1. Introduction
In order to achieve the goal of global carbon reduction, countries are actively developing various types of renewable energy. At present, solar energy is one of the most attractive types of energy. An important issue in research on solar photovoltaic (PV) systems is the reduction of the cost of PV ribbons, in addition to the improvement of the photoelectric conversion efficiency of solar cell materials [
1,
2].
In developing low-cost PV ribbons, Sn–Cu alloys are considered promising lead-free alloy solders due to their good weldability and conductivity [
3,
4]. However, the excessive formation of an intermetallic compound (IMC) at the interface between the Sn–Cu alloy and the Cu substrate during a prolonged heating is problematic [
5], and the Sn–Cu alloy has a higher melting point than the Sn–Pb alloy. Our previous report showed that the series resistance of the PV module increased by 52% due to the rapid growth of IMCs and the overconsumption of the Ag electrode after bias for a long time [
3]. Note that adding a small amount of Ag or Zn into an Sn–Cu alloy can reduce the above problems [
6,
7]. Adding Ag into a Sn–Cu solder can reduce the consumption of the Ag electrode in the module after reflow. However, the addition of Ag in the solder increases the module cost and also the proportion of a high-resistance phase in the solder alloy [
8]. Considering the cost of the solder, a Zn-containing Sn–Cu solder is examined as a candidate solder to improve the properties of the Sn–Cu alloy. The phase diagram of the Cu–Zn alloy showed that the solid solubility of Zn in Cu can reach 37 wt% at 300 °C [
9]. Related studies indicated that Zn atoms will accumulate in the Cu–Zn solid solution alloy at the interface between the solder and Cu substrate and form a Cu–Zn solid solution alloy [
6,
10]. This Zn accumulation layer could effectively retard the diffusion of Cu atoms into the solder alloy, reducing the growth of IMCs at the interface.
In the present study, an Sn–0.7Cu alloy solder [
3] with 0.2 wt% Zn (Sn–0.7Cu–0.2Zn) is used for the fabrication of a PV ribbon, and the contribution of Zn to the series resistance and interfacial microstructure of the module is investigated.
3. Results and Discussion
Figure 1 shows interfacial images (Cu/solder/Ag) of the Sn–0.7Cu–0.2Zn PV module after reflow for 5, 7, and 10 s. Cu
6Sn
5 and Ag
3Sn IMCs appeared at the Cu/solder and solder/Ag interfaces, respectively, in all PV modules. The solder matrix was composed of a eutectic structure (β-Sn + Cu
6Sn
5 + Cu
5Zn
8). Note that the IMC thickness (Cu
6Sn
5, Ag
3Sn) increased with the increasing reflow time, which is related to a static heat-induced (reflowed, hot-dipped) growth reaction [
2]. The peel strength of the PV ribbons reflowed onto an Ag electrode on a Si solar cell for various reflow times (5–10 s) is shown in
Figure 2. For the reflow time of 5 s, the peeling force of the PV module was lower than a standard value of 100 gf. This was attributed to the smaller IMC growth at the solder/Ag interface, which reduced the bond strength of the PV module. From the statistical results of ImageJ calculations (
Figure 2), the thicknesses of the Cu
6Sn
5 and Ag
3Sn IMCs increased with increasing reflow time. This was attributed to the formation of a too small amount of Ag
3Sn (≤ 1 μm) in the reflow time of 5 s which was insufficient to connect the PV ribbon to the solar cell. Under different reflow conditions (300 °C for 10 s), the IMCs thickness of the Sn–0.7Cu–0.2Zn PV module was lower than that of the Sn–0.7Cu one [
3]. This indicated that adding less Zn to the Sn–0.7Cu solder can retard the overgrowth of IMCs in the module.
Considering the bonding strength and residual Ag electrode, the PV module with reflow time of 7 s was subjected to the biasing test.
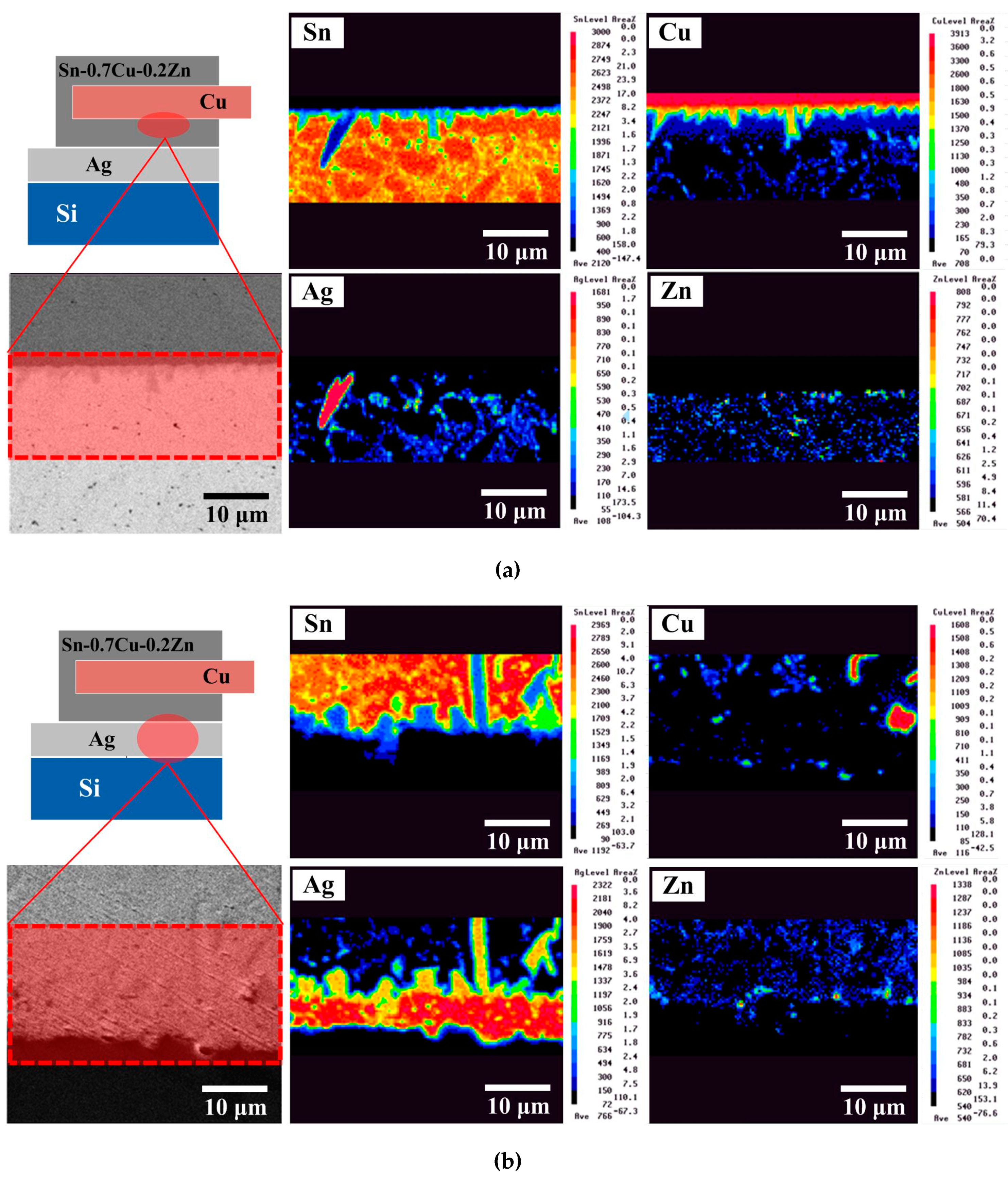
Figure 3 shows EPMA images of the Cu/Sn–0.7Cu–0.2Zn solder/Ag structure obtained under a given set of reflow conditions. The Cu
6Sn
5 compound formed at the Cu/solder interface during the hot-dipping process (
Figure 3a). The solder contained a β-Sn matrix and a eutectic structure (β-Sn + Cu
6Sn
5 + Ag
3Sn + (Ag, Cu)
5Zn
8) composed of Sn, Cu, Ag, and Zn elements. Note that a higher amount of Zn was present at the solder/Cu interface. According to a previous report [
6], the accumulating Zn atoms might be a Cu–Zn solid-solution layer near the Cu metal surface. This Zn accumulation layer could retard the diffusion of Cu into the solder matrix, slowing the growth of IMCs. At the solder/Ag interface (
Figure 3b), Ag atoms combined with Sn to form Ag
3Sn IMC after reflow and also diffused into the solder matrix to form the eutectic structure, which was attributed to reflow-induced thermal diffusion. In addition, a Zn accumulation layer also existed at the solder/Ag interface and could retard the growth of Ag
3Sn IMC. After biasing for 72 h (
Figure 4), a small amount of Ag
3Sn phase appeared at the Cu/solder interface (
Figure 4a), while a Cu
6Sn
5 phase was detected at the solder/Ag interface (
Figure 4b). This is typical of a diffusion behavior in two different directions and allowed to exclude electromigration, which occurs in a single direction [
2]. The growth of Ag
3Sn and Cu
6Sn
5 IMCs was related to an elemental thermomigration. This was attributed to a bias-induced Joule heat which increased the diffusion rate between the atoms, causing the IMCs (Ag
3Sn and Cu
6Sn
5) to migrate to another interface. Compared with the unbiased module, the 72 h-biased module showed increased consumption of the Ag electrode, while the Cu
6Sn
5 IMC did not display obvious growth. This result confirmed that a Zn accumulation layer existed at the Cu/solder interface, decreasing the diffusion of Cu atoms and retarding the overgrowth of Cu
6Sn
5 IMC.
The measurements of IMCs thickness and series resistance of the PV modules before and after biasing are shown in
Figure 5 [
3]. The series resistance of an unbiased Sn–0.7Cu–0.2Zn PV module (2.1 × 10
−2 Ω) was lower than that of an unbiased Sn–0.7Cu module (2.3 × 10
−2 Ω). This result was attributed to the Sn–0.7Cu–0.2Zn PV module having a thinner Cu
6Sn
5 and Ag
3Sn IMC layer. Adding Zn into the Sn–0.7Cu solder could retard the growth of Cu
6Sn
5 IMC, and the shorter reflow time (7 s) decreased the growth of Ag
3Sn. After biasing for 72 h, the Cu
6Sn
5 and Ag
3Sn IMCs of the Sn–0.7Cu–0.2Zn PV module did not significantly increase compared with those of the Sn–0.7Cu PV module. The growth of Cu
6Sn
5 and Ag
3Sn IMCs in the Sn–0.7Cu–0.2Zn PV module increased by 1% and 34%, respectively, while that in the Sn–0.7Cu PV module increased by 108% and 105%, respectively [
3]. In addition, the peeling force of the 72 h-biased Sn–0.7Cu PV module (~30 gf ± 20 gf) was much smaller than that of the reflowed Sn–0.7Cu PV module (~90 gf ± 10 gf), while that of the 72 h-biased Sn–0.7Cu–0.2Zn PV module only dropped to ~160 gf. This indicates that the overgrown IMCs decreased the module resistance and weakened the peeling force of the module. The chemical reaction between the Cu ribbon and the solders mainly dominated the growth behavior of Cu
6Sn
5 IMC (
Figure 6). Some Zn atoms in the Sn–0.7Cu–0.2Zn solder accumulated at the Cu/solder interface to form the Cu–Zn solid-solution layer during the hot-dip process. These Zn atoms could retard the thermal migration and electromigration of Cu atoms and their reaction with the Sn atoms of the solder. This indicates that the Zn accumulation layer could effectively retard the growth of IMCs, and thus the series resistance of the Sn–0.7Cu–0.2Zn PV module only increased by 9%.