A Milled Microdevice to Advance Glia-Mediated Therapies in the Adult Nervous System
Abstract
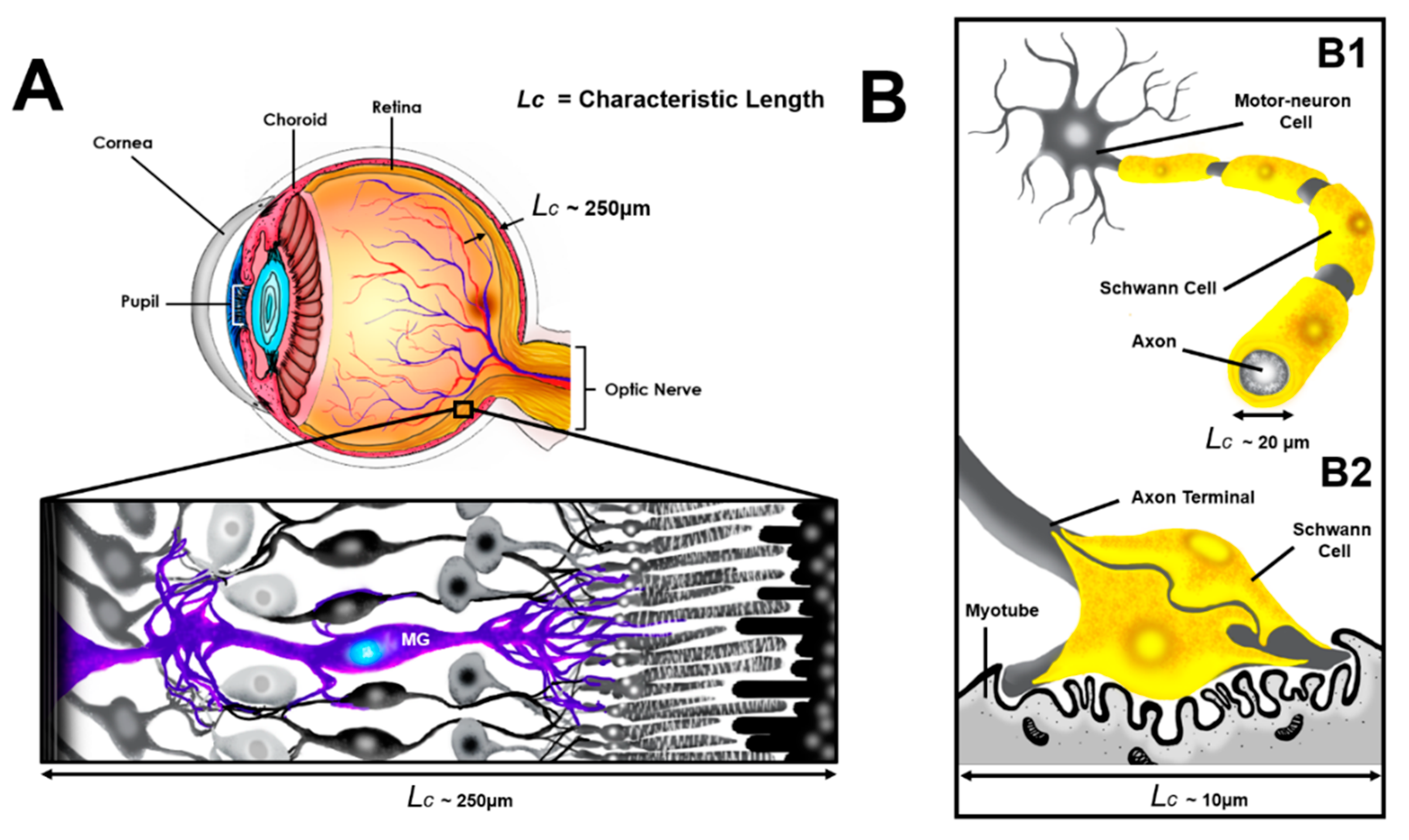
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Reagents
2.3. The Glia-Line System (gLL)
2.3.1. Design of the gLL
2.3.2. Transport Modeling and Validation
2.3.3. Manufacture and Assembly
2.3.4. gLL Operation
2.4. Measurement of Cell Viability, Proliferation, and Morphology
2.5. Measurement of Cell Migration in the gLL
2.6. Imaging and Analysis
2.7. Statistical Analysis
3. Results
3.1. The gLL System
3.2. Transport within the gLL Microchannel
3.3. Glial Survival
3.4. Cell Proliferation
3.5. Cell Morphology
3.6. Cell Migration
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Martin, J.B. Molecular basis of the neurodegenerative disorders. N. Engl. J. Med. 1999, 340, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Goldman, D. Muller glial cell reprogramming and retina regeneration. Nat. Rev. Neurosci. 2014, 15, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Chohan, A.; Singh, U.; Kumar, A.; Kaur, J. Muller stem cell dependent retinal regeneration. Clin. Chim. Acta 2017, 464, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Lorenzelli, L.; Margesin, B.; Martinoia, S.; Tedesco, M.T.; Valle, M. Bioelectrochemical signal monitoring of in-vitro cultured cells by means of an automated microsystem based on solid state sensor-array. Biosens. Bioelectron. 2003, 18, 621–626. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Hoh, J.; Liu, J. Muller cells in pathological retinal angiogenesis. Transl. Res. 2019, 207, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Shen, S.Q.; Jui, J.; Rupp, A.C.; Byrne, L.C.; Hattar, S.; Flannery, J.G.; Corbo, J.C.; Kefalov, V.J. CRALBP supports the mammalian retinal visual cycle and cone vision. J. Clin. Investig. 2015, 125, 727–738. [Google Scholar] [CrossRef]
- Rao, M.B.; Didiano, D.; Patton, J.G. Neurotransmitter-Regulated Regeneration in the Zebrafish Retina. Stem Cell Rep. 2017, 8, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Reichenbach, A.; Bringmann, A. Role of Purines in Muller Glia. J. Ocul. Pharm. 2016, 32, 518–533. [Google Scholar] [CrossRef]
- Singh, T.; Vazquez, M. Time-Dependent Addition of Neuronal and Schwann Cells Increase Myotube Viability and Length in an In Vitro Tri-culture Model of the Neuromuscular Junction. Regen. Eng. Transl. Med. 2019. [Google Scholar] [CrossRef]
- Griffin, J.W.; Thompson, W.J. Biology and pathology of nonmyelinating Schwann cells. Glia 2008, 56, 1518–1531. [Google Scholar] [CrossRef]
- Darlot, F.; Artuso, A.; Lautredou-Audouy, N.; Casellas, D. Topology of Schwann cells and sympathetic innervation along preglomerular vessels: A confocal microscopic study in protein S100B/EGFP transgenic mice. Am. J. Physiol. Ren. Physiol. 2008, 295, F1142–F1148. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The origin and development of glial cells in peripheral nerves. Nat. Rev. Neurosci. 2005, 6, 671–682. [Google Scholar] [CrossRef]
- De Hoz, R.; Rojas, B.; Ramirez, A.I.; Salazar, J.J.; Gallego, B.I.; Trivino, A.; Ramirez, J.M. Retinal Macroglial Responses in Health and Disease. Biomed. Res. Int. 2016, 2016, 2954721. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, Z.; Li, C.; Zheng, Y. Morphological and migratory alterations in retinal Muller cells during early stages of hypoxia and oxidative stress. Neural Regen. Res. 2012, 7, 31–35. [Google Scholar] [CrossRef] [PubMed]
- McGill, T.J.; Lund, R.D.; Douglas, R.M.; Wang, S.; Lu, B.; Prusky, G.T. Preservation of vision following cell-based therapies in a model of retinal degenerative disease. Vis. Res. 2004, 44, 2559–2566. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lawrence, J.M.; Sauve, Y.; Keegan, D.J.; Coffey, P.J.; Hetherington, L.; Girman, S.; Whiteley, S.J.; Kwan, A.S.; Pheby, T.; Lund, R.D. Schwann cell grafting into the retina of the dystrophic RCS rat limits functional deterioration. Royal College of Surgeons. Investig. Ophthalmol. Vis. Sci. 2000, 41, 518–528. [Google Scholar]
- Jorstad, N.L.; Wilken, M.S.; Grimes, W.N.; Wohl, S.G.; VandenBosch, L.S.; Yoshimatsu, T.; Wong, R.O.; Rieke, F.; Reh, T.A. Stimulation of functional neuronal regeneration from Muller glia in adult mice. Nature 2017, 548, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Ueki, Y.; Wilken, M.S.; Cox, K.E.; Chipman, L.; Jorstad, N.; Sternhagen, K.; Simic, M.; Ullom, K.; Nakafuku, M.; Reh, T.A. Transgenic expression of the proneural transcription factor Ascl1 in Muller glia stimulates retinal regeneration in young mice. Proc. Natl. Acad. Sci. USA 2015, 112, 13717–13722. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudzadeh, R.; Heidari-Keshel, S.; Lashay, A. Schwann Cell-Mediated Preservation of Vision in Retinal Degenerative Diseases via the Reduction of Oxidative Stress: A Possible Mechanism. Med. Hypothesis Discov. Innov. Ophthalmol. 2016, 5, 47–52. [Google Scholar]
- Becker, S.; Eastlake, K.; Jayaram, H.; Jones, M.F.; Brown, R.A.; McLellan, G.J.; Charteris, D.G.; Khaw, P.T.; Limb, G.A. Allogeneic Transplantation of Muller-Derived Retinal Ganglion Cells Improves Retinal Function in a Feline Model of Ganglion Cell Depletion. Stem Cells Transl. Med. 2016, 5, 192–205. [Google Scholar] [CrossRef]
- Koh, S.; Chen, W.J.; Dejneka, N.S.; Harris, I.R.; Lu, B.; Girman, S.; Saylor, J.; Wang, S.; Eroglu, C. Subretinal Human Umbilical Tissue-Derived Cell Transplantation Preserves Retinal Synaptic Connectivity and Attenuates Muller Glial Reactivity. J. Neurosci. 2018, 38, 2923–2943. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Gao, L.; Xu, H.; Duan, P.; Zeng, Y.; Liu, Y.; Yin, Z.Q. Combined transplantation of human mesenchymal stem cells and human retinal progenitor cells into the subretinal space of RCS rats. Sci. Rep. 2017, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Lorber, B.; Chew, D.J.; Hauck, S.M.; Chong, R.S.; Fawcett, J.W.; Martin, K.R. Retinal glia promote dorsal root ganglion axon regeneration. PLoS ONE 2015, 10, e0115996. [Google Scholar] [CrossRef] [PubMed]
- Zujovic, V.; Bachelin, C.; Baron-Van Evercooren, A. Remyelination of the central nervous system: A valuable contribution from the periphery. Neuroscientist 2007, 13, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Fouad, K.; Schnell, L.; Bunge, M.B.; Schwab, M.E.; Liebscher, T.; Pearse, D.D. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J. Neurosci. 2005, 25, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Hoke, A.; Redett, R.; Hameed, H.; Jari, R.; Zhou, C.; Li, Z.B.; Griffin, J.W.; Brushart, T.M. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J. Neurosci. 2006, 26, 9646–9655. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Guenard, V.; Kleitman, N.; Aebischer, P.; Bunge, M.B. A combination of BDNF and NT-3 promotes supraspinal axonal regeneration into Schwann cell grafts in adult rat thoracic spinal cord. Exp. Neurol. 1995, 134, 261–272. [Google Scholar] [CrossRef]
- Menei, P.; Montero-Menei, C.; Whittemore, S.R.; Bunge, R.P.; Bunge, M.B. Schwann cells genetically modified to secrete human BDNF promote enhanced axonal regrowth across transected adult rat spinal cord. Eur. J. Neurosci. 1998, 10, 607–621. [Google Scholar] [CrossRef]
- Thakur, A.; Mishra, S.; Pena, J.; Zhou, J.; Redenti, S.; Majeska, R.; Vazquez, M. Collective adhesion and displacement of retinal progenitor cells upon extracellular matrix substrates of transplantable biomaterials. J. Tissue Eng. 2018, 9. [Google Scholar] [CrossRef]
- Old, E.A.; Malcangio, M. Chemokine mediated neuron-glia communication and aberrant signalling in neuropathic pain states. Curr. Opin. Pharm. 2012, 12, 67–73. [Google Scholar] [CrossRef]
- Mishra, S.; Vazquez, M. A Gal-MmicroS Device to Evaluate Cell Migratory Response to Combined Galvano-Chemotactic Fields. Biosensors (Basel) 2017, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Faustino, V.; Catarino, S.O.; Lima, R.; Minas, G. Biomedical microfluidic devices by using low-cost fabrication techniques: A review. J. Biomech. 2016, 49, 2280–2292. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Chen, D.; Wang, X.; Jiang, J. Milling Positive Master for Polydimethylsiloxane Microfluidic Devices: The Microfabrication and Roughness Issues. Micromachines (Basel) 2017, 8, 287. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, N.; Urrios, A.; Kang, S.; Folch, A. The upcoming 3D-printing revolution in microfluidics. Lab Chip 2016, 16, 1720–1742. [Google Scholar] [CrossRef] [PubMed]
- Noiphung, J.; Nguyen, M.P.; Punyadeera, C.; Wan, Y.; Laiwattanapaisal, W.; Henry, C.S. Development of Paper-Based Analytical Devices for Minimizing the Viscosity Effect in Human Saliva. Theranostics 2018, 8, 3797–3807. [Google Scholar] [CrossRef] [PubMed]
- Channon, R.B.; Nguyen, M.P.; Scorzelli, A.G.; Henry, E.M.; Volckens, J.; Dandy, D.S.; Henry, C.S. Rapid flow in multilayer microfluidic paper-based analytical devices. Lab Chip 2018, 18, 793–802. [Google Scholar] [CrossRef]
- Coltro, W.K.; de Jesus, D.P.; da Silva, J.A.; do Lago, C.L.; Carrilho, E. Toner and paper-based fabrication techniques for microfluidic applications. Electrophoresis 2010, 31, 2487–2498. [Google Scholar] [CrossRef]
- Oliveira, K.A.; de Souza, F.R.; de Oliveira, C.R.; da Silveira, L.A.; Coltro, W.K. Microfluidic toner-based analytical devices: Disposable, lightweight, and portable platforms for point-of-care diagnostics with colorimetric detection. Methods Mol. Biol. 2015, 1256, 85–98. [Google Scholar] [CrossRef]
- Guckenberger, D.J.; de Groot, T.E.; Wan, A.M.; Beebe, D.J.; Young, E.W. Micromilling: A method for ultra-rapid prototyping of plastic microfluidic devices. Lab Chip 2015, 15, 2364–2378. [Google Scholar] [CrossRef]
- Sarthy, V.P.; Brodjian, S.J.; Dutt, K.; Kennedy, B.N.; French, R.P.; Crabb, J.W. Establishment and characterization of a retinal Muller cell line. Investig. Ophthalmol. Vis. Sci. 1998, 39, 212–216. [Google Scholar]
- Greenberg, K.P.; Geller, S.F.; Schaffer, D.V.; Flannery, J.G. Targeted transgene expression in muller glia of normal and diseased retinas using lentiviral vectors. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1844–1852. [Google Scholar] [CrossRef] [PubMed]
- Hahn, P.; Qian, Y.; Dentchev, T.; Chen, L.; Beard, J.; Harris, Z.L.; Dunaief, J.L. Disruption of ceruloplasmin and hephaestin in mice causes retinal iron overload and retinal degeneration with features of age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2004, 101, 13850–13855. [Google Scholar] [CrossRef]
- Du, Y.; Miller, C.M.; Kern, T.S. Hyperglycemia increases mitochondrial superoxide in retina and retinal cells. Free Radic. Biol. Med. 2003, 35, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.A. Isolation and Expansion of Schwann Cells from Transgenic Mouse Models. Methods Mol. Biol. 2018, 1739, 39–48. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Z.; Liu, J.; He, Q.; Zhou, Y.; Shao, G.; Sun, X.; Cao, X.; Gong, A.; Jiang, P. A fibrin matrix promotes the differentiation of EMSCs isolated from nasal respiratory mucosa to myelinating phenotypical Schwann-like cells. Mol. Cells 2015, 38, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Toolbox, T.E. Hydraulic Diameter of Pipes and Ducts. Available online: https://www.engineeringtoolbox.com/hydraulic-equivalent-diameter-d_458.html (accessed on 31 July 2019).
- Truskey, G.A.; Yuan, F.; Katz, D.F. Transport Phenomena in Biological Systems; Pearson/Prentice Hall: Upper Saddle River, NJ, USA, 2004. [Google Scholar]
- Kong, Q.; Majeska, R.J.; Vazquez, M. Migration of connective tissue-derived cells is mediated by ultra-low concentration gradient fields of EGF. Exp. Cell Res. 2011, 317, 1491–1502. [Google Scholar] [CrossRef]
- Venturoli, D.; Rippe, B. Ficoll and dextran vs. globular proteins as probes for testing glomerular permselectivity: Effects of molecular size, shape, charge, and deformability. Am. J. Physiol. Ren. Physiol. 2005, 288, F605–F613. [Google Scholar] [CrossRef]
- Segel, L.A.; Slemrod, M. The Quasi-Steady-State Assumption: A Case Study in Perturbation. Siam Rev. 1989, 31, 446–477. [Google Scholar] [CrossRef]
- Dudu, V.; Able, R.A., Jr.; Rotari, V.; Kong, Q.; Vazquez, M. Role of Epidermal Growth Factor-Triggered PI3K/Akt Signaling in the Migration of Medulloblastoma-Derived Cells. Cell Mol. Bioeng. 2012, 5, 402–413. [Google Scholar] [CrossRef]
- Rico-Varela, J.; Singh, T.; McCutcheon, S.; Vazquez, M. EGF as a New Therapeutic Target for Medulloblastoma Metastasis. Cell Mol. Bioeng. 2015, 8, 553–565. [Google Scholar] [CrossRef]
- Hessler, J.A.; Budor, A.; Putchakayala, K.; Mecke, A.; Rieger, D.; Banaszak Holl, M.M.; Orr, B.G.; Bielinska, A.; Beals, J.; Baker, J., Jr. Atomic force microscopy study of early morphological changes during apoptosis. Langmuir 2005, 21, 9280–9286. [Google Scholar] [CrossRef] [PubMed]
- Versaevel, M.; Grevesse, T.; Gabriele, S. Spatial coordination between cell and nuclear shape within micropatterned endothelial cells. Nat. Commun. 2012, 3, 671. [Google Scholar] [CrossRef] [PubMed]
- Hartig, S.M. Basic image analysis and manipulation in ImageJ. Curr. Protoc. Mol. Biol. 2013. [Google Scholar] [CrossRef]
- Pena, J.; Dulger, N.; Singh, T.; Zhou, J.; Majeska, R.; Redenti, S.; Vazquez, M. Controlled microenvironments to evaluate chemotactic properties of cultured Muller glia. Exp. Eye Res. 2018, 173, 129–137. [Google Scholar] [CrossRef]
- Altintas, Y. Manufacturing Automation: Metal Cutting Mechanics, Machine Tool Vibrations, and CNC Design; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Daignan-Fornier, B.; Sagot, I. Proliferation/Quiescence: When to start? Where to stop? What to stock? Cell Div. 2011, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Kaven, C.W.; Spraul, C.W.; Zavazava, N.K.; Lang, G.K.; Lang, G.E. Growth factor combinations modulate human retinal pigment epithelial cell proliferation. Curr. Eye Res. 2000, 20, 480–487. [Google Scholar] [CrossRef]
- Ozturk, B.T.; Bozkurt, B.; Kerimoglu, H.; Okka, M.; Kamis, U.; Gunduz, K. Effect of serum cytokines and VEGF levels on diabetic retinopathy and macular thickness. Mol. Vis. 2009, 15, 1906–1914. [Google Scholar]
- Wilhelm, J.C.; Xu, M.; Cucoranu, D.; Chmielewski, S.; Holmes, T.; Lau, K.S.; Bassell, G.J.; English, A.W. Cooperative roles of BDNF expression in neurons and Schwann cells are modulated by exercise to facilitate nerve regeneration. J. Neurosci. 2012, 32, 5002–5009. [Google Scholar] [CrossRef]
- Jha, M.K.; Lee, W.H.; Suk, K. Functional polarization of neuroglia: Implications in neuroinflammation and neurological disorders. Biochem. Pharm. 2016, 103, 1–16. [Google Scholar] [CrossRef]
- Streets, A.M.; Huang, Y. Chip in a lab: Microfluidics for next generation life science research. Biomicrofluidics 2013, 7, 11302. [Google Scholar] [CrossRef]
- Halldorsson, S.; Lucumi, E.; Gomez-Sjoberg, R.; Fleming, R.M.T. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens. Bioelectron. 2015, 63, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Distler, C.; Dreher, Z. Glia cells of the monkey retina-II. Muller cells. Vis. Res. 1996, 36, 2381–2394. [Google Scholar] [CrossRef]
- Salzer, J.L. Schwann cell myelination. Cold Spring Harb. Perspect. Biol. 2015, 7, a020529. [Google Scholar] [CrossRef] [PubMed]
- Ziganshin, R.H.; Ivanova, O.M.; Lomakin, Y.A.; Belogurov, A.A., Jr.; Kovalchuk, S.I.; Azarkin, I.V.; Arapidi, G.P.; Anikanov, N.A.; Shender, V.O.; Piradov, M.A.; et al. The Pathogenesis of the Demyelinating Form of Guillain-Barre Syndrome (GBS): Proteo-peptidomic and Immunological Profiling of Physiological Fluids. Mol. Cell Proteom. 2016, 15, 2366–2378. [Google Scholar] [CrossRef] [PubMed]
- Manganelli, F.; Nolano, M.; Pisciotta, C.; Provitera, V.; Fabrizi, G.M.; Cavallaro, T.; Stancanelli, A.; Caporaso, G.; Shy, M.E.; Santoro, L. Charcot-Marie-Tooth disease: New insights from skin biopsy. Neurology 2015, 85, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Ponath, G.; Park, C.; Pitt, D. The Role of Astrocytes in Multiple Sclerosis. Front. Immunol. 2018, 9, 217. [Google Scholar] [CrossRef]
- Fischer, A.J.; Reh, T.A. Muller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat. Neurosci. 2001, 4, 247–252. [Google Scholar] [CrossRef]
- Dyer, M.A.; Cepko, C.L. Control of Muller glial cell proliferation and activation following retinal injury. Nat. Neurosci. 2000, 3, 873–880. [Google Scholar] [CrossRef]
- Humphrey, M.F.; Constable, I.J.; Chu, Y.; Wiffen, S. A quantitative study of the lateral spread of Muller cell responses to retinal lesions in the rabbit. J. Comp. Neurol. 1993, 334, 545–558. [Google Scholar] [CrossRef]
- Kolb, H. Simple Anatomy of the Retina. In Webvision: The Organization of the Retina and Visual System; Kolb, H., Fernandez, E., Nelson, R., Eds.; University of Utah School of Medicine: Salt Lake City, UT, USA, 1995. [Google Scholar]
- Song, A.P.; Wu, X.Y.; Wang, J.R.; Liu, W.; Sun, Y.; Yu, T. Measurement of retinal thickness in macular region of high myopic eyes using spectral domain OCT. Int. J. Ophthalmol. 2014, 7, 122–127. [Google Scholar] [CrossRef]
- Susuki, K. Myelin: A Specialized Membrane for Cell Communication. Nat. Educ. 2010, 3, 59. [Google Scholar]
- Owens, G.C.; Bunge, R.P. Schwann cells depleted of galactocerebroside express myelin-associated glycoprotein and initiate but do not continue the process of myelination. Glia 1990, 3, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Able, R.A., Jr.; Dudu, V.; Vazquez, M. A microfluidic device to establish concentration gradients using reagent density differences. J. Biomech. Eng. 2010, 132, 121012. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, M.; Marte, O.; Barba, J.; Hubbard, K. An Approach to Integrating Health Disparities within Undergraduate Biomedical Engineering Education. Ann. Biomed. Eng. 2017, 45, 2703–2715. [Google Scholar] [CrossRef] [PubMed]
- Pena, J.S.; Vazquez, M. Reducing health disparities in adult vision loss via interfaces with emerging technology. Eye 2019, 33, 532–533. [Google Scholar] [CrossRef] [PubMed]
- Young, E.W.; Beebe, D.J. Fundamentals of microfluidic cell culture in controlled microenvironments. Chem. Soc. Rev. 2010, 39, 1036–1048. [Google Scholar] [CrossRef]
- Bringmann, A.; Wiedemann, P. Muller glial cells in retinal disease. Ophthalmologica 2012, 227, 1–19. [Google Scholar] [CrossRef]
- Fawcett, J.W.; Asher, R.A. The glial scar and central nervous system repair. Brain Res. Bull. 1999, 49, 377–391. [Google Scholar] [CrossRef]
- Bringmann, A.; Reichenbach, A. Role of Muller cells in retinal degenerations. Front. Biosci. 2001, 6, E72–E92. [Google Scholar] [CrossRef]
- Tassoni, A.; Gutteridge, A.; Barber, A.C.; Osborne, A.; Martin, K.R. Molecular Mechanisms Mediating Retinal Reactive Gliosis Following Bone Marrow Mesenchymal Stem Cell Transplantation. Stem Cells 2015, 33, 3006–3016. [Google Scholar] [CrossRef]
- Zelinka, C.P.; Volkov, L.; Goodman, Z.A.; Todd, L.; Palazzo, I.; Bishop, W.A.; Fischer, A.J. mTor signaling is required for the formation of proliferating Muller glia-derived progenitor cells in the chick retina. Development 2016, 143, 1859–1873. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, C.; Zhou, T.; Huang, Z.; Zhou, L.; Liu, X. NGF increases VEGF expression and promotes cell proliferation via ERK1/2 and AKT signaling in Muller cells. Mol. Vis. 2016, 22, 254–263. [Google Scholar] [PubMed]
- Ha, Y.; Shanmugam, A.K.; Markand, S.; Zorrilla, E.; Ganapathy, V.; Smith, S.B. Sigma receptor 1 modulates ER stress and Bcl2 in murine retina. Cell Tissue Res. 2014, 356, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.J.; Anderson, N.M.; Jiang, Y.; Bahouth, S.; Steinle, J.J. Role of beta-adrenergic receptor regulation of TNF-alpha and insulin signaling in retinal Muller cells. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9527–9533. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.A.; Mindos, T.; Parkinson, D.B. Plastic fantastic: Schwann cells and repair of the peripheral nervous system. Stem Cells Transl. Med. 2013, 2, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zheng, H.; Chen, Z.L.; Xiao, H.L.; Shen, Z.J.; Zhou, G.M. Preferential regeneration of photoreceptor from Muller glia after retinal degeneration in adult rat. Vis. Res. 2008, 48, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, H.; Jones, M.F.; Eastlake, K.; Cottrill, P.B.; Becker, S.; Wiseman, J.; Khaw, P.T.; Limb, G.A. Transplantation of photoreceptors derived from human Muller glia restore rod function in the P23H rat. Stem Cells Transl. Med. 2014, 3, 323–333. [Google Scholar] [CrossRef]
- Sparling, J.S.; Bretzner, F.; Biernaskie, J.; Assinck, P.; Jiang, Y.; Arisato, H.; Plunet, W.T.; Borisoff, J.; Liu, J.; Miller, F.D.; et al. Schwann cells generated from neonatal skin-derived precursors or neonatal peripheral nerve improve functional recovery after acute transplantation into the partially injured cervical spinal cord of the rat. J. Neurosci. 2015, 35, 6714–6730. [Google Scholar] [CrossRef]
- Ramon-Cueto, A.; Plant, G.W.; Avila, J.; Bunge, M.B. Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. J. Neurosci. 1998, 18, 3803–3815. [Google Scholar] [CrossRef]
- Rasouli, A.; Bhatia, N.; Suryadevara, S.; Cahill, K.; Gupta, R. Transplantation of preconditioned schwann cells in peripheral nerve grafts after contusion in the adult spinal cord. Improvement of recovery in a rat model. J. Bone Jt. Surg. Am. 2006, 88, 2400–2410. [Google Scholar] [CrossRef]
- Gupta, N.; Mansoor, S.; Sharma, A.; Sapkal, A.; Sheth, J.; Falatoonzadeh, P.; Kuppermann, B.; Kenney, M. Diabetic retinopathy and VEGF. Open Ophthalmol. J. 2013, 7, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Coorey, N.J.; Shen, W.; Chung, S.H.; Zhu, L.; Gillies, M.C. The role of glia in retinal vascular disease. Clin. Exp. Optom. 2012, 95, 266–281. [Google Scholar] [CrossRef]
- Belecky-Adams, T.L.; Chernoff, E.C.; Wilson, J.M.; Dharmarajan, S. Reactive Muller Glia as Potential Retinal Progenitors. In Neural Stem Cells; Bonfanti, L., Ed.; IntechOpen: London, UK, 2013; pp. 73–117. [Google Scholar]
- Yi, S.; Yuan, Y.; Chen, Q.; Wang, X.; Gong, L.; Liu, J.; Gu, X.; Li, S. Regulation of Schwann cell proliferation and migration by miR-1 targeting brain-derived neurotrophic factor after peripheral nerve injury. Sci. Rep. 2016, 6, 29121. [Google Scholar] [CrossRef] [PubMed]
- Minev, I.R.; Moshayedi, P.; Fawcett, J.W.; Lacour, S.P. Interaction of glia with a compliant, microstructured silicone surface. Acta Biomater. 2013, 9, 6936–6942. [Google Scholar] [CrossRef] [PubMed]
- West, E.L.; Pearson, R.A.; Tschernutter, M.; Sowden, J.C.; MacLaren, R.E.; Ali, R.R. Pharmacological disruption of the outer limiting membrane leads to increased retinal integration of transplanted photoreceptor precursors. Exp. Eye Res. 2008, 86, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Salzer, J.L. Axonal regulation of Schwann cell ensheathment and myelination. J. Peripher. Nerv. Syst. 2012, 17, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Wuestefeld, R.; Chen, J.; Meller, K.; Brand-Saberi, B.; Theiss, C. Impact of vegf on astrocytes: Analysis of gap junctional intercellular communication, proliferation, and motility. Glia 2012, 60, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, O.; Quinones-Hinojosa, A. Dose-dependent effect of EGF on migration and differentiation of adult subventricular zone astrocytes. Glia 2010, 58, 975–983. [Google Scholar] [CrossRef]
- Wang, Y.; Teng, H.L.; Gao, Y.; Zhang, F.; Ding, Y.Q.; Huang, Z.H. Brain-derived Neurotrophic Factor Promotes the Migration of Olfactory Ensheathing Cells Through TRPC Channels. Glia 2016, 64, 2154–2165. [Google Scholar] [CrossRef]






| Axis Travel | Parameter |
|---|---|
| X-, Y-, and Z-axes travel (cm) | 81.3 × 40.6 × 59.7 |
| Table working area (cm) | 125 × 23 |
| Table weight (kg) | ~1452 |
| Feed per tooth (fz) | 0.0965 |
| Spindle motor (kw) | 2.2371 |
| Spindle speed (rpm) | 40–600, 300–5000 |
| Cutting feed rate (mm/min) | 1346 |
| Position precision (mm) | 0.127 |
| Position repeatability (mm) | 0.127 |
| Lubrication | Yes |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peña, J.S.; Robles, D.; Zhang, S.; Vazquez, M. A Milled Microdevice to Advance Glia-Mediated Therapies in the Adult Nervous System. Micromachines 2019, 10, 513. https://doi.org/10.3390/mi10080513
Peña JS, Robles D, Zhang S, Vazquez M. A Milled Microdevice to Advance Glia-Mediated Therapies in the Adult Nervous System. Micromachines. 2019; 10(8):513. https://doi.org/10.3390/mi10080513
Chicago/Turabian StylePeña, Juan S., Denise Robles, Stephanie Zhang, and Maribel Vazquez. 2019. "A Milled Microdevice to Advance Glia-Mediated Therapies in the Adult Nervous System" Micromachines 10, no. 8: 513. https://doi.org/10.3390/mi10080513
APA StylePeña, J. S., Robles, D., Zhang, S., & Vazquez, M. (2019). A Milled Microdevice to Advance Glia-Mediated Therapies in the Adult Nervous System. Micromachines, 10(8), 513. https://doi.org/10.3390/mi10080513





