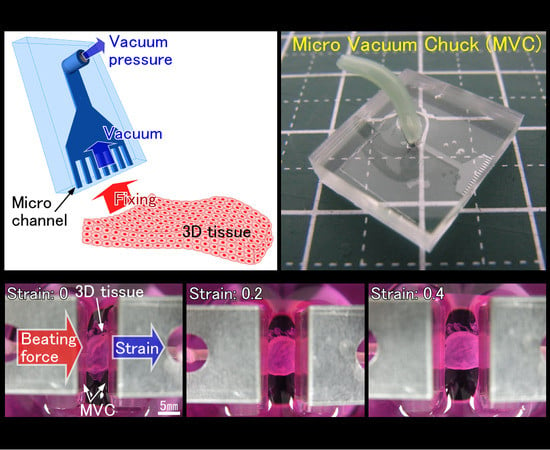
Micro Vacuum Chuck and Tensile Test System for Bio-Mechanical Evaluation of 3D Tissue Constructed of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes (hiPS-CM)
Abstract
1. Introduction
2. Materials and Methods
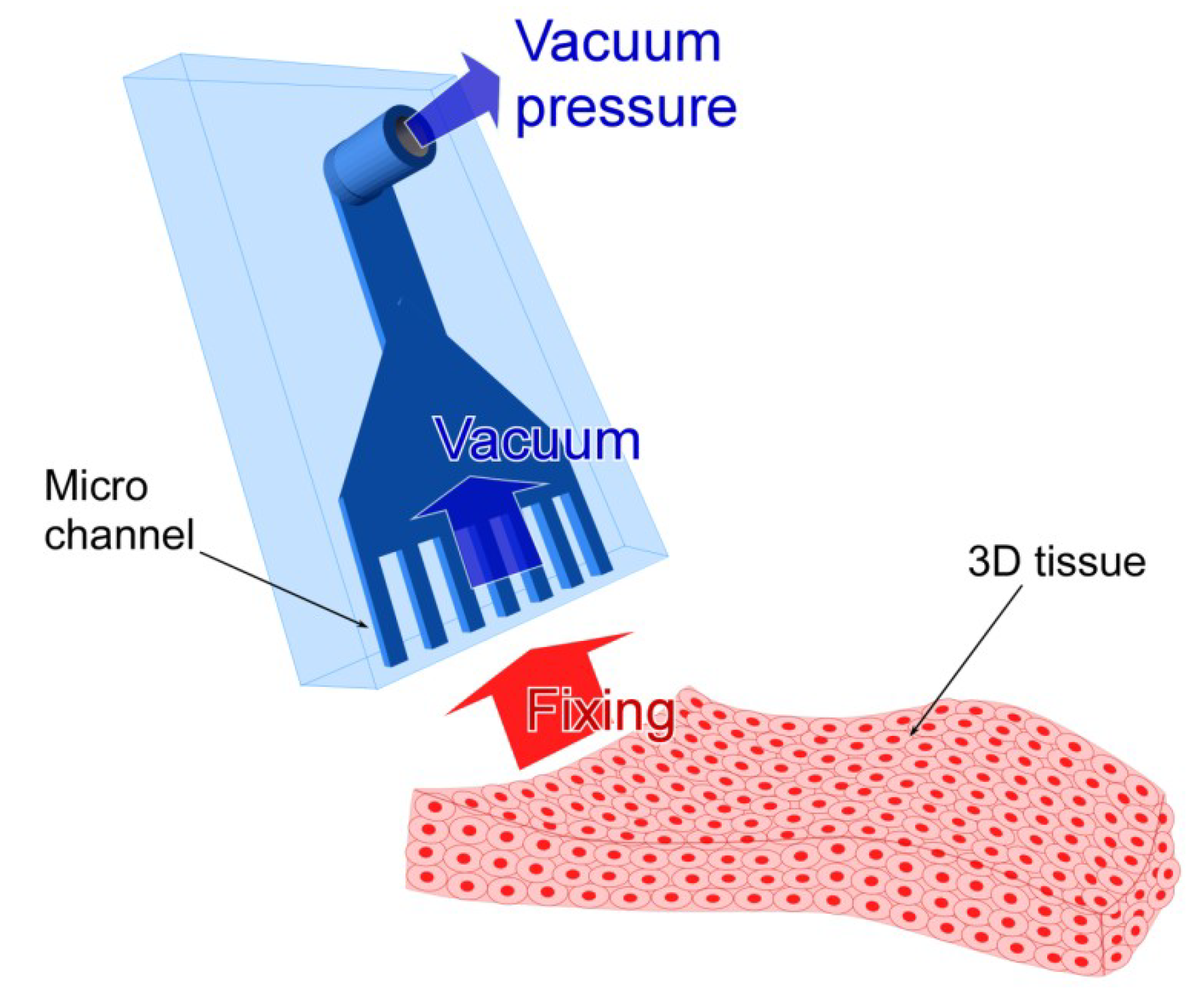
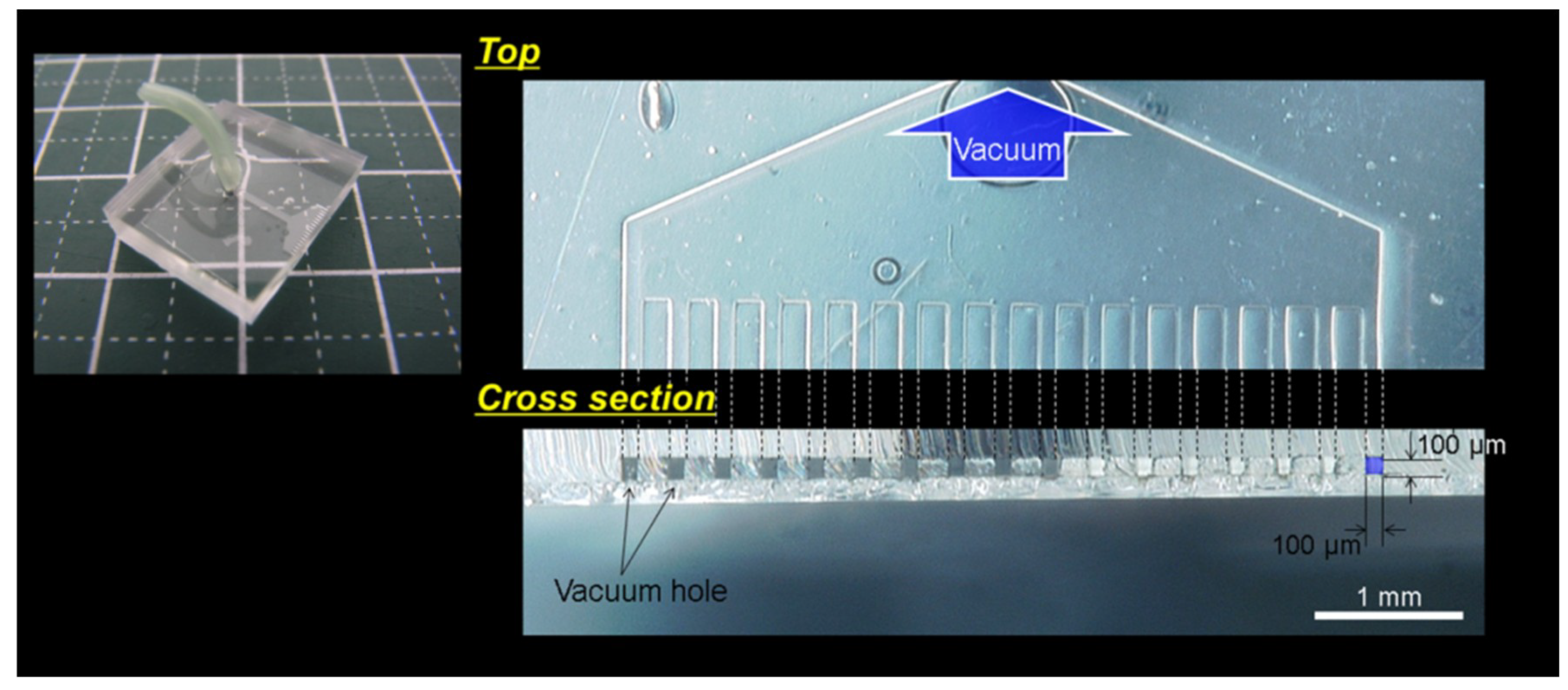
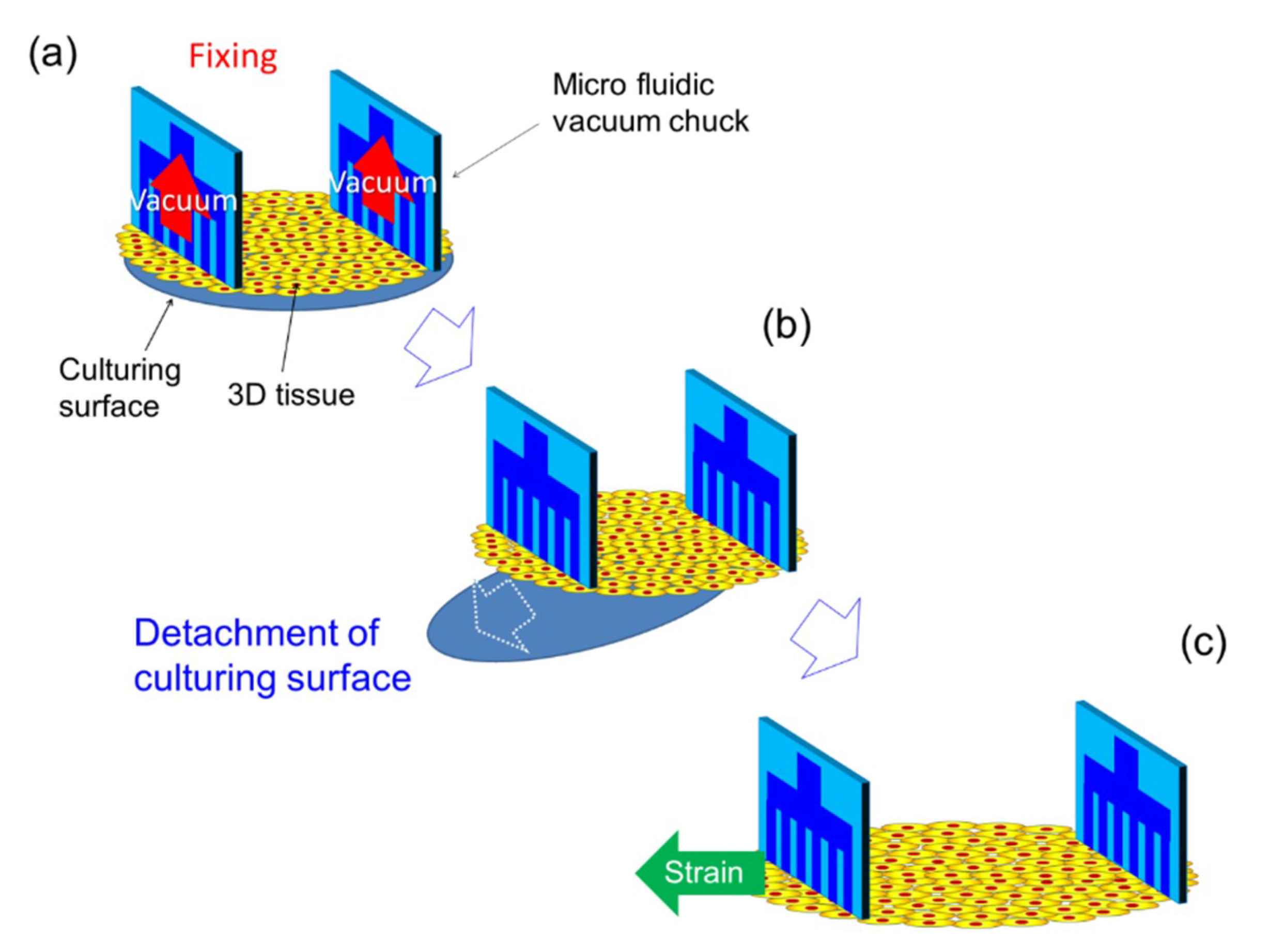
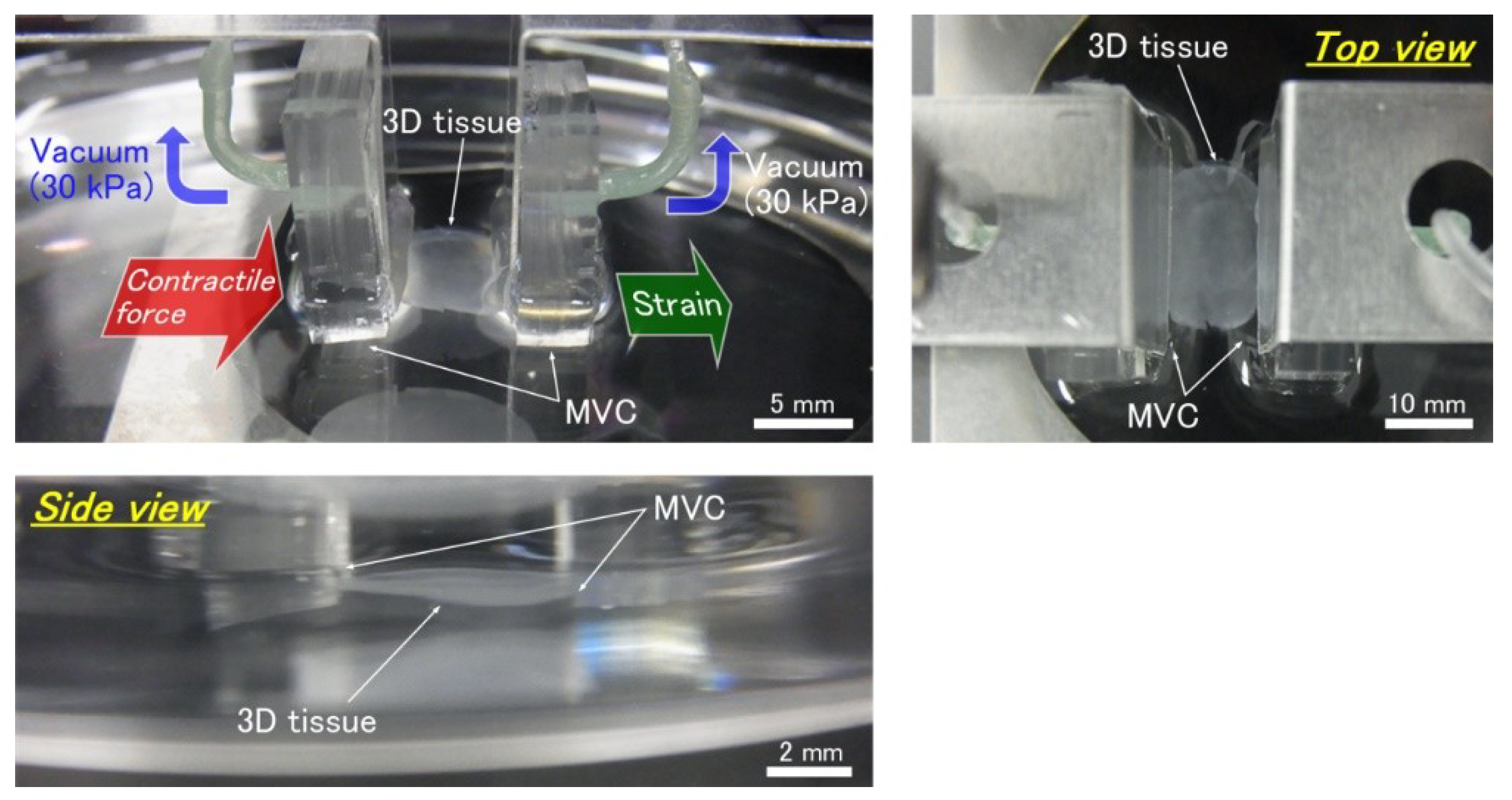
2.1. Design of the Micro Vacuum Chuck (MVC)
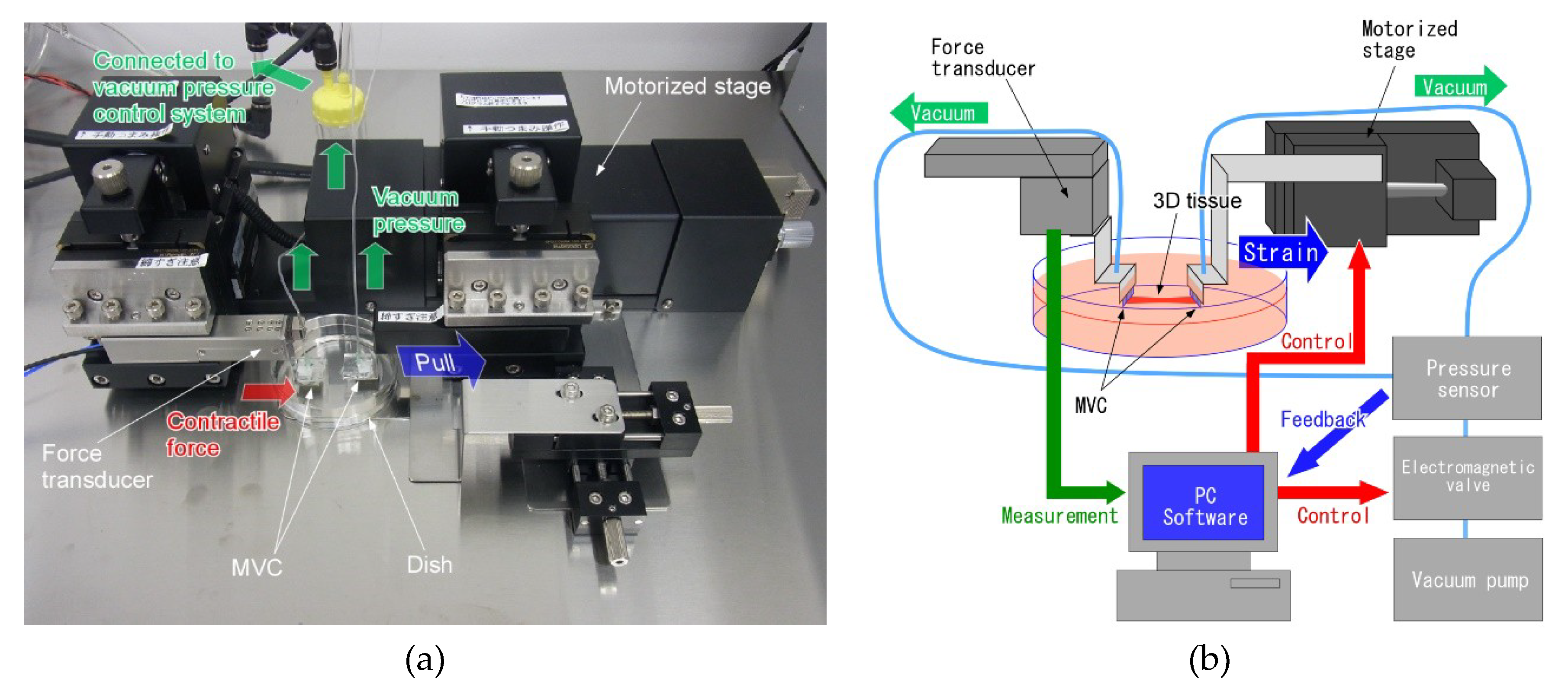
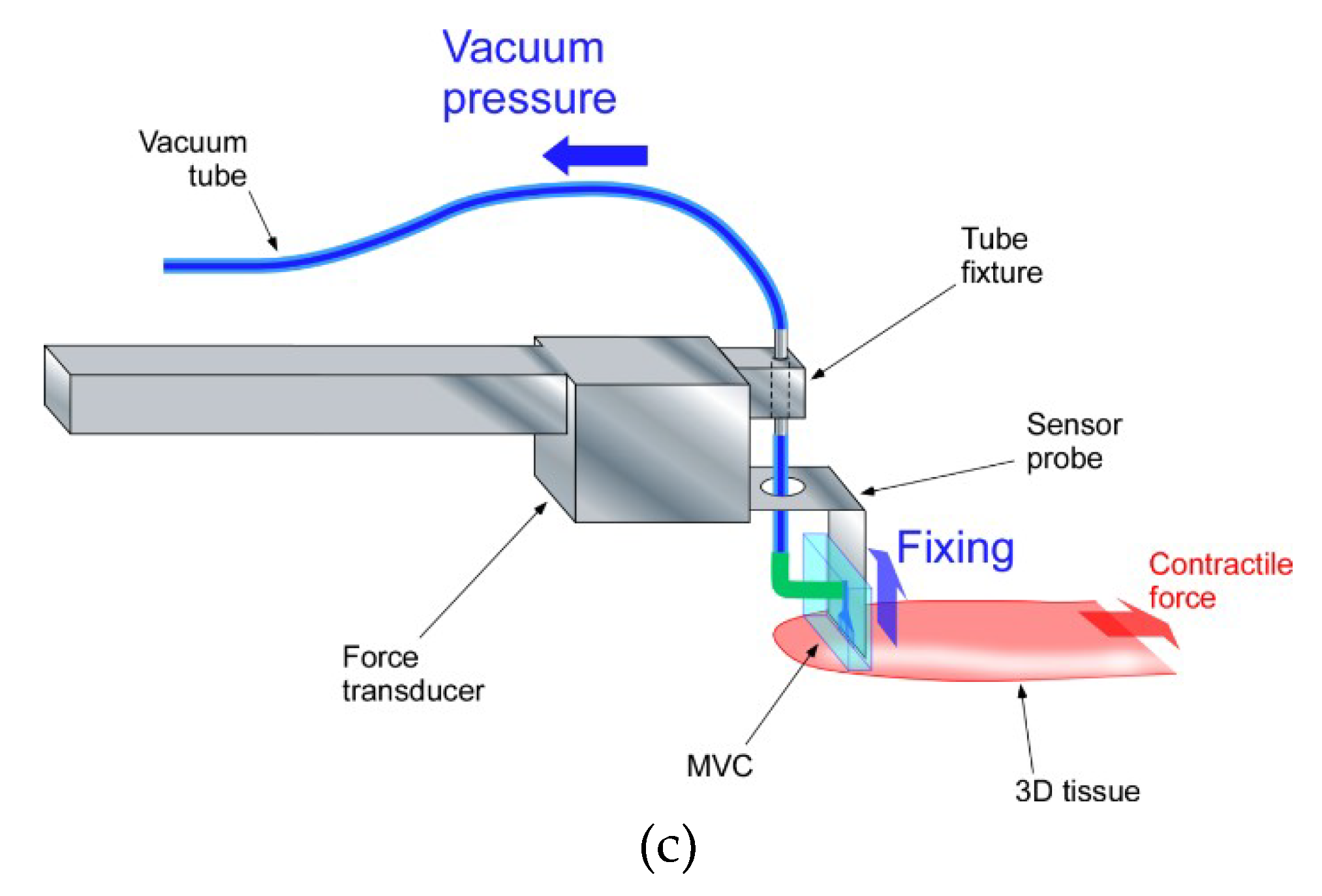
2.2. Design of Tensile Test System
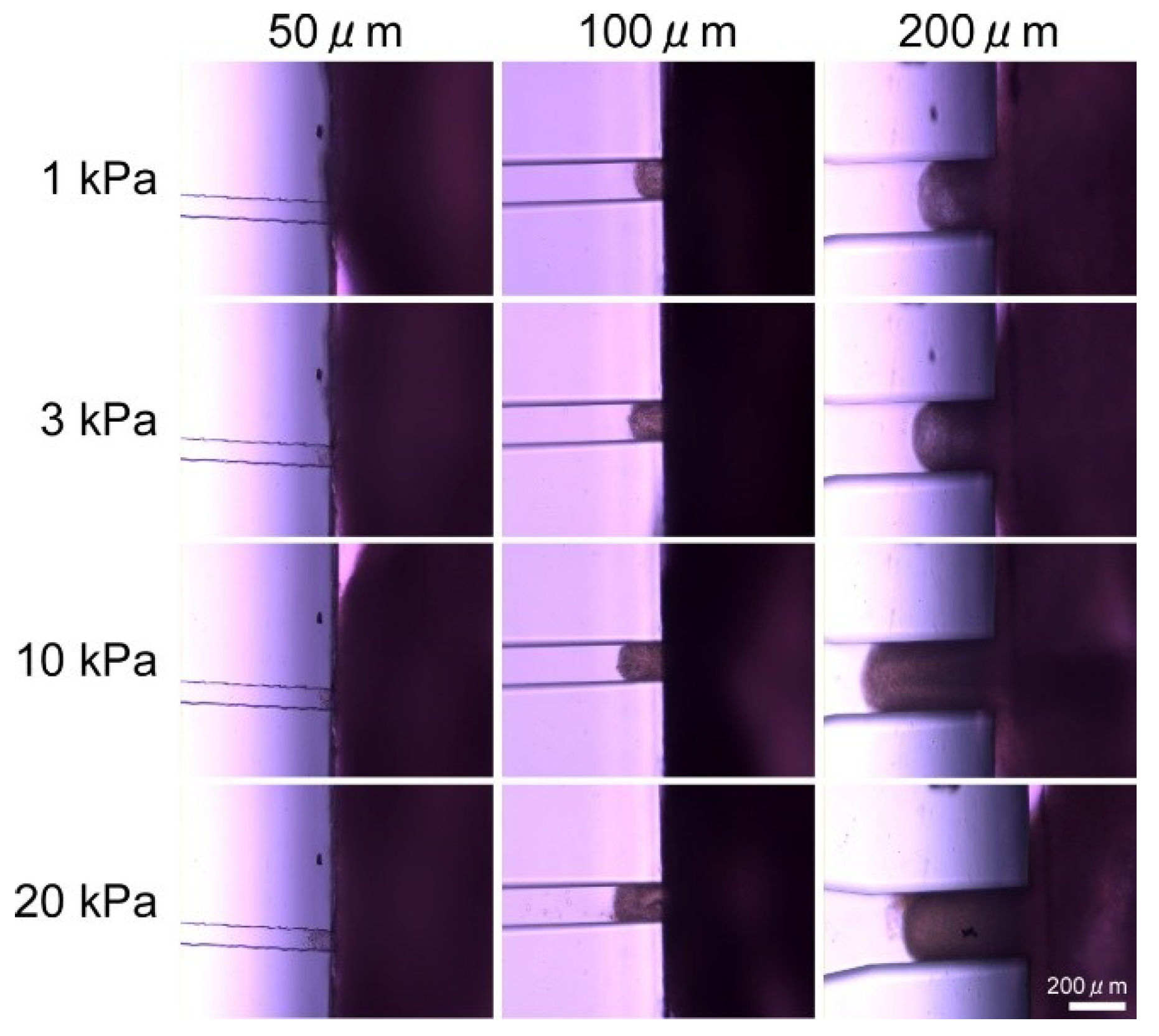
2.3. Confirmation Experiment on the Effect of Vacuum Hole Size and Vacuum Pressure
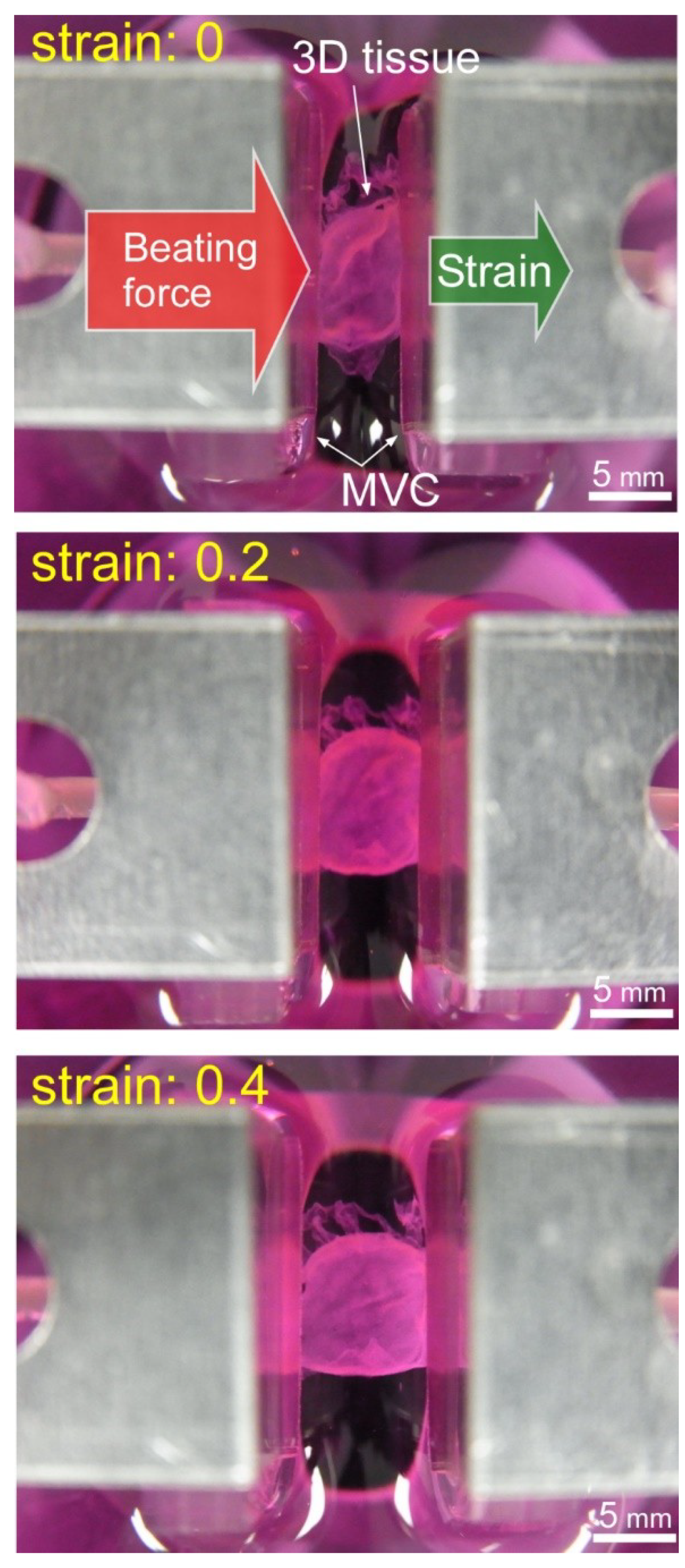
2.4. Tensile Test of 3D Tissue Constructed of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes (hiPS-CM)
3. Results
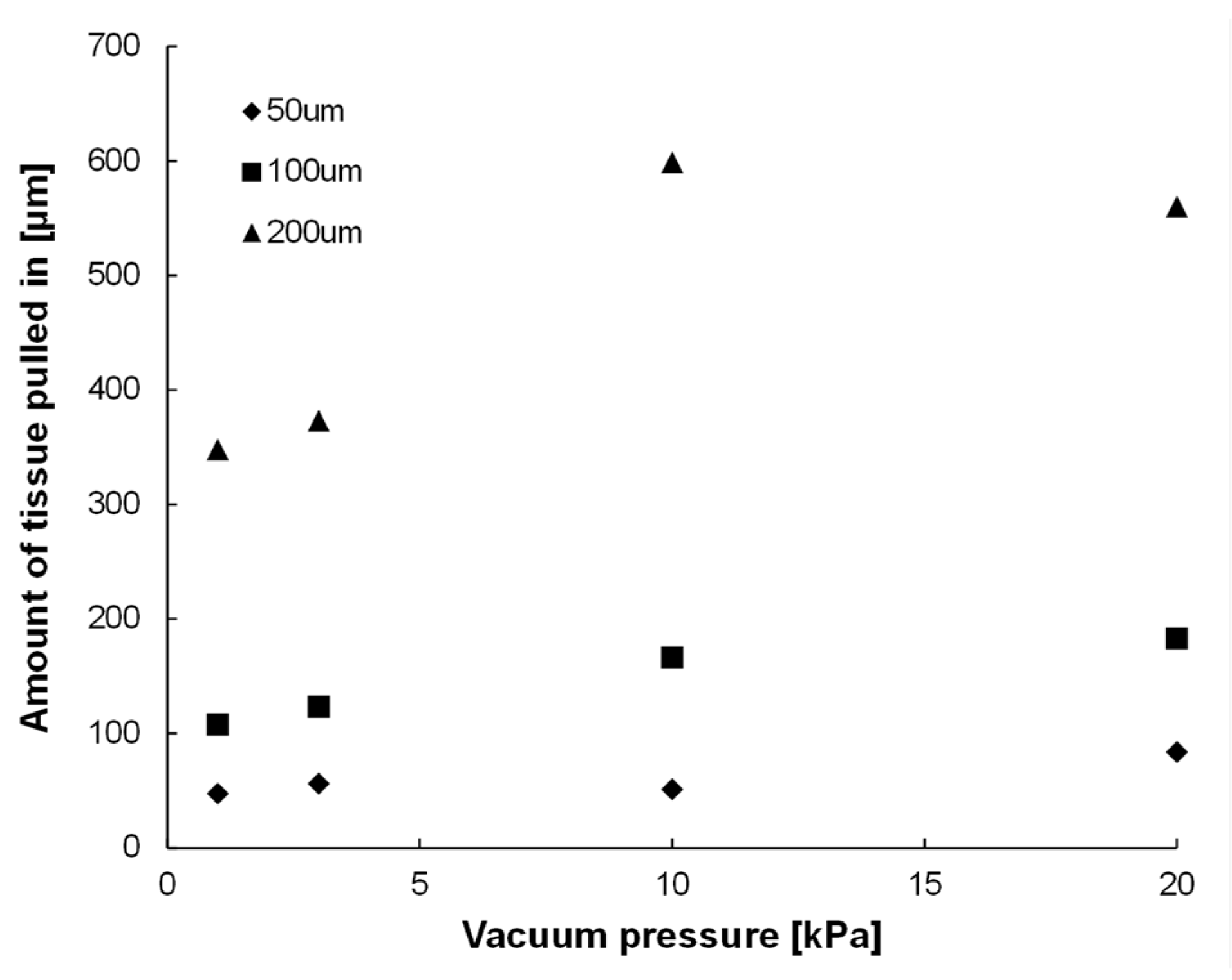
3.1. Results of Confirmation Experiment on Effects of Vacuum Hole Size and Vacuum Pressure
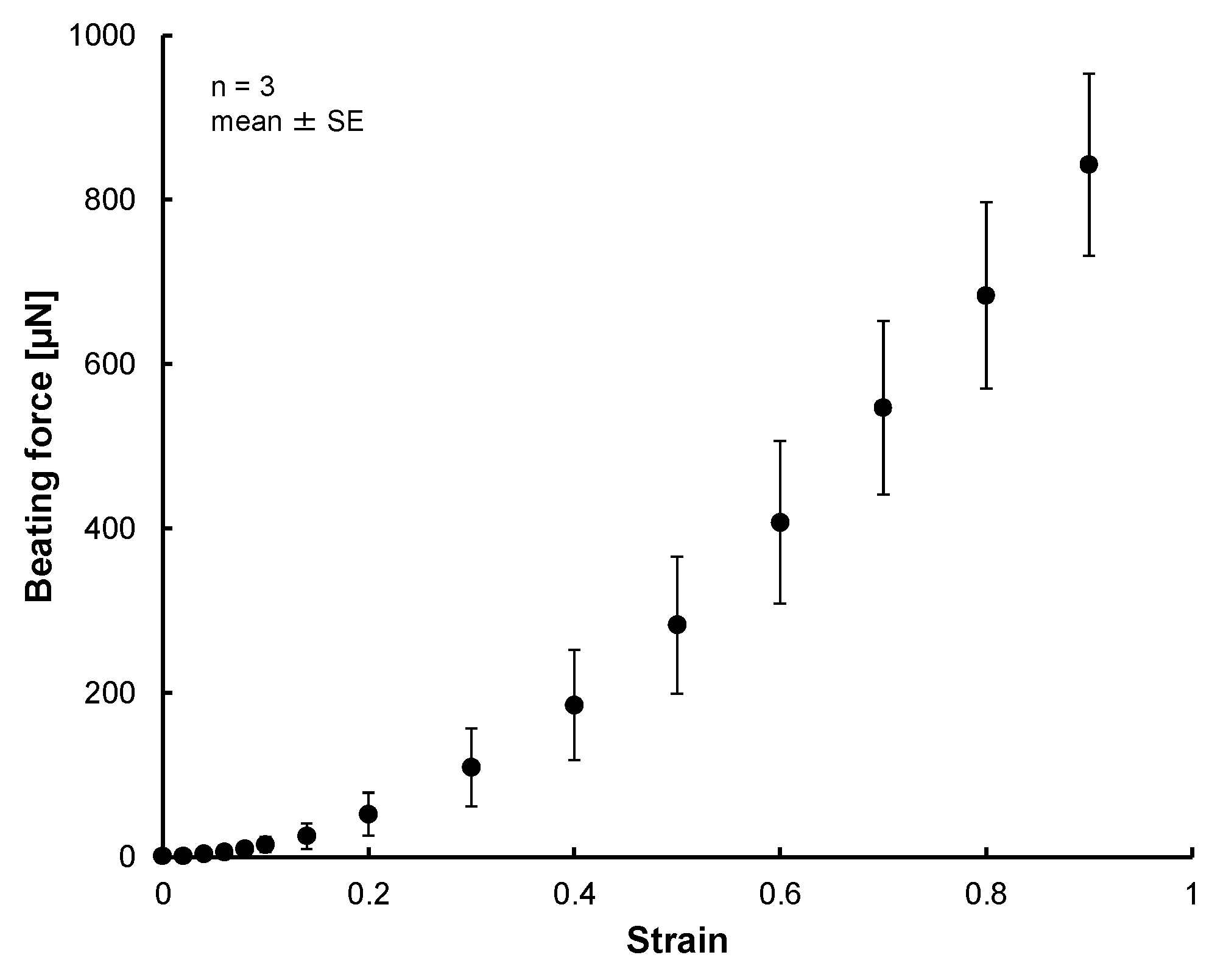
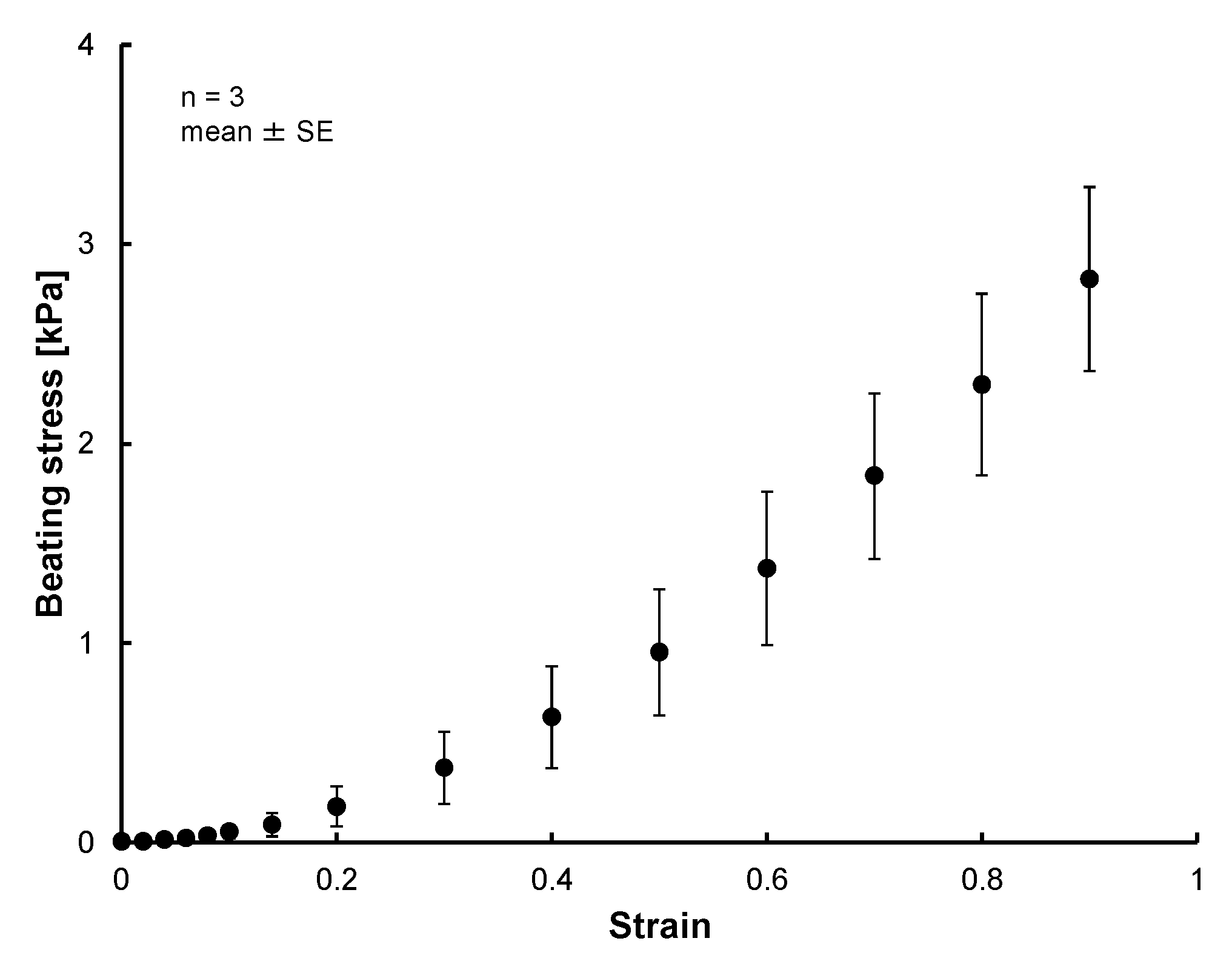
3.2. Results of Tensile Test of 3D Tissue Constructed of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes (hiPS-CM)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Gribova, V.; Liu, C.Y.; Nishiguchi, A.; Matsusaki, M.; Boudou, T.; Picart, C.; Akashi, M. Construction and myogenic differentiation of 3D myoblast tissues fabricated by fibronectin-gelatin nanofilm coating. Biochem. Biophys. Res. Commun. 2016, 47, 515–521. [Google Scholar] [CrossRef]
- Zimmermann, W.H.; Schneiderbanger, K.; Schubert, P.; Didié, M.; Münzel, F.; Heubach, J.F.; Kostin, S.; Neuhuber, W.L.; Eschenhagen, T. Tissue engineering of a differentiated cardiac muscle construct. Circ. Res. 2002, 90, 223–230. [Google Scholar] [CrossRef]
- Eschenhagen, T.; Didié, M.; Münzel, F.; Schubert, P.; Schneiderbanger, K.; Zimmermann, W.H. 3D engineered heart tissue for replacement therapy. Basic Res. Cardiol. 2002, 97, 146–152. [Google Scholar] [CrossRef]
- Zimmermann, W.H.; Melnychenko, I.; Eschenhagen, T. Engineered heart tissue for regeneration of diseased hearts. Biomaterials 2004, 25, 1639–1647. [Google Scholar] [CrossRef]
- Pillekamp, F.; Reppel, M.; Rubenchyk, O.; Pfannkuche, K.; Matzkies, M.; Bloch, W.; Sreeram, N.; Brockmeier, K.; Hescheler, J. Force Measurements of Human Embryonic Stem Cell-Derived Cardiomyocytes in an In Vitro Transplantation Model. Stem Cells 2007, 25, 174–180. [Google Scholar] [CrossRef]
- Xi, J.; Khalil, M.; Shishechian, N.; Hannes, T.; Pfannkuche, K.; Liang, H.; Fatima, A.; Haustein, M.; Suhr, F.; Bloch, W.; et al. Comparison of contractile behavior of native murine ventricular tissue and cardiomyocytes derived from embryonic or induced pluripotent stem cells. FASEB J. 2010, 24, 2739–2751. [Google Scholar] [CrossRef]
- Tulloch, N.L.; Muskheli, V.; Razumova, M.V.; Korte, F.S.; Regnier, M.; Hauch, K.D.; Pabon, L.; Reinecke, H.; Murry, C.E. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ. Res. 2011, 109, 47–59. [Google Scholar] [CrossRef]
- Schaaf, S.; Shibamiya, A.; Mewe, M.; Eder, A.; Stöhr, A.; Hirt, M.N.; Rau, T.; Zimmermann, W.H.; Conradi, L.; Eschenhagen, T.; et al. Human Engineered Heart Tissue as a Versatile Tool in Basic Research and Preclinical Toxicology. PLoS ONE 2011, 6, e26397. [Google Scholar] [CrossRef]
- Masumoto, H.; Nakane, T.; Tinney, J.P.; Yuan, F.; Ye, F.; Kowalski, W.J.; Minakata, K.; Sakata, R.; Yamashita, J.K.; Keller, B.B. The myocardial regenerative potential of three-dimensional engineered cardiac tissues composed of multiple human iPS cell-derived cardiovascular cell lineages. Sci. Rep. 2016, 6, 29933. [Google Scholar] [CrossRef]
- Jackman, C.P.; Carlson, A.L.; Bursac, N. Dynamic culture yields engineered myocardium with near-adult functional output. Biomaterials 2016, 111, 66–79. [Google Scholar] [CrossRef]
- Ruan, J.L.; Tulloch, N.L.; Razumova, M.V.; Saiget, M.; Muskheli, V.; Pabon, L.; Reinecke, H.; Regnier, M.; Murry, C.E. Mechanical Stress Conditioning and Electrical Stimulation Promote Contractility and Force Maturation of Induced Pluripotent Stem Cell-Derived Human Cardiac Tissue. Circulation 2016, 134, 1557–1567. [Google Scholar] [CrossRef]
- Nakane, T.; Masumoto, H.; Tinney, J.P.; Yuan, F.; Kowalski, W.J.; Ye, F.; LeBlanc, A.J.; Sakata, R.; Yamashita, J.K.; Keller, B.B. Impact of Cell Composition and Geometry on Human Induced Pluripotent Stem Cells-Derived Engineered Cardiac Tissue. Sci. Rep. 2017, 7, 45641. [Google Scholar] [CrossRef]
- Tiburcy, M.; Hudson, J.E.; Balfanz, P.; Schlick, S.; Meyer, T.; Chang Liao, M.L.; Levent, E.; Raad, F.; Zeidler, S.; Wingender, E.; et al. Defined Engineered Human Myocardium with Advanced Maturation for Applications in Heart Failure Modeling and Repair. Circulation 2017, 135, 1832–1847. [Google Scholar] [CrossRef]
- Sasaki, D.; Matsuura, K.; Seta, H.; Haraguchi, Y.; Okano, T.; Shimizu, T. Contractile force measurement of human induced pluripotent stem cell-derived cardiac cell sheet-tissue. PLoS ONE 2018, 13, e0198026. [Google Scholar] [CrossRef]
- Soliman, S.; Pagliari, S.; Rinaldi, A.; Forte, G.; Fiaccavento, R.; Pagliari, F.; Franzese, O.; Minieri, M.; Di Nardo, P.; Licoccia, S.; et al. Multiscale three-dimensional scaffolds for soft tissue engineering via multimodal electrospinning. Acta Biomater. 2010, 6, 1227–1237. [Google Scholar] [CrossRef]
- Liu, X.; Shen, H.; Song, S.; Chen, W.; Zhang, Z. Accelerated Biomineralization of Graphene Oxide—Incorporated Cellulose Acetate Nanofibrous Scaffolds for Mesenchymal Stem Cell Osteogenesis. Colloids Surf. B Biointerfaces 2017, 159, 251–258. [Google Scholar] [CrossRef]
- Janjanin, S.; Li, W.J.; Morgan, M.T.; Shanti, R.M.; Tuan, R.S. Mold-shaped, nanofiber scaffold-based cartilage engineering using human mesenchymal stem cells and bioreactor. J. Surg. Res. 2008, 149, 47–56. [Google Scholar] [CrossRef]
- Bhumiratana, S.; Eton, R.E.; Oungoulian, S.R.; Wan, L.Q.; Ateshian, G.A.; Vunjak-Novakovic, G. Large, stratified, and mechanically functional human cartilage grown in vitro by mesenchymal condensation. Proc. Natl. Acad. Sci. USA 2014, 111, 6940–6945. [Google Scholar] [CrossRef]
- Barber, J.G.; Handorf, A.M.; Allee, T.J.; Li, W.J. Braided nanofibrous scaffold for tendon and ligament tissue engineering. Tissue Eng. Part A 2013, 19, 1265–1274. [Google Scholar] [CrossRef]
- Chainani, A.; Hippensteel, K.J.; Kishan, A.; Garrigues, N.W.; Ruch, D.S.; Guilak, F.; Little, D. Multilayered Electrospun Scaffolds for Tendon Tissue Engineering. Tissue Eng. Part A 2013, 19, 2594–2604. [Google Scholar] [CrossRef]
- Orr, S.B.; Chainani, A.; Hippensteel, K.J.; Kishan, A.; Gilchrist, C.; Garrigues, N.W.; Ruch, D.S.; Guilak, F.; Little, D. Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater. 2015, 24, 117–126. [Google Scholar] [CrossRef]
- Tosun, Z.; Villegas-Montoya, C.; McFetridge, P.S. The influence of early-phase remodeling events on the biomechanical properties of engineered vascular tissues. J. Vasc. Surg. 2011, 54, 1451–1460. [Google Scholar] [CrossRef][Green Version]
- Arasteh, S.; Kazemnejad, S.; Khanjani, S.; Heidari-Vala, H.; Akhondi, M.M.; Mobini, S. Fabrication and characterization of nano-fibrous bilayer composite for skin regeneration application. Methods 2016, 99, 3–12. [Google Scholar] [CrossRef]
- Jayarama Reddy, V.; Radhakrishnan, S.; Ravichandran, R.; Mukherjee, S.; Balamurugan, R.; Sundarrajan, S.; Ramakrishna, S. Nanofibrous structured biomimetic strategies for skin tissue regeneration. Wound Repair Regen. 2013, 21, 1–16. [Google Scholar] [CrossRef]
- Amano, Y.; Nishiguchi, A.; Matsusaki, M.; Iseoka, H.; Miyagawa, S.; Sawa, Y.; Seo, M.; Yamaguchi, T.; Akashi, M. Development of vascularized iPSC derived 3D-cardiomyocyte tissues by filtration Layer-by-Layer technique and their application for pharmaceutical assays. Acta Biomater. 2016, 33, 110–121. [Google Scholar] [CrossRef]
- Matsusaki, M.; Sakaue, K.; Kadowaki, K.; Akashi, M. Three-dimensional human tissue chips fabricated by rapid and automatic inkjet cell printing. Adv. Healthc. Mater. 2013, 2, 534–539. [Google Scholar] [CrossRef]
- Yoshida, H.; Matsusaki, M.; Akashi, M. Multilayered blood capillary analogs in biodegradable hydrogels for in vitro drug permeability assays. Adv. Funct. Mater. 2013, 23, 1736–1742. [Google Scholar] [CrossRef]
- Nishiguchi, A.; Yoshida, H.; Matsusaki, M.; Akashi, M. Rapid construction of three-dimensional multilayered tissues with endothelial tube network by the cell-accumulation technique. Adv. Mater. 2011, 23, 3506–3510. [Google Scholar] [CrossRef]
- Matsusaki, M.; Case, C.P.; Akashi, M. Three-dimensional cell culture technique and pathophysiology. Adv. Drug Deliv. Rev. 2014, 74, 95–103. [Google Scholar] [CrossRef]
- Takeda, M.; Miyagawa, S.; Fukushima, S.; Saito, A.; Ito, E.; Harada, A.; Matsuura, R.; Iseoka, H.; Sougawa, N.; Mochizuki-Oda, N.; et al. Development of In Vitro Drug-Induced Cardiotoxicity Assay by Using Three-Dimensional Cardiac Tissues Derived from Human Induced Pluripotent Stem Cells. Tissue Eng. Part C Methods 2017, 24, 56–67. [Google Scholar] [CrossRef]
- Eschenhagen, T.; Fink, C.; Remmers, U.; Scholz, H.; Wattchow, J.; Weil, J.; Zimmermann, W.; Dohmen, H.H.; Schäfer, H.; Bishopric, N.; et al. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: A new heart muscle model system. FASEB J. 1997, 11, 683–694. [Google Scholar] [CrossRef]
- Vandenburgh, H.; Shansky, J.; Benesch-Lee, F.; Barbata, V.; Reid, J.; Thorrez, L.; Valentini, R.; Crawford, G. Drug-screening platform based on the contractility of tissue-engineered muscle. Muscle Nerve 2008, 37, 438–447. [Google Scholar] [CrossRef]
- Hansen, A.; Eder, A.; Bönstrup, M.; Flato, M.; Mewe, M.; Schaaf, S.; Aksehirlioglu, B.; Schwoerer, A.P.; Uebeler, J.; Eschenhagen, T. Development of a drug screening platform based on engineered heart tissue. Circ. Res. 2010, 107, 35–44. [Google Scholar] [CrossRef]
- Zhang, D.; Shadrin, I.Y.; Lam, J.; Xian, H.Q.; Snodgrass, H.R.; Bursac, N. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials 2013, 34, 5813–5820. [Google Scholar] [CrossRef]
- Turnbull, I.C.; Karakikes, I.; Serrao, G.W.; Backeris, P.; Lee, J.J.; Xie, C.; Senyei, G.; Gordon, R.E.; Li, R.A.; Akar, F.G.; et al. Advancing functional engineered cardiac tissues toward a preclinical model of human myocardium. FASEB J. 2014, 28, 644–654. [Google Scholar] [CrossRef]
- Huebsch, N.; Loskill, P.; Deveshwar, N.; Spencer, C.I.; Judge, L.M.; Mandegar, M.A.; Fox, C.B.; Mohamed, T.M.; Ma, Z.; Mathur, A.; et al. Miniaturized iPS-Cell-Derived Cardiac Muscles for Physiologically Relevant Drug Response Analyses. Sci. Rep. 2016, 6, 24726. [Google Scholar] [CrossRef]
- Pati, F.; Gantelius, J.; Svahn, H.A. 3D Bioprinting of Tissue/Organ Models. Angew. Chem. Int. Ed. 2016, 55, 4650–4665. [Google Scholar] [CrossRef]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef]
- Knowlton, S.; Onal, S.; Yu, C.H.; Zhao, J.J.; Tasoglu, S. Bioprinting for cancer research. Trends Biotechnol. 2015, 33, 504–513. [Google Scholar] [CrossRef]
- Ki-Hwan Nam, K.H.; Smith, A.S.; Lone, S.; Kwon, S.; Kim, D.H. Biomimetic three-dimensional tissue models for advanced high-throughput drug screening. J. Lab. Autom. 2015, 20, 201–215. [Google Scholar]
- Mironov, V.; Trusk, T.; Kasyanov, V.; Little, S.; Swaja, R.; Markwald, R. Biofabrication: A 21st century manufacturing paradigm. Biofabrication 2009, 1, 022001. [Google Scholar] [CrossRef]
- Fink, C.; Ergün, S.; Kralisch, D.; Remmers, U.; Weil, J.; Eschenhagen, T. Chronic stretch of engineered heart tissue induces hypertrophy and functional improvement. FASEB J. 2000, 14, 669–679. [Google Scholar] [CrossRef]
- Zimmermann, W.H.; Fink, C.; Kralisch, D.; Remmers, U.; Weil, J.; Eschenhagen, T. Three-dimensional engineered heart tissue from neonatal rat cardiac myocytes. Biotechnol. Bioeng. 2000, 68, 106–114. [Google Scholar] [CrossRef]
- Hinds, S.; Bian, W.; Dennis, R.G.; Bursac, N. The role of extracellular matrix composition in structure and function of bioengineered skeletal muscle. Biomaterials 2011, 32, 575–583. [Google Scholar] [CrossRef]
- Tchao, J.; Kim, J.J.; Lin, B.; Salama, G.; Lo, C.W.; Yang, L.; Tobita, K. Engineered Human Muscle Tissue from Skeletal Muscle Derived Stem Cells and Induced Pluripotent Stem Cell Derived Cardiac Cells. Int. J. Tissue Eng. 2013, 98762. [Google Scholar] [CrossRef]
- Horiguchi, H.; Imagawa, K.; Hoshino, T.; Akiyama, Y.; Morishima, K. Fabrication and Evaluation of Reconstructed Cardiac Tissue and Its Application to Bio-actuated Microdevices. IEEE Trans. Nanobiosci. 2009, 8, 349–355. [Google Scholar] [CrossRef]
- Akiyama, Y.; Terada, R.; Hashimoto, M.; Hoshino, T.; Furukawa, Y.; Morishima, K. Rod-shaped Tissue Engineered Skeletal Muscle with Artificial Anchors to Utilize as a Bio-Actuator. J. Biomech. Sci. Eng. 2010, 5, 236–244. [Google Scholar] [CrossRef][Green Version]
- Hoshino, T.; Imagawa, K.; Akiyama, Y.; Morishima, K. Cardiomyocyte-driven gel network for bio mechano-informatic wet robotics. Biomed. Microdevices 2012, 14, 969–977. [Google Scholar] [CrossRef]
- Kabumoto, K.; Hoshino, T.; Akiyama, Y.; Morishima, K. Voluntary Movement Controlled by the Surface EMG Signal for Tissue-Engineered Skeletal Muscle on a Gripping Tool. Tissue Eng. Part A 2013, 19, 1695–1703. [Google Scholar] [CrossRef]
- Cvetkovic, C.; Raman, R.; Chan, V.; Williams, B.J.; Tolish, M.; Bajaj, P.; Sakar, M.S.; Asada, H.H.; Saif, M.T.; Bashir, R. Three-dimensionally printed biological machines powered by skeletal muscle. Proc. Natl. Acad. Sci. USA 2014, 111, 10125–10130. [Google Scholar] [CrossRef]
- Catts, O.; Zurr, I. Growing for different ends. Int. J. Biochem. Cell Biol. 2014, 56, 20–29. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Bhat, H.F. Animal-free Meat Biofabrication. Am. J. Food Technol. 2011, 6, 441–459. [Google Scholar] [CrossRef]
- Arshad, M.S.; Javed, M.; Sohaib, M.; Saeed, F.; Imran, A.; Amjad, Z. Tissue engineering approaches to develop cultured meat from cells: A mini review. Cogent Food Agric. 2017, 3, 1320814. [Google Scholar] [CrossRef]
- McHugh, S. Real artificial: Tissue-cultured Meat, Genetically Modified Farm Animals, and Fictions. Configurations 2010, 18, 181–197. [Google Scholar] [CrossRef]
- Catts, O.; Zurr, I. Semi-Living Art. In Signs of Life: Bio Art and Beyond, 1st ed.; Kac, E., Ed.; The MIT Press: Cambridge, MA, USA, 1932; pp. 231–247. [Google Scholar]
- Catts, O.; Zurr, I. Growing Semi-Living Sculptures: The Tissue Culture & Art Project. Leonardo 2002, 35, 365–370. [Google Scholar]
- Rees, J. Exhibition: Cultures in the capital. Nature 2008, 451, 891. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Davis, J.; Coskun, A.F.; Church, G.M.; Yun, S.H. Bioart. Trends Biotechnol. 2015, 33, 724–734. [Google Scholar] [CrossRef]
- Vaage, N.S. What Ethics for Bioart? Nanoethics 2016, 10, 87–104. [Google Scholar] [CrossRef]
- Stelarc. Extra Ear: Ear on the Arm Blender. Diacritics 2006, 36, 117–119. [Google Scholar]
- Art Imitates Life: Artist Bioengineers Replica of van Gogh’s Ear. CNN: Carina Storrs. 2015. Available online: https://edition.cnn.com/2015/11/13/health/van-gogh-ear-art-science/index.html (accessed on 11 July 2018).
- Hayashi, R.; Yamato, M.; Takayanagi, H.; Oie, Y.; Kubota, A.; Hori, Y.; Okano, T.; Nishida, K. Validation system of tissue-engineered epithelial cell sheets for corneal regenerative medicine. Tissue Eng. Part C Methods 2009, 16, 553–560. [Google Scholar] [CrossRef]
- Even-Ram, S.; Artym, V.; Yamada, K.M. Matrix Control of Stem Cell Fate. Cell 2006, 126, 645–647. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Uesugi, K.; Akiyama, Y.; Yamato, M.; Okano, T.; Hoshino, T.; Morishima, K. Micro handling tool of cell sheet for measurement of adhesion force. In Proceedings of the µTAS 2009, Jeju, Korea, 27–31 October 2009; pp. 1844–1846. [Google Scholar]
- Uesugi, K.; Akiyama, Y.; Yamato, M.; Okano, T.; Hoshino, T.; Morishima, K. Development of cell-sheet handling tool for measurement of cell sheet adhesion force. In Proceedings of the MHS 2009, Nagoya, Japan, 8–11 November 2009; pp. 614–619. [Google Scholar]
- Uesugi, K.; Akiyama, Y.; Hoshino, T.; Akiyama, Y.; Yamato, M.; Okano, T.; Morishima, K. Measuring adhesion force of a cell sheet by the ninety-degree peel test using a multi hook type fixture. J. Biomech. Sci. Eng. 2013, 8, 129–138. [Google Scholar] [CrossRef]
- Uesugi, K.; Akiyama, Y.; Hoshino, T.; Akiyama, Y.; Yamato, M.; Okano, T.; Morishima, K. Measuring mechanical properties of cell sheet by tensile test using self-attachable fixture. J. Robot. Mechatron. 2013, 25, 603–610. [Google Scholar] [CrossRef]
- Uesugi, K.; Akiyama, Y.; Hoshino, T.; Akiyama, Y.; Yamato, M.; Okano, T.; Morishima, K. Measurement system for biomechanical properties of cell sheet. In Proceedings of the IROS 2013, Tokyo, Japan, 3–7 November 2013; pp. 1010–1015. [Google Scholar]
- Uesugi, K.; Nishiguchi, A.; Matsusaki, M.; Akashi, M.; Morishima, K. Evaluation system for mechanobiology of three-dimensional tissue multilayered in vitro. In Proceedings of the MHS 2015, Nagoya, Japan, 23–25 November 2015; pp. 336–338. [Google Scholar]
- Uesugi, K.; Fukumoto, K.; Shima, F.; Miyagawa, S.; Sawa, Y.; Akashi, M.; Morishima, K. Micro fluidic vacuum chuck system for handling of regenerative three dimensional tissue. In Proceedings of the µTAS 2016, Dublin, Ireland, 9–13 October 2016; pp. 671–672. [Google Scholar]
- Allen, D.G.; Kentish, J.C. The cellular basis of the length-tension relation in cardiac muscle. J. Mol. Cell. Cardiol. 1985, 17, 821–840. [Google Scholar] [CrossRef]
- Fuchs, F.; Smith, S.H. Calcium, cross-bridges, and the Frank-Starling relationship. News Physiol. Sci. 2001, 16, 5–10. [Google Scholar] [CrossRef]
- Moss, R.L.; Fitzsimons, D.P. Frank-Starling relationship: Long on importance, short on mechanism. Circ. Res. 2002, 90, 11–13. [Google Scholar] [CrossRef]
- Shiels, H.A.; White, E. The Frank-Starling mechanism in vertebrate cardiac myocytes. J. Exp. Biol. 2008, 211, 2005–2013. [Google Scholar] [CrossRef]
- Hasenfuss, G.; Mulieri, L.A.; Blanchard, E.M.; Holubarsch, C.; Leavitt, B.J.; Ittleman, F.; Alpert, N.R. Energetics of isometric force development in control and volume-overload human myocardium. Comparison with animal species. Circ. Res. 1991, 68, 836–846. [Google Scholar] [CrossRef]
- van der Velden, J.; Klein, L.J.; van der Bijl, M.; Huybregts, M.A.; Stooker, W.; Witkop, J.; Eijsman, L.; Visser, C.A.; Visser, F.C.; Stienen, G.J. Force production in mechanically isolated cardiac myocytes from human ventricular muscle tissue. Cardiovasc. Res. 1998, 38, 414–423. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uesugi, K.; Shima, F.; Fukumoto, K.; Hiura, A.; Tsukamoto, Y.; Miyagawa, S.; Sawa, Y.; Akagi, T.; Akashi, M.; Morishima, K. Micro Vacuum Chuck and Tensile Test System for Bio-Mechanical Evaluation of 3D Tissue Constructed of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes (hiPS-CM). Micromachines 2019, 10, 487. https://doi.org/10.3390/mi10070487
Uesugi K, Shima F, Fukumoto K, Hiura A, Tsukamoto Y, Miyagawa S, Sawa Y, Akagi T, Akashi M, Morishima K. Micro Vacuum Chuck and Tensile Test System for Bio-Mechanical Evaluation of 3D Tissue Constructed of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes (hiPS-CM). Micromachines. 2019; 10(7):487. https://doi.org/10.3390/mi10070487
Chicago/Turabian StyleUesugi, Kaoru, Fumiaki Shima, Ken Fukumoto, Ayami Hiura, Yoshinari Tsukamoto, Shigeru Miyagawa, Yoshiki Sawa, Takami Akagi, Mitsuru Akashi, and Keisuke Morishima. 2019. "Micro Vacuum Chuck and Tensile Test System for Bio-Mechanical Evaluation of 3D Tissue Constructed of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes (hiPS-CM)" Micromachines 10, no. 7: 487. https://doi.org/10.3390/mi10070487
APA StyleUesugi, K., Shima, F., Fukumoto, K., Hiura, A., Tsukamoto, Y., Miyagawa, S., Sawa, Y., Akagi, T., Akashi, M., & Morishima, K. (2019). Micro Vacuum Chuck and Tensile Test System for Bio-Mechanical Evaluation of 3D Tissue Constructed of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes (hiPS-CM). Micromachines, 10(7), 487. https://doi.org/10.3390/mi10070487