3D Sugar Printing of Networks Mimicking the Vasculature
Abstract
1. Introduction
- fabricating both in-plane bifurcations and merging points;
- implementing this multiple times to form complex networks;
- having control over fibre diameter not only per fibre but also during printing;
- printing both geometric shapes as well as organic shapes.
2. Materials and Methods
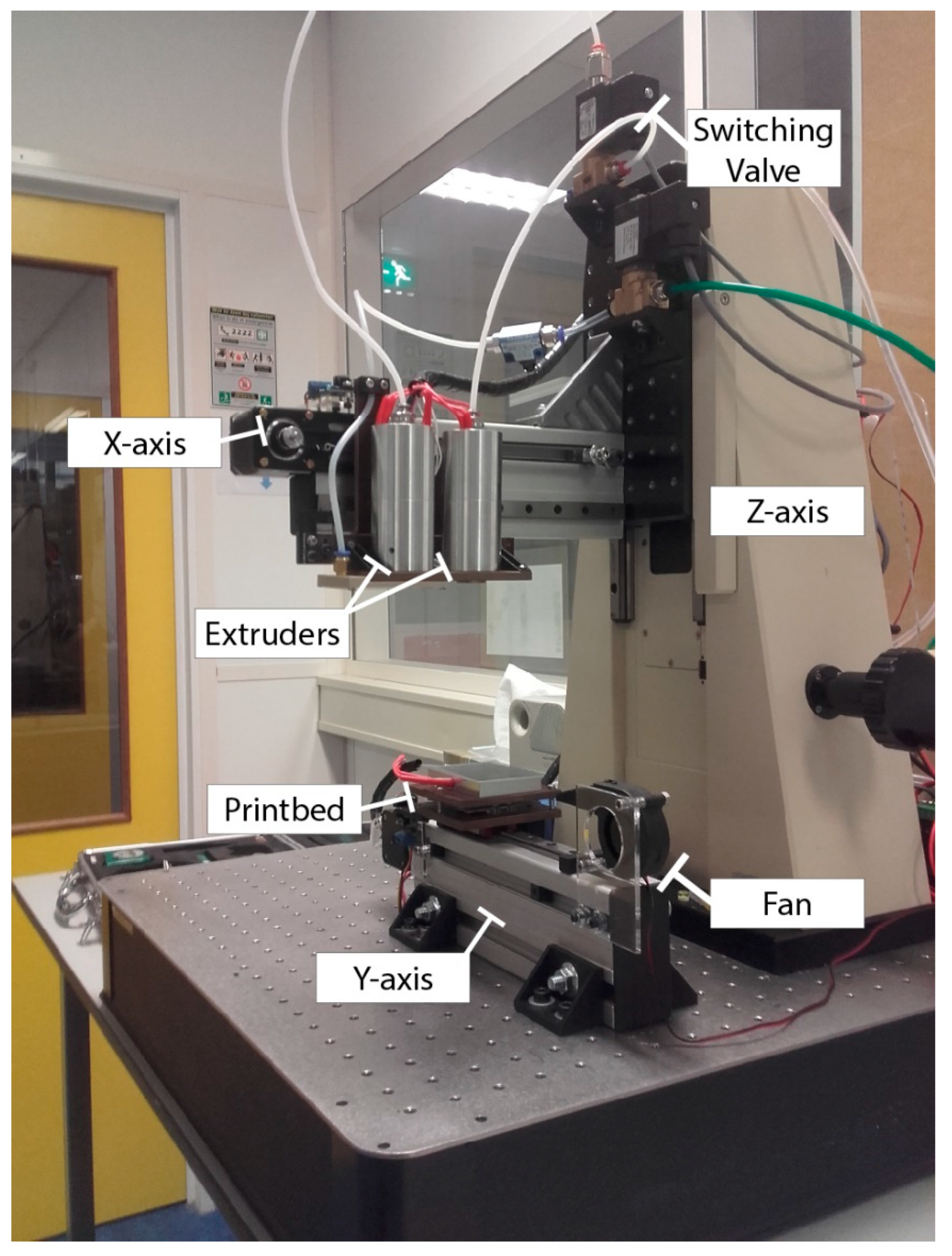
2.1. Printing Setup
2.2. Carbohydrate Glass Preparation and Characterisation
2.3. Fibre and Network Fabrication
2.4. Flow Experiments
3. Results
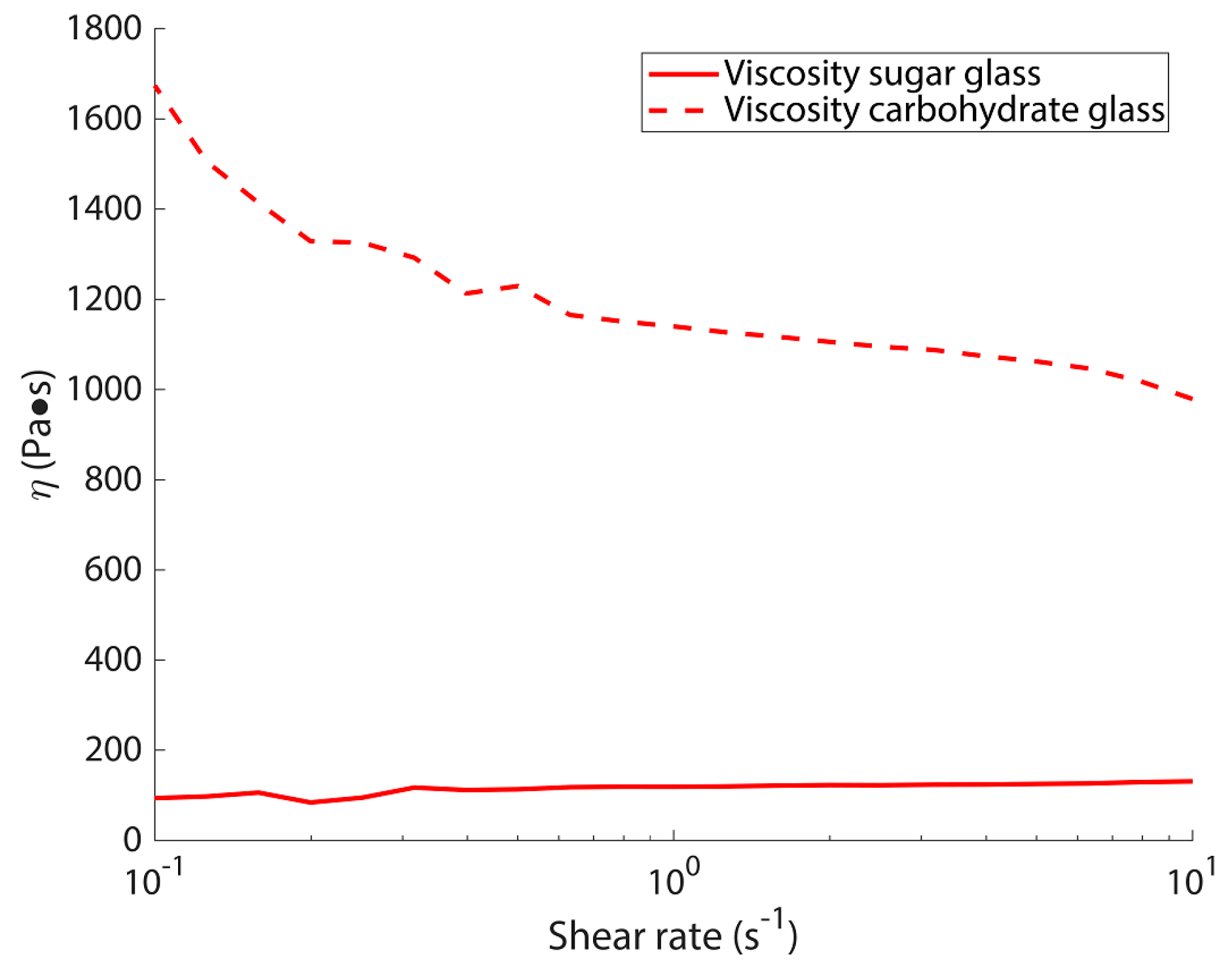
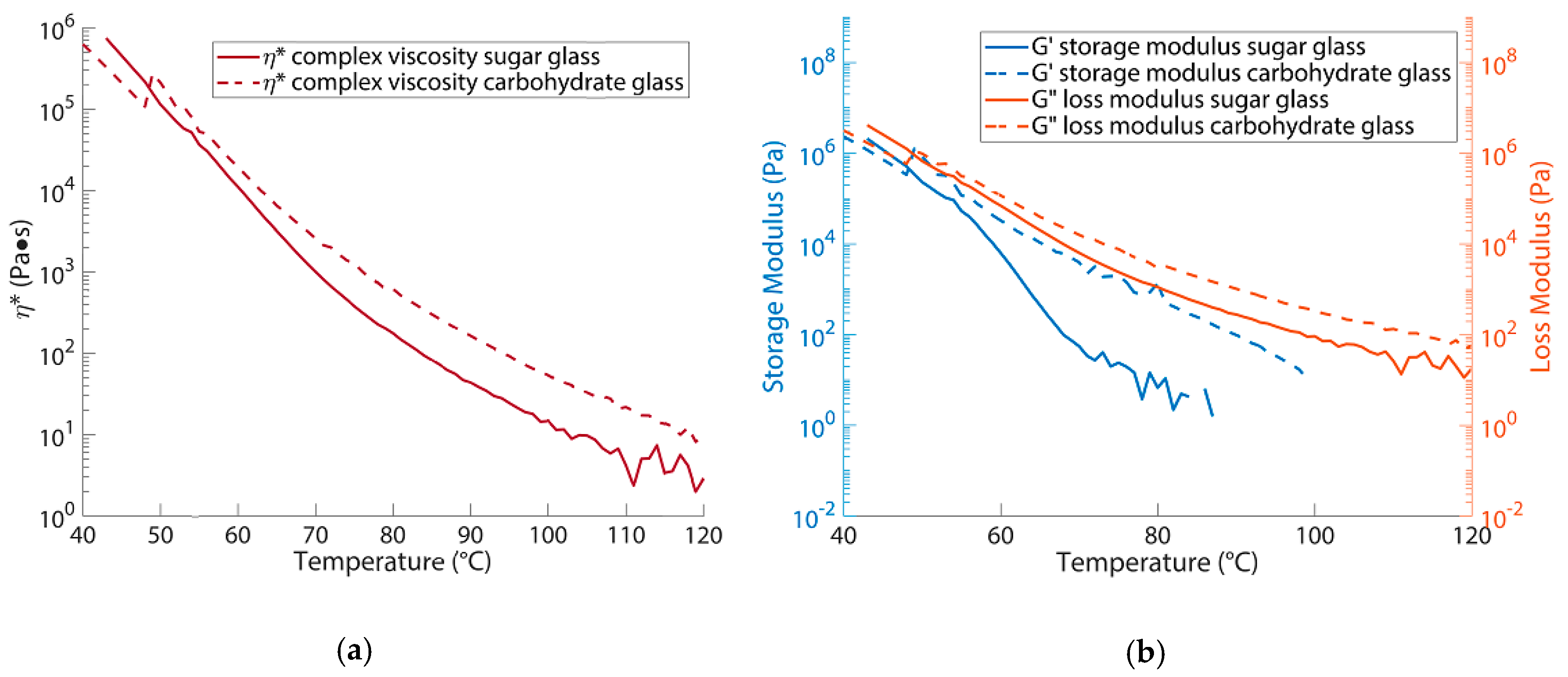
3.1. Rheology Results
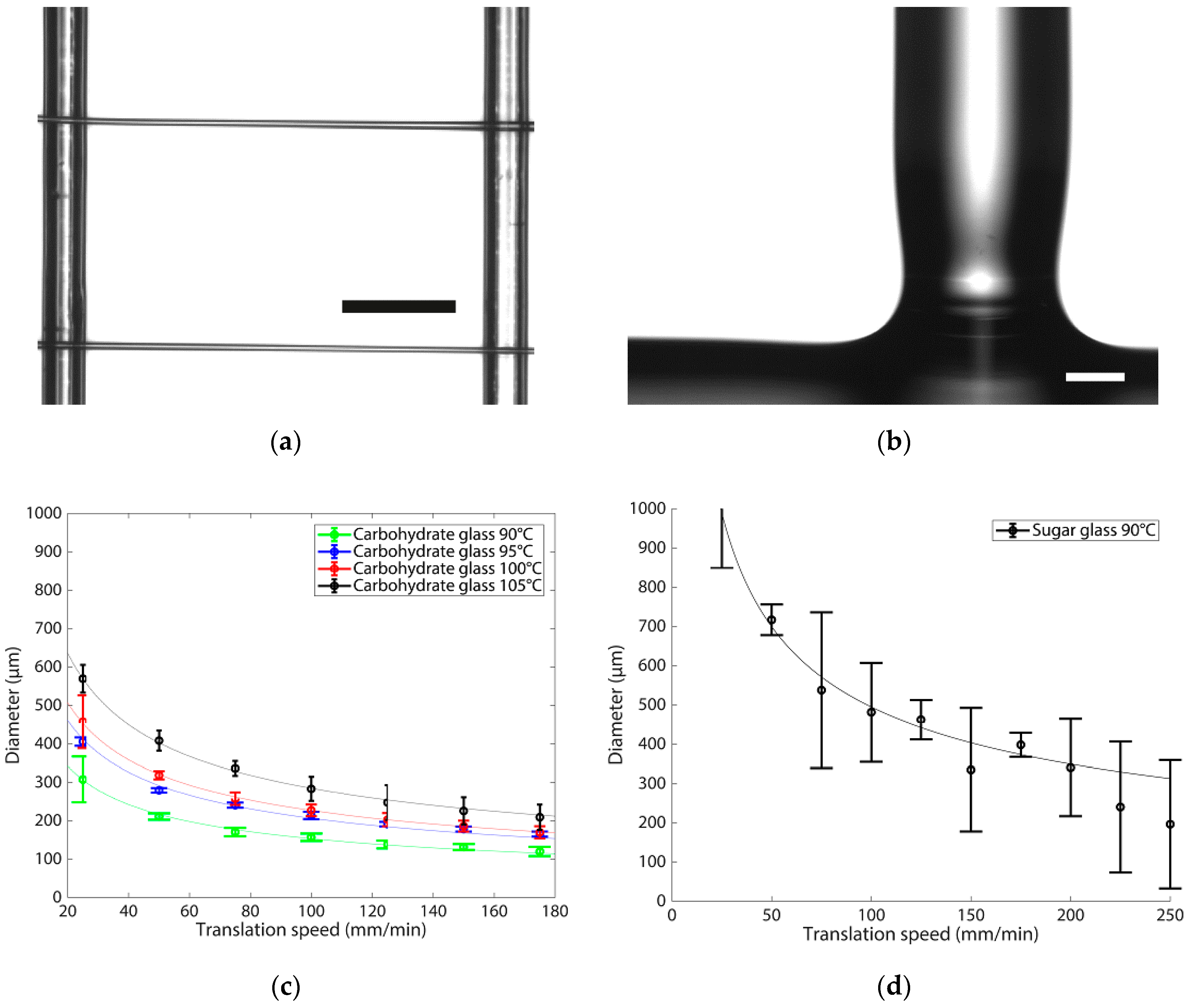
3.2. Printing Behaviour
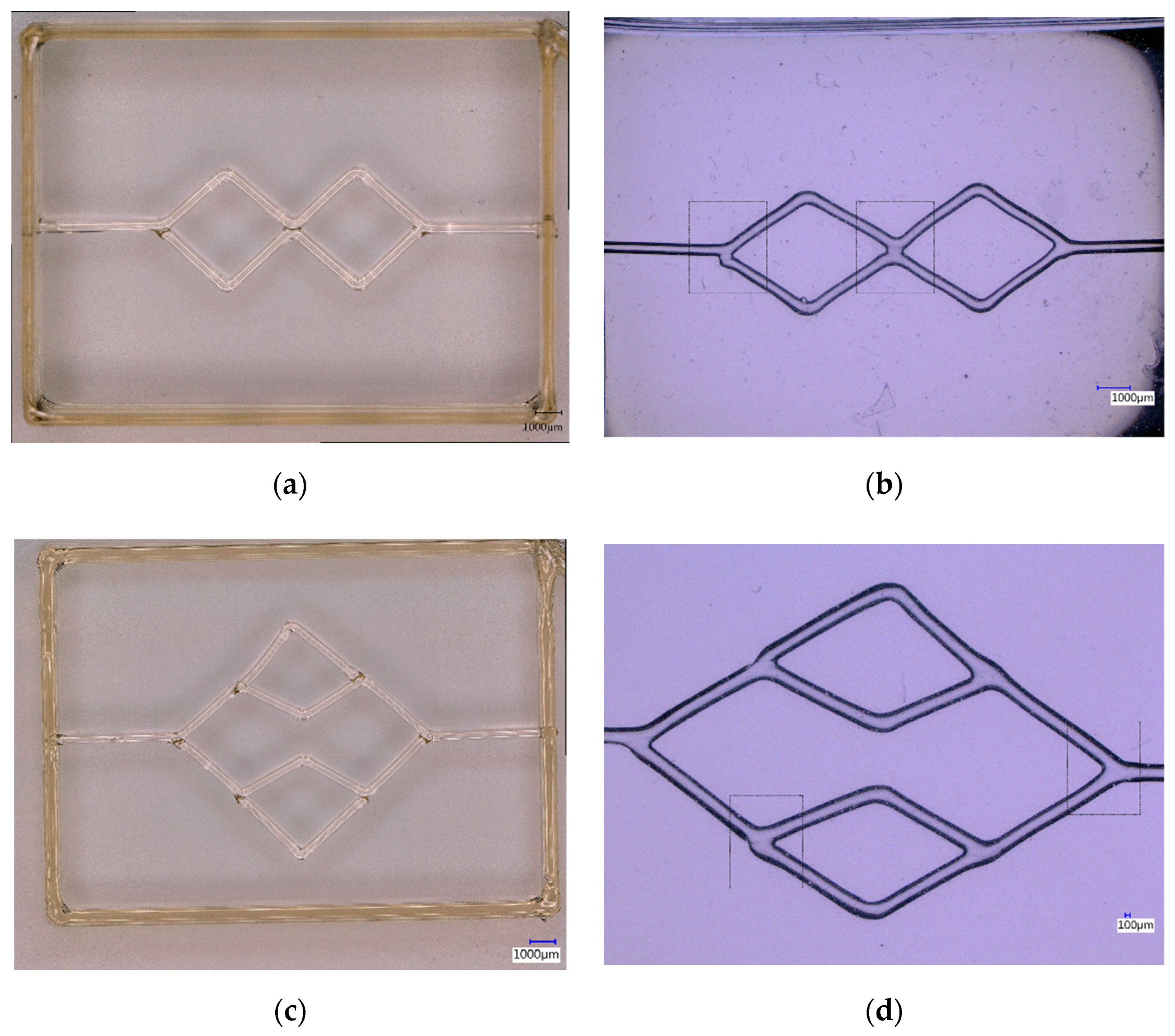
3.3. Printing and Casting Network Structures
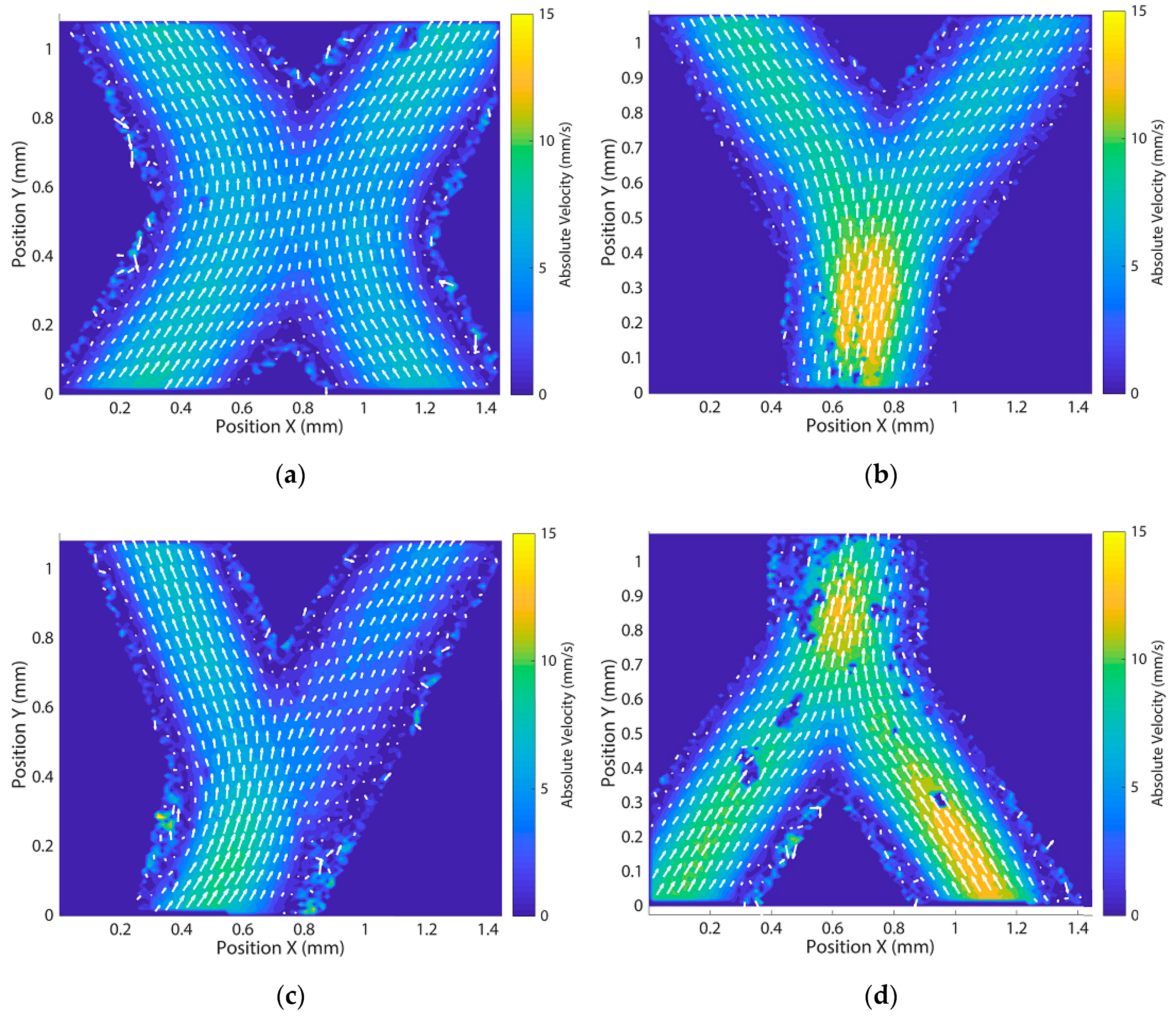
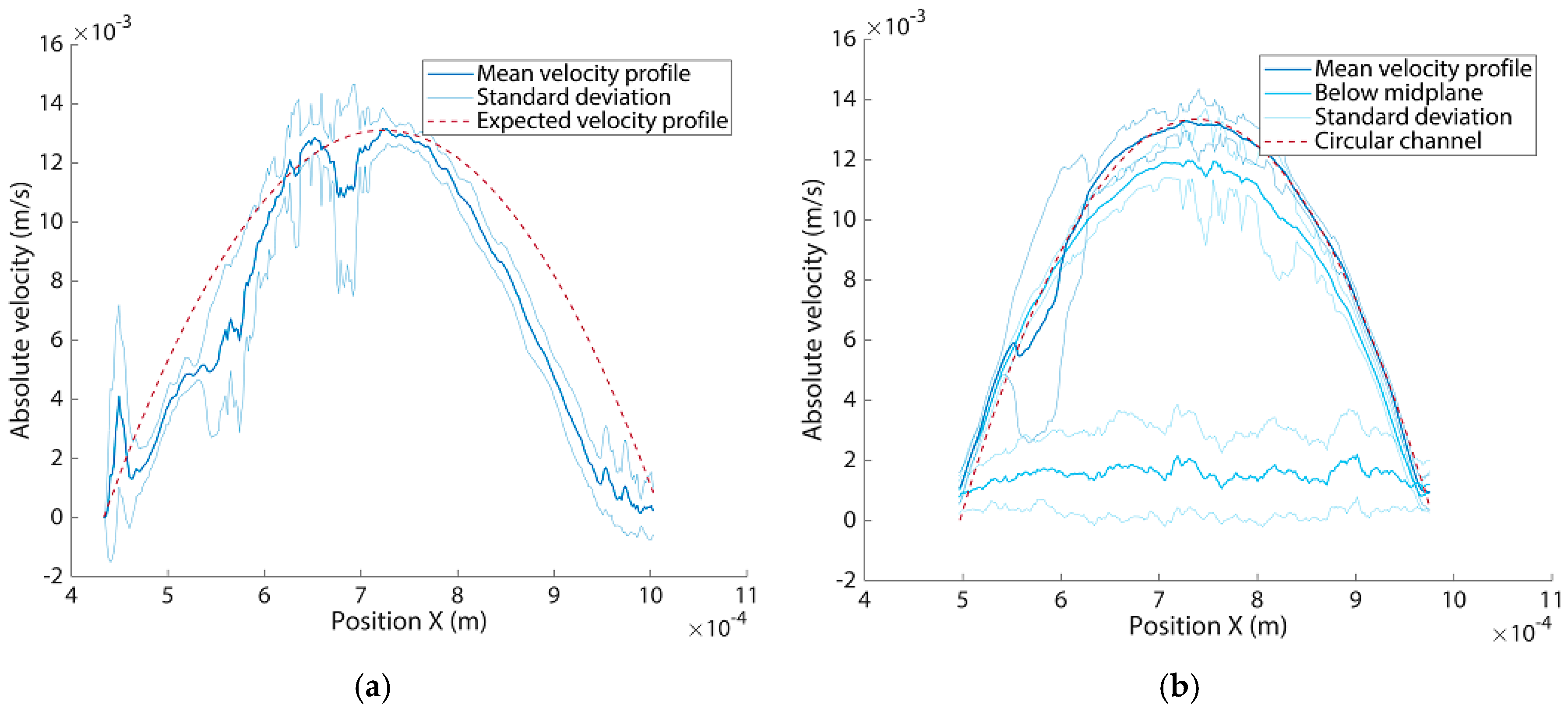
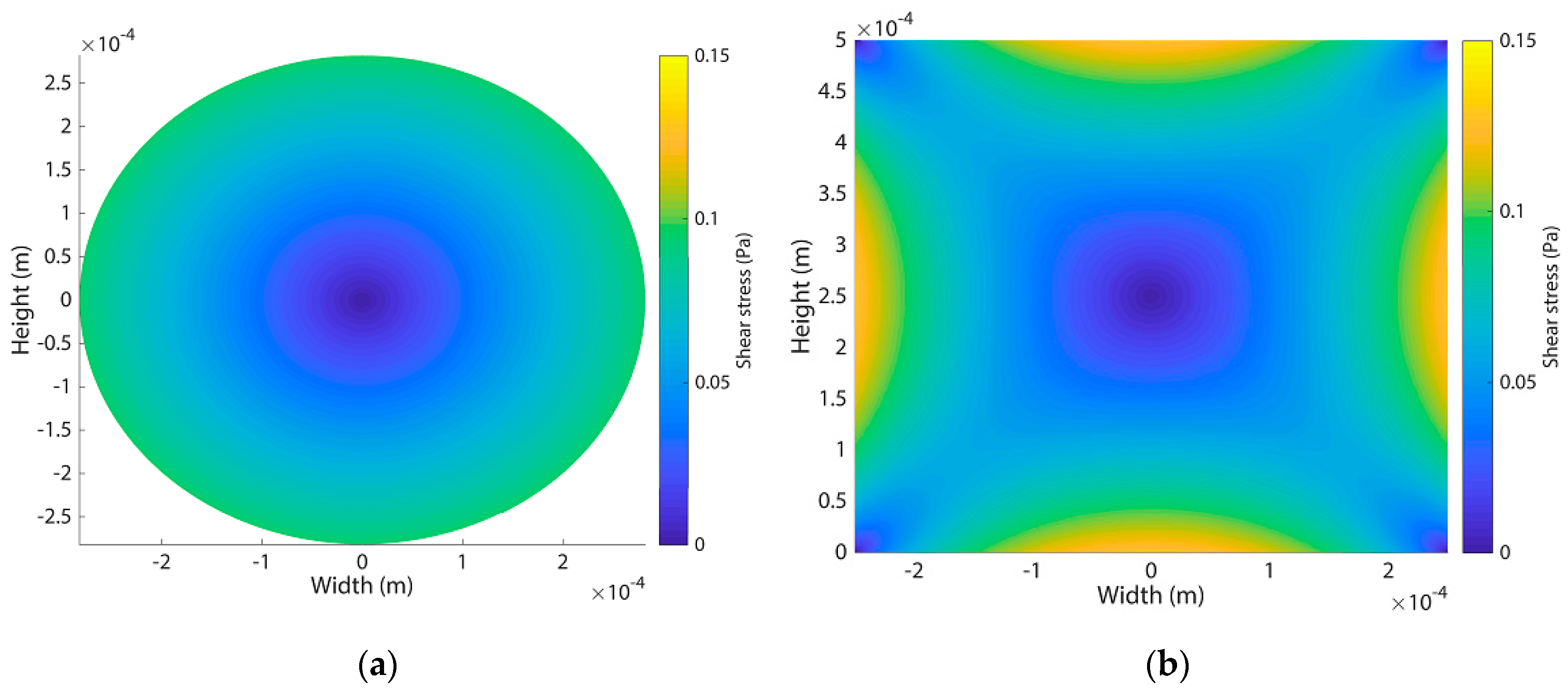
3.4. Flow Experiments
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B
Appendix C
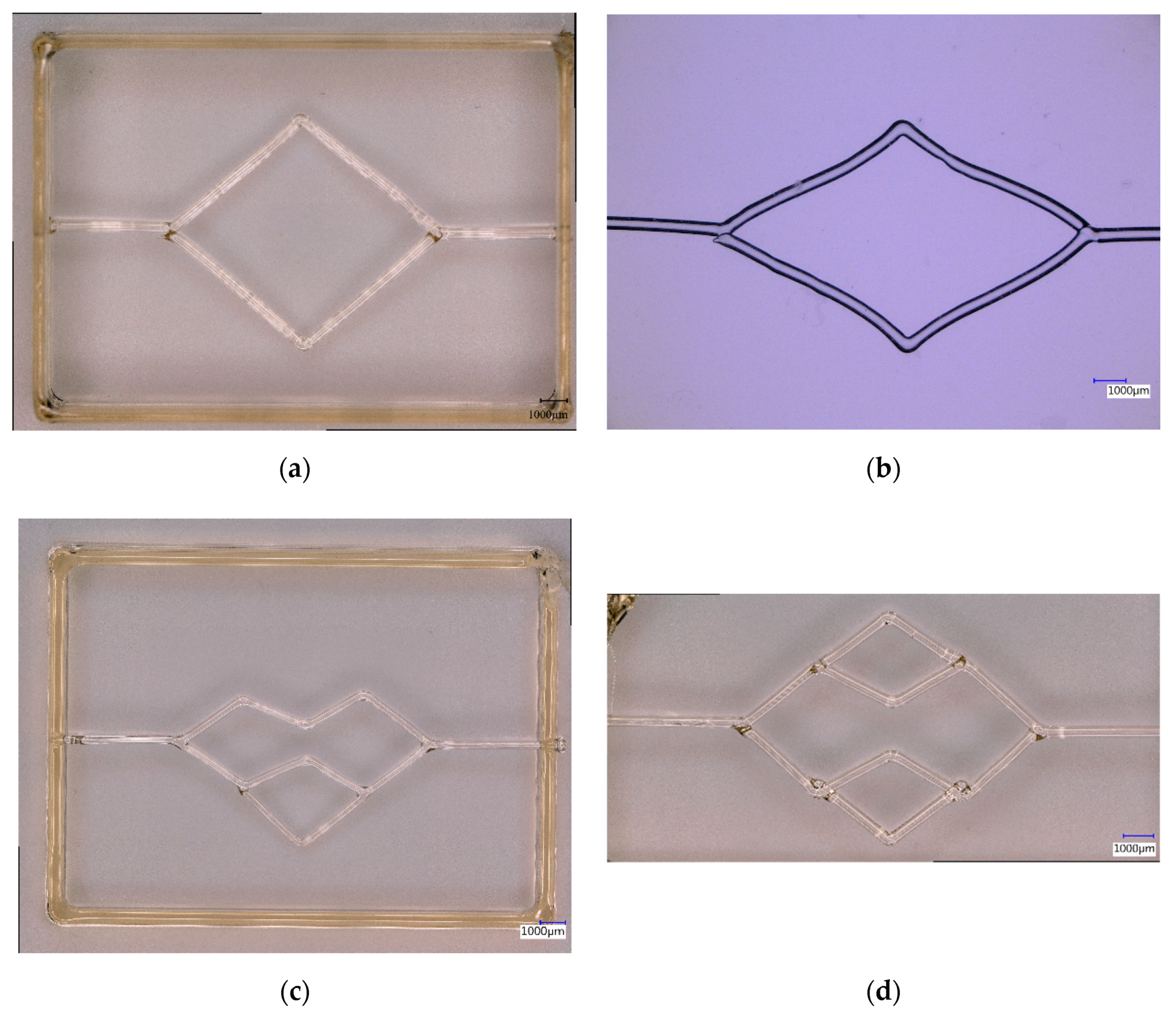
Appendix D
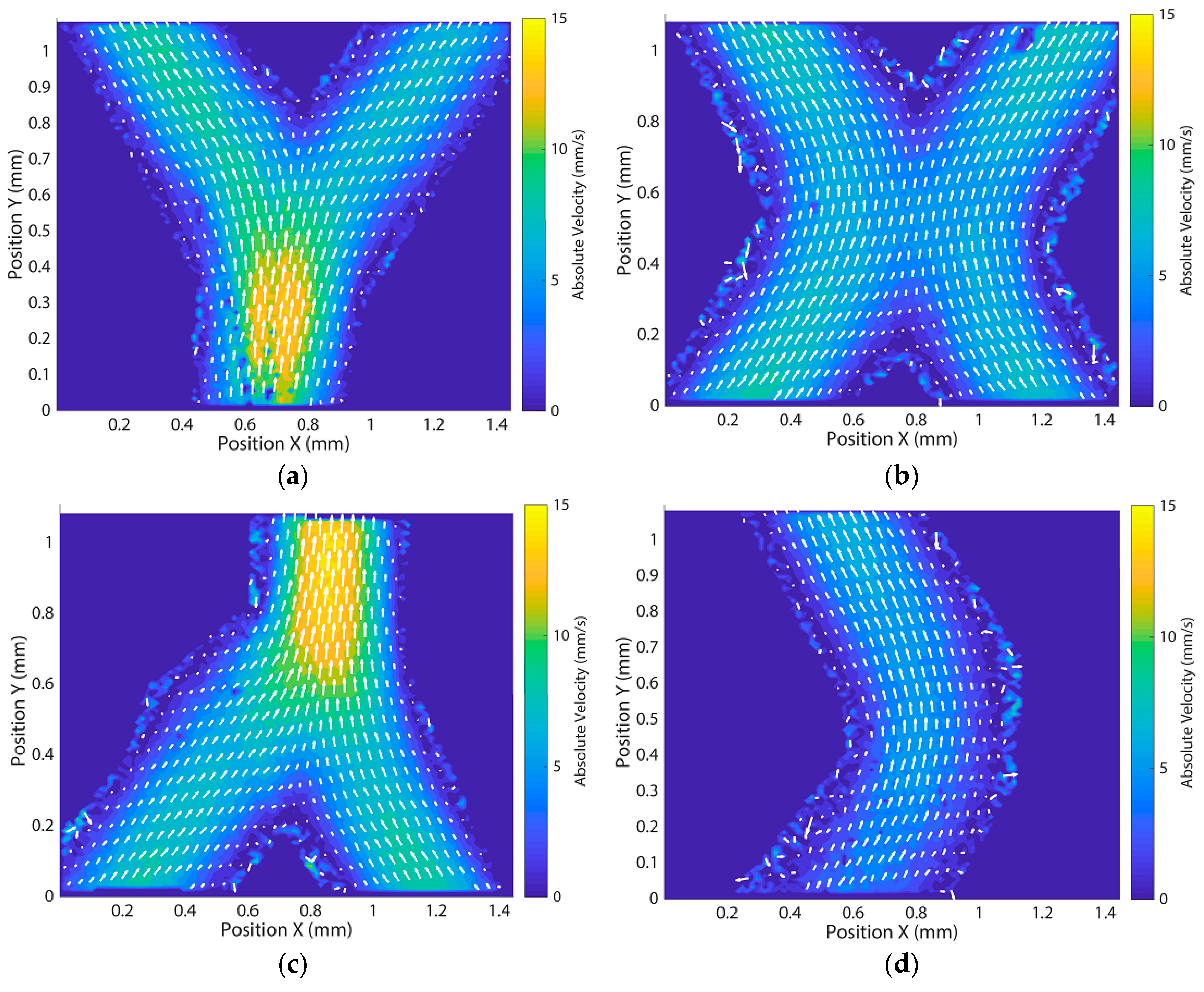

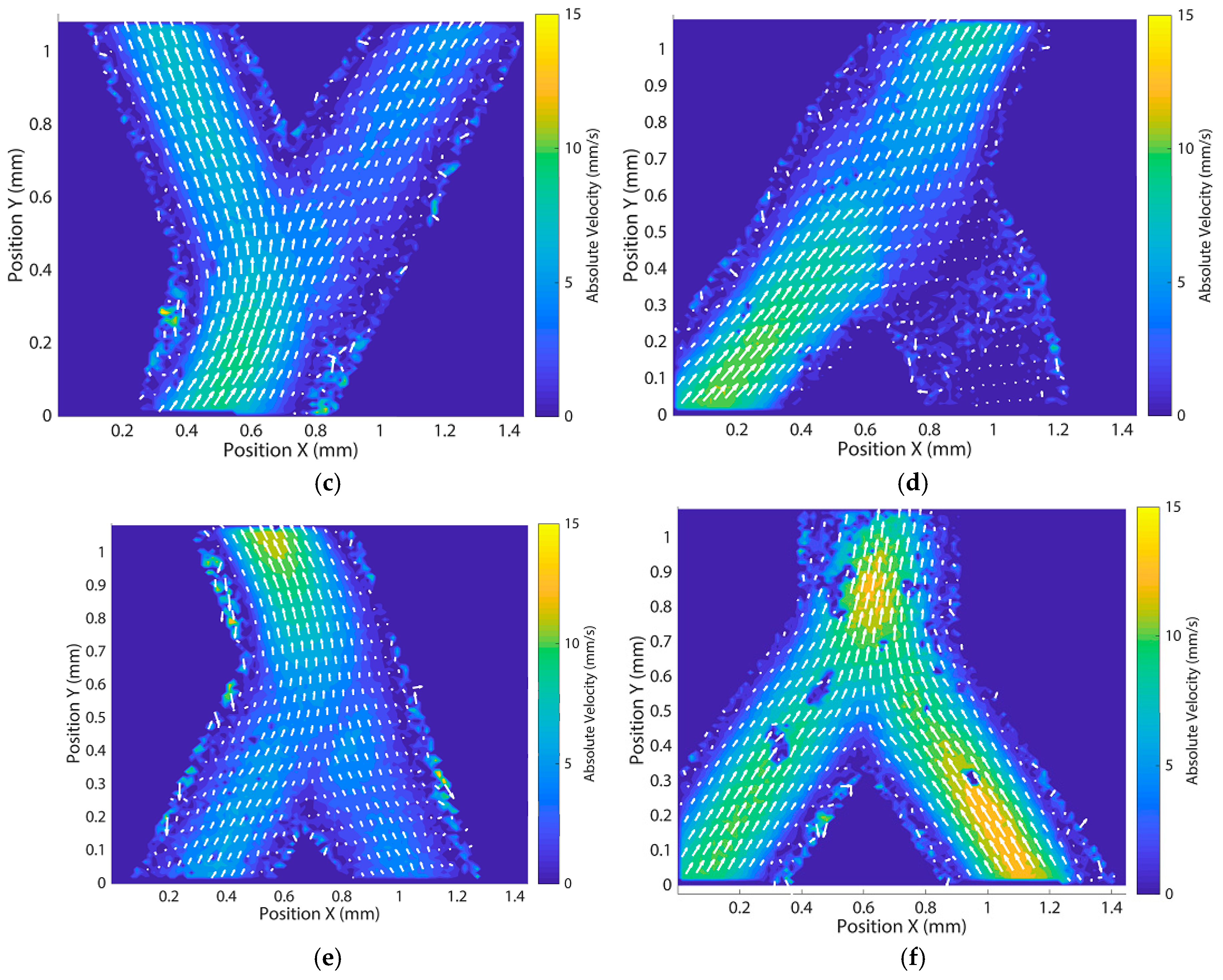
Appendix E
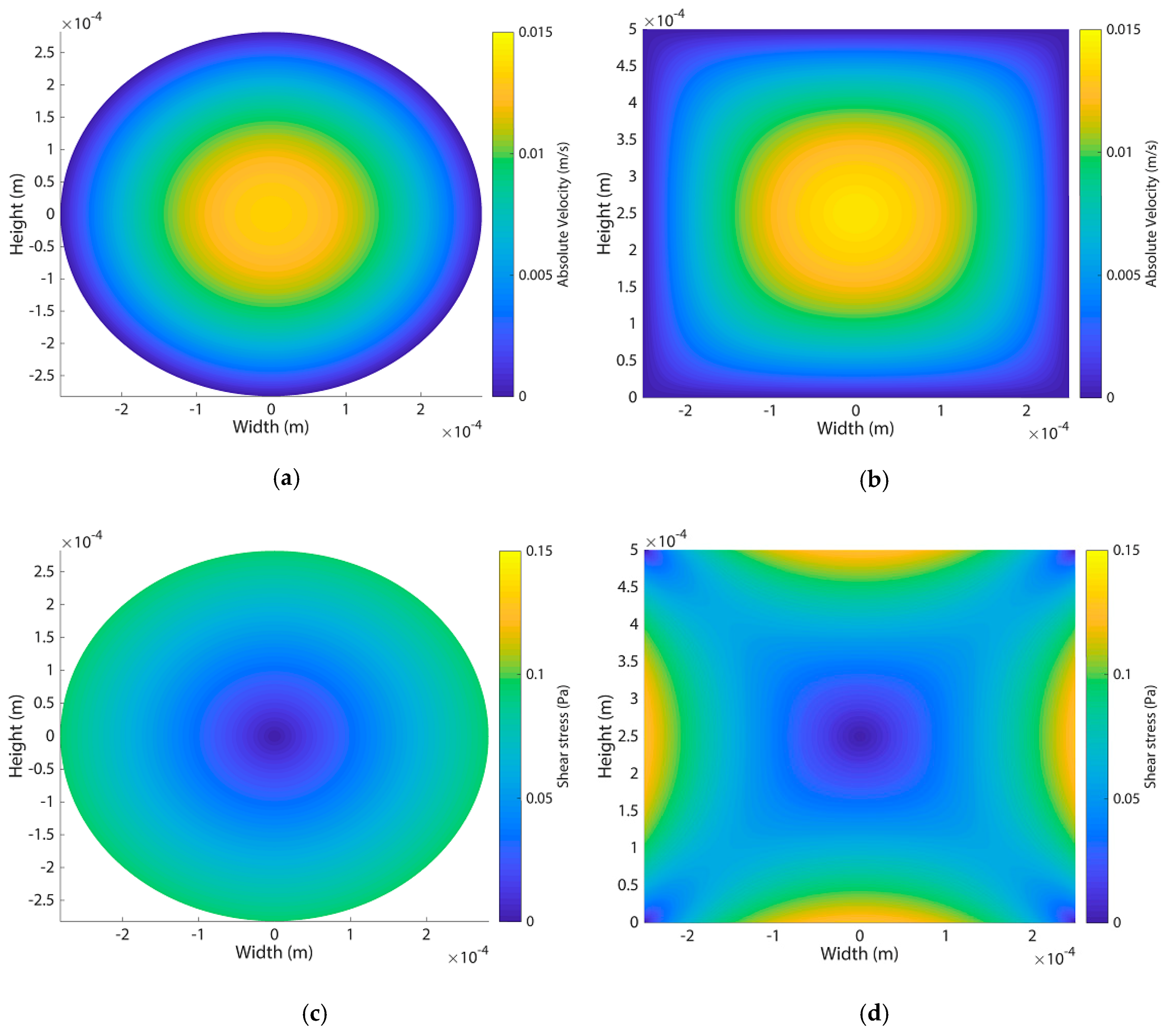
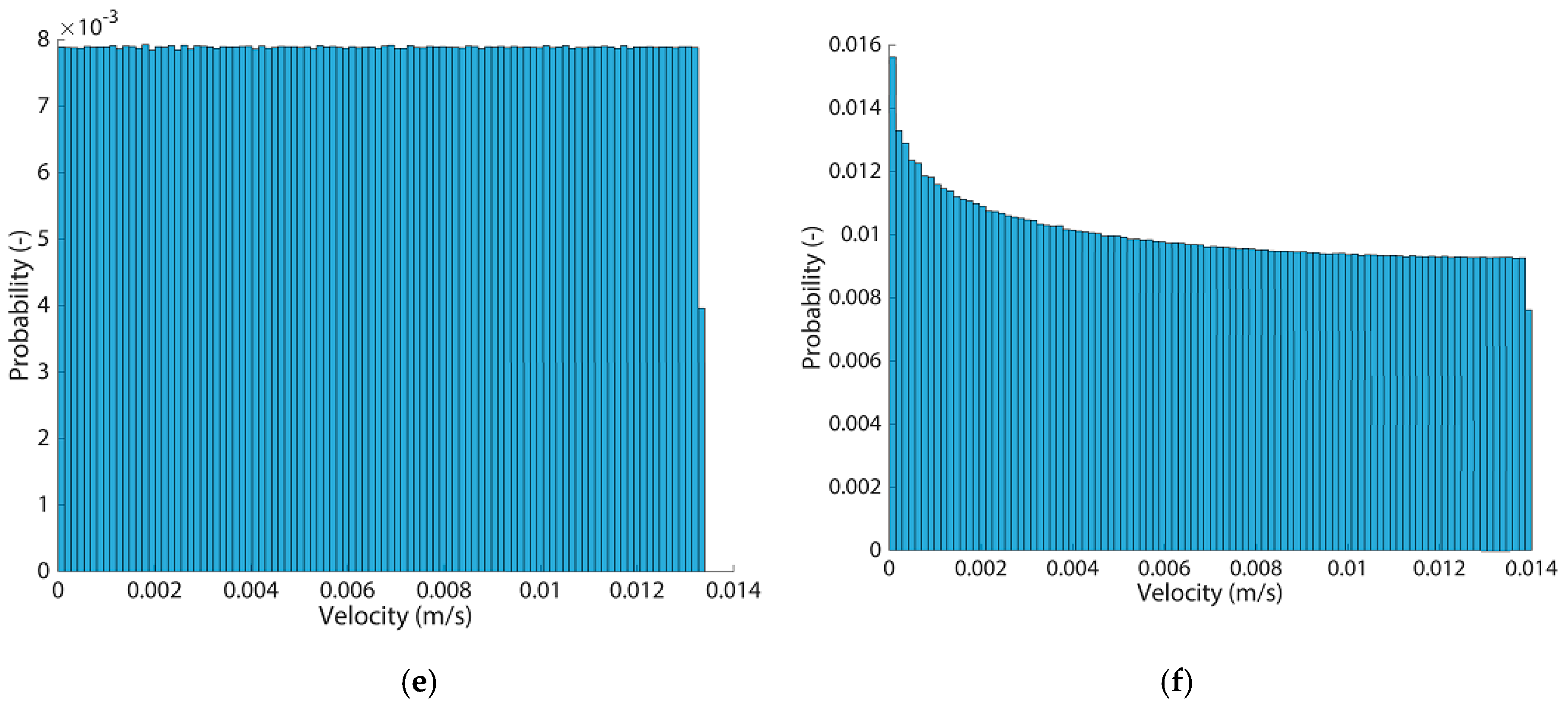
Appendix F
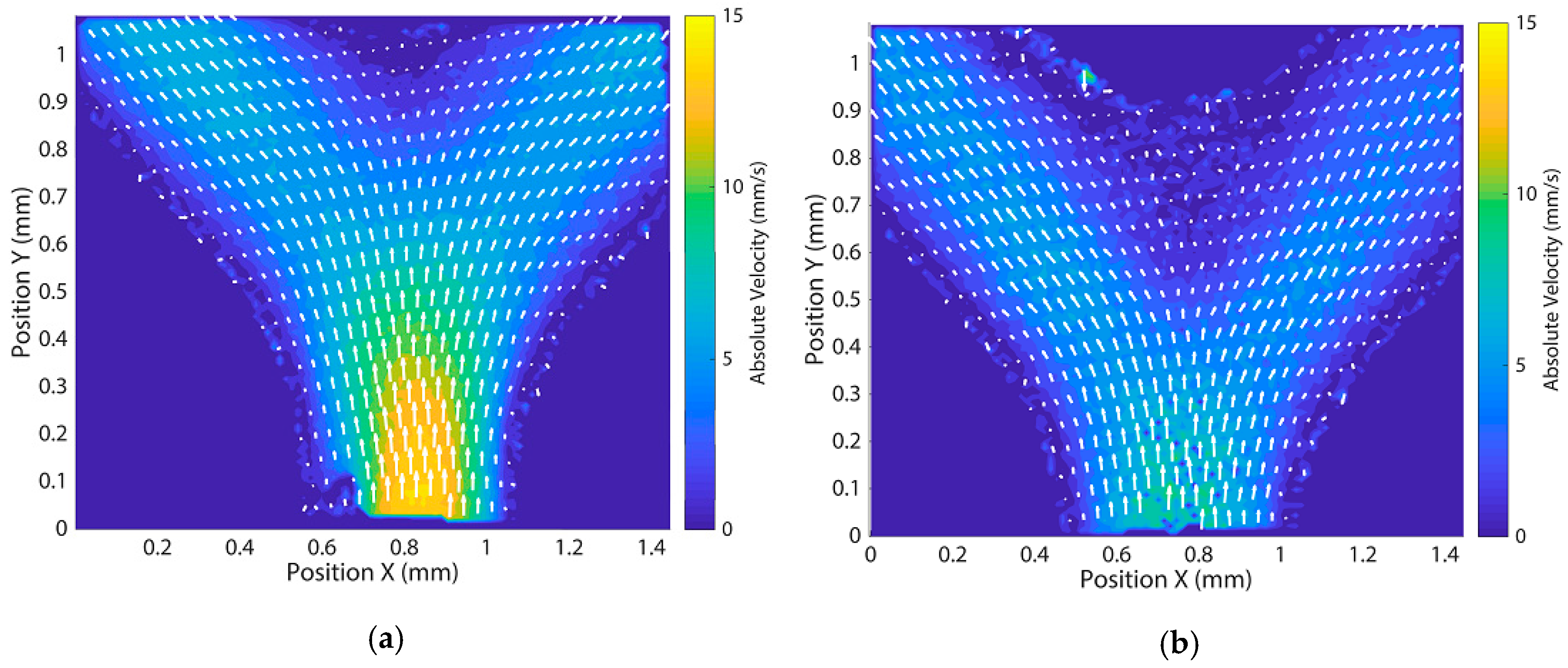
References
- Bogorad, M.I.; DeStefano, J.; Karlsson, J.; Wong, A.D.; Gerecht, S.; Searson, P.C. Review: In vitro microvessel models. Lab Chip 2015, 15, 4242–4255. [Google Scholar] [CrossRef] [PubMed]
- Papetti, M.; Herman, I.M. Mechanisms of normal and tumor-derived angiogenesis. Am. J. Physiol. Physiol. 2002, 282, C947–C970. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Paul, A.; Vrana, N.E.; Zhao, X.; Memic, A.; Hwang, Y.-S.; Dokmeci, M.R.; Khademhosseini, A. Microfluidic techniques for development of 3D vascularized tissue. Biomaterials 2014, 35, 7308–7325. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Rouwkema, J.; Khademhosseini, A. Vascularization and Angiogenesis in Tissue Engineering: Beyond Creating Static Networks. Trends Biotechnol. 2016, 34, 733–745. [Google Scholar] [CrossRef]
- Forster, J.C.; Harriss-Phillips, W.M.; Douglass, M.J.; Bezak, E. A review of the development of tumor vasculature and its effects on the tumor microenvironment. Hypoxia 2017, 5, 21–32. [Google Scholar] [CrossRef]
- Doshi, N.; Zahr, A.S.; Bhaskar, S.; Lahann, J.; Mitragotri, S. Red blood cell-mimicking synthetic biomaterial particles. Proc. Natl. Acad. Sci. USA 2009, 106, 21495–21499. [Google Scholar] [CrossRef]
- Merkel, T.J.; Jones, S.W.; Herlihy, K.P.; Kersey, F.R.; Shields, A.R.; Napier, M.; Luft, J.C.; Wu, H.; Zamboni, W.C.; Wang, A.Z.; et al. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc. Natl. Acad. Sci. USA 2011, 108, 586–591. [Google Scholar] [CrossRef]
- Bogorad, M.I.; DeStefano, J.; Wong, A.D.; Searson, P.C. Tissue-engineered 3D microvessel and capillary network models for the study of vascular phenomena. Microcirculation 2017, 24, e12360. [Google Scholar] [CrossRef]
- Heintz, K.A.; Mayerich, D.; Slater, J.H. Image-guided, Laser-based Fabrication of Vascular-derived Microfluidic Networks. J. Vis. Exp. 2017, 55101. [Google Scholar] [CrossRef] [PubMed]
- Brandenberg, N.; Lutolf, M.P. In Situ Patterning of Microfluidic Networks in 3D Cell-Laden Hydrogels. Adv. Mater. 2016, 28, 7450–7456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Montgomery, M.; Chamberlain, M.D.; Ogawa, S.; Korolj, A.; Pahnke, A.; Wells, L.A.; Massé, S.; Kim, J.; Reis, L.; et al. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat. Mater. 2016, 15, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Larsen, N.B. Stereolithographic hydrogel printing of 3D culture chips with biofunctionalized complex 3D perfusion networks. Lab Chip 2017, 17, 4273–4282. [Google Scholar] [CrossRef] [PubMed]
- Bischel, L.L.; Lee, S.-H.; Beebe, D.J. A practical method for patterning lumens through ECM hydrogels via viscous finger patterning. J. Lab. Autom. 2012, 17, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Saggiomo, V.; Velders, A.H. Simple 3D Printed Scaffold-Removal Method for the Fabrication of Intricate Microfluidic Devices. Adv. Sci. 2015, 2, 1500125. [Google Scholar] [CrossRef]
- Wu, W.; Deconinck, A.; Lewis, J.A. Omnidirectional Printing of 3D Microvascular Networks. Adv. Mater. 2011, 23, H178–H183. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Miller, J.S.; Stevens, K.R.; Yang, M.T.; Baker, B.M.; Nguyen, D.-H.T.; Cohen, D.M.; Toro, E.; Chen, A.A.; Galie, P.A.; Yu, X.; et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 2012, 11, 768–774. [Google Scholar] [CrossRef]
- Bégin-Drolet, A.; Dussault, M.-A.; Fernandez, S.A.; Larose-Dutil, J.; Leask, R.L.; Hoesli, C.A.; Ruel, J. Design of a 3D printer head for additive manufacturing of sugar glass for tissue engineering applications. Addit. Manuf. 2017, 15, 29–39. [Google Scholar] [CrossRef]
- Gelber, M.; Hurst, G.; Comi, T.; Bhargava, R. Model-guided design and characterization of a high-precision 3D printing process for carbohydrate glass. Addit. Manuf. 2018, 22, 38–50. [Google Scholar] [CrossRef]
- Marlin. Available online: http://marlinfw.org/ (accessed on 18 October 2018).
- Repetier-Host. Available online: https://www.repetier.com/ (accessed on 19 October 2018).
- Hadjikinova, R.; Marudova, M. Thermal behaviour of confectionary sweeteners’ blends. Bulg. Chem. Commun. 2016, 48, 446–450. [Google Scholar]
- Hartel, R.W.; Ergun, R.; Vogel, S. Phase/state transitions of confectionery sweeteners: Thermodynamic and kinetic aspects. Compr. Rev. Food Sci. Food Saf. 2011, 10, 17–32. [Google Scholar] [CrossRef]
- Larsen, B.S.; Skytte, J.; Svagan, A.J.; Meng-Lund, H.; Grohganz, H.; Löbmann, K. Using dextran of different molecular weights to achieve faster freeze-drying and improved storage stability of lactate dehydrogenase. Pharm. Dev. Technol. 2019, 24, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Doufas, A.K.; Rice, L.; Thurston, W. Shear and extensional rheology of polypropylene melts: Experimental and modeling studies. J. Rheol. 2011, 55, 95. [Google Scholar] [CrossRef]
- Kassab, G.S. Scaling laws of vascular trees: Of form and function. Am. J. Physiol. Circ. Physiol. 2006, 290, H894–H903. [Google Scholar] [CrossRef]
- Di Carlo, D. Inertial microfluidics. Lab Chip 2009, 9, 3038. [Google Scholar] [CrossRef]
- Sebastian, B.; Dittrich, P.S. Microfluidics to Mimic Blood Flow in Health and Disease. Annu. Rev. Fluid Mech. 2018, 50, 483–504. [Google Scholar] [CrossRef]
- Bischel, L.L.; Sung, K.E.; Jiménez-Torres, J.A.; Mader, B.; Keely, P.J.; Beebe, D.J. The importance of being a lumen. FASEB J. 2014, 28, 4583–4590. [Google Scholar] [CrossRef]
- Datta, S.; Ghosal, S. Characterizing dispersion in microfluidic channels. Lab Chip 2009, 9, 2537–2550. [Google Scholar] [CrossRef]
- Yang, X.; Forouzan, O.; Burns, J.M.; Shevkoplyas, S.S. Traffic of leukocytes in microfluidic channels with rectangular and rounded cross-sections. Lab Chip 2011, 11, 3231. [Google Scholar] [CrossRef] [PubMed]
- Pries, A.R.; Secomb, T.W. Blood Flow in Microvascular Networks. Microcirculation 2008, 3, 3–36. [Google Scholar]
- Secomb, T.W. Hemodynamics. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2016; pp. 975–1003. [Google Scholar]
- Akbari, E.; Spychalski, G.B.; Song, J.W. Microfluidic approaches to the study of angiogenesis and the microcirculation. Microcirculation 2017, 24, e12363. [Google Scholar] [CrossRef] [PubMed]
- Chary, S.R.; Jain, R.K. Direct measurement of interstitial convection and diffusion of albumin in normal and neoplastic tissues by fluorescence photobleaching. Proc. Natl. Acad. Sci. USA 1989, 86, 5385–5389. [Google Scholar] [CrossRef]
- Kohn, J.C.; Zhou, D.W.; Zhou, A.L.; Mason, B.N.; Mitchell, M.J.; King, M.R.; Reinhart-king, C.A. Article cooperative effects of matrix stiffness and fluid shear stress on endothelial cell behavior. Biophys. J. 2015, 108, 471–478. [Google Scholar] [CrossRef]
- Prabhakarpandian, B.; Shen, M.-C.; Pant, K.; Kiani, M.F. Microfluidic devices for modeling cell-cell and particle-cell interactions in the microvasculature. Microvasc. Res. 2011, 82, 210–220. [Google Scholar] [CrossRef]
- Toksvang, L.N.; Berg, R.M.G. Using a classic paper by Robin Fåhraeus and Torsten Lindqvist to teach basic hemorheology. Adv. Physiol. Educ. 2013, 37, 129–133. [Google Scholar] [CrossRef]
- Chebbi, R. Dynamics of blood flow: Modeling of the Fåhræus–Lindqvist effect. J. Biol. Phys. 2015, 41, 313–326. [Google Scholar] [CrossRef]
- Secomb, T.W. Blood flow in the microcirculation. Annu. Rev. Fluid Mech. 2016, 49, 443–461. [Google Scholar] [CrossRef]
- Pries, A.R.; Secomb, T.W. Microvascular blood viscosity in vivo and the endothelial surface layer. Am. J. Physiol. Circ. Physiol. 2005, 289, H2657–H2664. [Google Scholar] [CrossRef]
- Wong, K.H.; Chan, J.M.; Kamm, R.D.; Tien, J. Microfluidic models of vascular functions. Annu. Rev. Biomed. Eng. 2012, 14, 205–230. [Google Scholar] [CrossRef] [PubMed]
- Bruus, H. Theoretical Microfluidics; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Mortensen, N.A.; Okkels, F.; Bruus, H. Reexamination of Hagen-Poiseuille flow: Shape dependence of the hydraulic resistance in microchannels. Phys. Rev. E 2005, 71, 057301. [Google Scholar] [CrossRef] [PubMed]

| Component | Reported Tg | Reference |
|---|---|---|
| Sucrose | 62–70 °C | [24,25,26] |
| Dextrose | 31 °C | [25] |
| Dextran | 150–225 °C * | [24,25,26] |
| Material | Temperature | 95% Confidence Interval | ||
|---|---|---|---|---|
| °C | × 10−6 | × 10−6 | × 10−6 | |
| Carbohydrate glass | 90 | 5.54 | (5.45–5.63) | 6.49 |
| 95 | 7.46 | (7.42–50) | 8.60 | |
| 100 | 8.20 | (8.09–8.31) | 11.28 | |
| 105 | 10.29 | (10.17–10.42) | 14.4 | |
| Sugar glass | 90 | 17.89 | (17.34–18.43) | 12.50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pollet, A.M.A.O.; Homburg, E.F.G.A.; Cardinaels, R.; den Toonder, J.M.J. 3D Sugar Printing of Networks Mimicking the Vasculature. Micromachines 2020, 11, 43. https://doi.org/10.3390/mi11010043
Pollet AMAO, Homburg EFGA, Cardinaels R, den Toonder JMJ. 3D Sugar Printing of Networks Mimicking the Vasculature. Micromachines. 2020; 11(1):43. https://doi.org/10.3390/mi11010043
Chicago/Turabian StylePollet, Andreas M. A. O., Erik F. G. A. Homburg, Ruth Cardinaels, and Jaap M. J. den Toonder. 2020. "3D Sugar Printing of Networks Mimicking the Vasculature" Micromachines 11, no. 1: 43. https://doi.org/10.3390/mi11010043
APA StylePollet, A. M. A. O., Homburg, E. F. G. A., Cardinaels, R., & den Toonder, J. M. J. (2020). 3D Sugar Printing of Networks Mimicking the Vasculature. Micromachines, 11(1), 43. https://doi.org/10.3390/mi11010043







