Selective Carbon Material Engineering for Improved MEMS and NEMS
Abstract
1. Introduction
2. Brief Review on Main MEMS and NEMS Characteristics
2.1. Early Stage of Microelectromechanical Systems (MEMS)
2.2. MEMS Fab
2.3. NEMS
2.3.1. Definition and General Features
2.3.2. Early NEMS Application Fundamentals
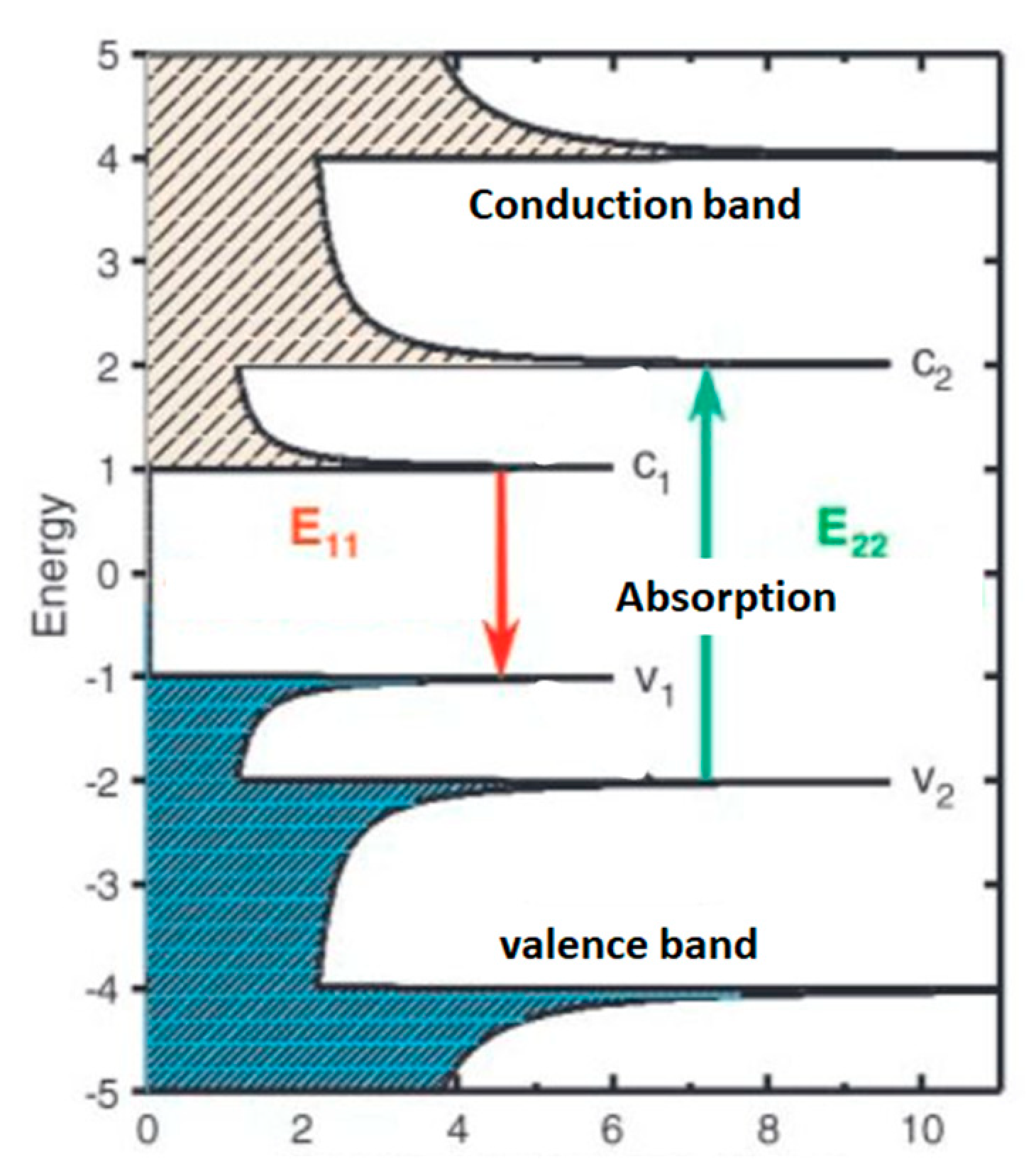
3. Increased NEMS Performances with Advanced Carbon Material
3.1. Progress in NEMS Technologies
3.2. Diamond and Related Materials
3.2.1. Different Categories of Diamond and Diamond-Like Materials
- (a)
- Polycrystalline diamond of different crystallite size, including the hexagonal and epitaxial diamond. To be observed that the denser and smoother micro- and nano-crystalline diamond is almost containing a significant part of graphitic material where more or less ordered/disordered diamond crystallites are imbedded. However, besides interesting tribological properties, those have generally reduced others (optical, optoelectronic, chemical and mechanical) [114,115,116,117,118,119].
- (b)
- (c)
- (d)
3.2.2. Upholding of Combined Properties
3.3. CNT and Graphene
3.3.1. Definition and Technologic Trends
3.3.2. Early Fundamentals on Graphene and CNT
4. Brief Review of Quantum Activated Atomic Rearrangement
4.1. Atomic Rearrangement during Synthesis of Carbon Materials
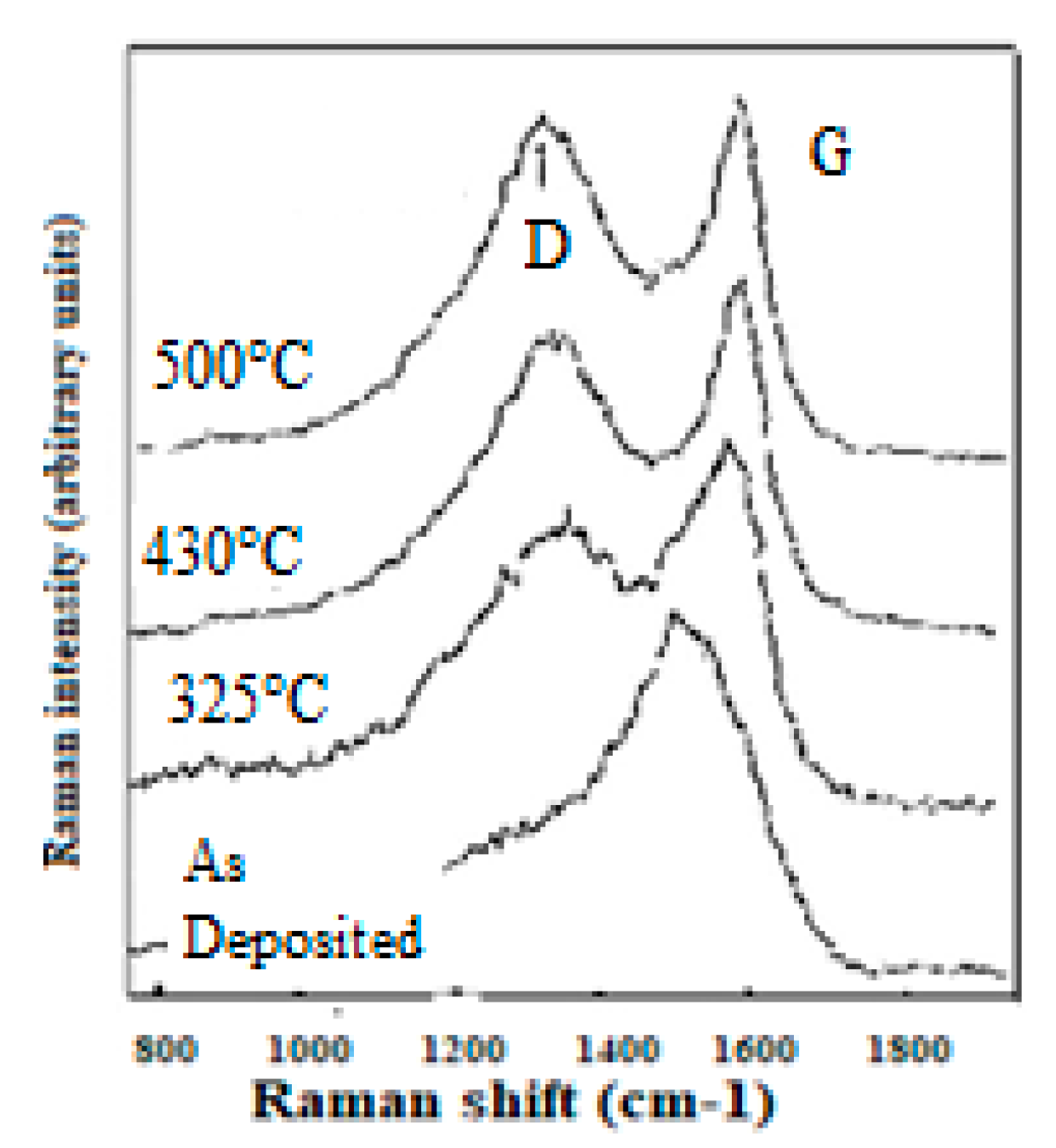
4.1.1. Graphitic Thermal Degradation and Diamond-Like Material Reforming
4.1.2. Surface Polarization and Diamond-Like Atomic Rearrangement
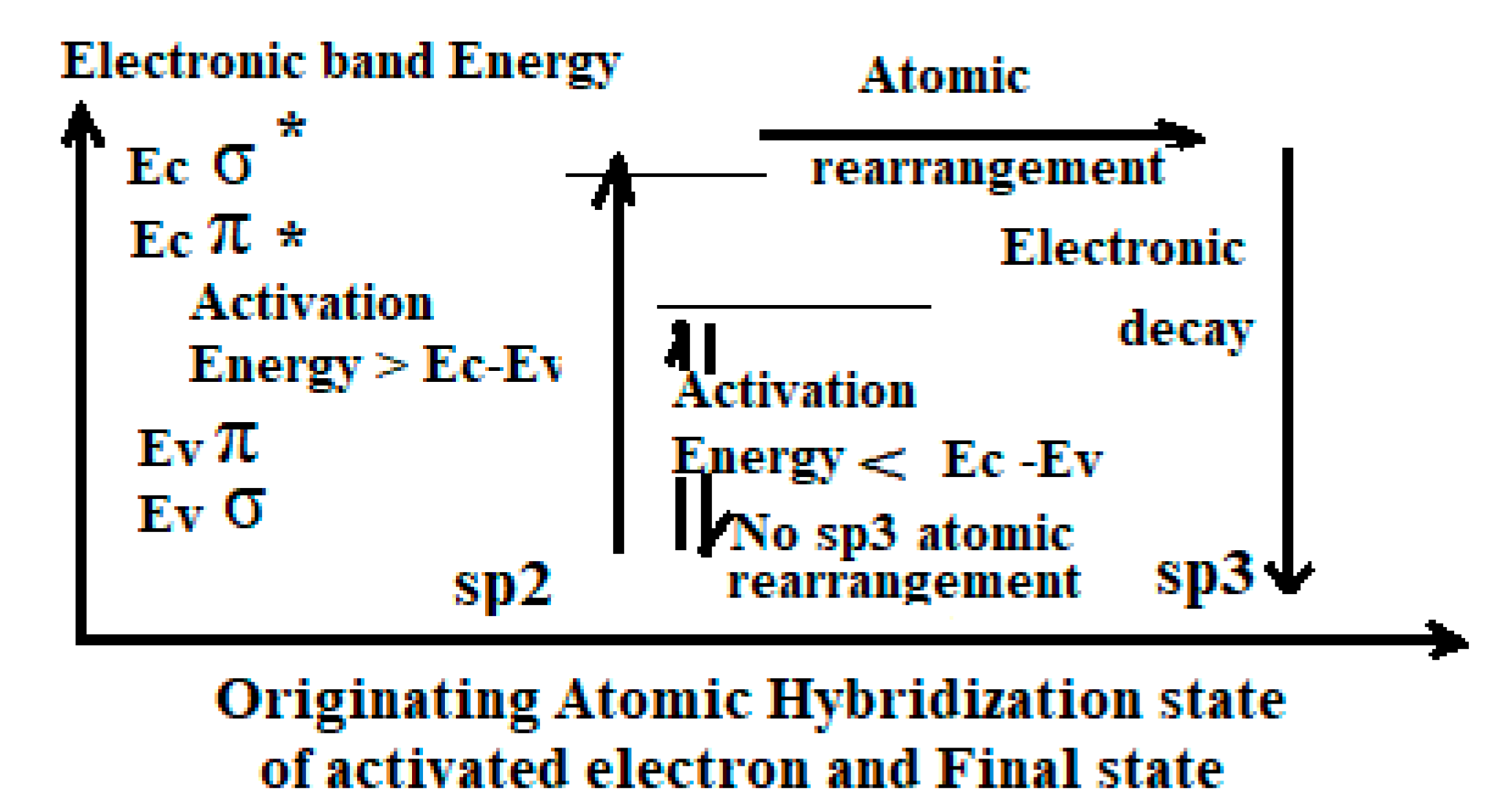
4.1.3. Criterion of Quantum Electronic Sp3 Activation
- (1)
- Valence band electrons must be excited up to the conduction band of the initial and final state.
- (2)
- More electrons must be activated than atoms to be rearranged.
- (3)
- Atomic rearrangement can only be achieved with its local (proximity) activated electrons.
- (4)
- The kinetic and density of activation events must be compatible with the decay and diffusion kinetic.
4.1.4. Ordered Atomic Rearrangement and Stress Modification
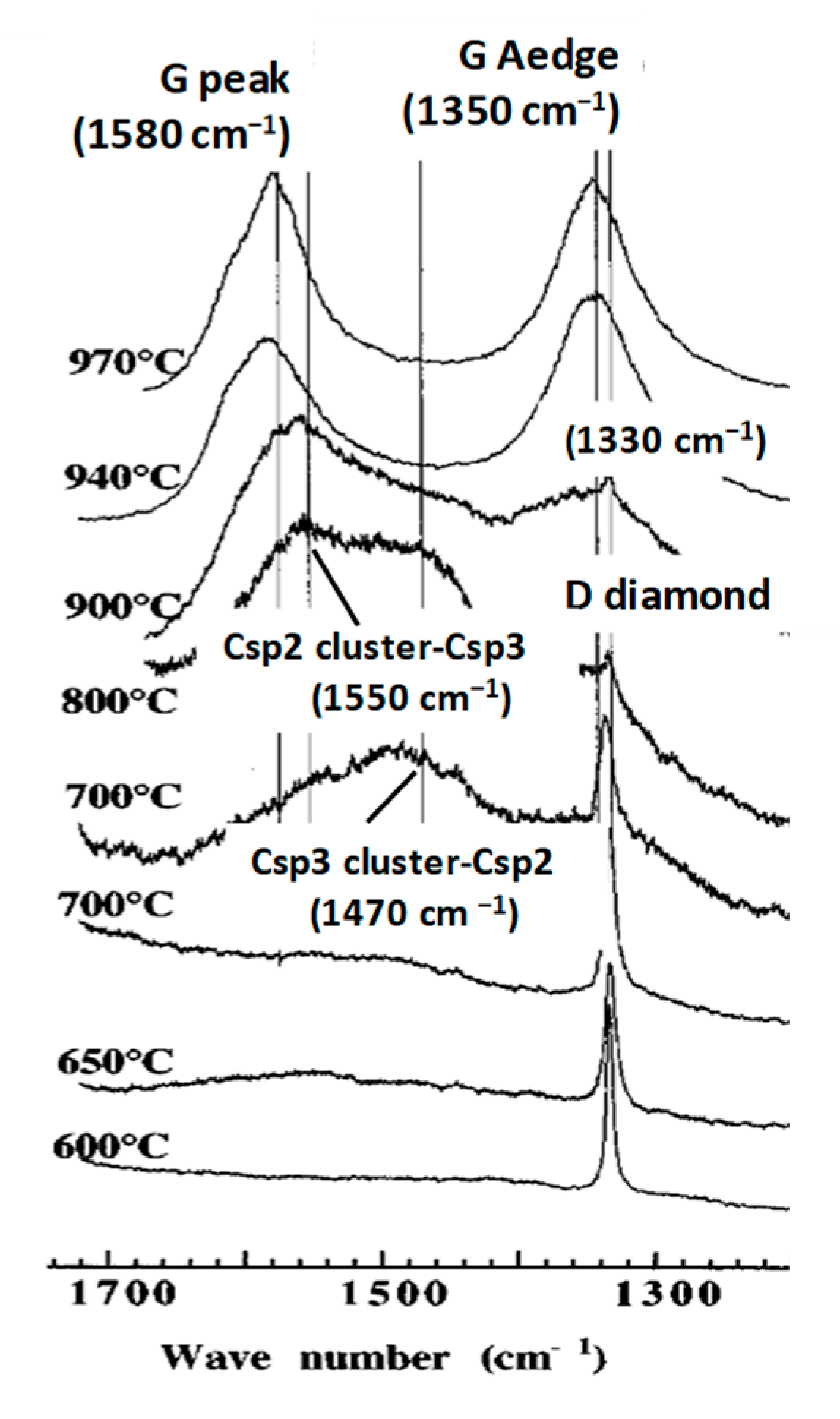
4.1.5. Examples of Diamond Like Atomic Rearrangement of Graphenic Material
CNx Annealing with Formation of Diamond Crystallites
Graphene Transformation into a Dielectric Material
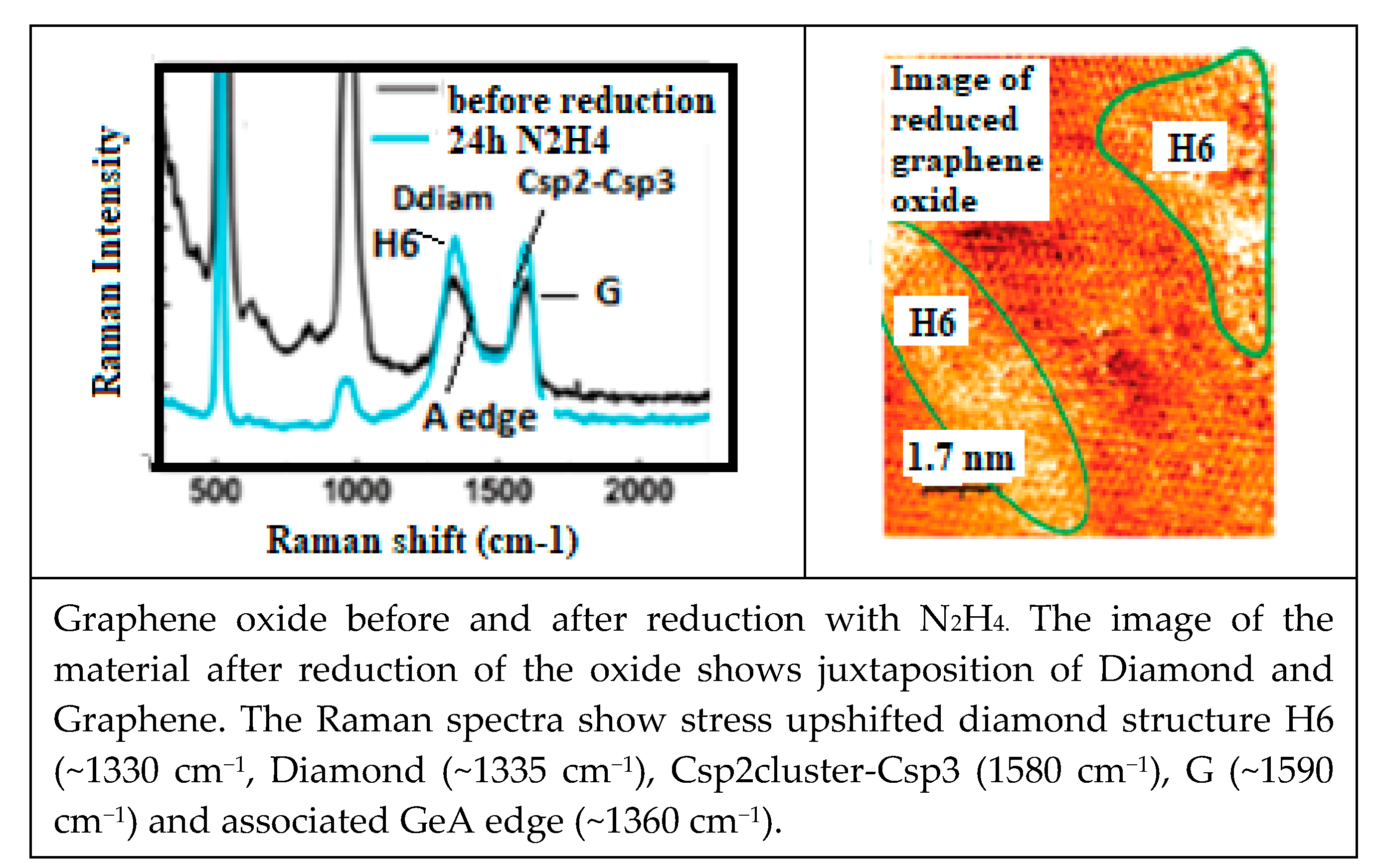
Transformation of Graphene into H6 Diamond with N and O
Oxygen Sensitivity of CNT Electric Conductivity
4.2. Importance of Diamond-Like Phase Transformation on Carbon Material Properties
4.2.1. Phase Transformation and Internal Stress Formation
4.2.2. Incidence on Carbon Film Nucleation
4.2.3. Incidence on Carbon-Based NEMS Engineering
5. Brief Review of Revisited Carbon Raman Spectroscopy
5.1. General Aspects of Carbon Raman Spectroscopy
5.2. Carbon Structures to Be Considered with Raman Spectroscopy
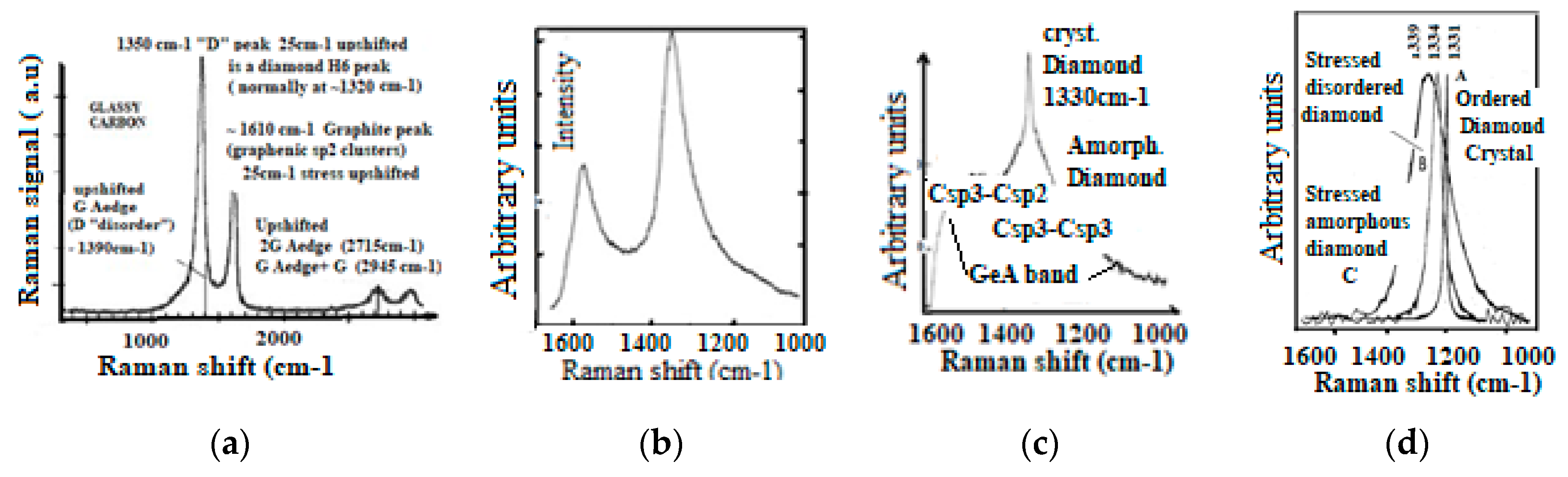
5.2.1. Comparison of Raman Spectra from Different Carbon Materials
5.2.2. Raman Stress Shift and Atomic Disorder Band Broadening
- (a)
- Sharp Raman peak corresponds always to an ordered structure.
- (b)
- Atomic disorder corresponds to peak broadening
- (c)
- Raman shift is proportional to internal stress.
5.2.3. Raman Peak Designation and Atomic Disorder
5.3. Revision Necessity of Common Raman Scattering Description
5.4. Refined Carbon Raman Spectroscopy
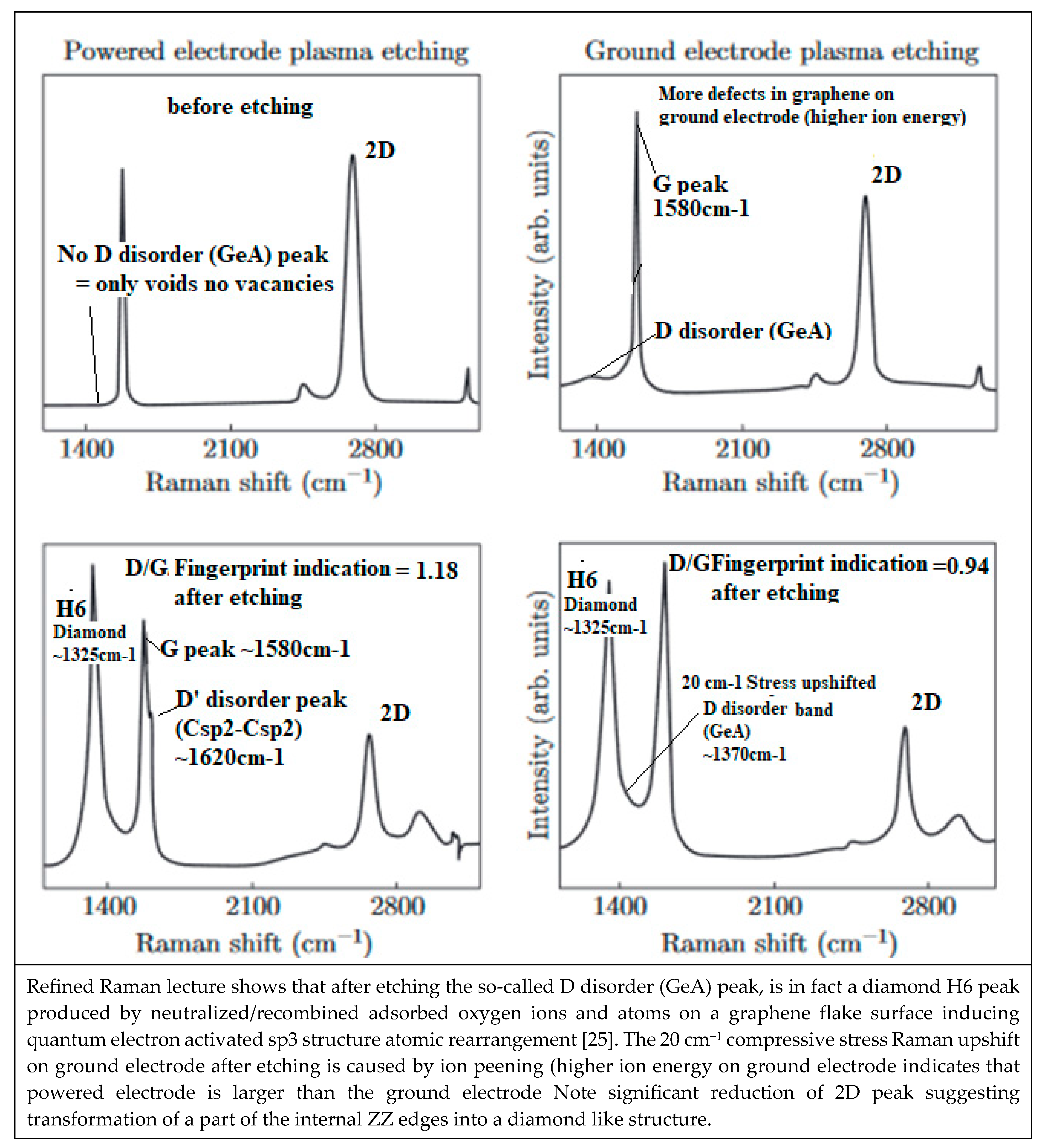
6. Defect Characterizing with Raman Spectroscopy
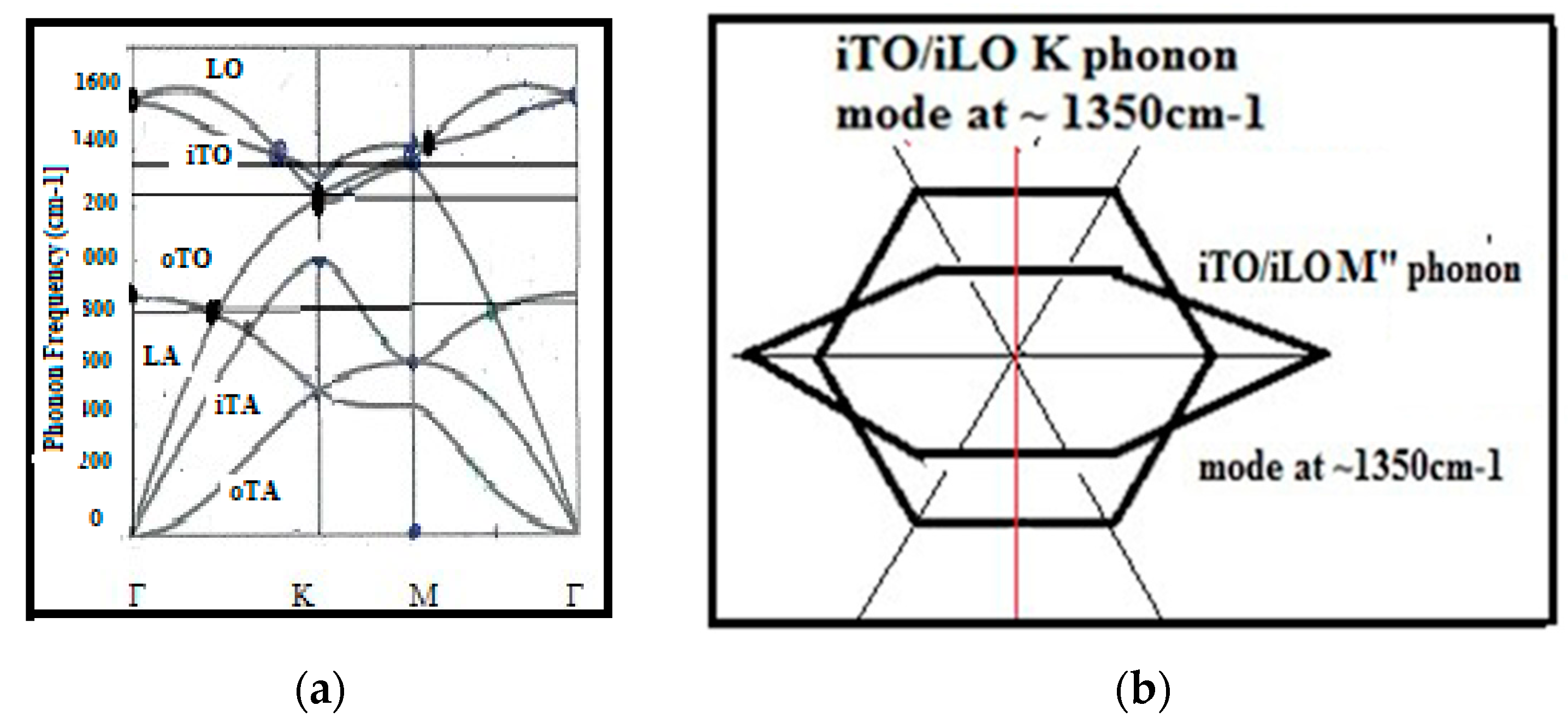
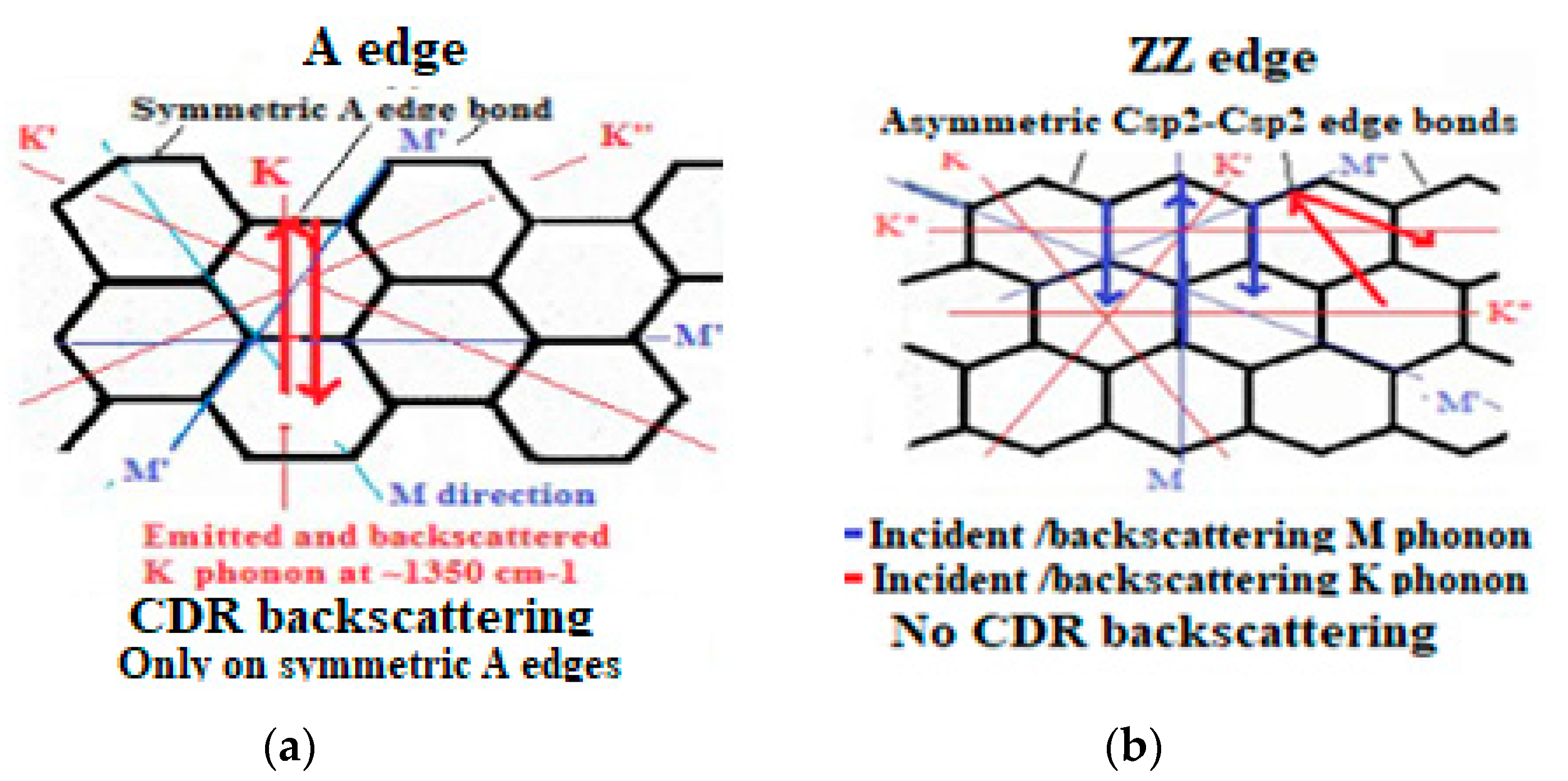
6.1. Phonon K Mode and M Mode Wave Scattering
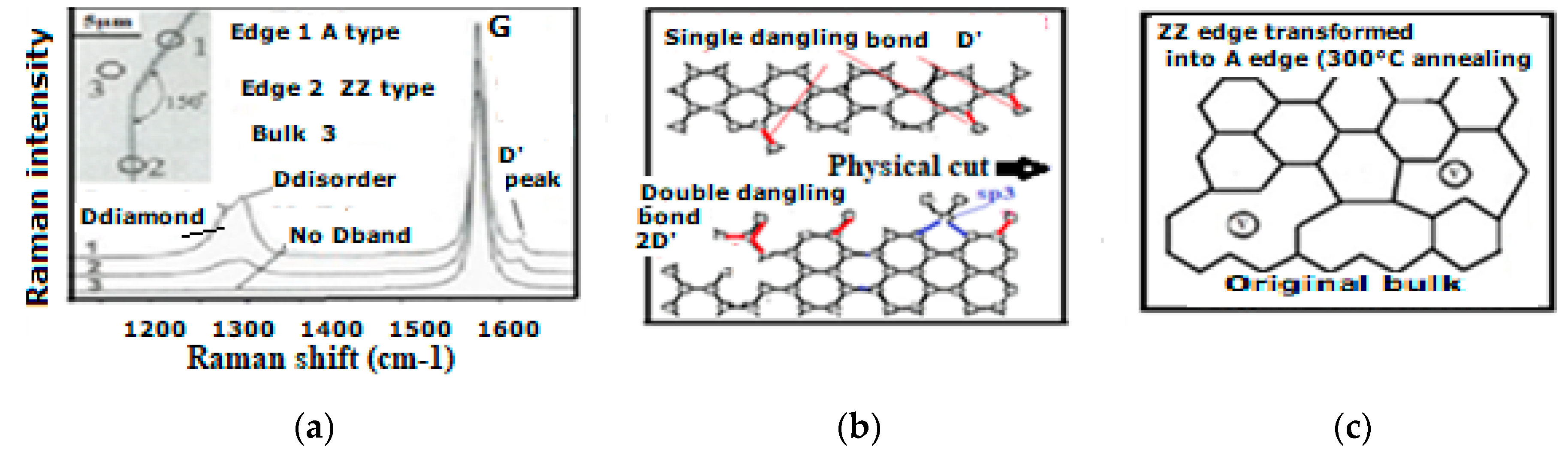
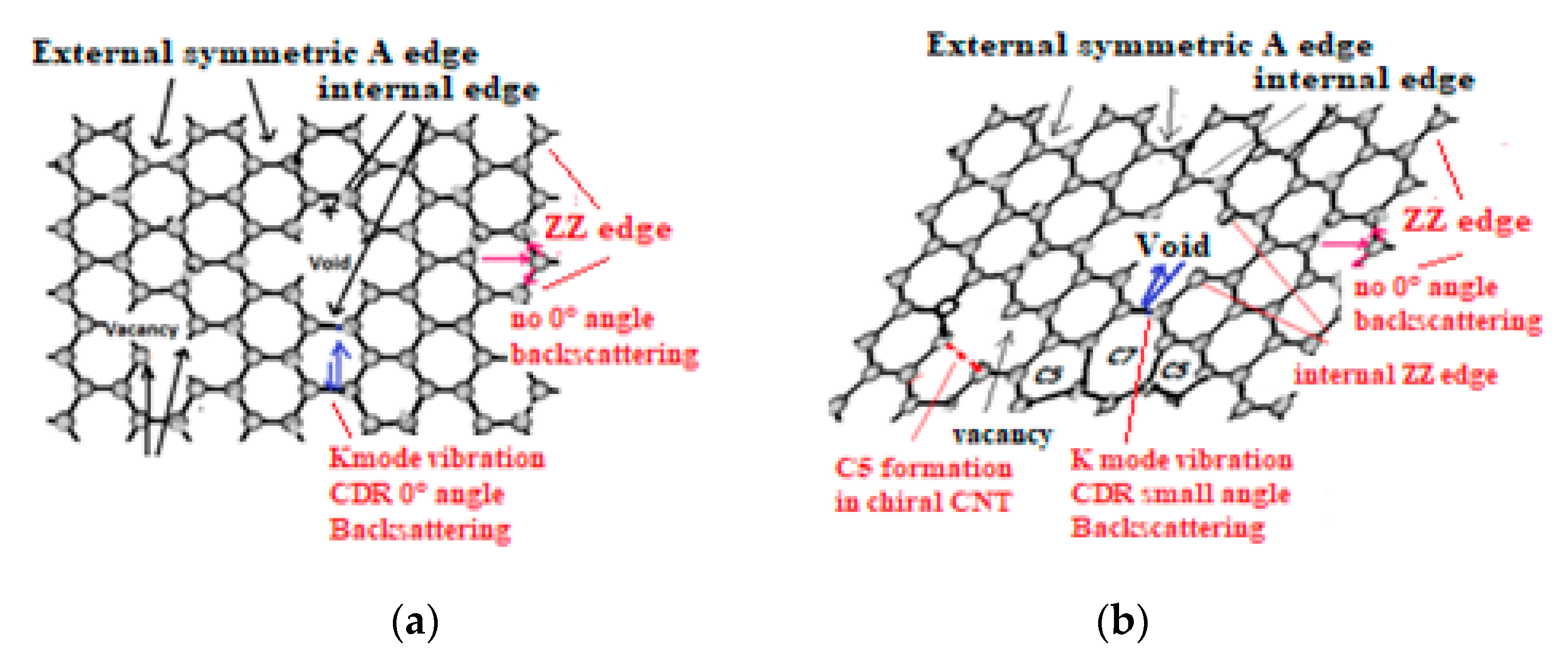
6.2. Defect Types to Be Considered for NEMS Engineering
- -
- -
- -
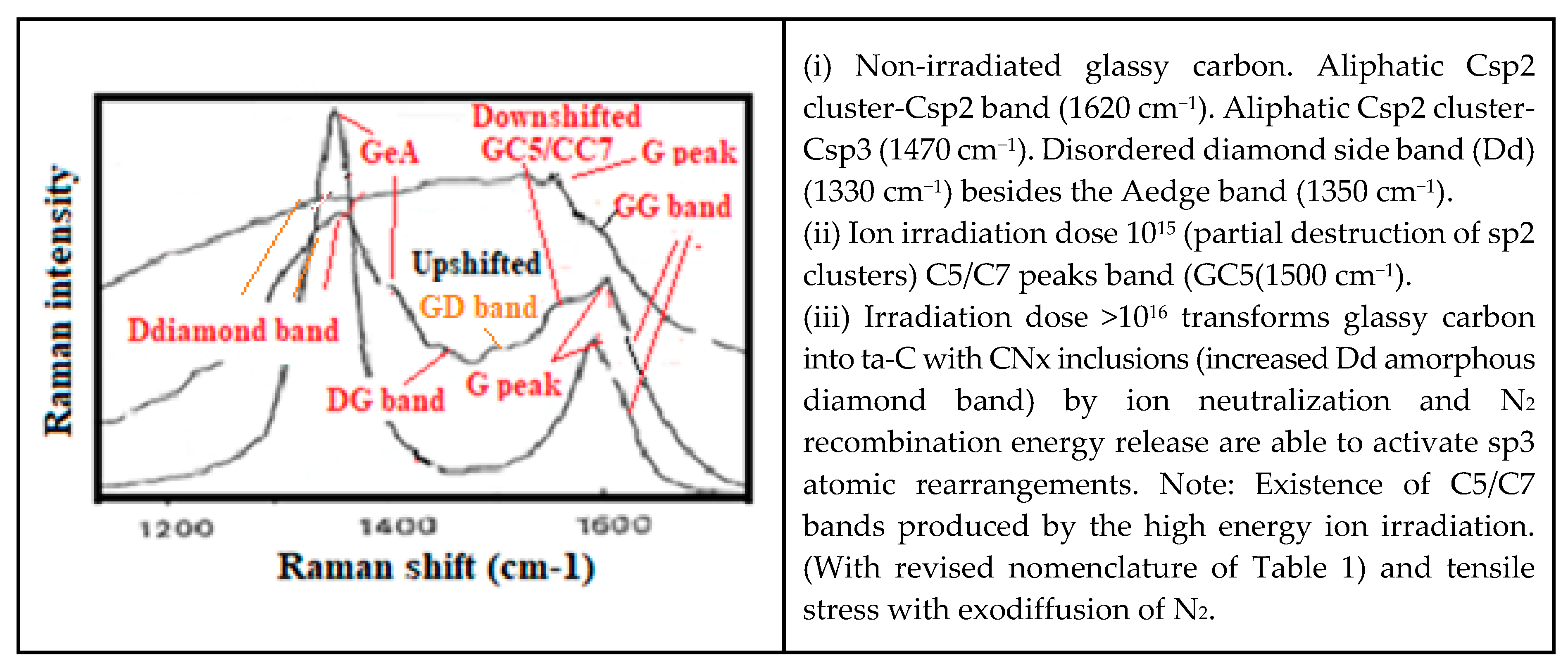
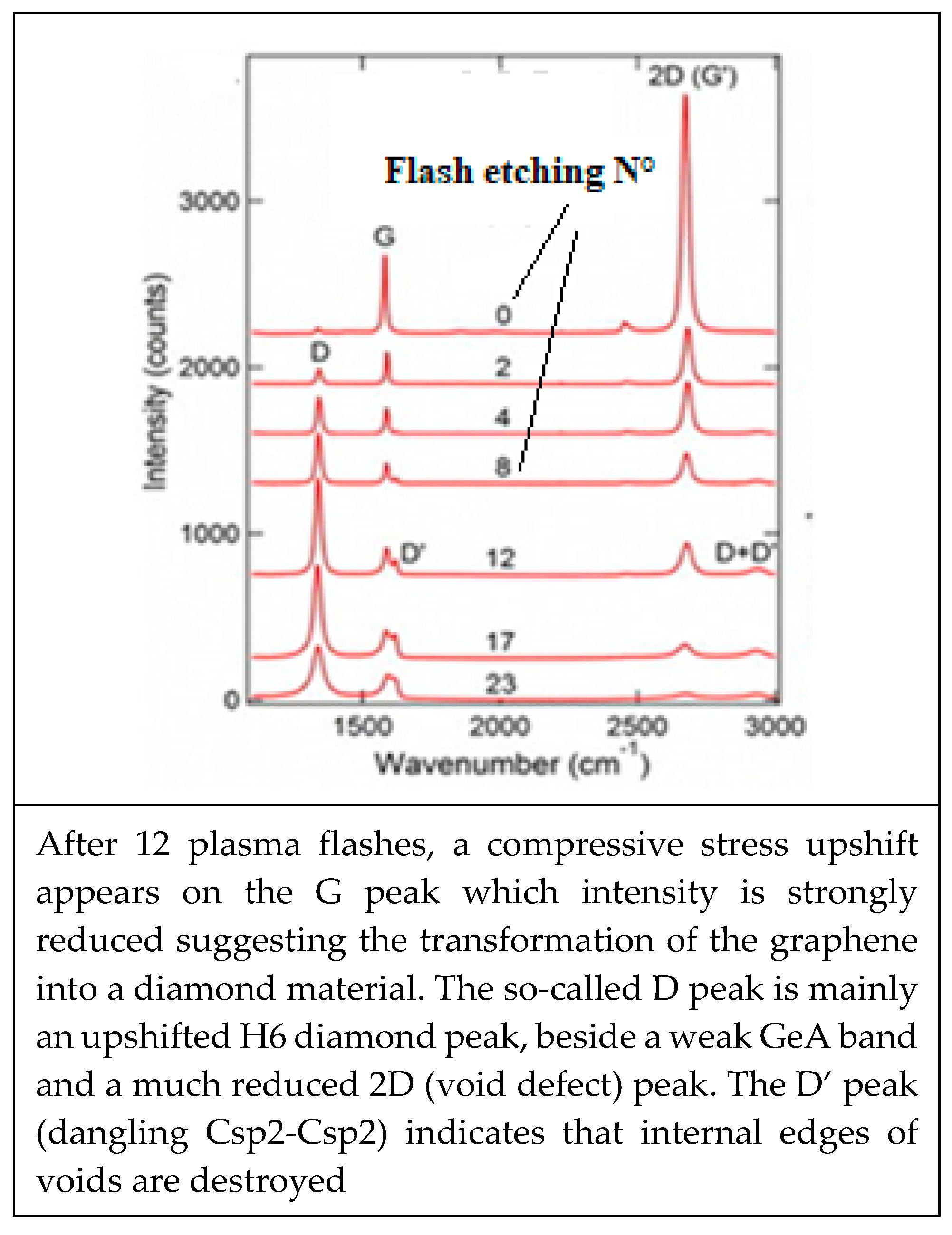
- Edge discontinuities resulting for instance from graphene plane cut [222] can produce on the cutting edge single and double aliphatic Csp2-Csp2 edge dangling bonds at ~1620 cm−1 and 3240 cm−1) and which are corresponding to so-called D’disorder peak (Figure 12a). Those can evidently modify chemical reactions and material rearrangement on graphenic external edges.
- -
6.3. Local Atomic H6 Diamond Rearrangement
7. Application Developments
7.1. Role of Interface Structure on Composite Material Properties
7.1.1. Anchoring and Adhesion of Graphenic Materials with Counter-Facing Materials
7.1.2. Modification of Intrinsic Mechanical, Electric and Optoelectronic Properties
7.1.3. Local Activation of Phase Transitions on Edges and in Graphene Bulk
- (a)
- With the differentiation between so-called “Ddisorder” peaks (GeA) and the neighbor collective vibration modes of the D diamond peaks and band (~1330 cm−1)
- (b)
- The related Csp3cluster-Csp2 (~1470 cm−1) and Csp3cluster-Csp3 structure (~1150 cm−1).
- (c)
- In considering some possible stress up- and down-shift which can be checked on the G peak.
- (d)
- The differentiated analysis of the so-called G band which is not always containing a nominal G peak (corresponding to ordered graphitic and graphenic Csp2 material at ~1580 cm−1 when not stress shifted and which can be eventually broadened by disorder). They can correspond to the superimposition of neighbor shifted other peaks and bands [26,180,181,182,208,209].
7.2. Friction and Wear
7.3. Yarn and Scaffolds
7.4. Micro and Nano Interconnecting and Thermal Management
7.5. Electronical and Optoelectronic Functions and Field Emission Effects
7.6. Solar Cells, Hydrogen and Energy Storage and Energy Conversion
7.6.1. Solar Cells and Energy Storage
7.6.2. Hydrogen Storage and Photocatalytic Production from Water
7.7. Sensors, Medical Applications and Miscellaneous
8. Conclusions
Funding
Conflicts of Interest
References
- Karumuri, S.R.; Srinivas, Y.; Sekhar, J.V.; Sravani, K.G. Review on Break through MEMS Technology. Sch. Res. Libr. Arch. Phys. Res. 2011, 2, 158–165. [Google Scholar]
- Mohr, M.; Caron, A.; Herbeck-Engel, P.; Bennewitz, R.; Gluche, P.; Brühne, K.; Fecht, H.J. Young’s modulus, fracture strength, and Poisson’s ratio of nanocrystalline diamond films. J. Appl. Phys. 2014, 116. [Google Scholar] [CrossRef]
- Vetter, J. 60 years of DLC coatings: Historical highlights and technical review of cathodic arc processes to synthesize various DLC types, and their evolution for industrial applications. Surf. Coat. Technol. 2014, 257, 213–240. [Google Scholar] [CrossRef]
- Tibrewala, A.; Peiner, E.; Bandorf, R.; Biehl, S.; Lüthje, H. The piezoresistive effect in diamond-like carbon films. J. Micromech. Microeng. 2007, 17, S77–S82. [Google Scholar] [CrossRef]
- Lu, J.X.; Cao, Z.; Aslam, D.M.; Sepulveda, N.; Sullivan, J.P. Diamond micro and nano-resonators using laser, capacitive or piezoresistive detection. In Proceedings of the 2008 3rd IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Sanya, China, 6–9 January 2008. [Google Scholar] [CrossRef]
- Kauth, C. Electronic Interfaces for Carbon Nanotube Electromechanical Oscillators and Sensors. Ph.D. Thesis, EPFL, Lausanne, Switzerland, 2014. [Google Scholar]
- Mashman, M.R.; Ehlert, G.J.; Dickinson, B.T.; Phillips, D.M.; Ray, C.W.; Reich, G.W.; Baur, J.W. Bioinspired Carbon Nanotube Fuzzy Fiber Hair Sensor for Air-Flow Detection. Adv. Mater. 2014, 26, 3230–3234. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.K.; Fu, Y.Q.; Le, H.R.A.; Williams, J.; Spearing, S.M.I.; Milne, W. Diamond and diamond-like carbon MEMS. J. Micromech. Microeng. 2007, 17, S147–S163. [Google Scholar] [CrossRef]
- Martin-Olmos, C.; Rasool, H.I.; Weiller, B.H.; Gimzewski, J.K. Graphene MEMS: AFM Probe Performance Improvement. ACS Nano 2013, 7, 4164–4170. [Google Scholar] [CrossRef]
- Jiang, S.; Shi, T.; Zhan, X.; Xi, S.; Long, H.; Gong, B.; Li, J.; Cheng, S.; Huang, Y.; Tang, Z. Scalable fabrication of carbon-based MEMS/NEMS and their applications: A review. J. Micromech. Microeng. 2015, 25, 113001. [Google Scholar] [CrossRef]
- Katz, E.; Willner, I. Biomolecule-Functionalized Carbon Nanotubes: Applications in Nano-bioelectronics. ChemPhysChem 2004, 5. [Google Scholar] [CrossRef]
- Kis, A.; Zettl, A. Nanomechanics of carbon nanotubes. Philos. Trans. R. Soc. A 2008, 366, 1591–1611. [Google Scholar] [CrossRef]
- Janas, D.; Koziol, K.K. A review of production methods of carbon nanotube and graphene thin films for electrothermal applications. Nanoscale 2014, 6, 3037. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Zhou, Q.; Chang, J.; Liu, Y.; Lin, L. Graphene and CNT in MEMS/NEMS applications. Microelectron. Eng. 2015, 132, 192–206. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, A.; Cho, Y.-K.; Madou, M. Increased Graphitization in Electrospun Single Suspended Carbon Nanowires Integrated with Carbon-MEMS and Carbon-NEMS Platforms. ACS Appl. Mater. Interfaces 2012, 4, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Varney, M.W.; Aslam, D.M.; Janoudi, A.; Chan, H.Y.; Wang, D.H. Polycrystalline-Diamond MEMS Biosensors Including Neural Microelectrode-Arrays. Biosensors 2011, 1, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Eun, Y.; Pyo, S.; Sim, J.; Kim, J. Vertically aligned carbon nanotube arrays as vertical comb structures for electrostatic torsional actuator. Microelectron. Eng. 2012, 98, 405–408. [Google Scholar] [CrossRef]
- Kim, M.-O.; Lee, K.; Na, H.; Kwon, D.-S.; Choi, J.; Lee, J.-I.; Baek, D.-H.; Kim, J. Highly sensitive cantilever type chemo-mechanical hydrogen sensor based on contact resistance of self-adjusted carbon nanotube arrays. Sens. Actuators B Chem. 2014, 197, 414–421. [Google Scholar] [CrossRef]
- Choi, J.; Kim, J. Defective carbon nanotube-silicon heterojunctions for photodetector and chemical sensor with improved responses. J. Micromech. Microeng. 2015, 25, 115004. [Google Scholar] [CrossRef]
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon Nanotube Chemical Sensors. Chem. Rev. 2019, 119, 599–663. [Google Scholar] [CrossRef]
- Lyshevski, S.E. MEMS and NEMS—Systems, Devices, and Structures (2001); CRC Press LLC Corporate Blvd: Boca Raton, FL, USA, 2000; ISBN 0-8493-1262-0. [Google Scholar]
- Loh, O.; Wei, X.; Ke, C.; Sullivan, J.; Espinosa, H.D. Robust carbon-nanotube-based nano-electromechanical devices: Understanding and eliminating prevalent failure modes using alternative electrode materials. Small 2011, 7, 79–86. [Google Scholar] [CrossRef]
- De Haan, S. NEMS—Emerging products and applications of nano-electro-mechanical systems. Nanotechnol. Percept. 2006, 2, 267–275. [Google Scholar] [CrossRef]
- Joshi, R.K.; Kumar, A. Diamond and Related Nanomaterials for MEMS/NEMS Applications. J. Nanomater. 2009, 2009. [Google Scholar] [CrossRef]
- Neuville, S. Quantum electronic mechanisms of atomic rearrangements during growth of hard carbon films. Surf. Coat. Technol. 2011, 206, 703–726. [Google Scholar] [CrossRef]
- Neuville, S. Refined Raman Spectroscopy Fundamentals for Improved Carbon Material Engineering; Lambert Academic Publishing: Kaiserslautern, Germany, 2014; 169p, ISBN 978-3-659-48909-9. [Google Scholar]
- Neuville, S.; Matthews, A. A perspective on the optimization of hard carbon and related coatings for engineering applications. Thin Solid Film 2007, 515, 6619–6655. [Google Scholar] [CrossRef]
- Neuville, S. Differentiated Carbon Material for Energy Storage and Conversion. Mater. Day Proc. 2018, 5, 13837–13845. [Google Scholar] [CrossRef]
- Neuville, S. Superconductivity described with Electron-Phonon Synchronic Coupling. Mater. Today Proc. 2018, 5, 13827–13836. [Google Scholar] [CrossRef]
- Bosseboeuf, A.; Mathias, H. Experimental techniques for damping Characterization of micro and nanostructures. In Advances in Multiphysic Simulation and Experimental Testing of MEMS; Frangi, A., Cercignagni, C., Mukherjee, S., Aluru, N., Eds.; Imperial College Press: London, UK, 2008. [Google Scholar]
- Fu, Y.; Du, H.; Huang, W.; Zhang, S.; Hu, M. TiNi-based thin films for MEMS applications. In Advanced Materials for Micro- and Nano-Systems (AMMNS); Singapore-MIT Alliance (SMA): Singapore, 2004. [Google Scholar]
- Saito, K.; Iwata, K.; Ishihara, Y.; Sugita, K.; Takato, M.; Uchikoba, F. Miniaturized Rotary Actuators Using Shape Memory Alloy for Insect-Type MEMS Microrobot. Micromachines 2016, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Karpelson, M.; Whitney, J.P.; Wei, G.Y.; Wood, R.J. Design and fabrication of ultralight high-voltage power circuits for flapping-wing robotic insects. In Proceedings of the 2011 Twenty-Sixth Annual IEEE Applied Power Electronics Conference and Exposition (APEC), Fort Worth, TX, USA, 6–11 March 2011. [Google Scholar]
- Zhang, M.; Llaser, N.; Mathias, H.; Dupret, A. High precision measurement of quality factor for MEMS resonators. Procedia Chem. 2009, 1, 827–830. [Google Scholar] [CrossRef][Green Version]
- Rotake, A.D. Heavy Metal Ion Detection in Water using MEMS Based Sensor. Mater. Today Proc. 2018, 5, 1530–1536. [Google Scholar] [CrossRef]
- Polster, T.; Hofmann, M. Aluminum Nitride based 3D piezoelectric sensors. Proc. Chem. 2009, 1, 144–147. [Google Scholar] [CrossRef]
- Stampfer, C.; Helbling, T.; Obergfell, D.; Schöberle, B.; Tripp, M.K.; Jungen, A.; Roth, S.; Bright, V.M.; Hierold, C. Fabrication of Single-Walled Carbon-Nanotube-Based Pressure Sensors. Nano Lett. 2006, 6, 233–237. [Google Scholar] [CrossRef]
- Stampfer, C.; Jungen, A.; Linderman, R.; Obergfell, D.; Roth, S.; Hierold, C. Nano-Electromechanical Displacement Sensing Based on Single-Walled Carbon Nanotubes. Nano Lett. 2006, 6, 1449–1453. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-I.; Song, Y.; Jung, H.; Choi, J.; Eun, Y.; Kim, J. Deformable Carbon Nanotube-Contact Pads for Inertial Microswitch to Extend Contact Time. IEEE Trans. Ind. Electron. 2012, 59, 4914–4920. [Google Scholar] [CrossRef]
- Kaul, A.B.; Wong, E.W.; Epp, L.; Hunt, B.D. Electromechanical Carbon Nanotube Switches for High-Frequency Applications. Nano Lett. 2006, 6, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Lee, D.S.; Morjan, R.E.; Jhang, S.H.; Sveningsson, M.; Nerushev, O.A.; Park, Y.W.; Campbell, E.E.B. A Three-Terminal Carbon Nano-relay. Nano-Lett. 2004, 4, 2027–2030. [Google Scholar] [CrossRef]
- Jang, J.E.; Cha, S.N.; Choi, Y.; Amaratunga, G.A.J.; Kang, D.J.; Hasko, D.G.; Jung, J.E.; Kim, J.M. Nanoelectromechanical switches with vertically aligned carbon nanotubes. Appl. Phys. Lett. 2005, 87, 163114. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Huang, X.Y.; Chuan, T.K. MEMS-micropumps: A review. J. Fluids Eng. 2002, 124, 384–392. [Google Scholar] [CrossRef]
- Roy, S.K. A Survey MEMS Micromotors Assemblies and applications. Int. J. Eng. Res. Gen. Sci. 2015, 3, 2091–2730. [Google Scholar]
- Büttgenbach, S. Review Electromagnetic Micromotors-Design, Fabrication and Applications. Micromachines 2014, 5, 929–942. [Google Scholar] [CrossRef]
- Hsieh, J.Y.; Kuo, P.H.; Huang, Y.C.; Huang, Y.-J.; Tsai, R.D.; Wang, T.; Chiu, H.W.; Wang, Y.H.; Lu, S.S. A Remotely-Controlled Locomotive IC Driven by Electrolytic Bubbles and Wireless Powering. IEEE Trans. Biomed. Circuits Syst. 2014, 8, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.M.; Pister, K. Planar two degree-of-freedom legs for walking microrobots. In Proceedings of the IARP Workshop on Micro & Nano Robotics, Paris, France, 23–24 October 2006. [Google Scholar]
- Kovac, M. Learning from nature how to land aerial robots. Science 2016, 352, 895–896. [Google Scholar] [CrossRef] [PubMed]
- Penskiy, I.; Bergbreiter, S. Optimized Electrostatic Inchworm Motors using a Flexible Driving Arm. J. Micromech. Microeng. 2013, 23, 015018. [Google Scholar] [CrossRef]
- Chollet, F.; Goedgebuer, J.-P. Improved LiNbO 3 Technology for Reducing Sidelobe Asymmetry in Mode Converter-Based Wavelength Filters. Jpn. J. Appl. Phys. 1998, 37, 979–981. [Google Scholar] [CrossRef]
- Liu, A.Q.; Zhang, X.M.; Murukeshan, V.M.; Zhang, Q.X.; Zou, Q.B.; Uppili, S. Optical Switch Using Draw-Bridge Micromirror for Large Array. In Transducers ‘01 EurosensorsXV, Proceedings of the11th International Conference on Solid-State Sensors and Actuators; Springer: Berlin/Heidelberg, Germany, 2001; pp. 1296–1299. [Google Scholar]
- Liu, A.; Zhao, B.; Chollet, F.; Zou, Q.; Asundi, A.; Fujita, H. Micro-opto-mechanical grating switches. Sens. Actuators A Phys. 2000, 86, 127–134. [Google Scholar] [CrossRef]
- Ostfeld, A.E.; Gaikwad, A.M.; Khan, Y.; Arias, A.C. High-performance flexible energy storage and harvesting system for wearable electronics. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Arun, A.; Le Poche, H.; Idda, T.; Acquaviva, D.; Badia, M.F.-B.; Pantigny, P.; Salet, P.; Ionescu, A.M. Tunable MEMS capacitors using vertical carbon nanotube arrays grown on metal lines. Nanotechnology 2010, 22, 025203. [Google Scholar] [CrossRef] [PubMed]
- Despont, M.; Brugger, J.; Drechsler, U.; Dürig, U.; Häberle, W.; Lutwyche, M.; Rothuizen, H.; Stutz, R.; Widmer, R.; Binnig, G.; et al. VLSI-NEMS chip for parallel AFM data storage. Sens. Actuators A Phys. 2000, 80, 100–107. [Google Scholar] [CrossRef]
- Waldner, J.P. Nano-computers and Swarm Intelligence; ISTE John Wiley &Sons: London, UK, 2008; p. 205. ISBN 978-1-84821-009-7. [Google Scholar]
- Shafagh, R.; Vastesson, A.; Guo, W.; van der Wijngaart, W.; Haraldsson, T. E-Beam Nano-structuring and Direct Click Biofunctionalization of Thiol–Ene Resist. ACS Nano. 2018, 12, 9940–9946. [Google Scholar] [CrossRef]
- Draz, M.S.; Kochehbyoki, K.M.; Vasan, A.; Battalapalli, D.; Sreeram, A.; Kanakasabapathy, M.K.; Kallakuri, S.; Tsibris, A.; Kuritzkes, D.R.; Shafiee, H. DNA engineered micromotors powered by metal nanoparticles for motion -based cellphone diagnostics. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef]
- Godssi, R.; Lin, P. MEMS Materials and Processes Handbook; Springer: Berlin, Germany, 2011; ISBN 978-0-387-47316-1. [Google Scholar]
- Zhang, S. Handbook of Nanostructured Thin Films and Coatings: Mechanical Properties; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2010; ISBN 13:978-1-4200-9403-9. [Google Scholar]
- Madou, M.J. Fundamentals of Microfabrication and Nanotechnology. In MEMS to Bio-MEMS. Manufacturing Techniques and Applications; CRC Press: Boca Raton, FL, USA, 2011; Volume III, p. 252. ISBN 143-989-5244. [Google Scholar]
- Rao, A.V.; Yadav, S.K. An introduction to Nano Electro Mechanical Systems. Int. J. Eng. Sci. (IJES) 2015, 4, 6–9. [Google Scholar]
- Ventra, M.D.; Evoy, S.; Heflin, J.R., Jr. Introduction to Nanoscale Science and Technology. In Nanostructure Science and Technology; Springer: Berlin, Germany, 2004; ISBN 978-1-4020-7720-3. [Google Scholar]
- Mahakud, R.; Panigrahi, M.; Rath, S. Various Technological Aspects of Nano Electro Mechanical Systems—A Review. Int. J. Anal. Exp. Finite Element Anal. (IJAEFEA) 2014, 1, 1–5. [Google Scholar]
- Peng, H.; Li, Q.; Chen, T. (Eds.) Industrial Applications of Carbon Nanotubes; Elsevier: Amsterdam, The Netherlands, 2017; 508p, ISBN 978-0-323-41481-4. [Google Scholar]
- Garcia, J.C.; Justo, J.F. Twisted ultrathin silicon nanowires: A possible torsion electromechanical nanodevice. EPL Europhys. Lett. 2014, 108, 36006. [Google Scholar] [CrossRef]
- Ke, C.; Espinosa, H.D. In Situ Electron Microscopy Electromechanical Characterization of a Bistable NEMS Device. Small 2006, 2, 1484–1489. [Google Scholar] [CrossRef] [PubMed]
- Ernst, T.; Hentz, S.; Arcamone, J.; Agache, V.; Duraffourg, L.; Ouerghi, I.; Ludurczak, W.; Ladner, C.; Ollier, E.; Andreucci, P.; et al. High performance NEMS devices for sensing applications. In Proceedings of the 2015 45th European Solid-State Device Research Conference (ESSDERC), Graz, Austria, 14–18 September 2015; pp. 31–35. [Google Scholar]
- Li, M.; Tang, H.X.; Roukes, M.L. Ultra-sensitive NEMS-based cantilevers for sensing, scanned probe and very high-frequency applications. Nat. Nanotechnol. 2007, 2, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Boss, J.M.; Moores, B.A.; Degen, C.L. Single-crystal diamond nanomechanical resonators with quality factors exceeding one million. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Davis, Z.J.; Abadal, G.; Helbo, B.; Hansen, O.; Campabadal, F.; Pérez-Murano, F.; Esteve, J.; Figueras, E.; Ruiz, R.; Barniol, N.; et al. High Mass and Spatial Resolution Mass Sensor based on Resonating Nano-Cantilevers Integrated with CMOS. In Transducers ’01 Eurosensors XV; Obermeier, E., Ed.; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Wang, Y.-H.; Lee, C.-Y.; Chiang, C.-M. A MEMS-based Air Flow Sensor with a Free-standing Micro-cantilever Structure. Sensors 2007, 7, 2389–2401. [Google Scholar] [CrossRef] [PubMed]
- Vajpayee, S.; Kumar, B.; Thakur, R.; Kumar, M. Design and Development of Nano pH Sensor and Interfacing with Arduino. Int. J. Electron. Electr. Comput. Syst. IJEECS 2017, 8, 6. [Google Scholar] [CrossRef]
- Wang, J. Nanomachines: Fundamental and Application; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Yadav, V.; Duan, W.; Butler, P.J.; Sen, A. Anatomy of Nanoscale Propulsion. Annu. Rev. Biophys. 2015, 44, 77–100. [Google Scholar] [CrossRef]
- Ren, L.; Zhou, D.; Mao, Z.; Xu, P.; Huang, T.J.; Mallouk, T.E. Rheotaxis of Bimetallic Micromotors Driven by Chemical–Acoustic Hybrid Power. ACS Nano 2017, 11, 10591–10598. [Google Scholar] [CrossRef]
- Das, S.; Garg, A.; Campbell, A.I.; Howse, J.; Sen, A.; Velegol, D.; Golestanian, R.; Ebbens, S.J. Boundaries can steer active Janus spheres. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef]
- Duan, W.; Ibele, M.; Liu, R.; Sen, A. Motion analysis of light-powered autonomous silver chloride nanomotors. Eur. Phys. J. E 2012, 35. [Google Scholar] [CrossRef]
- Wong, F.; Sen, A. Progress Towards Light-Harvesting Self-Electrophoretic Motors: Highly Efficient Bimetallic Nanomotors and Micropumps in Halogen Media. ACS Nano 2016, 10, 7172–7179. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Wang, W.; Das, S.; Yadav, V.; Mallouk, T.E.; Sen, A. Synthetic Nano- and Micromachines in Analytical Chemistry: Sensing, Migration, Capture, Delivery, and Separation. Annu. Rev. Anal. Chem. 2015, 8, 311–333. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Sánchez, B.; Wang, J. Micromotors for environmental applications: A review. Environ. Sci. Nano Issue 2018, 5, 1530–1544. [Google Scholar] [CrossRef]
- Ghosh, A.; Dasgupta, D.; Pal, M.; Morozov, K.; Lehshansky, A.; Ghosh, A. Helical Nano-machines as Mobile Viscometers. Adv. Funct. Mater. 2018, 28, 1705687. [Google Scholar] [CrossRef]
- Hindi, S.S.Z. Molecular Machines: I. An Overview of Biological and Synthetic Angstromic Devices. Nanosci. Nanotechnol. Res. 2017, 4, 98–105. [Google Scholar]
- Bamrungsap, S.; Phillips, J.A.; Xiong, X.; Kim, Y.; Wang, H.; Liu, H.; Hebard, A.; Tan, W. Magnetically driven single DNA nanomotor. Small 2011, 7, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Somalwar, N.; Singh, A.; Bhat, R.; Eswarappa, S.M.; Saini, D.K.; Ghosh, A. Maneuverability of Magnetic Nanomotors Inside Living Cells. Adv. Mater. 2018, 30, 1800429. [Google Scholar] [CrossRef] [PubMed]
- Dietrich-Buchecker, C.O.; Jimenez-Molero, M.C.; Sartor, V.; Sauvage, J.-P. Rotaxanes and catenanes as prototypes of molecular machines and motors. Pure Appl. Chem. 2003, 75, 1383–1393. [Google Scholar] [CrossRef]
- Kudernac, T.; Ruangsupapichat, N.; Parschau, M.; Maciá, B.; Katsonis, N.; Harutyunyan, S.R.; Ernst, K.H.; Feringa, B.L. Electrically driven directional motion of a four-wheeled molecule on a metal surface. Nature 2011, 479, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Coskun, A.; Banaszak, M.; Astumian, R.D.; Stoddart, J.F.; Grzybowski, B.A. Great expectations: Can artificial molecular machines deliver on their promise? Chem. Soc. Rev. 2012, 41, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Bianco, B.; Moggia, E.; Giordano, S.; Rocchia, W.; Chiabrera, A. Friction and noise in quantum mechanics: A model for the interactions between a system and a thermal bath. Il Nuovo Cim. B 2001, 116, 155–165. [Google Scholar]
- Farias, M.B.; Fosco, C.D.; Lombardo, F.C.; Mazzitelli, F.D. Quantum friction between graphene sheets. Phys. Rev. D 2017, 95, 065012. [Google Scholar] [CrossRef]
- Jiang, Q.D.; Wilczek, F. Chiral Casimir forces: Repulsive, enhanced, tunable. Phys. Rev. B 2019, 99, 125403. [Google Scholar] [CrossRef]
- Bennett, S.D.; Clerk, A.A. Laser-like instabilities in quantum nano-electromechanical systems. Phys. Rev. B 2006, 74, 201301. [Google Scholar] [CrossRef]
- Cao, A.; Baskaran, R.; Frederick, M.; Turner, K.; Ajayan, P.; Ramanath, G. Direction-Selective and Length-Tunable In-Plane Growth of Carbon Nanotubes. Adv. Mater. 2003, 15, 1105–1109. [Google Scholar] [CrossRef]
- Choi, J.; Pyo, S.; Baek, D.-H.; Lee, J.-I.; Kim, J. Thickness-, alignment- and defect-tunable growth of carbon nanotube arrays using designed mechanical loads. Carbon 2014, 66, 126–133. [Google Scholar] [CrossRef]
- Hutchison, D.N.; Morrill, N.B.; Aten, Q.; Turner, B.W.; Jensen, B.D.; Howell, L.L.; Vanfleet, R.R.; Davis, R.C. Carbon Nanotubes as a Framework for High-Aspect-Ratio MEMS Fabrication. J. Microelectromech. Syst. 2010, 19, 75–82. [Google Scholar] [CrossRef]
- Choi, J.; Lee, J.-I.; Eun, Y.; Kim, M.-O.; Kim, J. Aligned Carbon Nanotube Arrays for Degradation-Resistant, Intimate Contact in Micromechanical Devices. Adv. Mater. 2011, 23, 2231–2236. [Google Scholar] [CrossRef]
- Choi, J.; Eun, Y.; Kim, J. Investigation of Interfacial Adhesion between the Top Ends of Carbon Nanotubes. ACS Appl. Mater. Interfaces 2014, 6, 6598–6605. [Google Scholar] [CrossRef]
- Eun, Y.; Lee, J.I.; Choi, J.; Song, Y.; Kim, J. Integrated Carbon Nanotube Array as Dry Adhesive for High-Temperature Silicon Processing. Adv. Mater. 2011, 23, 2231–2236. [Google Scholar] [CrossRef]
- Goold, J.; Huber, M.; Riera, A.; Del Rio, L.; Skrzypczyk, P. The role of quantum information in thermodynamics—A topical review. J. Phys. A Math. Theor. 2016, 49, 143001. [Google Scholar] [CrossRef]
- Neuville, S. Transient Transversal Electric Field by Adsorbtion on Semiconductors. SNB 2007, 121, 436. [Google Scholar]
- Caschera, D.; Federici, F.; Zane, D.; Focanti, F.; Curulli, A.; Padeletti, G. Gold nanoparticles modified GC electrodes: Electrochemical behavior dependence of different neurotransmitters and molecules of biological interest on the particles size and shape. J. Nanoparticle Res. 2009, 11, 1925. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Jagan, E.; Rajasekar, S.; Selvakumar, P.; Kalaichelvan, P.; Mohan, N. Synthesis of silver nanoparticles using Acalypha Indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf. B Biointerfaces 2010, 76, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Radovic-Moreno, A.F.; Wu, J.; Shi, R.L.J. Nano-medicine in the management of microbial infection overview and perspectives. Nano Today 2014, 9, 478–498. [Google Scholar] [CrossRef]
- Waqar, A.; Abdelbary, E.; Vinod, D.; Karthikeyan, S. Emerging Nanotechnologies in Dentistry (Second Edition), Chapter 18—Carbon nanotubes: Applications in cancer therapy and drug delivery research. Micro Nano Technol. 2018, 371–389. [Google Scholar] [CrossRef]
- Sharifi, S.; Behzadi, S.; Laurent, S.; Forrest, M.L.; Stroeve, P.; Mahmoudi, M. Toxicity of nanomaterials. Chem. Soc. Rev. 2012, 41, 2323–2343. [Google Scholar] [CrossRef]
- Ramsden, J.J. Assessing the toxic risks of the nanotechnology industry. Nanotechnol. Percept. 2013, 9, 119–134. [Google Scholar] [CrossRef]
- Madani, S.Y.; Mandel, A.; Seifalian, A.M. A concise review of carbon nanotube’s toxicology. Nano Rev. 2013, 4. [Google Scholar] [CrossRef]
- Choi, J.; Kim, J. Batch-processed carbon nanotube wall as pressure and flow sensor. Nanotechnology 2010, 21, 105502. [Google Scholar] [CrossRef]
- Hayamizu, Y.; Yamada, T.; Mizuno, K.; Davis, R.C.; Futaba, D.N.; Yumura, M.; Hata, K. Integrated three-dimensional micro-electromechanical devices from processable carbon nanotube wafers. Nat. Nanotechnol. 2008, 3, 289–294. [Google Scholar] [CrossRef]
- Lee, J.I.; Eun, Y.; Choi, J.; Kwon, D.S.; Kim, J. Using Confined Self-Adjusting Carbon Nanotube Arrays as High-Sensitivity Displacement Sensing Element. ACS Appl. Mater. Interfaces 2014, 6, 10181–10187. [Google Scholar] [CrossRef]
- Silva, S.P.R.; Carey, J.D.; Kahn, R.U.A.; Gerstner, E.G.; Anguita, J.V. Amorphous carbon thin films. In Handbook of Thin Film Materials; Nalwa, H.S., Ed.; Academic Press: New York, NY, USA, 2002; Chapter 9; Volume 4, pp. 403–505. [Google Scholar]
- Casiraghi, C.; Robertson, J.; Ferrari, A. Review. Diamond Like Carbon for data storage and beer storage. Mater. Day 2007, 10, 44–53. [Google Scholar]
- Huong, P.V. Structural studies of diamond films and ultrahard materials by Raman spectroscopy. Diam. Relat. Mater. 1991, 1, 31–41. [Google Scholar] [CrossRef]
- Bachmann, P.K.; van Enckevort, W. Diamond deposition technologies. Diam. Relat. Mater. 1992, 1, 1021–1034. [Google Scholar] [CrossRef]
- Nimmagadda, R.R.; Joshi, J.; Hsu, L. Role of microstructure on the oxidation behavior of microwave plasma synthetized diamond and diamond like carbon. J. Mater. Res. 1990, 5, 2445–2450. [Google Scholar] [CrossRef]
- McNamara, K.; Gleason, K.; Vestyck, D.; Butler, J.; Butler, J. Evaluation of diamond films by nuclear magnetic resonance and Raman spectroscopy. Diam. Relat. Mater. 1992, 1, 1145–1155. [Google Scholar] [CrossRef]
- Fayette, L.; Marcus, B.; Mermoux, M.; Tourillon, G.; Laffon, K.; Parent, P.; Le Normand, F. Local order in CVD diamond films: Comparative Raman, x-ray-diffraction, and x-ray-absorption near-edge studies. Phys. Rev. B 1998, 57, 14123–14132. [Google Scholar] [CrossRef]
- Hanada, K.; Nishiyama, T.; Yoshitake, T.; Nagayama, K. Time-Resolved Observation of Deposition Process of Ultra-nanocrystalline Diamond/Hydrogenated Amorphous Carbon Composite Films in Pulsed Laser Deposition. Hindawi J. Nanomater. 2009, 2009, 901241. [Google Scholar]
- Hernberg, R.; Lepisto, T.; Mantyla, T.; Stenberg, T.; Vattulainen, J. Diamond film synthesis on Mo in thermal RF plasma. Diam. Relat. Mater. 1992, 1, 255–261. [Google Scholar] [CrossRef]
- Kim, T.-Y.; Lee, C.S.; Lee, Y.J.; Lee, K.-R.; Chae, K.-H.; Oh, K.H. Reduction of the residual compressive stress of tetrahedral amorphous carbon film by Ar background gas during the filtered vacuum arc process. J. Appl. Phys. 2007, 101, 023504. [Google Scholar] [CrossRef]
- Neuville, S. New applications perspectives on ta-C coating. QScience Connect 2014, 2014, 8. [Google Scholar] [CrossRef]
- Reisse, G.; Weissmantel, S.; Rost, D. Preparation of super-hard coatings by pulsed laser deposition. Appl. Phys. A 2004, 79, 1275–1278. [Google Scholar] [CrossRef]
- Shi, X.; Tay, B.K.; Lau, S.P. The Double Bent Filtered Cathodic Arc Technology and its Applications. Int. Mod. Phys. B 2000, 14, 136–153. [Google Scholar] [CrossRef]
- Schultrich, B. Tetrahedrally Bonded Amorphous Carbon Films I: Basics, Structure and Preparation; Springer Nature: Berlin, Germany, 2018; 752p, ISBN 978-3-662-55927-7. [Google Scholar]
- Charitidis, C.A. Nanomechanical and nanotribological properties of carbon-based thin films: A review. Int. J. Refract. Met. 2010, 28, 51–70. [Google Scholar] [CrossRef]
- Sullivan, J.P.; Friedmann, T.A.; de Boer, M.P.; la Van, D.A.; Hohlfelder, R.J.; Ashby, C.I.H.; Dugger, M.T.; Mitchell, M.; Dunn, R.G.; Magerkurth, A.J. Developing a new material for MEMS: Amorphous diamond. In Proceedings of the Materials Research Society of MRS Fall Meating 2000 Symposium, Boston, MA, USA, USA, 27 November–1 December 2001; Volume 657. [Google Scholar]
- Mel’nichenko, V.M.; Sladkov, A.M.; Nikulin, Y.N. Structure of Polymeric Carbon. In Russian Chemical Reviews; The British Library: London, UK, 1982; Volume 51. [Google Scholar]
- Li, X.; Masters, A.F.; Maschmeyer, T. Polymeric carbon nitride for solar hydrogen production. Chem. Commun. 2017, 53, 7438–7446. [Google Scholar] [CrossRef]
- Bredas, J.L.; Street, G.B. Electronic structure of amorphous hydrogenated carbon films. In Proceedings of the Vol. XVII MRS Strasbourg Meeting, Strasbourg, France, 2 June 1987; Koidl, P., Oelhafen, P., Eds.; Les Editions de la Physique: Paris, France, 1987; pp. 237–250. [Google Scholar]
- Wagner, J.; Ramsteiner, M.; Wild, C. Amorphous hydrogenated carbon films. In Proceedings of the Vol. XVII. MRS Strasbourg Meeting, Strasbourg, France, 2 June 1987; Koidl, P., Oelhafen, P., Eds.; Les Editions de la Physique: Paris, France, 1987; pp. 219–228. [Google Scholar]
- Santra, T.S.; Bhattacharyya, T.K.; Patel, P.; Tseng, F.G.; Barik, T.K. Diamond, Diamond-Like Carbon (DLC) and Diamond-Like Nanocomposite (DLN) Thin Films for MEMS Applications. Microelectromech. Syst. D Devices 2012, 28, 459–480. [Google Scholar]
- Badzian, A.R.; Bachmann, P.K.; Hartnett, T.; Badzian, T.; Messier, R. Diamond thin films prepared by PACVD processes. In Proceedings of the Vol. XVII MRS Strasbourg Meeting, Strasbourg, France, 2 June 1987; Koidl, P., Oelhafen, P., Eds.; Les Editions de la Physique: Paris, France, 1987; pp. 63–77. [Google Scholar]
- Schueller, O.J.A.; Brittain, S.T.; Marzolin, C.; Whitesides, G.M. Fabrication and Characterization of Glassy Carbon MEMS. Chem. Mater. 1997, 9, 1399–1406. [Google Scholar] [CrossRef]
- Chaudhury, S.; Sinha, S.K. Carbon Nanotube and Nanowires for Future Semiconductor Devices Applications. In Nanoelectronics; Kaush, B.K., Ed.; Devices, Circuits and Systems; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Chapter 12; pp. 375–398. ISBN 978-0-12-813353. [Google Scholar]
- Spencer, J.H.; Nesbitt, J.M.; Trewhitt, H.; Kashtiban, R.J.; Bell, G.; Ivanov, V.G.; Faulques, E.; Sloan, J.; Smith, D.C. Raman Spectroscopy of Optical Transitions and Vibrational Energies of ∼1 nm HgTe Extreme Nanowires within Single Walled Carbon Nanotubes. ACS Nano 2014, 8, 9044–9052. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Wei, H.W.; Leou, K.C.; Lin, H.; Tung, C.H.; Wei, M.T.; Lin, C.; Tsai, C.H. Experimental characterization of an inductively coupled acetylene/hydrogen plasma for Carbon Nano Fiber synthesis (CNF). J. Vac. Sci. Technol. B 2006, 24, 97–112. [Google Scholar] [CrossRef]
- Iijima, S. Synthesis of Carbon Nanotubes. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Endo, M.; Strano, M.S.; Ajayan, P.M. Potential Applications of Carbon Nanotubes. In Carbon Nanotubes; Jorio, A., Dresselhaus, G., Dresselhaus, M.S., Eds.; Topics in Applied Physics; Springer: New York, NY, USA, USA, 2007; pp. 13–62. ISBN 978-3-540-72864-1. [Google Scholar]
- Endo, M.; Iijima, S.; Dresselhaus, M.S. Carbon Nanotubes; Elsevier: Amsterdam, The Netherlands, 1997; 198p, ISBN 9780080426822. [Google Scholar]
- Küttel, O.M.; Gröning, O.; Emmenegger, C.; Nilsson, L.; Maillard, E.; Diederich, L.; Schlapbach, L. Field emission from diamond, diamond-like and nanostructured carbon films. Carbon 1999, 37, 745–752. [Google Scholar] [CrossRef]
- Giubileo, F.; di Bartolomeo, A.; Iemmo, L.; Luongo, G.; Urban, F. Field Emission from Carbon Nanostructures. Appl. Sci. 2018, 8, 526. [Google Scholar] [CrossRef]
- Fedoseeva, Y.V.; Bulusheva, L.G.; Okotrub, A.V.; Kanygin, M.A.; Gorodetskiy, D.V.; Asanov, I.P.; Vyalikh, D.V.; Puzyr, A.P.; Bondar, V.S. Field Emission Luminescence of Nano-Diamonds Deposited on the Aligned Carbon Nanotube Array. Sci. Rep. 2015, 5, 9379. [Google Scholar] [CrossRef]
- Bonard, J.M.; Weiss, N.; Kind, H.; Stöckli, T.; Forró, L.; Kern, K.; Châtelain, A. Tuning the Field Emission Properties of Patterned Carbon Nanotube Films. Adv. Mater. 2001, 13, 184–188. [Google Scholar] [CrossRef]
- Clark, I.T.; Yoshimura, M. Electronic properties of carbon nanotube. In Fabrication of Carbon Nanotubes for High-Performance Scanning Probe Microscopy; Intechopen: London, UK, 2011; pp. 91–104. [Google Scholar]
- Tanaka, K.; Yoshimura, M.; Ueda, K. High-Resolution Magnetic Force Microscopy Using Carbon Nanotube Probes Fabricated Directly by Microwave Plasma-Enhanced Chemical Vapor Deposition. J. Nanomater. 2009, 2009. [Google Scholar] [CrossRef]
- Laocharoensuk, R.; Burdick, J.; Wang, J. Carbon-Nanotube-Induced Acceleration of Catalytic Nanomotors. ACS Nano 2008, 2, 1069–1075. [Google Scholar] [CrossRef]
- Inami, N.; Shikoh, E.; Mohamed, M.A.; Fujiwara, A. Synthesis-condition dependence of carbon nanotube growth by alcohol catalytic chemical vapor deposition method. Sci. Technol. Adv. Mater. 2007, 8, 292–295. [Google Scholar] [CrossRef]
- Hermann, S.; Ecke, R.; Schulz, S.; Gessner, T. Controlling the formation of nanoparticles for definite growth of carbon nanotubes for interconnect applications. Microelectron. Eng. 2008, 85, 1979–1983. [Google Scholar] [CrossRef]
- Costa, S.; Borowiak-Palen, E.; Kruszynska, M.; Bachmatiuk, A.; Kalenczuk, R.J. Characterization of carbon nanotubes by Raman spectroscopy. Mater. Sci. Pol. 2008, 26, 433–441. [Google Scholar]
- Dresselhaus, M.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Ritter, K.A.; Lyding, J.W. The influence of edge structure on the electronic properties of graphene quantum dots and nanoribbons. Nat. Mater. 2009, 8, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Begliarbekov, M.; Sul, O.; Kalliakos, S.; Yang, E.-H.; Strauf, S. Determination of edge purity in bilayer graphene using μ-Raman spectroscopy. Appl. Phys. Lett. 2010, 97, 31908. [Google Scholar] [CrossRef]
- You, Y.; Ni, Z.; Yu, T.; Shen, Z. Edge chirality determination of graphene by Raman spectroscopy. Appl. Phys. Lett. 2008, 93, 163112. [Google Scholar] [CrossRef]
- Xu, Y.N.; Zhan, D.; Liu, L.; Suo, H.; Ni, Z.H.; Nguyen, T.T.; Zhao, C.; Shen, Z.X. Thermal Dynamics of Graphene Edges Investigated by Polarized Raman Spectroscopy. ACS Nano 2011, 5, 142–152. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iakoubovskii, K.; Minami, N.; Ueno, T.; Kazaoui, S.; Kataura, H. Optical Characterization of Double-Wall Carbon Nanotubes: Evidence for Inner Tube Shielding. J. Phys. Chem. C 2008, 112, 11194–11198. [Google Scholar] [CrossRef]
- Mogensen, K.B.; Kutter, J.P. Carbon nanotube based stationary phases for microchip chromatography. Lab Chip 2012, 12, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Bachilo, S.M. Structure-Assigned Optical Spectra of Single-Walled Carbon Nanotubes. Science 2002, 298, 2361–2366. [Google Scholar] [CrossRef] [PubMed]
- Cherukuri, P.; Bachilo, S.M.; Litovsky, S.H.; Weisman, R.B. Near-Infrared Fluorescence Microscopy of Single-Walled Carbon Nanotubes in Phagocytic Cells. J. Am. Chem. Soc. 2004, 126, 15638–15639. [Google Scholar] [CrossRef]
- Yum, K.; McNicholas, T.P.; Mu, B.; Strano, M.S. Single-Walled Carbon Nanotube-Based Near-Infrared Optical Glucose Sensors toward In Vivo Continuous Glucose Monitoring. J. Diabetes Sci. Technol. 2013, 7, 72–87. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, X.; Nanot, S.; Cong, K.; Jiang, Q.; Kane, A.A.; Goldsmith, J.E.M.; Hauge, R.H.; Leonard, F.; Kono, J. Photo-thermoelectric p-n junction photodetector with intrinsic broadband polarimetry based on macroscopic carbon nanotube films. ACS Nano 2013, 7, 7271–7277. [Google Scholar] [CrossRef] [PubMed]
- Janas, D.; Herman, A.P.; Boncel, S.; Koziol, K.K. Iodine monochloride as a powerful enhancer of electrical conductivity of carbon nanotube wires. Carbon 2014, 73, 225–233. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, J.; Vajtai, R.; Ajayan, P.M.; Barrera, E.V. Iodine doped carbon nanotube cables exceeding specific electrical conductivity of metals. Sci. Rep. 2011, 1. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, C.; Yamada, T.; Kobashi, K.; Sekiguchi, A.; Futaba, D.N.; Yumura, M.; Hata, K. One-hundred-fold increase in current carrying capacity in a carbon nanotube–copper composite. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.T.; Tannenbaum, A.; Tannenbaum, R. Anisotropic conductivity of magnetic carbon nanotubes embedded in epoxy matrices. Carbon 2011, 49, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-S.; Ren, W.; Xu, L.; Li, F.; Cheng, H.-M. Doped Graphene Sheets as Anode Materials with Superhigh Rate and Large Capacity for Lithium Ion Batteries. ACS Nano 2011, 5, 5463–5471. [Google Scholar] [CrossRef]
- Javey, A.; Guo, J.; Farmer, D.B.; Wang, Q.; Yenilmez, E.; Gordon, R.G.; Lundstrom, M.; Dai, H. Self-Aligned Ballistic Molecular Transistors and Electrically Parallel Nanotube Arrays. Nano Lett. 2004, 4, 1319–1322. [Google Scholar] [CrossRef]
- Hasan, S.; Salahuddin, S.; Vaidyanathan, M.; Alam, M.A. High frequency performance projections for ballistic carbon nano-tube transistors. IEEE Trans. Nanotechnol. 2006, 5, 14–22. [Google Scholar] [CrossRef]
- Ritter, U.; Scharff, P.; Sigmund, C.; Dimmytrenko, O.; Kulish, N.; Prylutskyy, Y.; Belyi, N.; Gubanov, V.; Komarova, L.; Lizunova, S.; et al. Radiation damage to multi-walled carbon nanotubes and their Raman vibrational modes. Carbon 2006, 44, 2694–2700. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Filho, A.G.S.; Saito, R. Defect characterization in graphene and carbon nanotubes using Raman spectroscopy. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 5355–5377. [Google Scholar] [CrossRef] [PubMed]
- Banhart, F.; Kotakoski, J.; Krasheninnikov, A.V. Structural Defects in Graphene. ACS Nano 2011, 5, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Campos-Delgado, J.; Kim, Y.A.; Hayashi, T.; Morelos-Gomez, A.; Hofmann, M.; Muramatsu, H.; Endo, M.; Terrones, H.; Shull, R.D.; Dresselhaus, M.S.; et al. Thermal stability studies of CVD-grown graphene nanoribbons: Defect annealing and loop formation. Chem.Phys. Lett. 2009, 469, 177–182. [Google Scholar] [CrossRef]
- Huong, P.V.; Marcus, B.; Mermoux, M.; Veirs, D.K.; Rosenblatt, G.M. Diamond-like films prepared by microwave plasma assisted chemical vapor deposition and by magnetron sputtering. Diam. Relat. Mater. 1992, 1, 869–873. [Google Scholar] [CrossRef]
- Flamant, G.; Guillard, R.; Laplaz, D. La synthese des fullerenes par energie solaire. Le Vide 2001, 2–4, 266. [Google Scholar]
- Sato, F. Diamond-like bonds in amorphous hydrogenated carbon films induced by x-ray irradiation. J. Vac. Sci. Technol. A 1998, 16, 2553–2555. [Google Scholar] [CrossRef]
- Balat-Pichelin, M.; Eck, J.; Sans, J. Thermal radiative properties of carbon materials under high temperature and vacuum ultra-violet (VUV) radiation for the heat shield of the Solar Probe Plus mission. Appl. Surf. Sci. 2012, 258, 2829–2835. [Google Scholar] [CrossRef]
- Matsui, Y.; Yabe, H.; Sugimoto, T.; Hirose, Y. The structure of acetylene flames for diamond synthesis. Diam. Relat. Mater. 1991, 1, 19–24. [Google Scholar] [CrossRef]
- Hinneberg, H.-J.; Eck, M.; Schmidt, K. Hot-filament-grown diamond films on Si: Characterization of impurities. Diam. Relat. Mater. 1992, 1, 810–813. [Google Scholar] [CrossRef]
- Smith, D.; Sevillano, E.; Besen, M.; Berkman, V.; Bourget, L. Large area diamond reactor using a high flow velocity microwave plasma jet. Diam. Relat. Mater. 1992, 1, 814–817. [Google Scholar] [CrossRef]
- Schultrich, B.; Scheibe, H.-J.; Drescher, D.; Ziegele, H. Deposition of superhard amorphous carbon films by pulsed vacuum arc deposition. Surf. Coat. Technol. 1998, 98, 1097–1101. [Google Scholar] [CrossRef]
- Liu, D.; Tu, J.; Hong, C.; Gu, C.; Mao, S. Two-phase nanostructured carbon nitride films prepared by direct current magnetron sputtering and thermal annealing. Surf. Coat. Technol. 2010, 205, 152–157. [Google Scholar] [CrossRef]
- Iwaki, M.; Takahashi, K.; Sekiguchi, A. Wear property and structure of implanted glassy carbon. J. Mater. Res. 1990, 5, 2562–2566. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Wang, Q.; Zhang, J. Annealing effect on the microstructure modification and tribological properties of amorphous carbon nitride films. J. Appl. Phys. 2008, 104, 073306. [Google Scholar] [CrossRef]
- Li, J.; Zheng, W.; Bian, H.J.; Lu, X.Y.; Jiang, Z.G.; Bai, Y.Z.; Jin, Z.S.; Zhao, Y.N. The effect of annealing on the field emission properties of amorphous CNx films. Acta Phys. Sin. 2003, 52, 1797–1801. [Google Scholar]
- Borroni-Bird, C.E.; King, D.A. An ultrahigh vacuum single crystal adsorption micro-calorimeter. Rev. Sci. Instrum. 1991, 62, 2177–2185. [Google Scholar] [CrossRef]
- Sankara-Reddy, K.S.; Satyam, M. Structural ordering of diamond like carbon films by applied electric field. Solid State Commun. 1995, 93, 797–799. [Google Scholar] [CrossRef]
- De Padova, P.; Quaresima, C.; Ottaviani, C.; Sheverdyaeva, P.M.; Moras, P.; Carbone, C.; Topwal, D.; Olivieri, B.; Kara, A.; Oughaddou, H.; et al. Evidence of graphene-like electronic signature in silicene nanoribbons. Appl. Phys. Lett. 2010, 96, 261905. [Google Scholar] [CrossRef]
- Endo, M.; Kim, Y.A.; Matusita, T.; Hayashi, T. From Vapor-Grown Carbon Fibers (VGCFs) to Carbon Nanotubes. In Carbon Filaments and Nanotubes: Common Origins, Differing Applications? NATO Science Series (Series E: Applied Sciences); Biró, L.P., Bernardo, C.A., Tibbetts, G.G., Lambin, P., Eds.; Springer: Dordrecht, The Netherlands, 2001; Volume 372, ISBN 978-0-7923-6908. [Google Scholar]
- Spear, K.E.; Phelps, A.W.; White, W.B.; Phelps, A. Diamond polytypes and their vibrational spectra. J. Mater. Res. 1990, 5, 2277–2285. [Google Scholar] [CrossRef]
- Phelps, A.W.; Howard, W.; White, W.B.; Spear, K.E.; Huang, D. Diamond, Boron Nitride, Silicon Carbide, and Related Wide Bandgap Semiconductors. In Proceedings of the Materials Research Society Symposium, Boston, MA, USA, 27 Novermber–1 December 1989; Glass, J.T., Messier, R.F., Fujimori, N., Eds.; Materials Research Society: Pittsburgh, PA, USA, 1989. [Google Scholar]
- Beeman, D.; Silverman, J.; Lynds, R.; Anderson, M.R. Modeling studies of amorphous carbon. Phys. Rev. B 1984, 30, 870–875. [Google Scholar] [CrossRef]
- Sønderby, S.; Berthelsen, A.; Almtoft, K.; Christensen, B.; Nielsen, L.; Bøttiger, J. Optimization of the mechanical properties of magnetron sputtered diamond-like carbon coatings. Diam. Relat. Mater. 2011, 20, 682–686. [Google Scholar] [CrossRef]
- Ohl, A.; Röpke, J. Mechanism of substrate heating in diamond-forming low-pressure microwave discharges. Diam. Relat. Mater. 1992, 1, 243–247. [Google Scholar] [CrossRef]
- Neuville, S. The enhancement of interconnected sp3 sites by chemical effects during ta-C film growth. Diam. Relat. Mater. 2002, 11, 1721–1730. [Google Scholar] [CrossRef]
- Wang, X.H.; Ma, G.H.M.; Zhu, W.; Glass, J.T.; Bergman, L.; Turner, K.F.; Nemanich, R.J. Effect of Boron doping on the surface morphology of and structural imperfection of diamond films. Diam. Relat. Mater. 1992, 1, 828–835. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, S.; Lou, J.; Wang, J.; Yang, Q.; Liu, C.; Huang, D.; Zhu, T. Graphene’s cousin: The present and future of graphane. Nanoscale Res. Lett. 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.C.; Nair, R.R.; Mohiuddin, T.M.; Morozov, S.V.; Blake, P.; Halsall, M.P.; Ferrari, A.C.; Boukhvalov, D.W.; Katsnelson, M.I.; Geim, A.K.; et al. Control of graphene’s properties by reversible hydrogenation: Evidence for graphane. Science 2009, 323, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Tolédano, J.C.; Tolédano, P. The Landau Theory of Phase Transitions; World Scientific: Singapore, 1987; ISBN 9971-50-025-6. [Google Scholar]
- Sofo, J.O.; Chaudhari, A.S.; Barber, G.D. Graphane: A two-dimensional hydrocarbon. Phys. Rev. B 2007, 75, 153401. [Google Scholar] [CrossRef]
- Podlivaev, A.I.; Openov, L.A. Elementary Defects in Graphane. JETP Lett. 2017, 106, 110–115. [Google Scholar] [CrossRef]
- Poh, H.L.; Šaněk, F.; Sofer, Z.; Pumera, M. High-pressure hydrogenation of graphene: Towards graphane. Nanoscale 2012, 4, 7006–7011. [Google Scholar] [CrossRef]
- Liu, A.Y.; Cohen, M.L. Structural properties and electronic structure of low-compressibility materials: β-Si3N4 and hypothetical β-C3N4. Phys. Rev. B 1990, 41, 10727. [Google Scholar] [CrossRef]
- Cao, S.; Yu, J. g-C 3 N 4 -Based Photocatalysts for Hydrogen Generation. J. Phys. Chem. Lett. 2014, 5, 2101–2107. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.G.; Bradley, K.; Ishigami, M.; Zettl, A. Extreme Oxygen Sensitivity of Electronic Properties of Carbon Nanotubes. Science 2000, 287, 1801–1804. [Google Scholar] [CrossRef] [PubMed]
- Al-Mumen, H.; Rao, F.; Li, W.; Dong, L. Singular Sheet Etching of Graphene with Oxygen Plasma. Nano- Micro Lett. 2014, 6, 116–124. [Google Scholar] [CrossRef]
- Czech, B.; Oleszczuk, P.; Wiacek, A.E.; Barczak, M. Cracking of CNT wall by UV. Environ. Sci. Pollut. Res. 2015, 22, 20198–20206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Neuville, S. Perspective of low energy Bethe nuclear fusion reactor with quantum electronic atomic rearrangement of carbon. Condens. Matter. Nucl. Sci. 2017, 22, 1–26. [Google Scholar]
- Rouzaud, J.; Oberlin, A.; Beny-Bassez, C. Carbon films: Structure and micro-texture (optical and electron microscopy, Raman spectroscopy). Thin Solid Film. 1983, 105, 75–96. [Google Scholar] [CrossRef]
- Anders, S.; Iii, J.W.A.; Pharr, G.M.; Tsui, T.Y.; Brown, I.G. Heat treatment of cathodic arc deposited amorphous hard carbon films. Thin Solid Film. 1997, 308, 186–190. [Google Scholar] [CrossRef]
- Prawer, S.; Nugent, K.; Lifshitz, Y.; Lempert, G.; Grossman, E.; Kulik, J.; Avigal, I.; Kalish, R. Systematic variation of the Raman spectra of DLC films as a function of sp2: sp3 composition. Diam. Relat. Mater. 1996, 5, 433–438. [Google Scholar] [CrossRef]
- Ferrari, A.C. Determination of bonding in diamond-like carbon by Raman spectroscopy. Diam. Relat. Mater. 2002, 11, 1053–1061. [Google Scholar] [CrossRef]
- Rammamurti, R.; Shanov, V.; Singh, R.N.; Mamedov, S.; Boolchand, P. Raman spectroscopy study of the influence of processing conditions on the structure of polycrystalline diamond films. J. Vac. Sci. Technol. A 2006, 24, 179. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman spectra of graphite. J. Phys. Chem. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Robertson, J.; O’Reilly, E.P. Electronic and atomic structure of amorphous carbon. Phys. Rev. B 1987, 35, 2946–2957. [Google Scholar] [CrossRef] [PubMed]
- Pauleau, Y. Residual Stresses in DLC Films and Adhesion to Various Substrates. In Tribology of DLC; Donnet, C., Erdemir, A., Eds.; Springer: New York, NY, USA, 2008; pp. 102–136. [Google Scholar]
- Maultzsch, J.; Reich, S.; Thomsen, C. Double-resonant Raman scattering in graphite: Interference effects, selection rules, and phonon dispersion. Phys. Rev. B 2004, 70, 155403. [Google Scholar] [CrossRef]
- Jorio, A. Raman Spectroscopy in Graphene-Based Systems: Prototypes for Nanoscience and Nanometrology. Int. Sch. Res. Netw. ISRN Nanotechnol. 2012, 2012, 234216. [Google Scholar] [CrossRef]
- Watanabe, E.; Conwill, A.; Tsuya, D.; Koide, Y. Low contact resistance metals for graphene-based devices. Diam. Relat. Mater. 2012, 24, 171–174. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulović, V.; Dresselhaus, M.S.; Kong, J. Large Area, Few-Layer Graphene Films on Arbitrary Substrates by Chemical Vapor Deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Wani, M.F. Synthesis and tribological properties of graphene: A review. J. Tribol. 2017, 13, 36–71. [Google Scholar]
- Penkov, O.; Kim, H.J.; Kim, H.J.; Kim, D.E. Tribology of Graphene: A Review. Int. J. Precis. Eng. Manuf. 2014, 15, 577–585. [Google Scholar] [CrossRef]
- Cançado, C.L.G.; Pimenta, M.A.; Neves, B.R.; Dantas, M.S.; Jorio, A. Influence of the Atomic Structure on the Raman Spectra of Graphite Edges. Phys. Rev. Lett. 2004, 93, 247401. [Google Scholar] [CrossRef] [PubMed]
- Dischler, B. Bonding and hydrogen incorporation in a-C: H studied by infrared spectroscopy. In EMRS XVII Proceedings; Koidl, P., Oelhafen, P., Eds.; Les Editions de la Physique: Paris, France, 1987; pp. 189–201. [Google Scholar]
- Marcus, B.; Fayette, L.; Mermoux, M.; Abello, L.; Lucazeau, G. Analysis of the structure of multi-component carbon films by resonant Raman scattering. J. Appl. Phys. 1994, 76, 3463–3470. [Google Scholar] [CrossRef]
- Singh, R.C.V. Raman and the Discovery of the Raman Effect. Phys. Perspect. 2002, 4, 399–420. [Google Scholar] [CrossRef]
- Saito, R.; Jorio, A.; Filho, A.G.S.; Dresselhaus, G.; Dresselhaus, M.S.; Pimenta, M.A. Probing Phonon Dispersion Relations of Graphite by Double Resonance Raman Scattering. Phys. Rev. Lett. 2001, 88, 027401. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, M.; Attaccalite, C.; Wirtz, L.; Mauri, F. Impact of the electron-electron correlation on phonon dispersion: Failure of LDA and GGA DFT functionals in graphene and graphite. Phys. Rev. B 2008, 78, 081406. [Google Scholar] [CrossRef]
- Dallas, T.E.J. Thesis on Structural Phases of Disordered Carbon Materials. Ph.D. Thesis, Texas Tech University, Lubbock, TX, USA, 1996. [Google Scholar]
- Chivilikhin, S.A.; Gusarov, V.V.; Yu-Popov, I. Flows in nanostructures: Hybrid classical-quantum models. Nano-Syst. Phys. Chem. Math. 2012, 3, 7–26. [Google Scholar]
- Piscanec, S.; Lazzeri, M.; Mauri, F.; Ferrari, A.C.; Robertson, J. Kohn Anomalies and Electron-Phonon Interactions in Graphite. Phys. Rev. Lett. 2004, 93, 185503. [Google Scholar] [CrossRef] [PubMed]
- Fakrach, B.; Rahmani, A.; Chadli, H.; Sbai, K.; Sauvajol, J.L. Raman spectrum of single-walled boron nitride nanotube. Physica E 2009, 41, 1800–1805. [Google Scholar] [CrossRef]
- Lee, S. Effect of Intrinsic Ripples on Elasticity of the Graphene Monolayer. Nanoscale Res. Lett. 2015, 10, 1704. [Google Scholar] [CrossRef]
- Nicholl, R.J.T.; Conley, H.J.; Lavrik, N.V.; Vlassiouk, I.; Puzyrev, Y.S.; Sreenivas, V.P.; Pantelides, S.T.; Bolotin, K.I. The effect of intrinsic crumpling on the mechanics of free-standing graphene. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef]
- Thompson-Flagg, R.C.; Moura, M.J.B.; Marder, M. Rippling of graphene. EPL (Europhys. Lett.) 2009, 85. [Google Scholar] [CrossRef]
- Tanigaki, K.; Ogi1, H.; Sumiya, H.; Kusakabe, K.; Nakamura, N.; Hirao, M.; Ledbetter, H. Observation of higher stiffness in nano-polycrystal diamond than monocrystal diamond. Nat. Commun. 2013. [Google Scholar] [CrossRef]
- Gómez-Navarro, C.; Weitz, R.T.; Bittner, A.M.; Scolari, M.; Mews, A.; Burghard, M.; Kern, K. Electronic Transport Properties of Individual Chemically Reduced Graphene Oxide Sheets. Nano Lett. 2007, 7, 3499–3503. [Google Scholar] [CrossRef] [PubMed]
- Childres, I.; A Jauregui, L.; Tian, J.; Chen, Y.P. Effect of oxygen plasma etching on graphene studied using Raman spectroscopy and electronic transport measurements. New J. Phys. 2011, 13, 25008. [Google Scholar] [CrossRef]
- Janas, D. Towards monochiral carbon nanotubes: A review of progress in the sorting of single-walled carbon nanotubes. Mater. Chem. Front. 2018, 2, 36–63. [Google Scholar] [CrossRef]
- Green, A.A.; Duch, M.C.; Hersam, M.C. Isolation of single-walled carbon nanotube enantiomers by density differentiation. Nano Res. 2009, 2, 69–77. [Google Scholar] [CrossRef]
- Neuville, S. Prebiotic mechanisms of life appearance near deep sea vents with early earth synthetized L-handed amino acids being adsorbed on SWCNT. Q. Phys. Rev. 2018, 4, 1–36. [Google Scholar]
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon Nanotubes: Present and Future Commercial Applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, Q.; Li, Z.; Fan, G.; Xiong, D.B.; Su, Y.; Zhang, J.; Zhang, D. Enhanced Mechanical Properties of Graphene (Reduced Graphene Oxide)/Aluminum Composites with a Bioinspired Nano-laminated Structure. Nano Lett. 2015, 15, 8077–8083. [Google Scholar] [CrossRef]
- Zhang, M.; Knoch, D.; Pascual-Leone, A.; Meyer, K.; Treyer, V.; Fehr, E. Strong, Transparent, Multifunctional, Carbon Nanotube Sheets. Science 2005, 309, 1215–1219. [Google Scholar] [CrossRef]
- Coleman, J.N.; Khan, U.; Blau, W.J.; Gun’Ko, Y.K. Small but strong: A review of the mechanical properties of carbon nanotube–polymer composites. Carbon 2006, 44, 1624–1652. [Google Scholar] [CrossRef]
- Kim, K.H.; Oh, Y.; Islam, M.F.; Islam, M. Graphene coating makes carbon nanotube aerogels superelastic and resistant to fatigue. Nat. Nanotechnol. 2012, 7, 562–566. [Google Scholar] [CrossRef]
- Poelma, R.H.; Morana, B.; Vollebregt, S.; Schlangen, E.; Van Zeijl, H.W.; Fan, X.; Zhang, G.Q. Tailoring the Mechanical Properties of High-Aspect-Ratio Carbon Nanotube Arrays using Amorphous Silicon Carbide Coatings. Adv. Funct. Mater. 2014, 24, 5737–5744. [Google Scholar] [CrossRef]
- Watcharotone, S.; Dikin, D.A.; Stankovich, S.; Piner, R.; Jung, I.; Dommett, G.H.B.; Evmenenko, G.; Wu, S.E.; Chen, S.F.; Liu, C.P.; et al. Graphene–Silica Composite Thin Films as Transparent Conductors. Nano Lett. 2007, 7, 1888–1892. [Google Scholar] [CrossRef] [PubMed]
- Susantyoko, R.A.; Alkindi, T.S.; Kanagaraj, A.B.; An, B.; Alshibli, H.; Choi, D.; Aldahmani, S.; Fadaq, H.; Almheiri, S. Performance optimization of freestanding MWCNT-LiFePO4 sheets as cathodes for improved specific capacity of lithium-ion batteries. RSC Adv. 2018, 8, 16566–16573. [Google Scholar] [CrossRef]
- Lalwani, G.; Kwaczala, A.T.; Kanakia, S.; Patel, S.C.; Judex, S.; Sitharaman, B. Fabrication and characterization of three-dimensional macroscopic all-carbon scaffolds. Carbon 2013, 53, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Van Der Zande, A.M.; Verbridge, S.S.; Frank, I.W.; Tanenbaum, D.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L.; Bunch, J.S. Electromechanical Resonators from Graphene Sheets. Science 2007, 315, 490–493. [Google Scholar]
- Xiao, L.; Chen, Z.; Feng, C.; Liu, L.; Bai, Z.-Q.; Wang, Y.; Qian, L.; Zhang, Y.; Li, Q.; Jiang, K.; et al. Flexible, Stretchable, Transparent Carbon Nanotube Thin Film Loudspeakers. Nano Lett. 2008, 8, 4539–4545. [Google Scholar] [CrossRef]
- Zhang, J.-C.; Tang, Y.-J.; Yi, Y.; Ma, K.-F.; Zhou, M.-J.; Wu, W.-D.; Wang, C.-Y. Large-scale synthesis of novel vertically-aligned helical carbon nanotube arrays. New Carbon Mater. 2016, 31, 568–573. [Google Scholar] [CrossRef]
- Aliev, A.E.; Oh, J.; Kozlov, M.E.; Kuznetsov, A.A.; Fang, S.; Fonseca, A.F.; Ovalle, R.; Lima, M.D.; Haque, M.H.; Gartstein, Y.N.; et al. Giant-Stroke, Superelastic Carbon Nanotube Aerogel Muscles. Science 2009, 323, 1575–1578. [Google Scholar] [CrossRef]
- Du, S. A microelectromechanical system based inertial system with rotating accelerometers and gyroscope for Land navigation systems. Int. J. Distrib. Sens. Netw. 2017, 13. [Google Scholar] [CrossRef]
- Miller, D.C.; Foster, R.R.; Zhang, Y.; Jen, S.H.; Bertrand, J.A.; Lu, Z.; Seghete, D.; O’Patchen, J.L.; Lee, R.Y.Y.C.; George, S.M.; et al. The mechanical robustness of atomic-layer- and molecular-layer-deposited coatings on polymer substrates. J. Appl. Phys. 2009, 105. [Google Scholar] [CrossRef]
- Berman, D.; Erdemir, A.; Sumant, A.V. Reduced wear and friction enabled by graphene layers on sliding steel surfaces in dry nitrogen. Carbon 2013, 59, 167–175. [Google Scholar] [CrossRef]
- Yan, C.; Kim, K.S.; Lee, S.K.; Bae, S.H.; Hong, B.H.; Kim, J.H.; Lee, H.J.; Ahn, J.H. Mechanical and Environmental Stability of Polymer Thin-Film-Coated Graphene. ACS Nano 2012, 6, 2096–2103. [Google Scholar] [CrossRef] [PubMed]
- Marchetto, D.; Held, C.; Hausen, F.; Wählisch, F.; Dienwiebel, M.; Bennewitz, R. Friction and Wear on Single-Layer Epitaxial Graphene in Multi-Asperity Contacts. Tribol. Lett. 2012, 48, 77–82. [Google Scholar] [CrossRef]
- Naraghi, M.; Filleter, T.; Moravsky, A.; Locascio, M.; Loutfy, R.O.; Espinosa, H.D. A Multiscale Study of High-Performance Double-Walled Nanotube−Polymer Fibers. ACS Nano 2010, 4, 6463–6476. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, T.; Gülseren, O.; Kılıç, C.; Ciraci, S. Pressure-induced interlinking of carbon nanotubes. Phys. Rev. B 2000, 62. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.; Zhang, Q.; Xie, H.; Qian, W.; Wei, F. Growth of Half-Meter Long Carbon Nanotubes Based on Schulz–Flory Distribution. ACS Nano 2013, 7, 6156–6161. [Google Scholar] [CrossRef] [PubMed]
- Motta, M.; Moisala, A.; Kinloch, I.A.; Windle, A.H. High Performance Fibers from ‘Dog Bone’ Carbon Nanotubes. Adv. Mater. 2007, 19, 3721–3726. [Google Scholar] [CrossRef]
- Dalton, A.B.; Collins, S.; Muñoz, E.; Razal, J.M.; Ebron, V.H.; Ferraris, J.P.; Coleman, J.N.; Kim, B.G.; Baughman, R.H. Super-tough carbon-nanotube fibers. Nature 2003, 423. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, X.; Shao, Z.; Zhou, P.; Porter, D.; Knight, D.P.; Vollrath, F. Toughness of Spider Silk at High and Low Temperatures. Adv. Mater. 2005, 17, 84–88. [Google Scholar] [CrossRef]
- Behabtu, N.; Young, C.C.; Tsentalovich, D.E.; Kleinerman, O.; Wang, X.; Ma, A.W.K.; Bengio, E.A.; Ter Waarbeek, R.F.; De Jong, J.J.; Hoogerwerf, R.E.; et al. Strong, Light, Multifunctional Fibers of Carbon Nanotubes with Ultrahigh Conductivity. Science 2013, 339, 182–186. [Google Scholar] [CrossRef]
- Liu, G.; Zheng, H.; Jiang, Z.; Zhao, J.; Wang, Z.; Pan, B.; Xing, B. Formation and Physicochemical Characteristics of Nano Biochar: Insight into Chemical and Colloidal Stability. Environ. Sci. Technol. 2018, 52, 10369–10379. [Google Scholar] [CrossRef] [PubMed]
- Giorcelli, M.; Savi, P.; Khan, A.; Tagliaferro, A. Analysis of biochar with different pyrolysis temperatures used as filler in epoxy resin composites. Biomass Bioenergy 2019, 122, 466–471. [Google Scholar] [CrossRef]
- Srivastava, N.; Joshi, R.; Banerjee, K. Carbon nanotube interconnects: Implications for performance, power dissipation and thermal management. In Proceedings of the IEEE International Electron Devices Meeting, Washington, DC, USA, 5 December 2005; IEDM Technical Digest. IEEE: Piscataway, NJ, USA, 2005; p. 249, ISBN 0-7803-9268-X. [Google Scholar]
- Coiffic, J.C.; Fayolle, M.; Maitrejean, S.; Torres, L.E.F.F.; Le Poche, H. Conduction regime in innovative carbon nanotube via interconnect architectures. Appl. Phys. Lett. 2007, 91, 252107. [Google Scholar] [CrossRef]
- Bauerdick, S.; Linden, A.; Stampfer, C.; Helbling, T.; Hierold, C. Direct wiring of carbon nanotubes for integration in nanoelectromechanical systems. J. Vac. Sci. Technol. B Microelectron. Nanometer. Struct. 2006, 24, 3144–3147. [Google Scholar] [CrossRef]
- Banerjee, K.; Srivastava, N. Are carbon nanotubes the future of VLSI interconnections? In Proceedings of the 39th conference on Design automation—DAC ’02 2006, San Francisco, CA, USA, 24–28 July 2006; p. 809. [Google Scholar]
- Naeemi, A.; Meindl, J.D. Carbon nanotube interconnects. In Proceedings of the 2007 International Symposium on Physical Design, ISPD ’07, Austin, TX, USA, 18–21 March 2007; p. 77. [Google Scholar]
- Chai, Y.; Chan, P.C.H. High electro-migration-resistant copper/carbon nanotube composite for Interconnect application. In Proceedings of the 2008 IEEE Electron Devices Meeting, San Francisco, CA, USA, 15–17 December 2008. [Google Scholar]
- Kordás, K.; TóTh, G.; Moilanen, P.; Kumpumäki, M.; Vähäkangas, J.; Uusimäki, A.; Vajtai, R.; Ajayan, P.M. Chip cooling with integrated carbon nanotube microfilm architectures. Appl. Phys. Lett. 2007, 90, 123105. [Google Scholar] [CrossRef]
- Li, J.; Ye, Q.; Cassell, A.; Ng, H.T.; Stevens, R.; Han, J.; Meyyappan, M. Bottom-up approach for carbon nanotube interconnects. Appl. Phys. Lett. 2003, 82, 2491–2493. [Google Scholar] [CrossRef]
- Collins, P.G. Nanotubes for Electronics. Sci. Am. 2000, 283, 67–69. [Google Scholar] [CrossRef]
- Westervelt, R.M. APPLIED PHYSICS: Graphene Nanoelectronics. Science 2008, 320, 324–325. [Google Scholar] [CrossRef]
- Potsma, H.W.C.; Teepen, T.; Yao, Z.; Grifoni, M.; Dekker, C. Carbon Nanotube Single-Electron Transistors at Room temperature. Science 2001, 293, 76–79. [Google Scholar]
- Chen, Z.; Sippel-Oakley, J.; Tang, J.; Wind, S.J.; Solomon, P.M.; Appenzeller, J.; Lin, Y.-M.; Rinzler, A.G.; Avouris, P. An Integrated Logic Circuit Assembled on a Single Carbon Nanotube. Science 2006, 311, 1735. [Google Scholar] [CrossRef]
- Wang, C.; Chien, J.-C.; Takei, K.; Takahashi, T.; Nah, J.; Niknejad, A.M.; Javey, A. Extremely Bendable, High-Performance Integrated Circuits Using Semiconducting Carbon Nanotube Networks for Digital, Analog, and Radio-Frequency Applications. Nano Lett. 2012, 12, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Appenzeller, J.; Lin, Y.-M.; Knoch, J.; Chen, Z.; Avouris, P. Comparing Carbon Nanotube Transistors—The Ideal Choice: A Novel Tunneling Device Design. IEEE Trans. Electron Devices 2005, 52, 2568–2576. [Google Scholar] [CrossRef]
- Parnell, A.J. Nano-technology and the potential for a renewable solar future. Nanotechnol. Percept. 2011, 7, 180–187. [Google Scholar] [CrossRef]
- Rasmussen, L. Electroactivity in Polymeric Materials; Springer Science & Business Media: Berlin, Germany, 2012; 162p. [Google Scholar]
- Jørgensen, M.; Carlé, J.E.; Søndergaard, R.R.; Lauritzen, M.; Dagnæs-Hansen, N.A.; Byskov, S.L.; Andersen, T.R.; Larsen-Olsen, T.T.; Böttiger, A.P.; Andreasen, B.; et al. The state of organic solar cells—A meta-analysis. Sol. Energy Mater. Sol. Cells 2013, 119, 84–93. [Google Scholar] [CrossRef]
- Chauhan, A.K.; Gusain, A.; Jha, P.; Koiry, S.P.; Saxena, V.; Veerender, P.; Aswal, D.K.; Gupta, S.K. Graphene composite for improvement in the conversion efficiency of flexible poly 3-hexyl-thiophene: [6,6]-phenyl C71 butyric acid methyl ester polymer solar cells. Appl. Phys. Lett. 2014, 104. [Google Scholar] [CrossRef]
- Guldi, D.M.; Rahman, G.M.A.; Prato, M.; Jux, N.; Qin, S.; Ford, W. Single-Wall Carbon Nanotubes as Integrative Building Blocks for Solar-Energy Conversion. Angew. Chem. 2005, 117, 2051–2054. [Google Scholar] [CrossRef]
- Neuville, S. Advanced ta-C coatings with up-dated fundamentals for energy production efficiency. Mater. Day Proc. 2018, 5, 13816–13826. [Google Scholar]
- Casas, C.D.L.; Li, W. A review of application of carbon nanotubes for lithium ion battery anode material. J. Power Sources 2012, 208, 74–85. [Google Scholar] [CrossRef]
- Banerjee, A.; Ziv, B.; Levi, E.; Shilina, Y.; Luski, S.; Aurbac, D. Single-Wall Carbon Nanotubes Embedded in Active Masses for High-Performance Lead-Acid Batteries. J. Electrochem. Soc. 2016, 163, A1518–A1526. [Google Scholar] [CrossRef]
- Fu, K.; Yildiz, Ö.; Bhanushali, H.; Wang, Y.; Stano, K.; Xue, L.; Zhang, X.; Bradford, P.D. Aligned Carbon Nanotube-Silicon Sheets: A Novel Nano-architecture for Flexible Lithium Ion Battery Electrodes. Adv. Mater. 2013, 25, 5109–5114. [Google Scholar] [CrossRef]
- Barghi, S.H.; Tsotsis, T.T.; Sahimi, M. Chemisorption, physisorption and hysteresis during hydrogen storage in carbon nanotubes. Int. J. Hydrog. Energy 2014, 39, 1390–1397. [Google Scholar] [CrossRef]
- Dillon, A.C.; Jones, K.M.; Bekkedahl, T.A.; Kiang, C.H.; Bethune, D.S.; Heben, M.J. Storage of hydrogen in single-walled carbon nanotubes. Nature 1997, 386, 377–379. [Google Scholar] [CrossRef]
- Safa, S.; Mojtahedzadeh, L.M.; Fathollahi, V.; Kakuee, O.R. Investigating hydrogen storage behavior of carbon nanotubes at ambient temperature by ion beam analysis. Nano 2010, 5, 341–347. [Google Scholar] [CrossRef]
- Yuca, N.; Karatepe, N. Hydrogen storage in single-walled carbon nanotubes purified by microwave digestion method. World Acad. Sci. Eng. Technol. 2011, 79, 605–610. [Google Scholar]
- Valenti, G.; Boni, A.; Melchionna, M.; Cargnello, M.; Nasi, L.; Bertoni, G.; Gorte, R.J.; Marcaccio, M.; Rapino, S.; Bonchio, M.; et al. Co-axial heterostructures integrating palladium/titanium dioxide with carbon nanotubes for efficient electrocatalytic hydrogen evolution. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Shao, T.; Kose, H.S.; Karanfil, T. Adsorption of aromatic compounds by carbonaceous absorbents: A comparative study on granular activated carbon, activated carbon fibers, and carbon nanotubes. Environ. Sci. Technol. 2010, 44, 6377–6383. [Google Scholar] [CrossRef] [PubMed]
- Apul, O.G.; Karanfil, T. Adsorption of synthetic organic contaminants by carbon nanotubes: A critical review. Water Res. 2015, 68, 34–55. [Google Scholar] [CrossRef] [PubMed]
- Polikarpova, N.P.; Zaporotskova, I.V.; Vilkeeva, D.E.; Polikarpov, D.I. Sensor properties of carboxyl-modified carbon nanotubes. Nanosyst. Phys. Chem. Math. 2014, 5, 101–106. [Google Scholar]
- Pötschke, P.; Andres, T.; Villmow, T.; Pegel, S.; Brünig, H.; Kobashi, K.; Fischer, D.; Häussler, L. Liquid sensing properties of fibers prepared by melt spinning from polylactic acid containing multi-walled carbon nanotubes. Compos. Sci. Technol. 2010, 70, 343–349. [Google Scholar] [CrossRef]
- Hashim, D.P.; Narayanan, N.T.; Romo-Herrera, J.M.; Cullen, D.A.; Hahm, M.G.; Lezzi, P.; Suttle, J.R.; Kelkhoff, D.; Muñoz-Sandoval, E.; Ganguli, S.; et al. Covalently bonded three-dimensional carbon nanotube solids via boron induced nanojunctions. Sci. Rep. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Mikael, P.E.; Nukavarapu, S.P. Functionalized Carbon Nanotube Composite Scaffolds for Bone Tissue Engineering: Prospects and Progress. J. Biomater. Tissue Eng. 2011, 1, 76–85. [Google Scholar] [CrossRef]
- Balavoine, F.; Schultz, P.; Richard, C.; Mallouh, V.; Ebbesen, T.W.; Mioskowski, C. Helical Crystallization of Proteins on Carbon Nanotubes: A First Step towards the Development of New Biosensors. Angew. Chem. Int. Ed. 1999, 38, 1912–1915. [Google Scholar] [CrossRef]
- Fasano, M.; Chiavazzo, E.; Asinari, P. Water transport control in carbon nanotube arrays. Nanoscale Res. Lett. 2014, 9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hesami, M.; Bagheri, R.; Masoomi, M. Combination effects of carbon nanotubes, MMT and phosphorus flame retardant on fire and thermal resistance of fiber-reinforced epoxy composites. Iran. Polym. J. 2014, 23, 469–476. [Google Scholar] [CrossRef]
- Lv, J.; Leong, E.S.; Jiang, X.; Kou, S.; Dai, H.; Lin, J.; Liu, Y.J.; Si, G. Plasmon-Enhanced Sensing: Current Status and Prospects. Hindawi J. Nanomater. 2015, 16. [Google Scholar] [CrossRef]
- Lamb, H. Sandvik Reveals 3D Printed Diamond Composite; Engineering and Technology: Hertfordshire, UK, 2019. [Google Scholar]






| Raman (cm−1) (Nominal) | Peak/Band | Type of Structure (Non-Stressed Structure) | Type of Bonds Energy in eV |
|---|---|---|---|
| ~1330 ~1325 ~1200/1400 | D peak DH6 peak Dd band | Ordered Diamond cubic Ordered hexagonal diamond Amorphous diamond, ta-C | Csp3-Csp3 ~7.02 ~7.015 eV Overlapping with DD band |
| ~1150 ~1470 | DD DG | Edges of Diamond crystallites. Diamond and sp2 edges | Aliphatic Csp3–Csp3 Aliphatic Csp3–Csp2 |
| ~1580 ~1560/1620 | G peak G band | In plane double degenerated Γ phonon mode stationary vibration of sp2 cyclic ring Atomic disorder broadening | Csp2-Csp2 ~7.03 eV collective bond vibration Superposition GG, DG, GC5/C7 |
| ~1620 ~1510 | GG GD | Csp2-Csp2 clusters edge in a-C and DLC Csp3-Csp2 cluster | Csp2-Csp2 ~7.03 eV collective bond vibration |
| ~1490 ~1540 | GC5 GC7 | C5 ring C7 ring | Fullerene C5 (~1550 cm−1) (upshift by plane curvature) |
| ~1350 ~1300/1400 | GeA GeA band | A edge 0° CDR scattering Voids internal A edges 1st, 2d order disorder on edge Broadening by edge disorder | Free edges: not active with ┴ vertical polarized laser light Bonded edges: all laser light polarization |
| ~2690 | G2P | 2 phonon CDR scattering Any polarized laser light | In plane 2K and 2M Preferential in-plane polarization |
| ~150 ~1600 ~1560 | RBM G+ G− | Breathing mode of CNT (radius dependent) CNT in plane Longitudinal CNT in plane Transversal | Collective phasic stretching Distorted Csp2-Csp2 by sp2 plane curvature of CNT |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neuville, S. Selective Carbon Material Engineering for Improved MEMS and NEMS. Micromachines 2019, 10, 539. https://doi.org/10.3390/mi10080539
Neuville S. Selective Carbon Material Engineering for Improved MEMS and NEMS. Micromachines. 2019; 10(8):539. https://doi.org/10.3390/mi10080539
Chicago/Turabian StyleNeuville, Stephane. 2019. "Selective Carbon Material Engineering for Improved MEMS and NEMS" Micromachines 10, no. 8: 539. https://doi.org/10.3390/mi10080539
APA StyleNeuville, S. (2019). Selective Carbon Material Engineering for Improved MEMS and NEMS. Micromachines, 10(8), 539. https://doi.org/10.3390/mi10080539




