A Passive Microfluidic Device for Chemotaxis Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
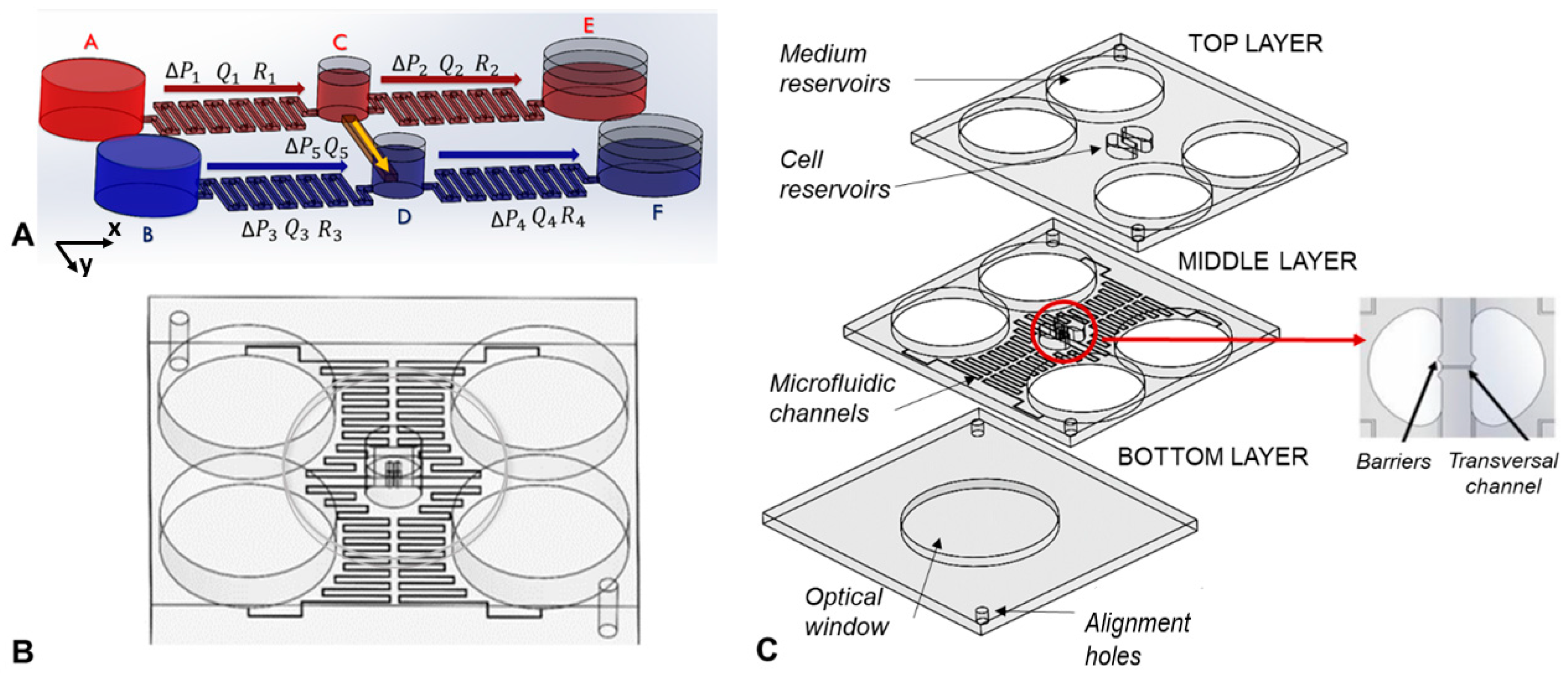
2.2. Working Principle
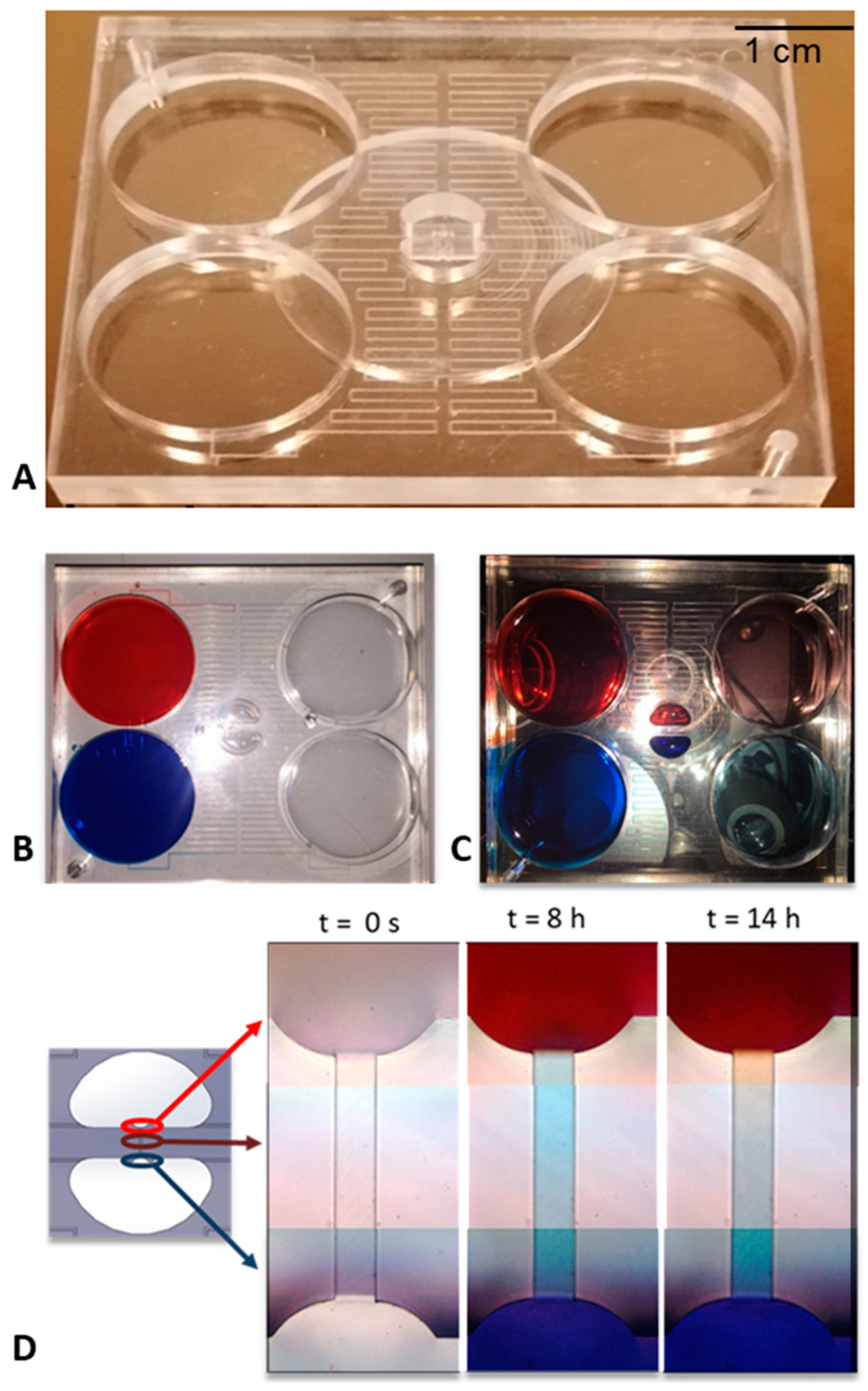
2.3. Device Fabrication
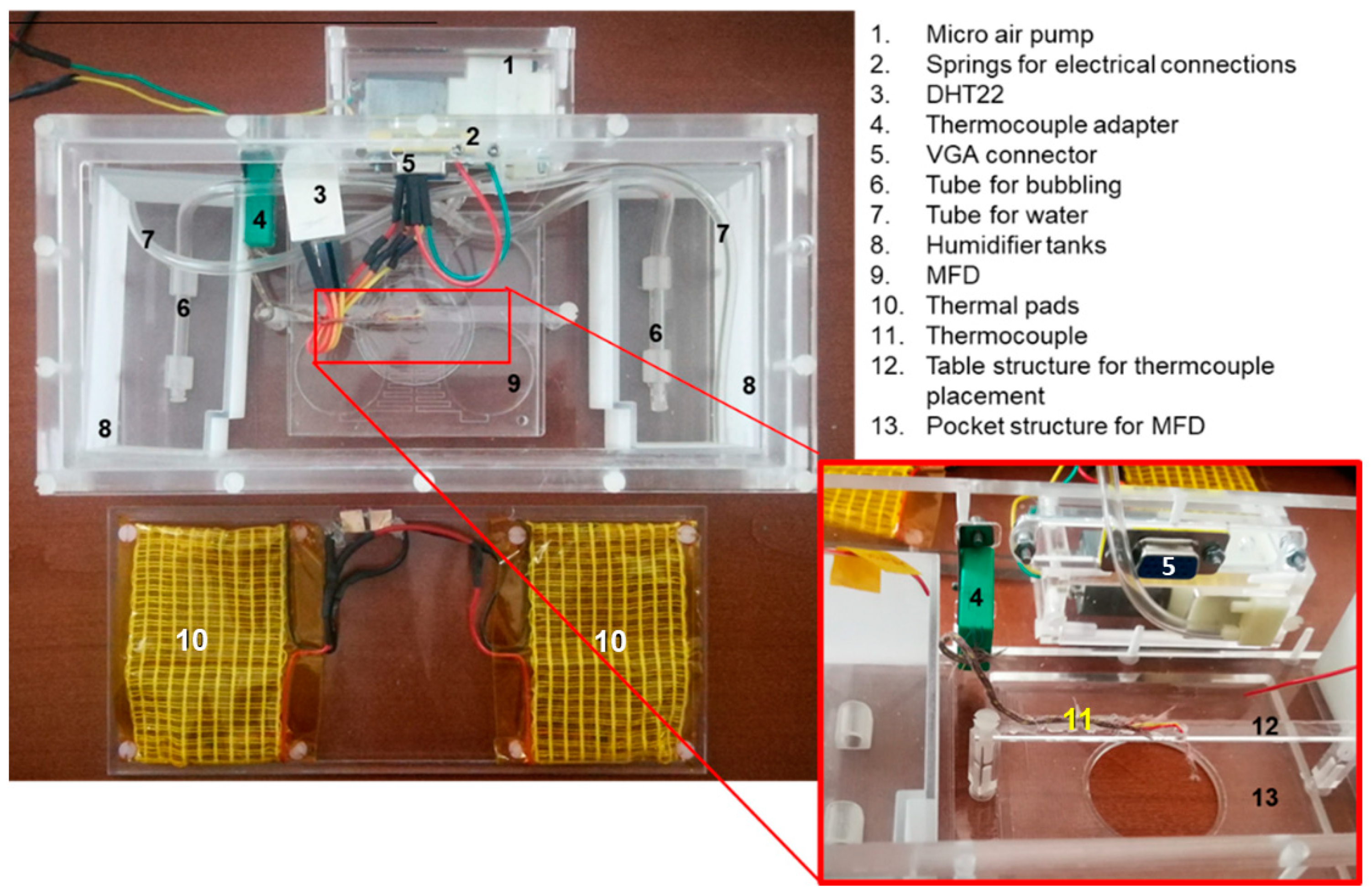
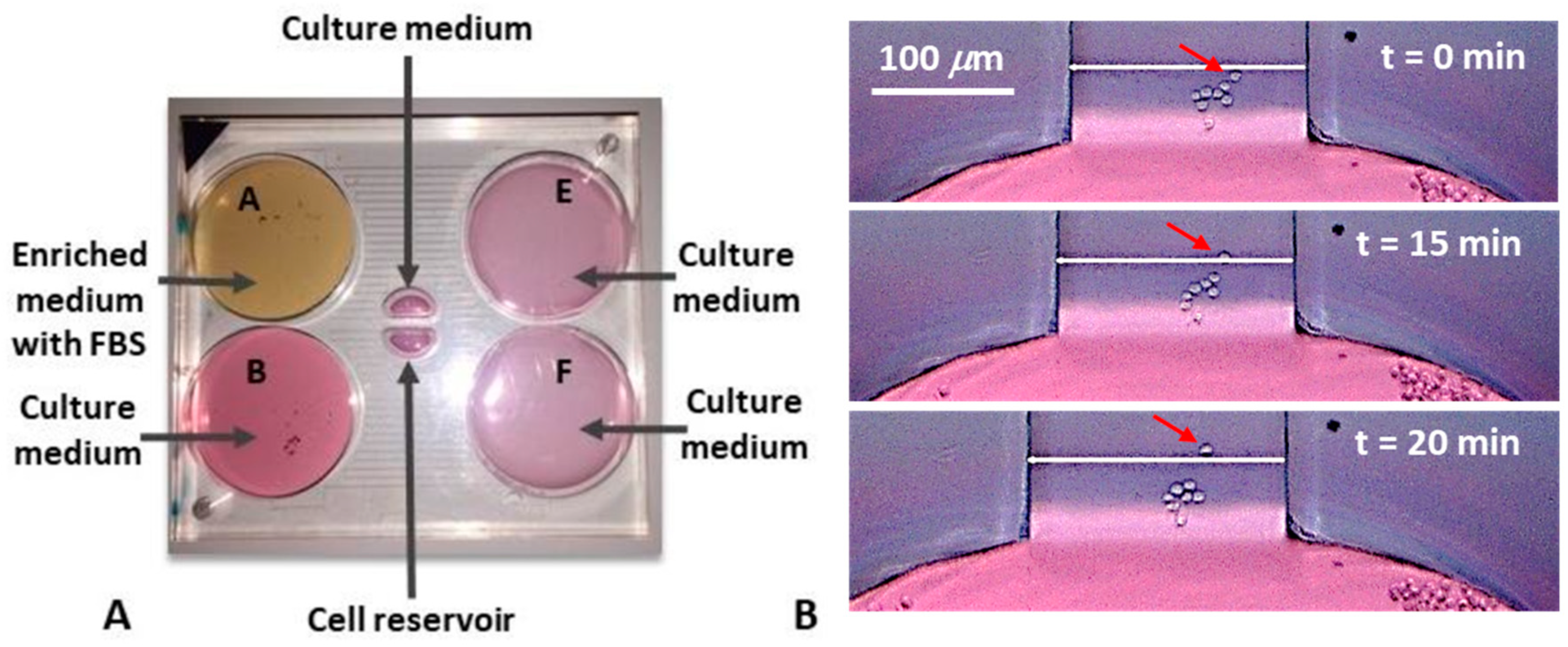
2.4. Experimental Set-Up
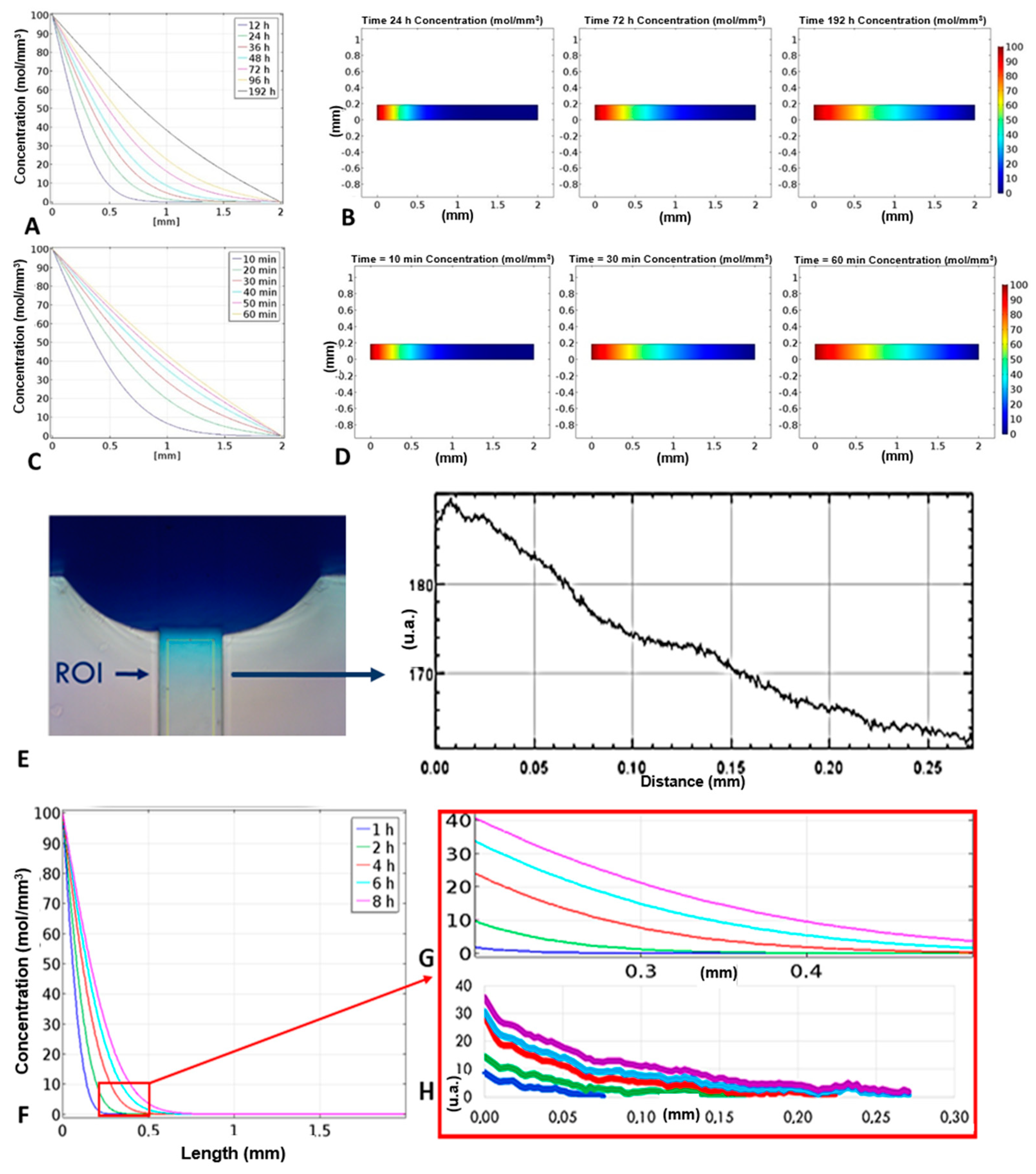
2.5. Simulation of the Concentration Gradient
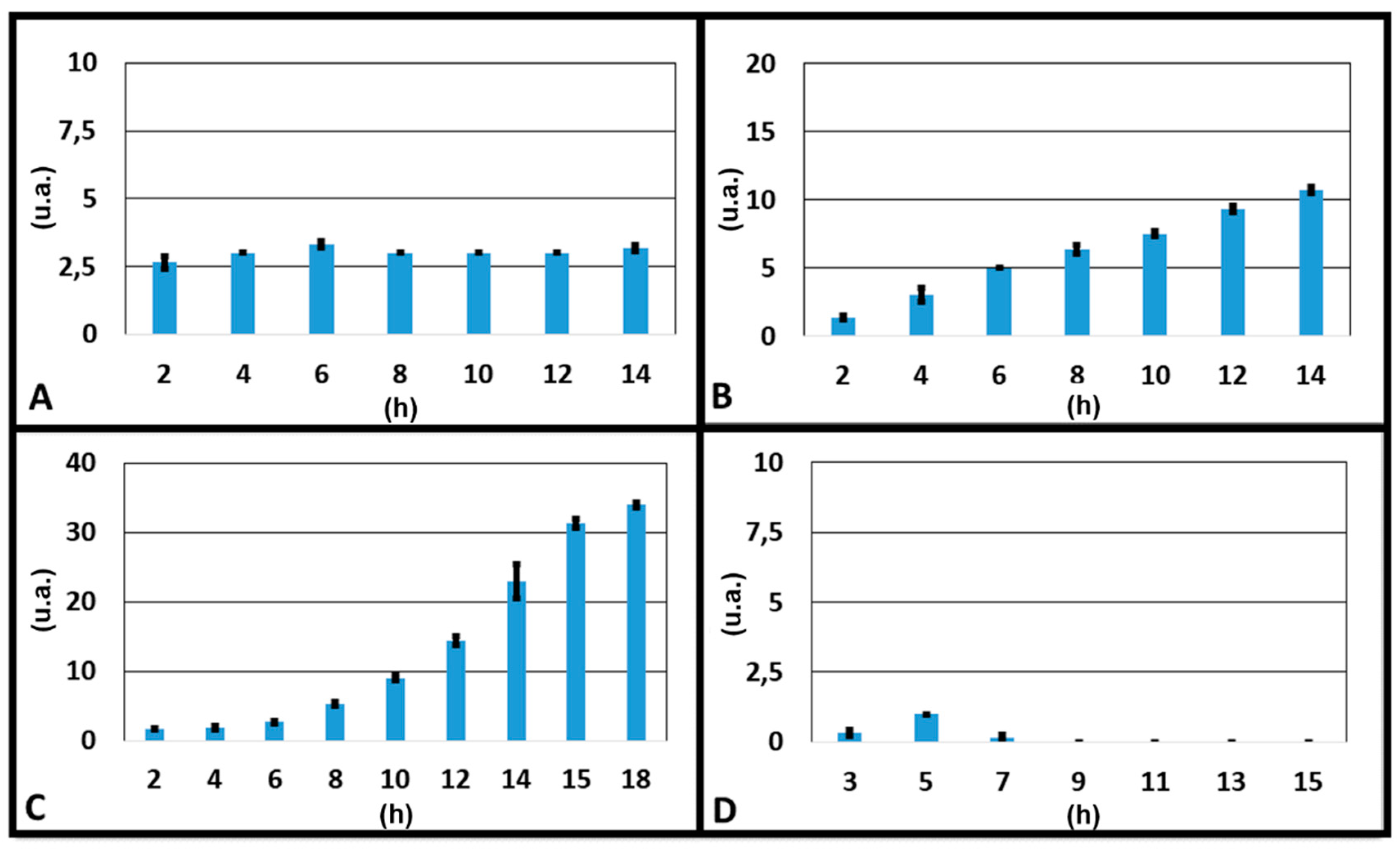
2.6. Device Characterization—Concentration Gradient
2.7. Device Characterization—Chemotaxis Experiments
- The channels were primed with the culture medium.
- The volumes accumulated in the reservoirs were emptied in the priming phase.
- The drain (E and F in Figure 1A) was filled with the appropriate volumes of culture media (1320.4 μL).
- The source (A and B in Figure 1A) was filled with the appropriate volumes: one (A) with the enriched medium (3750 μL), the other (B) with the culture medium (3961 μL).
- The central reservoirs were filled: One with culture medium, the other with cells. For this step a multi-channel pipette was used, to introduce simultaneously the same volumes inside the reservoirs, in order to avoid cell flows inside the transverse channel.
3. Results and Discussion
3.1. Simulation Results
3.2. Concentration Gradient Characterization
3.3. Chemotaxis Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kim, S.; Kim, H.J.; Jeon, N.L. Biological applications of microfluidic gradient devices. Integr. Biol. 2010, 2, 584–603. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.G.; Choo, J. Microfluidic gradient platforms for controlling cellular behavior. Electrophoresis 2010, 31, 3014–3027. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.W.; Luster, A.D. Targeting cells in motion: Migrating toward improved therapies. Eur. J. Immunol. 2013, 43, 1430–1435. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.J.; Von Andrian, U.H.; Wagers, A.J. Stem cell trafficking in tissue development, growth, and disease. Cell 2008, 132, 612–630. [Google Scholar] [CrossRef] [PubMed]
- Condeelis, J.; Segall, J.E. Intravital imaging of cell movement in tumours. Nat. Rev. Cancer 2003, 3, 921–930. [Google Scholar] [CrossRef]
- Keenan, T.M.; Folch, A. Biomolecular gradients in cell culture systems. Lab Chip 2008, 8, 34–57. [Google Scholar] [CrossRef]
- Li, J.; Lin, F. Microfluidic devices for studying chemotaxis and electrotaxis. Trends Cell Biol. 2011, 21, 489–497. [Google Scholar] [CrossRef]
- Kőhidai, L. Method for determination of chemoattraction in Tetrahymena pyriformis. Curr. Microbiol. 1995, 30, 251–253. [Google Scholar] [CrossRef]
- Boyden, S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J. Exp. Med. 1962, 115, 453–466. [Google Scholar] [CrossRef]
- Zigmond, S.H. Ability of polymorphonuclear leukocytes to orient in gradients of chemotactic factors. J. Cell Biol. 1997, 75, 606–616. [Google Scholar] [CrossRef]
- Zicha, D.; Dunn, G.; Jones, G. Analyzing chemotaxis using the Dunn direct-viewing chamber. Meth. Mol. Biol. 1997, 75, 449–457. [Google Scholar]
- Perozziello, G.; Møllenbach, J.; Laursen, S.; Di Fabrizio, E.; Gernaey, K.; Krühne, U. Lab on a chip automates in vitro cell culturing. Microelectron. Eng. 2012, 98, 655–658. [Google Scholar] [CrossRef] [Green Version]
- Simone, G.; Perozziello, G.; Battista, E.; De Angelis, F.; Candeloro, P.; Gentile, F.; Malara, N.; Manz, A.; Carbone, E.; Netti, P.; et al. Cell rolling and adhesion on surfaces in shear flow. A model for an antibody-based microfluidic screening system. Microelectron. Eng. 2012, 98, 668–691. [Google Scholar] [CrossRef]
- Keramas, G.; Perozziello, G.; Geschke, O.; Christensen, C.B.V. Development of a multiplex microarray microsystem. Lab Chip 2004, 4, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Simone, G.; Malara, N.; Trunzo, V.; Perozziello, G.; Neuzil, P.; Francardi, M.; Roveda, L.; Renne, M.; Prati, U.; Mollace, V.; et al. Protein–carbohydrate complex reveals circulating metastatic cells in a microfluidic assay. Small 2013, 9, 2152–2161. [Google Scholar] [CrossRef] [PubMed]
- Perozziello, G.; Candeloro, P.; De Grazia, A.; Esposito, F.; Allione, M.; Coluccio, M.L.; Tallerico, R.; Valpapuram, I.; Tirinato, L.; Das, G.; et al. Microfluidic device for continuous single cells analysis via Raman spectroscopy enhanced by integrated plasmonic nanodimers. Opt. Exp. 2016, 24, A180–A190. [Google Scholar] [CrossRef]
- Perozziello, G.; Catalano, R.; Francardi, M.; Rondanina, E.; Pardeo, F.; De Angelis, F.; Malara, N.; Candeloro, P.; Morrone, G.; Di Fabrizio, E. A microfluidic device integrating plasmonic nanodevices for Raman spectroscopy analysis on trapped single living cells. Microelectron. Eng. 2013, 111, 314–319. [Google Scholar] [CrossRef]
- Perozziello, G.; Bundgaard, F.; Geschke, O. Fluidic interconnections for microfluidic systems: A new integrated fluidic interconnection allowing plug‘n’play functionality. Sens. Actuators B 2008, 130, 947–953. [Google Scholar] [CrossRef]
- Simone, G.; Perozziello, G.; Sardella, G.; Disegna, I.; Tori, S.; Manaresi, N.; Medoro, G. A microvalve for hybrid microfluidic systems. Microsyst. Technol. 2010, 16, 1269–1276. [Google Scholar] [CrossRef]
- Snakenborg, D.; Perozziello, G.; Klank, H.; Geschke, O.; Kutter, J.P. Direct milling and casting of polymerbased optical waveguides for improved transparency in the visible range. J. Micromech. Microeng. 2006, 16, 375–381. [Google Scholar] [CrossRef]
- Simone, G.; Perozziello, G. UV/VIS transparent optical waveguides fabricated using organic–inorganic nanocomposite layers. J. Nanosci. Nanotechnol. 2011, 11, 2057–2063. [Google Scholar] [CrossRef] [PubMed]
- Perozziello, G.; Candeloro, P.; Gentile, F.; Nicastri, A.; Perri, A.M.; Coluccio, M.L.; Parrotta, E.; De Grazia, A.; Tallerico, R.; Pardeo, F.; et al. A microfluidic dialysis device for complex biological mixture SERS analysis. Microelectron. Eng. 2015, 144, 37–41. [Google Scholar] [CrossRef]
- Perozziello, G.; La Rocca, R.; Cojoc, G.; Liberale, C.; Malara, N.; Simone, G.; Candeloro, P.; Anichini, A.; Tirinato, L.; Gentile, F.; et al. Microfluidic devices module tumor cell line susceptibility to NK cell recognition. Small 2012, 8, 2886–2894. [Google Scholar] [CrossRef] [PubMed]
- Simone, G.; Perozziello, G. Ca2+ mediates the adhesion of breast cancer cells in self-assembled multifunctional microfluidic chip prepared with carbohydrate beads. Micro Nanosyst. 2010, 2, 261–268. [Google Scholar] [CrossRef]
- Yang, K.; Wu, J.; Zhu, L.; Liu, Y.; Lin, F. A triple-unit microfluidic device (D3-chip) for cell migration research. Protoc. Exch. 2017. [Google Scholar] [CrossRef]
- Song, J.; Zhang, Y.; Zhang, C.; Du, X.; Guo, Z.; Kuang, Y.; Wang, Y.; Wu, P.; Zou, K.; Zou, L.; et al. A microfluidic device for studying chemotaxis mechanism of bacterial cancer targeting. Sci. Rep. 2018, 8, 6394. [Google Scholar] [CrossRef]
- Perozziello, G.; Candeloro, P.; Gentile, F.; Nicastri, A.; Perri, A.M.; Coluccio, M.L.; Adamo, A.; Pardeo, F.; Catalano, R.; Parrotta, E.; et al. Microfluidics & Nanotechnology: Towards fully integrated analytical devices for the detection of cancer biomarkers. RSC Adv. 2014, 4, 55590–55598. [Google Scholar]
- Moghe, P.V.; Nelson, R.D.; Tranquillo, R.T. Cytokine-stimulated chemotaxis of human neutrophils in a 3-D conjoined fibrin gel assay. J. Immunol Meth. 1995, 180, 193–211. [Google Scholar] [CrossRef]
- Yu, X.; Schmidt, A.R.; Bello-Perez, L.A.; Schmidt, S.J. Determination of the bulk moisture diffusion coefficient for corn starch using an automated water sorption instrument. J. Agric. Food Chem. 2008, 56, 50–58. [Google Scholar] [CrossRef]
- Okada, T.; Miller, M.J.; Parker, I.; Krummel, M.F.; Neighbors, M.; Hartley, S.B.; O’Garra, A.; Cahalan, M.D.; Cyster, J.G. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PloS Biol. 2005, 36, 1047–1061. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coluccio, M.L.; D’Attimo, M.A.; Cristiani, C.M.; Candeloro, P.; Parrotta, E.; Dattola, E.; Guzzi, F.; Cuda, G.; Lamanna, E.; Carbone, E.; et al. A Passive Microfluidic Device for Chemotaxis Studies. Micromachines 2019, 10, 551. https://doi.org/10.3390/mi10080551
Coluccio ML, D’Attimo MA, Cristiani CM, Candeloro P, Parrotta E, Dattola E, Guzzi F, Cuda G, Lamanna E, Carbone E, et al. A Passive Microfluidic Device for Chemotaxis Studies. Micromachines. 2019; 10(8):551. https://doi.org/10.3390/mi10080551
Chicago/Turabian StyleColuccio, Maria Laura, Maria Antonia D’Attimo, Costanza Maria Cristiani, Patrizio Candeloro, Elvira Parrotta, Elisabetta Dattola, Francesco Guzzi, Giovanni Cuda, Ernesto Lamanna, Ennio Carbone, and et al. 2019. "A Passive Microfluidic Device for Chemotaxis Studies" Micromachines 10, no. 8: 551. https://doi.org/10.3390/mi10080551
APA StyleColuccio, M. L., D’Attimo, M. A., Cristiani, C. M., Candeloro, P., Parrotta, E., Dattola, E., Guzzi, F., Cuda, G., Lamanna, E., Carbone, E., Krühne, U., Di Fabrizio, E., & Perozziello, G. (2019). A Passive Microfluidic Device for Chemotaxis Studies. Micromachines, 10(8), 551. https://doi.org/10.3390/mi10080551







