Micropatterning Method for Porous Materials Using the Difference of the Glass Transition Temperature between Exposed and Unexposed Areas of a Thick-Photoresist
Abstract
1. Introduction
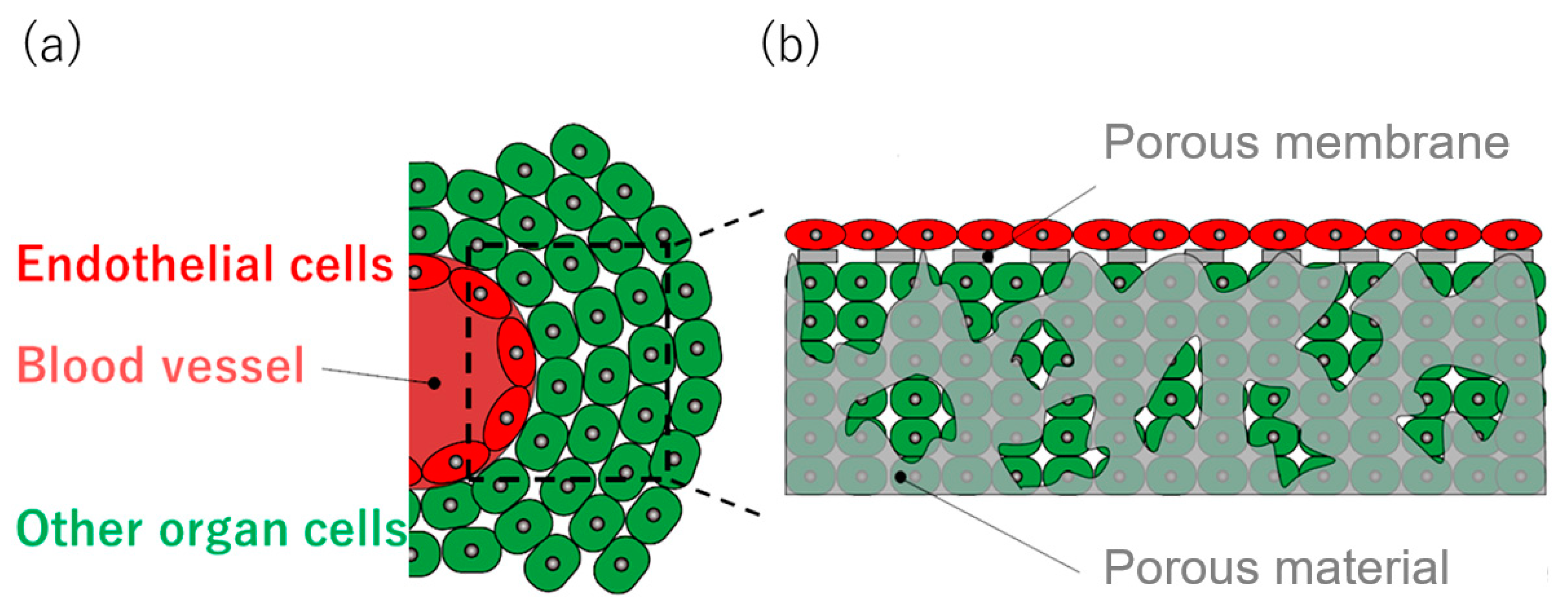
2. Tissue on Artificial Scaffold
3. Materials and Methods
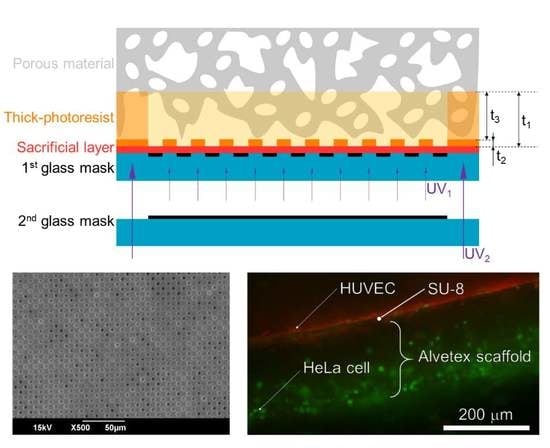
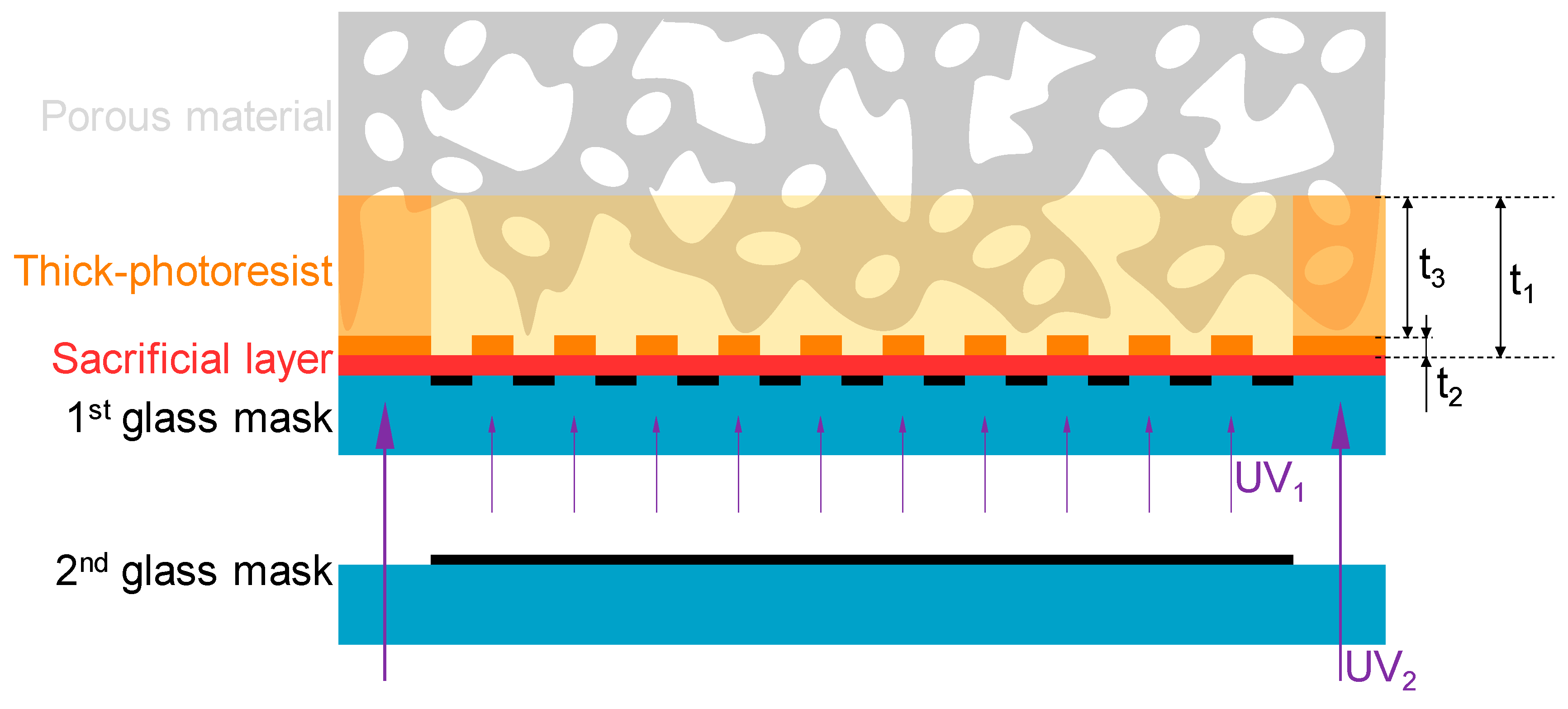
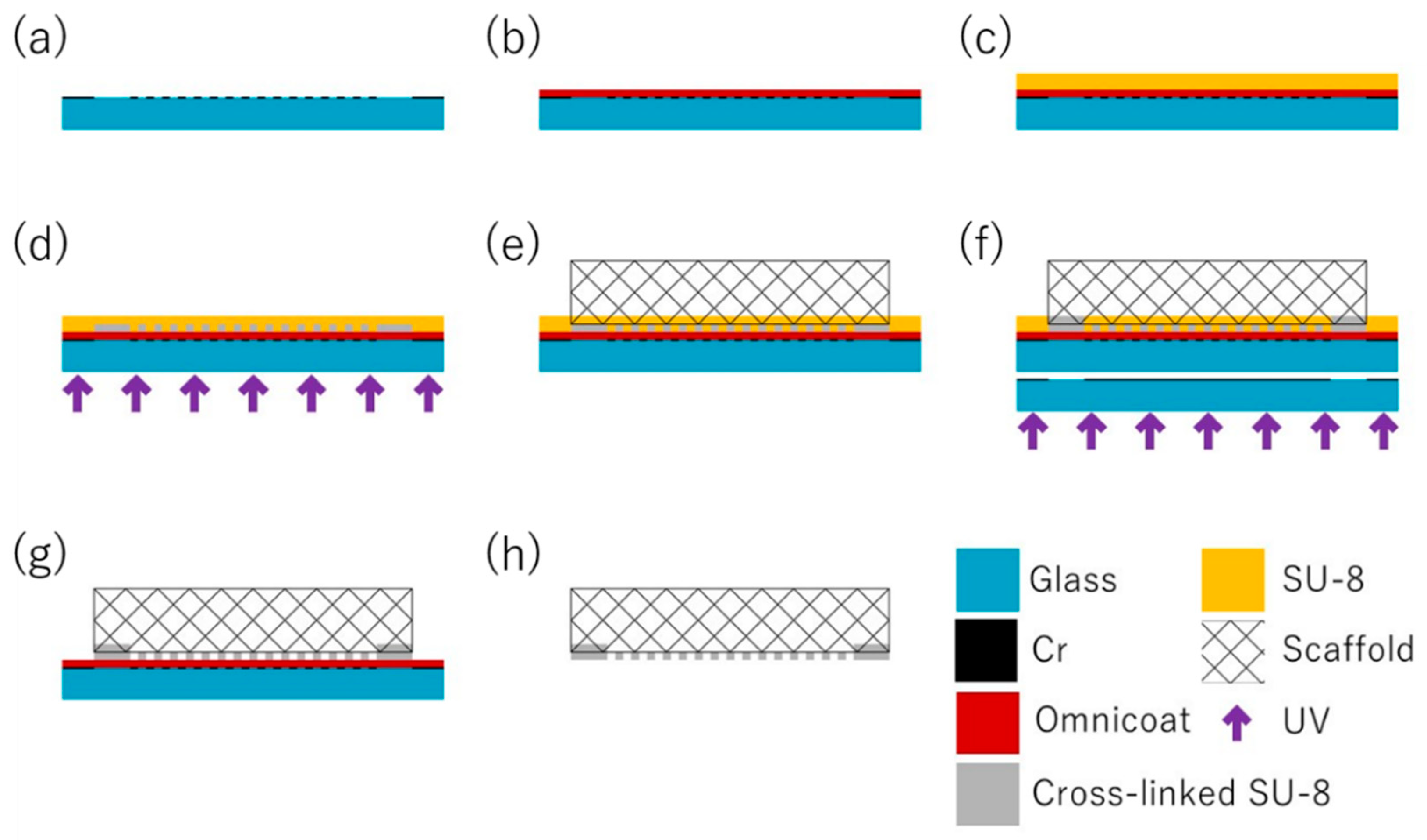
3.1. Principles of the Micropatterning Method
3.2. Materials
3.3. Process Flow of the Micropatterning Method
3.4. Cell Culturing on the MIS
4. Results
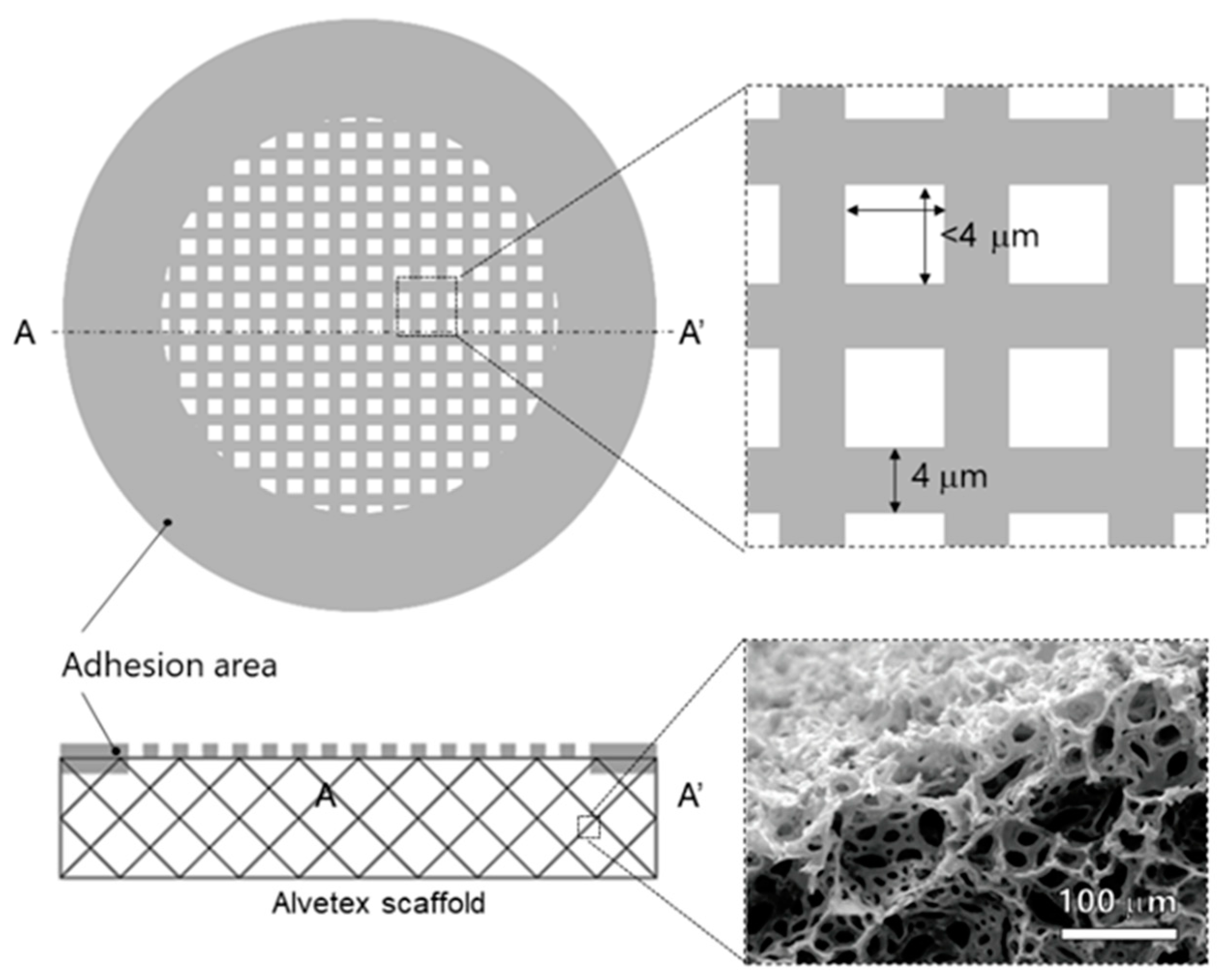
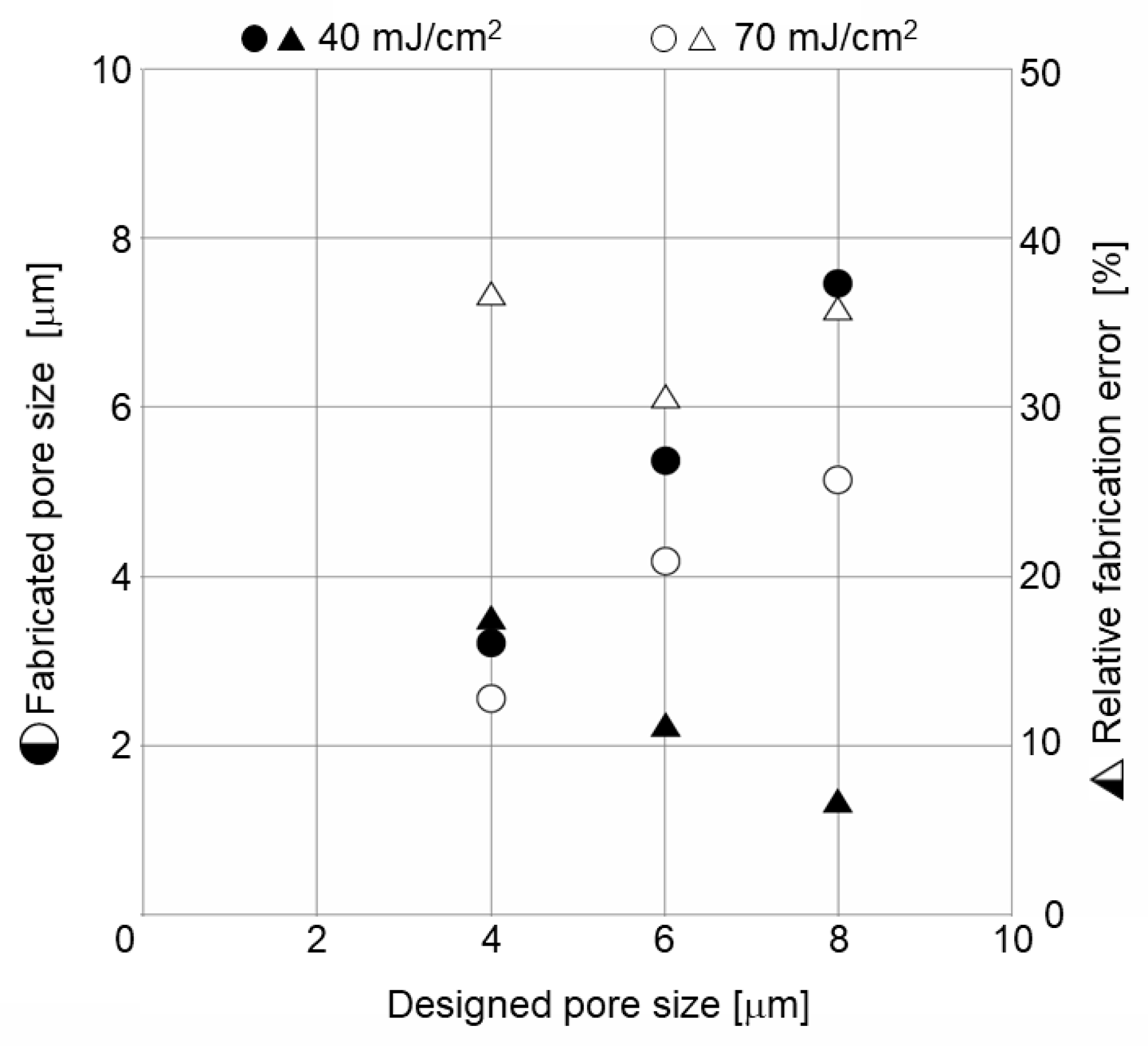
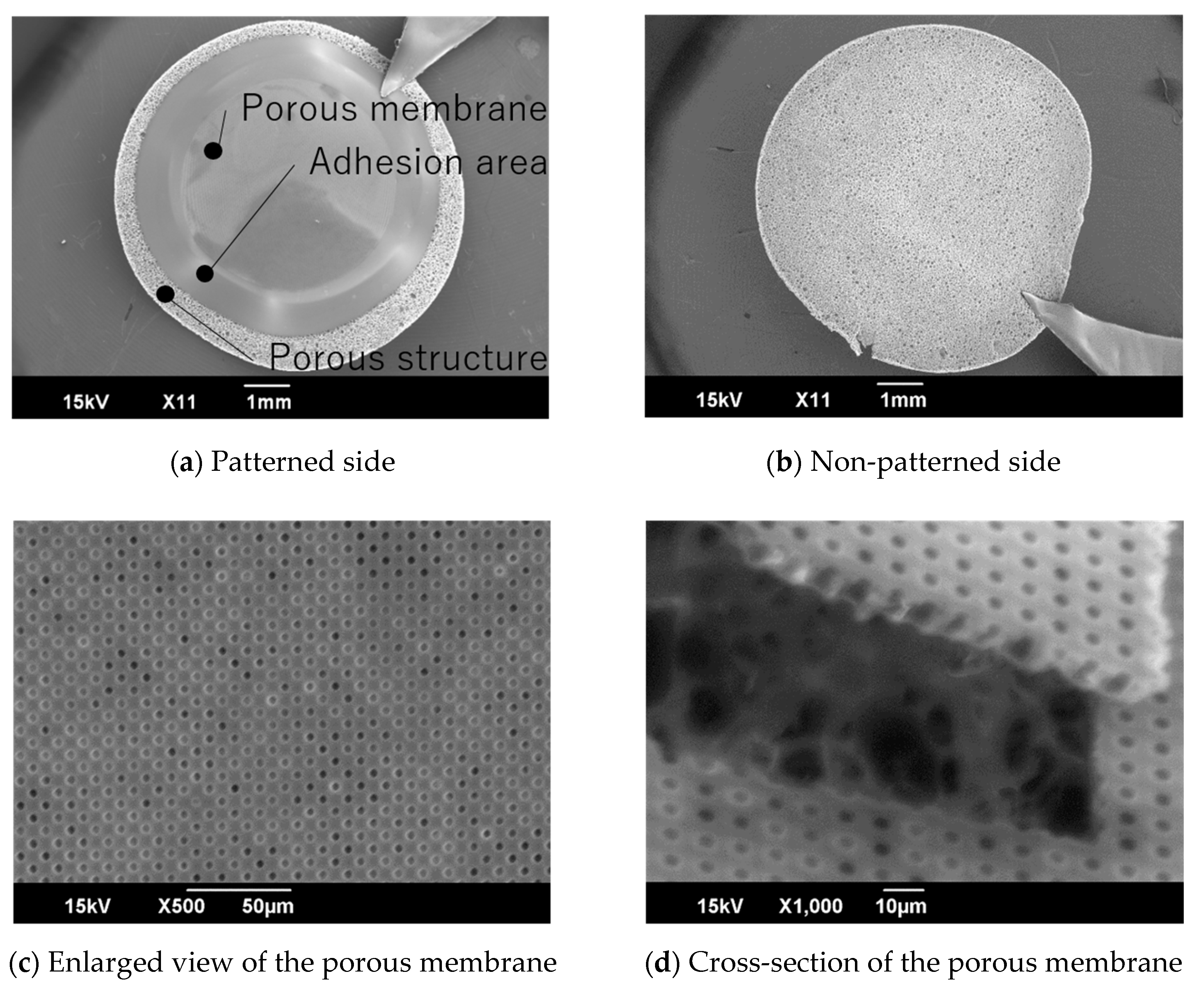
4.1. Micropatterning on Porous Material
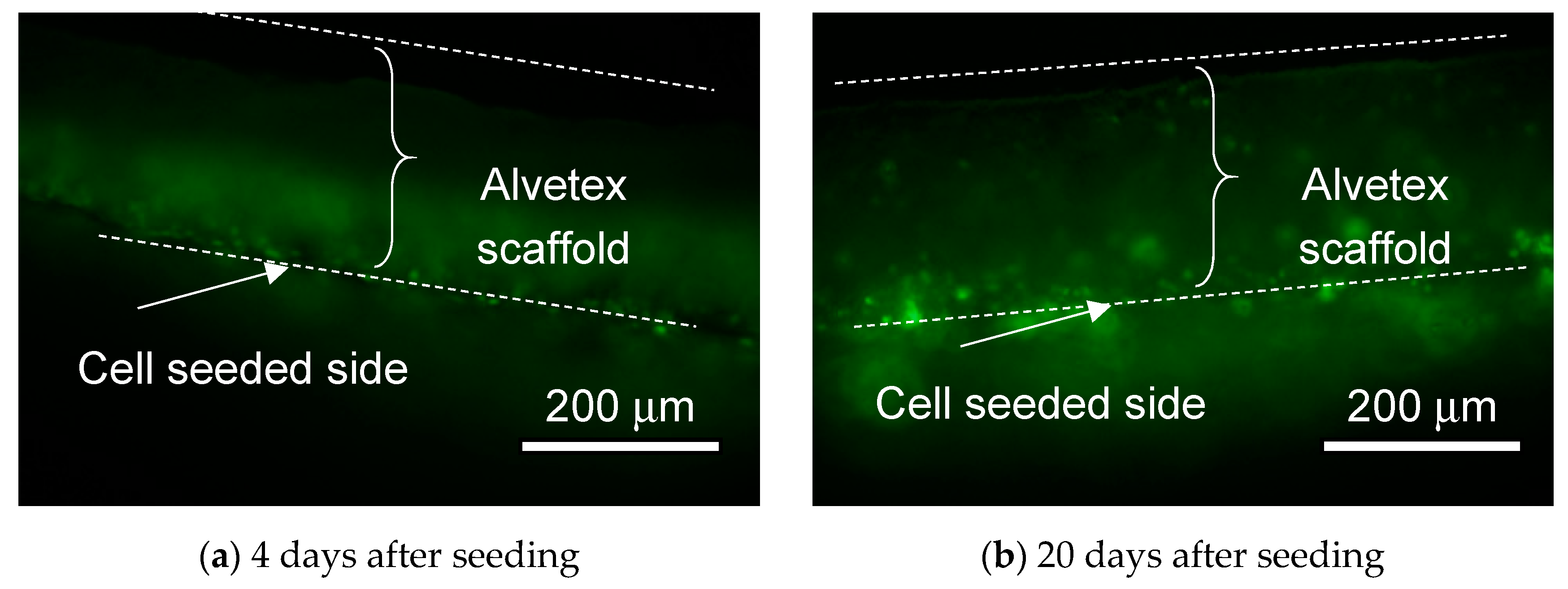
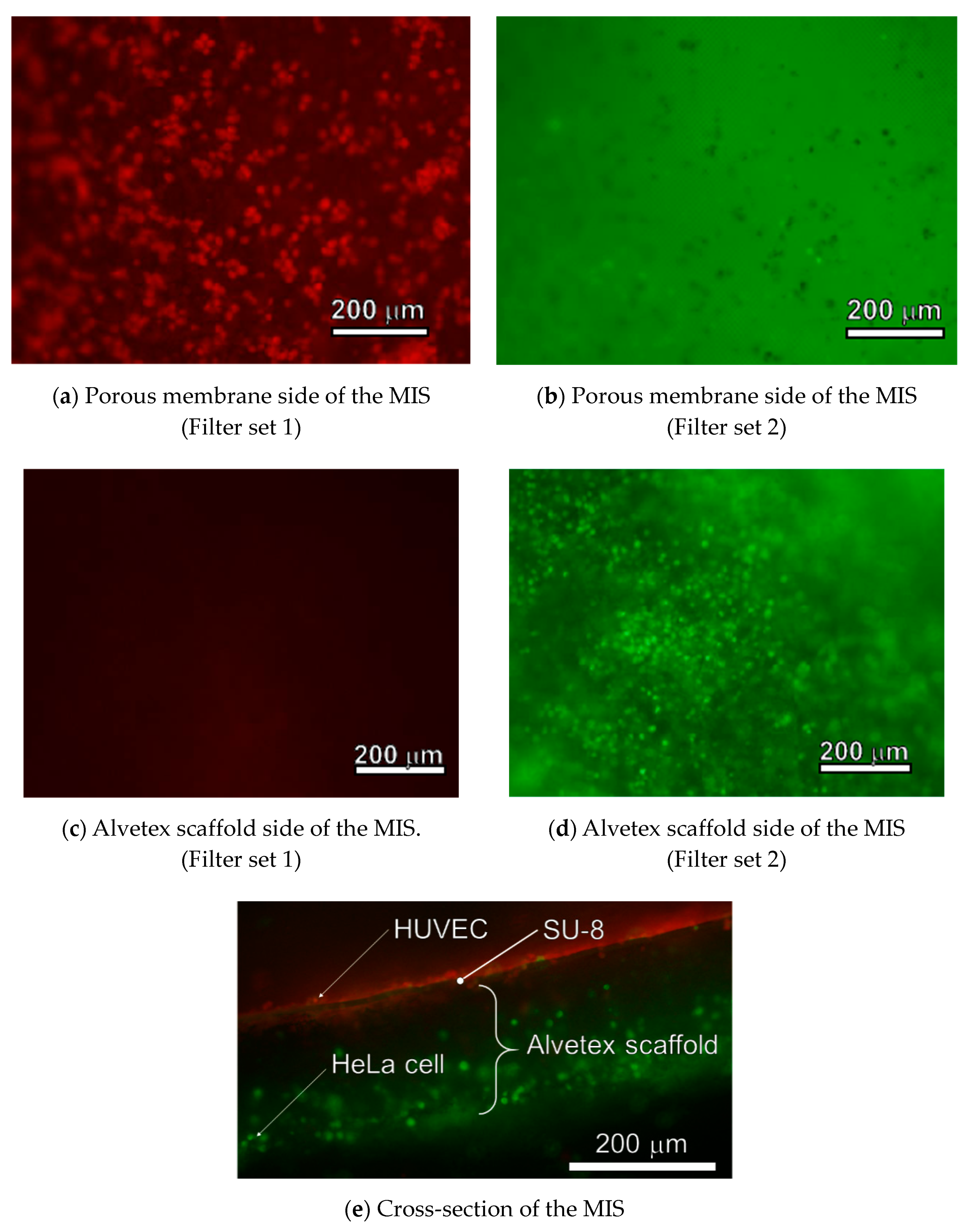
4.2. Cell Culturing on the MIS
5. Discussion
5.1. Comparison with Other Micropatterning Methods
5.2. Cellular Tissue Cultured on an MIS Made of Photoresist and Polystyrene
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schindler, M.; Ahmed, I.; Kamal, J.; Nur-E-Kamal, A.; Grafe, T.H.; Chung, H.Y.; Meiners, S. A synthetic nanofibrillar matrix promotes in vivo-like organization and morphogenesis for cells in culture. Biomaterials 2005, 26, 5624–5631. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Sun, Y.; Finne-Wistrand, A.; Mustafa, K.; Albertsson, A. Electroactive porous tubular scaffolds with degradability and non-cytotoxicity for neural tissue regeneration. Acta Biomater. 2012, 8, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.M.; Leong, K.W. Myogenic induction of aligned mesenchymal stem cell sheets by culture on thermally responsive electrospun nanofibers. Adv. Maters. 2007, 19, 2775–2779. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, M.T.; Nava, M.M.; Eaton, S.M.; Bernasconi, A.; Vishnubhatla, K.C.; Cerullo, G.; Osellame, R. Optimization of femtosecond laser polymerized structural niches to control mesenchymal stromal cell fate in culture. Micromachines 2014, 5, 341–358. [Google Scholar] [CrossRef]
- Malinauskas, M.; Rekštyte, S.; Lukoševicius, L.; Butkus, S.; Balciunas, E.; Peciukaityte, M.; Baltriukiene, D.; Bukelskiene, V.; Butkevicius, A.; Kucevicius, P.; et al. 3D microporous scaffolds manufactured via combination of fused filament fabrication and direct laser writing ablation. Micromachines 2014, 5, 839–858. [Google Scholar] [CrossRef]
- Edington, C.D.; Chen, W.L.K.; Geishecker, E.; Kassis, T.; Soenksen, L.R.; Bhushan, B.M.; Freake, D.; Kirschner, J.; Maass, C.; Tsamandouras, N.; et al. Interconnected microphysiological systems for quantitative biology and pharmacology studies. Sci. Rep. 2018, 8, 4530. [Google Scholar] [CrossRef]
- Satoh, T.; Sugiura, S.; Shin, K.; Onuki-Nagasaki, R.; Ishida, S.; Kikuchi, K.; Kakiki, M.; Kanamori, T. A multi-throughput multi-organ-on-a-chip system on a plate formatted pneumatic pressure-driven medium circulation platform. Lab Chip 2017, 18, 115–125. [Google Scholar] [CrossRef]
- Kim, M.Y.; Li, J.; Pham, L.K.; Wong, B.W.; Hui, E.E. Microfabrication of high-resolution porous membranes for cell culture. J. Membr. Sci. 2014, 452, 460–469. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Mochida, J.; Sakai, D.; Nakai, T.; Nishimura, K.; Kawada, H.; Hotta, T. Upregulation of the viability of nucleus pulposus cells by bone marrow-derived stromal cells: Significance of direct cell-to-cell contact in coculture system. SPINE 2004, 29, 1508–1514. [Google Scholar] [CrossRef]
- Bartolo, L.D.; Rende, M.; Morelli, S.; Giusi, G.; Salerno, S.; Piscioneri, A.; Gordano, A.; Vito, A.D.; Canonaco, M.; Drioli, E. Influence of membrane surface properties on the growth of neuronal cells isolated from hippocampus. J. Membr. Sci. 2008, 325, 139–149. [Google Scholar] [CrossRef]
- Liu, W.; Wang, D.; Huang, J.; Wei, Y.; Xiong, J.; Zhu, W.; Duan, L.; Chen, J.; Sun, R.; Wang, D. Low-temperature deposition manufacturing: A novel and promising rapid prototyping technology for the fabrication of tissue-engineered scaffold. Mater. Sci. Eng. 2017, 70, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Li, W.; Li, A.; Zhan, Z.; Wang, L.; Sun, D. Design and manufacturing of a passive pressure sensor based on LC resonance. Micromachines 2016, 7, 87. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, Z.; Yu, Y.; Sizdahkhani, S.; Ho, W.S.; Yin, F.; Wang, L.; Zhu, G.; Zhang, M.; Jiang, L.; et al. A dynamic in vivo-like organotypic blood-brain barrier model to probe metastatic brain tumors. Sci. Rep. 2016, 6, 36670. [Google Scholar] [CrossRef] [PubMed]
- Salazar, G.T.; Wang, Y.; Young, G.; Bachman, M.; Sims, C.E.; Li, G.P.; Allbritton, N.L. Micropallet arrays for the separation of single, adherent cells. Anal. Chem. 2007, 79, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Maltman, D.J.; Przyborski, S.A. Developments in three-dimensional cell culture technology aimed at improving the accuracy of in vitro analyses. Biochem. Soc. Trans. 2010, 38, 1072–1075. [Google Scholar] [CrossRef]
- Ueno, H.; Yamada, K.; Suzuki, T. Integration method of microchannel and vertical micromesh structure for three-dimensional cell culture using inclined exposure and inclined oxygen ashing. Micromachines 2018, 9, 681. [Google Scholar] [CrossRef]
- Futai, N.; Tamura, M.; Ogawa, T.; Tanaka, M. Microfluidic long-term gradient generator with axon separation prototyped by 185 nm diffused light photolithography of SU-8 photoresist. Micromachines 2018, 10, 9. [Google Scholar] [CrossRef]
- Campo, A.; Greiner, C. SU-8: A photoresist for high-aspect-ratio and 3D submicron lithography. J. Micromech. Microeng. 2007, 17, 81–95. [Google Scholar] [CrossRef]
- Martinez-Duarte, R.; Madou, M.J. SU-8 Photolithography and Its Impact on Microfluidics, 1st ed.; CRS Press Taylor & Francis Group: Abingdon, UK, 2011; pp. 233–238. [Google Scholar]
- Takei, T.; Kitazono, Z.; Ozuno, Y.; Yoshinaga, T.; Nishimata, H.; Yoshida, M. Vascular-like network prepared using hollow hydrogel microfibers. J. Biosci. Bioeng. 2016, 121, 336–340. [Google Scholar] [CrossRef]
- Lamas-Ardisana, P.J.; Martinez-Paredes, G.; Anorga, L.; Grande, H.J. Glucose biosensor based on disposable electrochemical paper-based transducers fully fabricated by screen-printing. Biosens. Bioelectron. 2018, 109, 8–12. [Google Scholar] [CrossRef]
- Baradai, O.E.; Beneventi, D.; Alloin, F.; Bultel, Y.; Chaussy, D. Use of cellulose nanofibers as an electrode binder for lithium ion battery screen printing on a paper separator. Nanomaterials 2018, 8, 982. [Google Scholar] [CrossRef] [PubMed]
- Esch, M.B.; Sung, J.H.; Yang, J.; Yu, C.; Yu, J.; March, J.C.; Shuler, M.L. On chip porous polymer membranes for integration of gastrointestinal tract epithelium with microfluidic ‘body-on-a-chip’ devices. Biomed. Microdevices 2012, 14, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011, 21, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Pearce, I.A.; Cambray-Deakin, M.A.; Burgoyne, R.D. Glutamate acting on NMDA receptors stimulates neurite outgrowth from cerebellar granule cells. FEBS Lett. 1987, 223, 143–147. [Google Scholar] [CrossRef]
- Hennemeyer, M.; Walther, F.; Kerstan, S.; Schürzinger, K.; Gigler, A.M.; Stark, R.W. Cell proliferation assays on plasma activated SU-8. Microelectron. Eng. 2008, 85, 1298–1301. [Google Scholar] [CrossRef]
- Tu, C.; Huang, B.; Zhou, J.; Liang, Y.; Tian, J.; Ji, L.; Liang, X.; Ye, X. A microfluidic chip for cell patterning utilizing paired microwells and protein patterns. Micromachines 2016, 8, 1. [Google Scholar] [CrossRef]
- Terryn, S.; Jouret, F.; Vandenabeele, F.; Smolders, I.; Moreels, M.; Devuyst, O.; Steels, P.; Kerkhove, E.V. A primary culture of mouse proximal tubular cells, established on collagen-coated membranes. Am. J. Physiol. Renal Physiol. 2007, 293, 476–485. [Google Scholar] [CrossRef]
- Hayman, M.W.; Smith, K.H.; Cameron, N.R.; Przyborski, S.A. Growth of human stem cell-derived neurons on solid three-dimensional polymers. J. Biochem. Biophys. Methods 2005, 62, 231–240. [Google Scholar] [CrossRef]
- Esch, M.B.; Ueno, H.; Applegate, D.R.; Shuler, M.L. Modular, pumpless body-on-a-chip platform for the co-culture of GI tract epithelium and 3D primary liver tissue. Lab Chip 2016, 16, 2719–2729. [Google Scholar] [CrossRef]
- Tamai, H.; Maruo, K.; Ueno, H.; Terao, K.; Kotera, H.; Suzuki, T. Development of low-fluorescence thick photoresist for high-aspect-ratio microstructure in bio-application. Biomicrofluidics 2015, 9, 022405. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueno, H.; Sato, K.; Yamada, K.; Suzuki, T. Micropatterning Method for Porous Materials Using the Difference of the Glass Transition Temperature between Exposed and Unexposed Areas of a Thick-Photoresist. Micromachines 2020, 11, 54. https://doi.org/10.3390/mi11010054
Ueno H, Sato K, Yamada K, Suzuki T. Micropatterning Method for Porous Materials Using the Difference of the Glass Transition Temperature between Exposed and Unexposed Areas of a Thick-Photoresist. Micromachines. 2020; 11(1):54. https://doi.org/10.3390/mi11010054
Chicago/Turabian StyleUeno, Hidetaka, Kiichi Sato, Kou Yamada, and Takaaki Suzuki. 2020. "Micropatterning Method for Porous Materials Using the Difference of the Glass Transition Temperature between Exposed and Unexposed Areas of a Thick-Photoresist" Micromachines 11, no. 1: 54. https://doi.org/10.3390/mi11010054
APA StyleUeno, H., Sato, K., Yamada, K., & Suzuki, T. (2020). Micropatterning Method for Porous Materials Using the Difference of the Glass Transition Temperature between Exposed and Unexposed Areas of a Thick-Photoresist. Micromachines, 11(1), 54. https://doi.org/10.3390/mi11010054