 905–922. [Google Scholar] [CrossRef]
- Shi, J.J.; Huang, H.; Stratton, Z.; Huang, Y.P.; Huang, T.J. Continuous particle separation in a microfluidic channel via standing surface acoustic waves (SSAW). Lab Chip 2009, 9, 3354–3359. [Google Scholar] [CrossRef]
- Nam, J.; Lee, Y.; Shin, S. Size-dependent microparticles separation through standing surface acoustic waves. Microfluid. Nanofluid. 2011, 11, 317–326. [Google Scholar] [CrossRef]
- Nam, J.; Lim, H.; Kim, C.; Kang, J.Y.; Shin, S. Density-dependent separation of encapsulated cells in a microfluidic channel by using a standing surface acoustic wave. Biomicrofluidics 2012, 6, 024120. [Google Scholar] [CrossRef]
- Xie, Y.; Mao, Z.; Bachman, H.; Li, P.; Zhang, P.; Ren, L.; Wu, M.; Huang, T.J. Acoustic Cell Separation Based on Density and Mechanical Properties. J. Biomech. Eng. 2020, 142, 031005. [Google Scholar] [CrossRef]
- Ren, L.Q.; Yang, S.J.; Zhang, P.R.; Qu, Z.G.; Mao, Z.M.; Huang, P.H.; Chen, Y.C.; Wu, M.X.; Wang, L.; Li, P. Standing Surface Acoustic Wave (SSAW)-Based Fluorescence-Activated Cell Sorter. Small 2018, 14, 1801996. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.A. Acoustofluidic Fluorescence Activated Cell Sorter. Anal. Chem. 2015, 87, 12051–12058. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hartman, J.H.; Chen, C.; Yang, S.; Li, Q.; Tian, Z.; Huang, P.-H.; Wang, L.; Meyer, J.N.; Huang, T.J. Fluorescence-based sorting of Caenorhabditis elegans via acoustofluidics. Lab Chip 2020, 20, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ding, X.; Guo, F.; Chen, Y.; Lapsley, M.I.; Lin, S.-C.S.; Wang, L.; McCoy, J.P.; Cameron, C.E.; Huang, T.J. An on-chip, multichannel droplet sorter using standing surface acoustic waves. Anal. Chem. 2013, 85, 5468–5474. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.K.; Bhethanabotla, V.R. Numerical analysis of wave generation and propagation in a focused surface acoustic wave device for potential microfluidics applications. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Chen, Y.; Li, P.; Mao, Z.; Huang, P.-H.; Rufo, J.; Guo, F.; Wang, L.; McCoy, J.P.; Levine, S.J. A high-throughput acoustic cell sorter. Lab Chip 2015, 15, 3870–3879. [Google Scholar] [CrossRef]
- Ding, X.Y.; Peng, Z.L.; Lin, S.C.S.; Geri, M.; Li, S.X.; Li, P.; Chen, Y.C.; Dao, M.; Suresh, S.; Huang, T.J. Cell separation using tilted-angle standing surface acoustic waves. Proc. Natl. Acad. Sci. USA 2014, 111, 12992–12997. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, M.; Yang, S.; Wu, Y.; Gu, Y.; Chen, C.; Ye, J.; Xie, Z.; Tian, Z.; Bachman, H. A disposable acoustofluidic chip for nano/microparticle separation using unidirectional acoustic transducers. Lab Chip 2020, 20, 1298–1308. [Google Scholar] [CrossRef] [PubMed]
- Rambach, R.W.; Skowronek, V.; Franke, T. Localization and shaping of surface acoustic waves using PDMS posts: Application for particle filtering and washing. Rsc Adv. 2014, 4, 60534–60542. [Google Scholar] [CrossRef]
- Shamloo, A.; Boodaghi, M. Design and simulation of a microfluidic device for acoustic cell separation. Ultrasonics 2018, 84, 234–243. [Google Scholar] [CrossRef]
- Li, S.X.; Ding, X.Y.; Mao, Z.M.; Chen, Y.C.; Nama, N.; Guo, F.; Li, P.; Wang, L.; Cameron, C.E.; Huang, T.J. Standing surface acoustic wave (SSAW)-based cell washing. Lab Chip 2015, 15, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Dykes, J.; Lenshof, A.; Åstrand-Grundström, B.; Laurell, T.; Scheding, S. Efficient removal of platelets from peripheral blood progenitor cell products using a novel micro-chip based acoustophoretic platform. PLoS ONE 2011, 6, e23074. [Google Scholar] [CrossRef] [PubMed]
- Phan, H.V.; Sesen, M.; Alan, T.; Neild, A. Single line particle focusing using a vibrating bubble. Appl. Phys. Lett. 2014, 105, 193507. [Google Scholar] [CrossRef]
- Shamloo, A.; Parast, F.Y. Simulation of Blood Particle Separation in a Trapezoidal Microfluidic Device by Acoustic Force. IEEE Trans. Electron. Devices 2019, 66, 1495–1503. [Google Scholar] [CrossRef]
- Gupta, T.; Ghosh, R.; Ganguly, R. Acoustophoretic separation of infected erythrocytes from blood plasma in a microfluidic platform using biofunctionalized, matched-impedance layers. Int. J. Numer. Methods Biomed. Eng. 2018, 34, e2943. [Google Scholar] [CrossRef]
- Lu, X.L.; Martin, A.D.; Soto, F.; Angsantikul, P.; Li, J.X.; Chen, C.R.; Liang, Y.Y.; Hu, J.H.; Zhang, L.F.; Wang, J. Parallel Label-Free Isolation of Cancer Cells Using Arrays of Acoustic Microstreaming Traps. Adv. Mater. Technol. 2019, 4, 1800374. [Google Scholar] [CrossRef]
- Collins, D.J.; Khoo, B.L.; Ma, Z.C.; Winkler, A.; Weser, R.; Schmidt, H.; Han, J.; Ai, Y. Selective particle and cell capture in a continuous flow using micro-vortex acoustic streaming. Lab Chip 2017, 17, 1769–1777. [Google Scholar] [CrossRef]
- Li, P.; Mao, Z.M.; Peng, Z.L.; Zhou, L.L.; Chen, Y.C.; Huang, P.H.; Truica, C.I.; Drabick, J.J.; El-Deiry, W.S.; Dao, M.; et al. Acoustic separation of circulating tumor cells. Proc. Natl. Acad. Sci. USA 2015, 112, 4970–4975. [Google Scholar] [CrossRef]
- Faridi, M.A.; Ramachandraiah, H.; Iranmanesh, I.; Grishenkov, D.; Wiklund, M.; Russom, A. MicroBubble activated acoustic cell sorting. Biomed. Microdevices 2017, 19, 23. [Google Scholar] [CrossRef]
- Yang, A.H.J.; Soh, H.T. Acoustophoretic Sorting of Viable Mammalian Cells in a Microfluidic Device. Anal. Chem. 2012, 84, 10756–10762. [Google Scholar] [CrossRef]
- Olofsson, K.; Hammarström, B.; Wiklund, M. Acoustic separation of living and dead cells using high density medium. Lab Chip 2020, 20, 1981–1990. [Google Scholar] [CrossRef] [PubMed]
- Hartono, D.; Liu, Y.; Tan, P.L.; Then, X.Y.S.; Yung, L.Y.L.; Lim, K.M. On-chip measurements of cell compressibility via acoustic radiation. Lab Chip 2011, 11, 4072–4080. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Bragheri, F.; Nava, G.; Chiodi, I.; Mondello, C.; Osellame, R.; Berg-Sorensen, K.; Cristiani, I.; Minzioni, P. A comprehensive strategy for the analysis of acoustic compressibility and optical deformability on single cells. Sci. Rep. 2016, 6, 23946. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.Y.; Zhou, W.; Lin, Z.G.; Cai, F.Y.; Li, F.; Wu, J.R.; Meng, L.; Niu, L.L.; Zheng, H.R. Sorting of tumour cells in a microfluidic device by multi-stage surface acoustic waves. Sens. Actuators B Chem. 2018, 258, 1174–1183. [Google Scholar] [CrossRef]
- Antfolk, M.; Magnusson, C.; Augustsson, P.; Lija, H.; Laurell, T. Acoustofluidic, Label-Free Separation and Simultaneous Concentration of Rare Tumor Cells from White Blood Cells. Anal. Chem. 2015, 87, 9322–9328. [Google Scholar] [CrossRef]
- Iranmanesh, I.; Ramachandraiah, H.; Russom, A.; Wiklund, M. On-chip ultrasonic sample preparation for cell based assays. Rsc Adv. 2015, 5, 74304–74311. [Google Scholar] [CrossRef]
- Norris, J.V.; Evander, M.; Horsman-Hall, K.M.; Nilsson, J.; Laurell, T.; Landers, J.P. Acoustic Differential Extraction for Forensic Analysis of Sexual Assault Evidence. Anal. Chem. 2009, 81, 6089–6095. [Google Scholar] [CrossRef]
- Xu, K.; Clark, C.P.; Poe, B.L.; Lounsbury, J.A.; Nilsson, J.; Lauren, T.; Landers, J.P. Isolation of a Low Number of Sperm Cells from Female DNA in a Glass-PDMS-Glass Microchip via Bead-Assisted Acoustic Differential Extraction. Anal. Chem. 2019, 91, 2186–2191. [Google Scholar] [CrossRef]
- Hao, H.C.; Chang, H.Y.; Wang, T.P.; Yao, D.J. Detection of Cells Captured with Antigens on Shear Horizontal Surface-Acoustic-Wave Sensors. J. Lab. Autom. 2013, 18, 69–76. [Google Scholar] [CrossRef]
- Lenshof, A.; Jamal, A.; Dykes, J.; Urbansky, A.; Astrand-Grundstrom, I.; Laurell, T.; Scheding, S. Efficient Purification of CD4+Lymphocytes from Peripheral Blood Progenitor Cell Products Using Affinity Bead Acoustophoresis. Cytom. A 2014, 85a, 933–941. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Diffley, J.F.X.; Cocker, J.H. Protein DNA Interactions at a Yeast Replication Origin. Nature 1992, 357, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Leake, M.C.; Greene, N.P.; Godun, R.M.; Granjon, T.; Buchanan, G.; Chen, S.; Berry, R.M.; Palmer, T.; Berks, B.C. Variable stoichiometry of the TatA component of the twin-arginine protein transport system observed by in vivo single-molecule imaging. Proc. Natl. Acad. Sci. USA 2008, 105, 15376–15381. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Destgeer, G.; Afzal, M.; Park, J.; Ahmed, H.; Jung, J.H.; Park, K.; Yoon, T.S.; Sung, H.J. Acoustic Wave-Driven Functionalized Particles for Aptamer-Based Target Biomolecule Separation. Anal. Chem. 2017, 89, 13313–13319. [Google Scholar] [CrossRef] [PubMed]
- Neumann, J.; Hennig, M.; Wixforth, A.; Manus, S.; Radler, J.O.; Schneider, M.F. Transport, Separation, and Accumulation of Proteins on Supported Lipid Bilayers. Nano Lett. 2010, 10, 2903–2908. [Google Scholar] [CrossRef]
- Fong, E.J.; Johnston, A.C.; Notton, T.; Jung, S.Y.; Rose, K.A.; Weinberger, L.S.; Shusteff, M. Acoustic focusing with engineered node locations for high-performance microfluidic particle separation. Analyst 2014, 139, 1192–1200. [Google Scholar] [CrossRef]
- Jung, B.; Fisher, K.; Ness, K.D.; Rose, K.A.; Mariella, R.P. Acoustic Particle Filter with Adjustable Effective Pore Size for Automated Sample Preparation. Anal. Chem. 2008, 80, 8447–8452. [Google Scholar] [CrossRef]
- Mach, A.J.; Adeyiga, O.B.; Di Carlo, D. Microfluidic sample preparation for diagnostic cytopathology. Lab Chip 2013, 13, 1011–1026. [Google Scholar] [CrossRef]
- Ai, Y.; Sanders, C.K.; Marrone, B.L. Separation of Escherichia coli Bacteria from Peripheral Blood Mononuclear Cells Using Standing Surface Acoustic Waves. Anal. Chem. 2013, 85, 9126–9134. [Google Scholar] [CrossRef]
- Dow, P.; Kotz, K.; Gruszka, S.; Holder, J.; Fiering, J. Acoustic separation in plastic microfluidics for rapid detection of bacteria in blood using engineered bacteriophage. Lab Chip 2018, 18, 923–932. [Google Scholar] [CrossRef]
- Silva, R.; Dow, P.; Dubay, R.; Lissandrello, C.; Holder, J.; Densmore, D.; Fiering, J. Rapid prototyping and parametric optimization of plastic acoustofluidic devices for blood-bacteria separation. Biomed. Microdevices 2017, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Li, S.X.; Ma, F.; Bachman, H.; Cameron, C.E.; Zeng, X.Q.; Huang, T.J. Acoustofluidic bacteria separation. J. Micromech. Microeng. 2017, 27, 015031. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, P.; Petersson, K.; Augustsson, P.; Laurell, T. Acoustic impedance matched buffers enable separation of bacteria from blood cells at high cell concentrations. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Carugo, D.; Octon, T.; Messaoudi, W.; Fisher, A.L.; Carboni, M.; Harris, N.R.; Hill, M.; Glynne-Jones, P. A thin-reflector microfluidic resonator for continuous-flow concentration of microorganisms: A new approach to water quality analysis using acoustofluidics. Lab Chip 2014, 14, 3830–3842. [Google Scholar] [CrossRef]
- Antfolk, M.; Muller, P.B.; Augustsson, P.; Bruus, H.; Laurell, T. Focusing of sub-micrometer particles and bacteria enabled by two-dimensional acoustophoresis. Lab Chip 2014, 14, 2791–2799. [Google Scholar] [CrossRef]
- Mitchenall, L.A.; Hipkin, R.E.; Piperakis, M.M.; Burton, N.P.; Maxwell, A. A rapid high-resolution method for resolving DNA topoisomers. Bmc Res. Notes 2018, 11, 37. [Google Scholar] [CrossRef]
- Iranmanesh, I.; Ohlin, M.; Ramachandraiah, H.; Ye, S.; Russom, A.; Wiklund, M. Acoustic micro-vortexing of fluids, particles and cells in disposable microfluidic chips. Biomed. Microdevices 2016, 18. [Google Scholar] [CrossRef]
- Taller, D.; Richards, K.; Slouka, Z.; Senapati, S.; Hill, R.; Go, D.B.; Chang, H.C. On-chip surface acoustic wave lysis and ion-exchange nanomembrane detection of exosomal RNA for pancreatic cancer study and diagnosis. Lab Chip 2015, 15, 1656–1666. [Google Scholar] [CrossRef]
- Miansari, M.; Friend, J.R. Acoustic Nanofluidics via Room-Temperature Lithium Niobate Bonding: A Platform for Actuation and Manipulation of Nanoconfined Fluids and Particles. Adv. Funct. Mater. 2016, 26, 7861–7872. [Google Scholar] [CrossRef]
- Lissandrello, C.; Dubay, R.; Kotz, K.T.; Fiering, J. Purification of lymphocytes by acoustic separation in plastic microchannels. Slas Technol. Transl. Life Sci. Innov. 2018, 23, 352–363. [Google Scholar] [CrossRef]
- Yazdi, A.A.; Popma, A.; Wong, W.; Nguyen, T.; Pan, Y.; Xu, J. 3D printing: An emerging tool for novel microfluidics and lab-on-a-chip applications. Microfluid. Nanofluid. 2016, 20, 50. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Wu, M.; Lin, Y.; Xu, J. Acoustic Microfluidic Separation Techniques and Bioapplications: A Review. Micromachines 2020, 11, 921. https://doi.org/10.3390/mi11100921
Gao Y, Wu M, Lin Y, Xu J. Acoustic Microfluidic Separation Techniques and Bioapplications: A Review. Micromachines. 2020; 11(10):921. https://doi.org/10.3390/mi11100921
Chicago/Turabian StyleGao, Yuan, Mengren Wu, Yang Lin, and Jie Xu. 2020. "Acoustic Microfluidic Separation Techniques and Bioapplications: A Review" Micromachines 11, no. 10: 921. https://doi.org/10.3390/mi11100921
APA StyleGao, Y., Wu, M., Lin, Y., & Xu, J. (2020). Acoustic Microfluidic Separation Techniques and Bioapplications: A Review. Micromachines, 11(10), 921. https://doi.org/10.3390/mi11100921





