Development of a Capsule Robot for Exploring the Colon
Abstract
:1. Introduction
2. Design Overview
2.1. Design Considerations
- The colon diameter ranges from 25 mm to 60 mm, therefore the body diameter of the ILCR should be less than 25 mm to reduce movement resistance, and the maximum expanding diameter of the EM should exceed 60 mm to ensure that the expanding and parking abilities can be implemented effectively.
- The colon is suspended by connecting to an abdominal wall with a soft mesentery and the colon tissue is viscoelastic, which are both adverse to the ILCR movement. To impair this adverseness, the design parameters of the ILCR must be carefully selected, and the design rule of maximizing the periodic stroke and minimizing the body length has been proven to be effective.
- The colon has right, left, and sigmoid flexures. To pass these flexures, the body length of the ILCR should not exceed 50 mm [11], or if exceeded, the body should be flexible.
- The soft colon tissue is easily damaged and the contact safety must be ensured when the ILCR explores the colon.
- The ILCR should be tether-less because a tether can cause friction and may abrade the colon tissue, therefore the ILCR is best to be wirelessly controlled and powered.
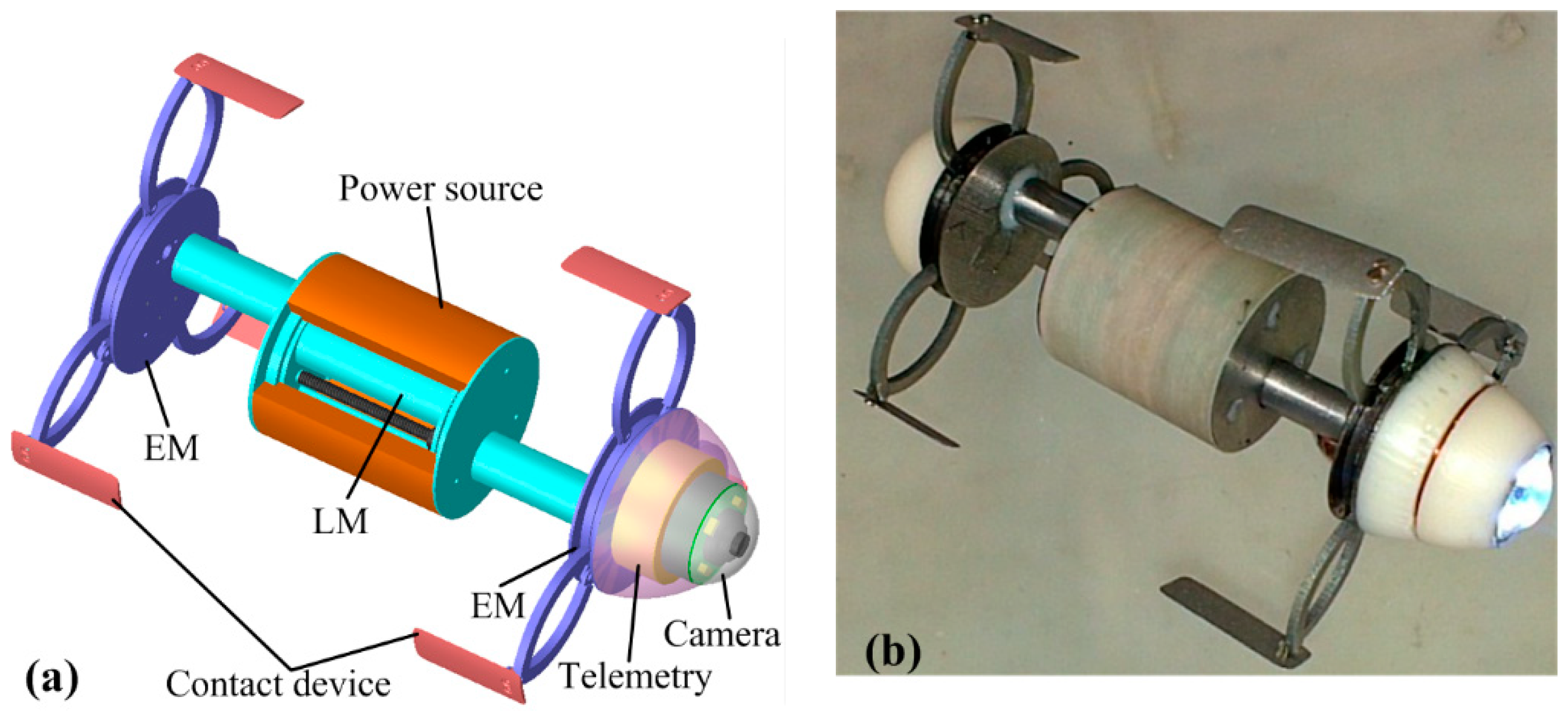
2.2. Overall Design of the Enhanced Inchworm-Like Capsule Robot (ILCR)
3. Design of the Enhanced ILCR
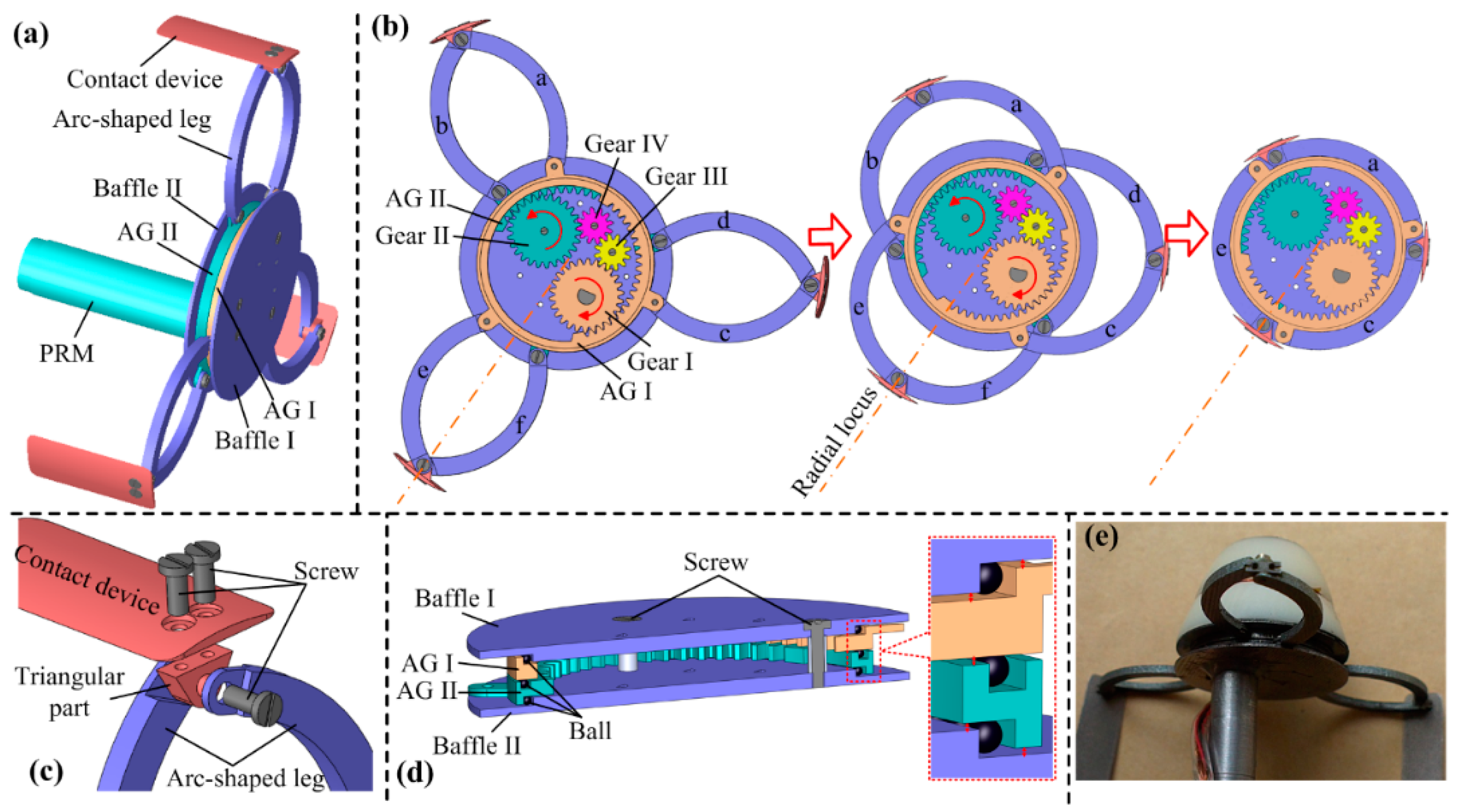
3.1. Design of the Expanding Mechanism (EM)
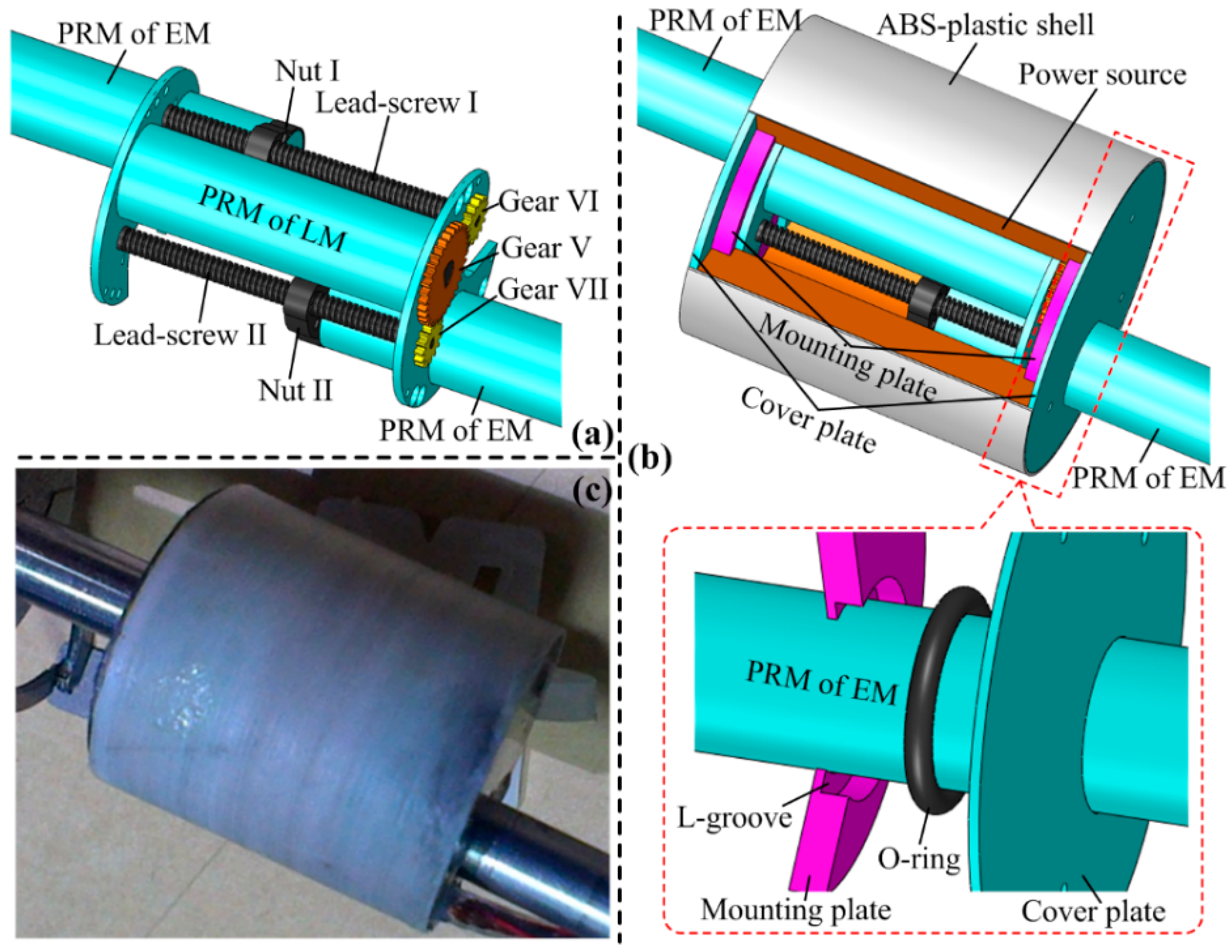
3.2. Design of the Linear Mechanism (LM)
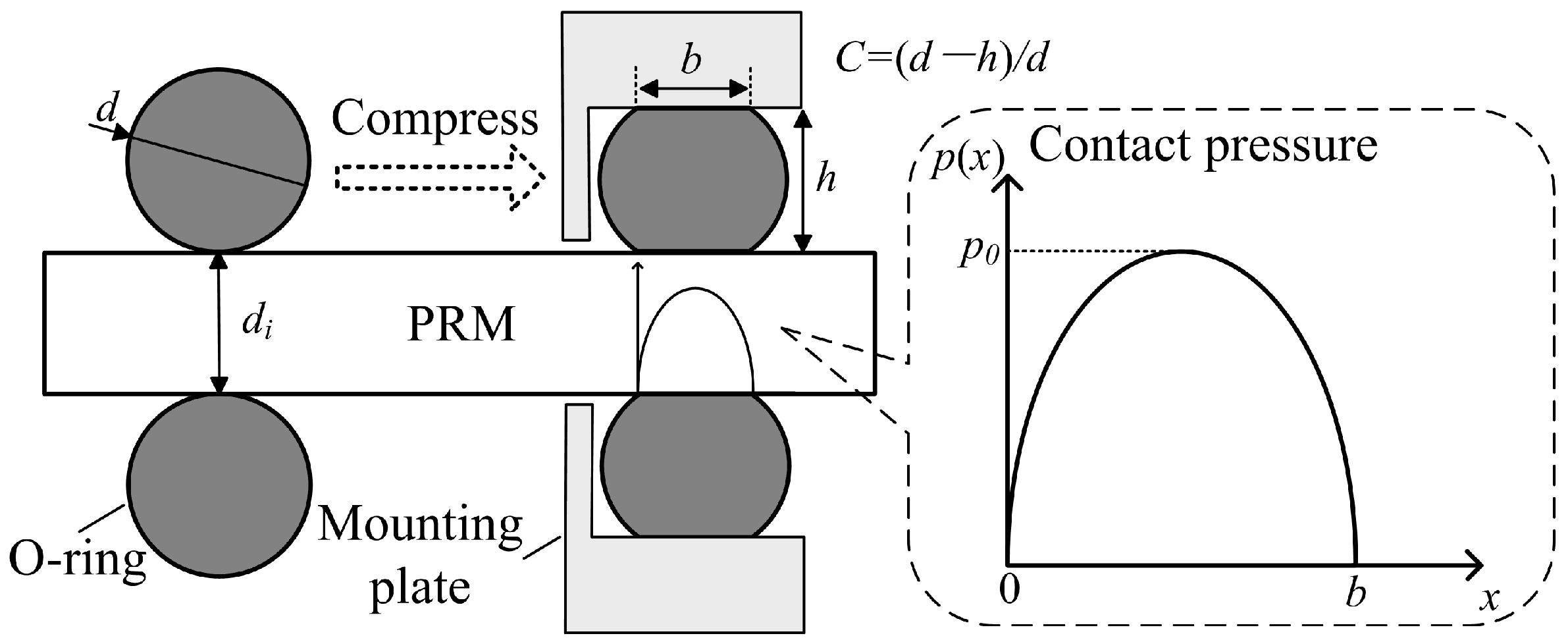
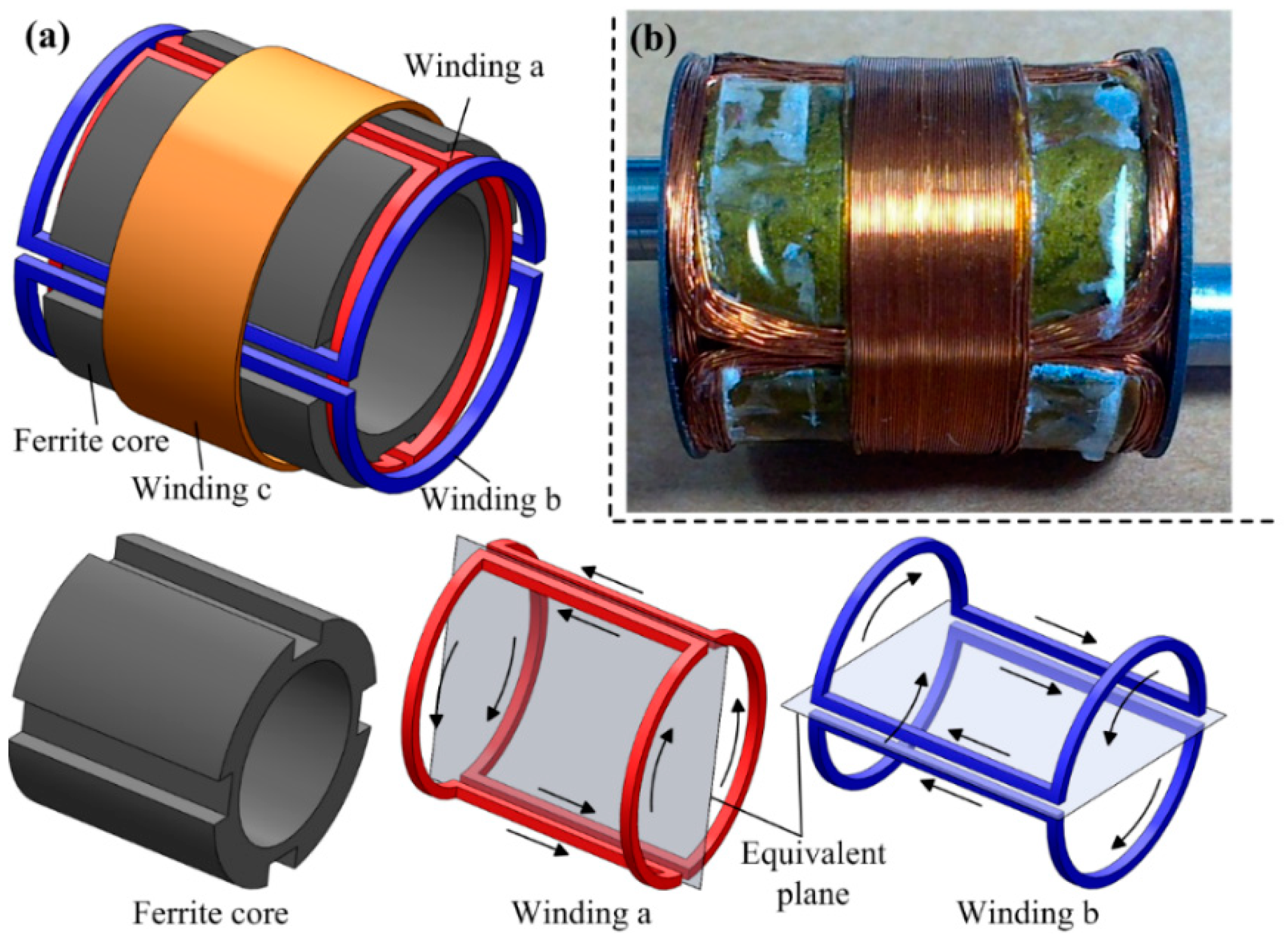
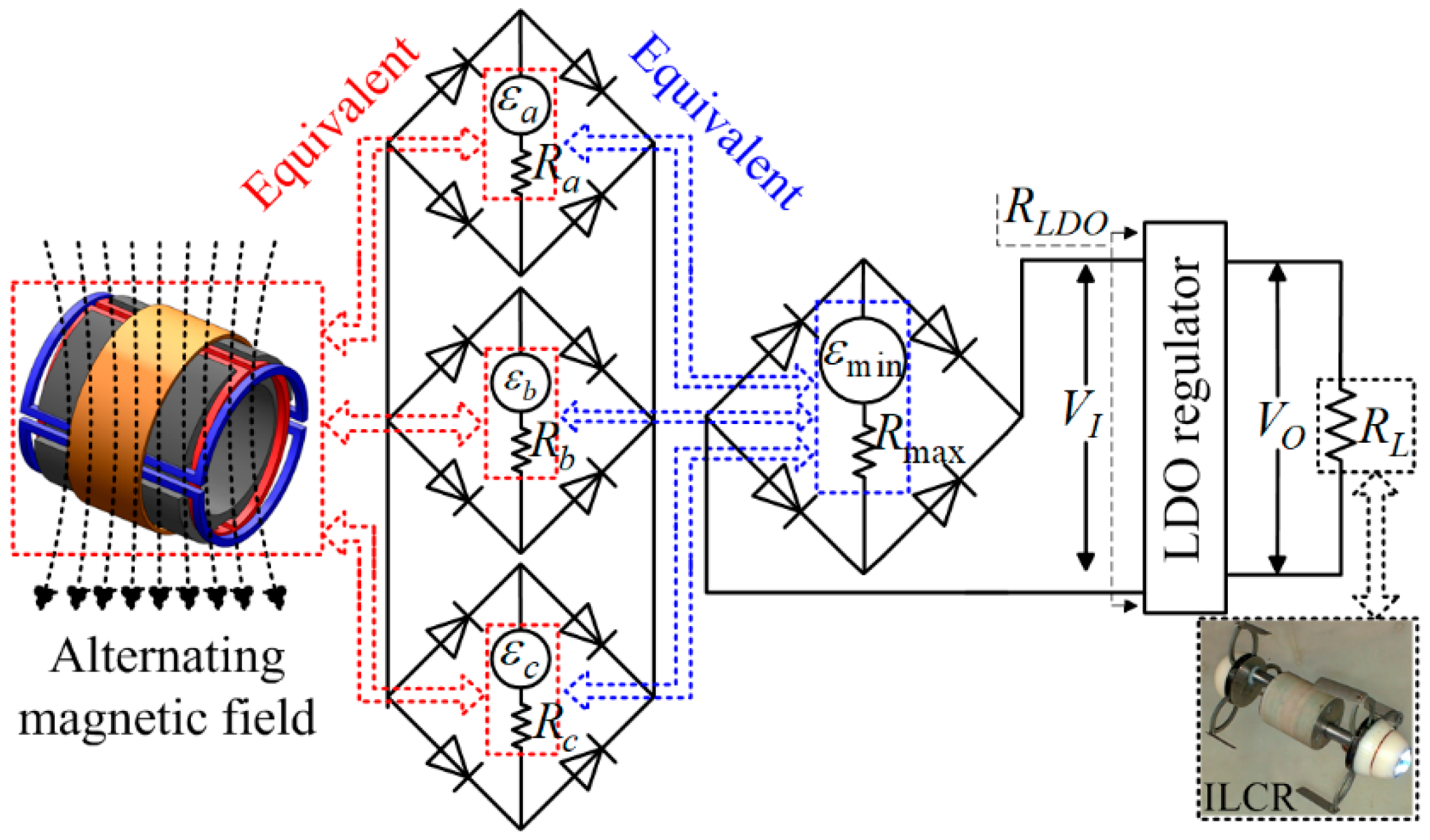
3.3. Design of the Power Source
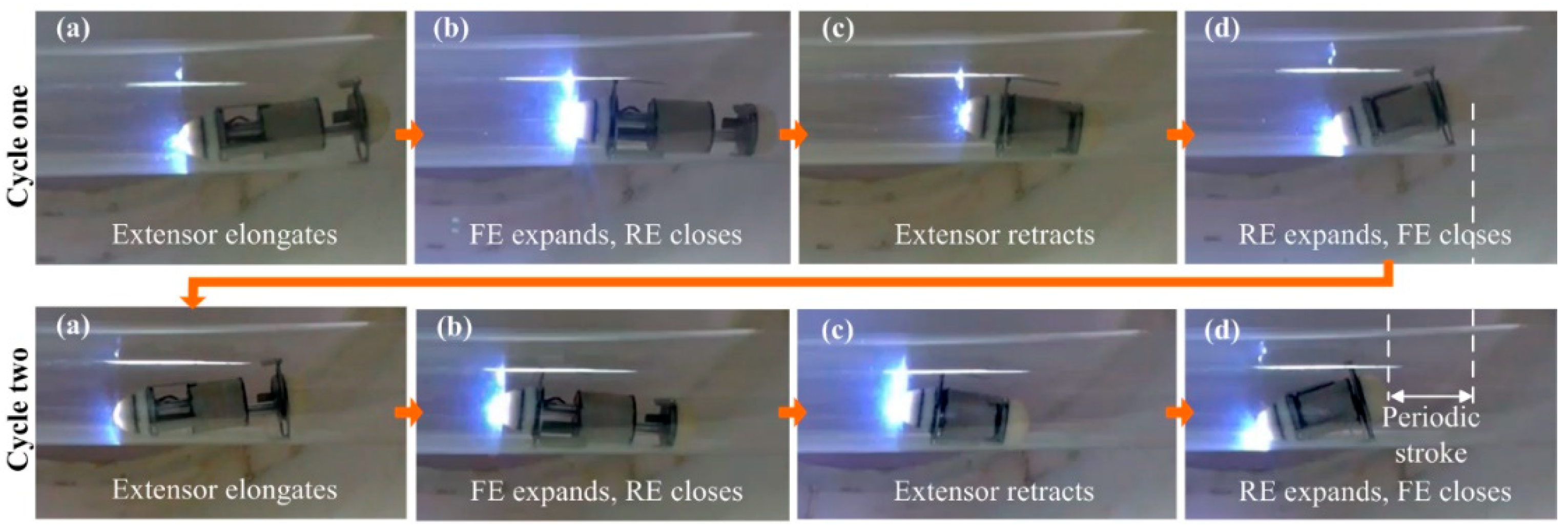
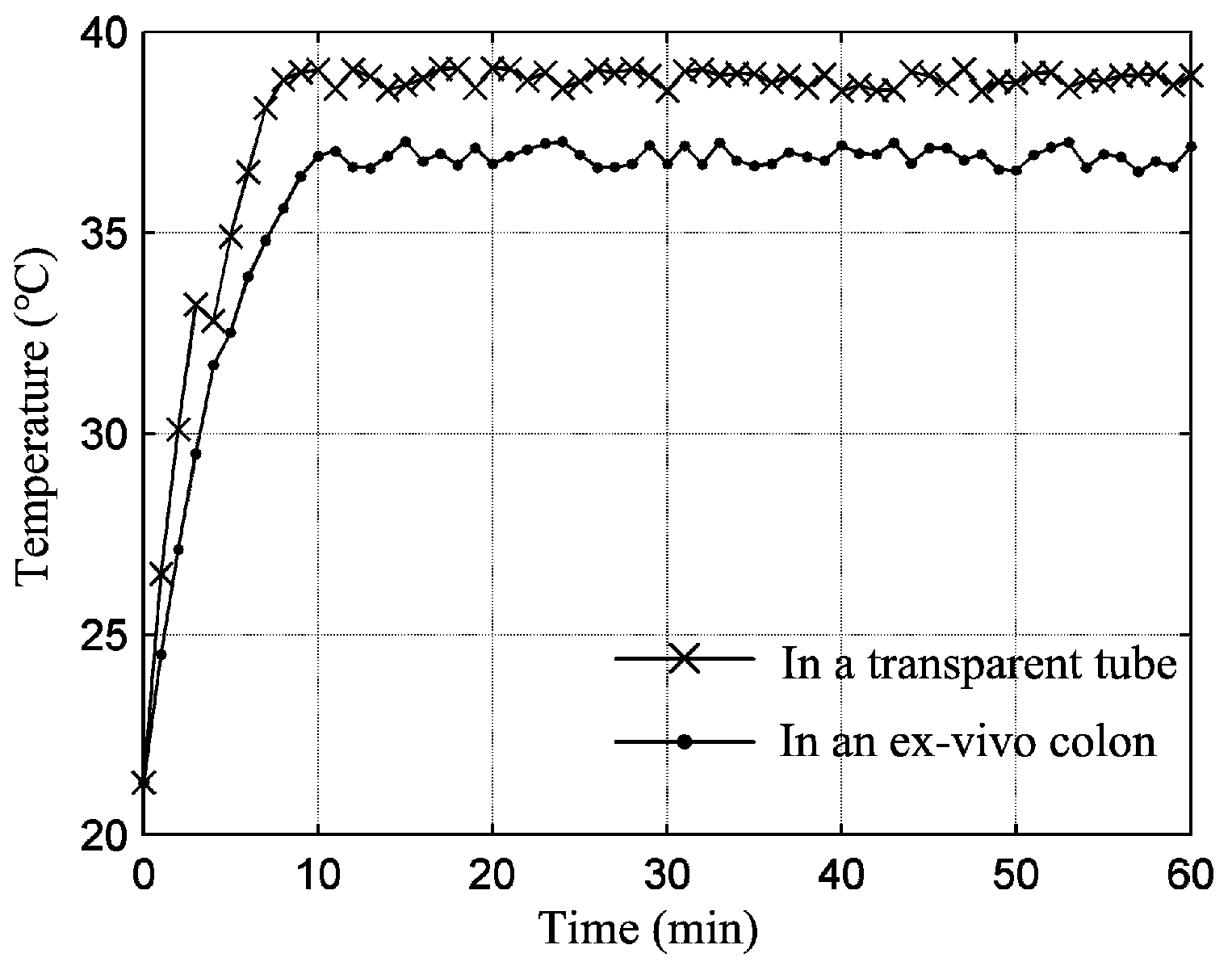
4. Experiments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yeung, B.P.M.; Chiu, P.W.Y. Application of robotics in gastrointestinal endoscopy: A review. World J. Gastroenterol. 2016, 22, 1811–1825. [Google Scholar] [CrossRef] [PubMed]
- Yim, S.; Sitti, M. Design and rolling locomotion of a magnetically actuated soft capsule endoscope. IEEE Trans. Robot. 2012, 28, 183–194. [Google Scholar] [CrossRef]
- Liao, Z.; Zou, W.; Li, Z.S. Clinical application of magnetically controlled capsule gastroscopy in gastric disease diagnosis: Recent advances. Sci. China Life Sci. 2018, 61, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Yang, Q.; Bai, L.; Zhao, Y. Development of multiple capsule robots in pipe. Micromachines 2018, 9, 259. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Zhang, S.; Guo, S.; Guo, J. Performance Evaluation of a Magnetically Actuated Capsule Microrobotic System for Medical Applications. Micromachines 2018, 9, 641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chi, M.; Su, Z. Critical Coupling Magnetic Moment of a Petal-Shaped Capsule Robot. IEEE Trans. Magn. 2016, 52, 1–9. [Google Scholar] [CrossRef]
- Valdastri, P.; Webster, R.J., III; Quaglia, C.; Quirini, Q.; Menciassi, A.; Dario, P. A new mechanism for mesoscale legged locomotion in compliant tubular environments. IEEE Trans. Robot. 2009, 25, 1047–1057. [Google Scholar] [CrossRef]
- Kim, H.M.; Yang, S.; Kim, J.; Park, S.; Cho, J.H.; Park, J.Y.; Bang, S. Active locomotion of a paddling-based capsule endoscope in an in vitro and in vivo experiment (with videos). Gastrointest. Endosc. 2010, 72, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Sliker, L.J.; Kern, M.D.; Rentschler, M.E. An automated traction measurement platform and empirical model for evaluation of rolling micropatterned wheels. IEEE-ASME Trans. Mechatron. 2015, 20, 1854–1862. [Google Scholar] [CrossRef]
- Gao, J.; Yan, G.; Wang, Z.; He, S.; Xu, F.; Jiang, P.; Liu, D. Design and testing of a motor-based capsule robot powered by wireless power transmission. IEEE/ASME Trans. Mechatron. 2016, 21, 683–693. [Google Scholar] [CrossRef]
- Gao, J.; Yan, G.; He, S.; Xu, F.; Wang, Z. Design, analysis, and testing of a motor-driven capsule robot based on a sliding clamper. Robotica 2017, 35, 521–536. [Google Scholar] [CrossRef]
- Wang, J.; Fei, Y.; Liu, Z. Locomotion modeling of a triangular closed-chain soft rolling robot. Mechatronics 2019, 57, 150–163. [Google Scholar] [CrossRef]
- Le, V.H.; Lee, C.; Go, G.; Park, J.O.; Park, S. Miniaturized biopsy module using gripper tool for active locomotive capsule endoscope. Mechatronics 2017, 44, 52–59. [Google Scholar] [CrossRef]
- Dario, P.; Ciarletta, P.; Menciassi, A.; Kim, B. Modeling and experimental validation of the locomotion of endoscopic robots in the colon. Int. J. Robot. Res. 2004, 23, 549–556. [Google Scholar] [CrossRef]
- Park, H.; Kim, D.; Kim, B. A robotic colonoscope with long stroke and reliable leg clamping. Int. J. Precis. Eng. Manuf. 2012, 13, 1461–1466. [Google Scholar] [CrossRef]
- Karagozler, M.E.; Cheung, E.; Kwon, J.; Sitti, M. Miniature endoscopic capsule robot using biomimetic micro-patterned adhesives. In Proceedings of the The First IEEE/RAS-EMBS International Conference on Biomedical Robotics and Biomechatronics, Pisa, Italy, 20–22 February 2006; pp. 105–111. [Google Scholar]
- Chen, W.; Yan, G.; He, S.; Ke, Q.; Wang, Z.; Liu, H.; Jiang, P. Wireless powered capsule endoscopy for colon diagnosis and treatment. Physiol. Meas. 2013, 34, 1545–1561. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Yan, G.; Wang, Z.; Jiang, P.; Liu, H. Microgroove cushion of robotic endoscope for active locomotion in the gastrointestinal tract. Int. J. Med. Robot. Comput. Assist. Surg. 2012, 8, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Phee, L.; Accoto, D.; Menciassi, A.; Stefanini, C.; Carrozza, M.C.; Dario, P. Analysis and development of locomotion devices for the gastrointestinal tract. IEEE Trans. Biomed. Eng. 2002, 49, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yan, G. Locomotion analysis of an inchworm-like capsule robot in the intestinal tract. IEEE Trans. Biomed. Eng. 2016, 63, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yan, G.; Wang, Z.; Xu, F.; Wang, W.; Jiang, P.; Liu, D. Locomotion enhancement of an inchworm-like capsule robot using long contact devices. Int. J. Med. Robot. Comput. Assist. Surg. 2017, 13, e1759. [Google Scholar] [CrossRef]
- Liu, G.; Yan, G.; Xu, W.; Kuang, S. Dual-head wireless powered video capsule based on new type of receiving coils. J. Med. Eng. Technol. 2015, 39, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Carta, R.; Sfakiotakis, M.; Pateromichelakis, N.; Thoné, J.; Tsakiris, D.P.; Puers, R. A multi-coil inductive powering system for an endoscopic capsule with vibratory actuation. Sens. Actuator A-Phys. 2011, 172, 253–258. [Google Scholar] [CrossRef]
- Xu, F.; Yan, G.; Zhao, K.; Lu, L.; Gao, J.; Liu, G. A wireless capsule system with ASIC for monitoring the physiological signals of the human gastrointestinal tract. IEEE Trans. Biomed. Circuit Syst. 2014, 8, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Basar, M.R.; Ahmad, M.Y.; Cho, J.; Ibrahim, F. Stable and high-efficiency wireless power transfer system for robotic capsule using a modified Helmholtz coil. IEEE Trans. Ind. Electron. 2017, 64, 1113–1122. [Google Scholar] [CrossRef]
- Jia, Z.; Yan, G.; Liu, H.; Wang, Z.; Jiang, P.; Shi, Y. The optimization of wireless power transmission: Design and realization. Int. J. Med. Robot. Comput. Assist. Surg. 2012, 8, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Basar, M.R.; Ahmad, M.Y.; Cho, J.; Ibrahim, F. Performance evaluation of power transmission coils for powering endoscopic wireless capsules. In Proceedings of the 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Milan, Italy, 25–29 August 2015; pp. 2263–2266. [Google Scholar]
- Shiba, K.; Morimasa, A.; Hirano, H. Design and development of low-loss transformer for powering small implantable medical devices. IEEE Trans. Biomed. Circuit Syst. 2010, 4, 77–85. [Google Scholar] [CrossRef] [PubMed]

| Driving Ways | Ref. | Tether | Power Supply | Communication and Control | Periodic Stroke (mm) | Body Length (mm) | Diameter (mm) | Horizontal Velocity (cm/min) |
|---|---|---|---|---|---|---|---|---|
| Pneumatic-driven | [14] | Yes | Air tube | 115 | 115 | 24 | 9 | |
| [15] | Yes | 140 | 110 | 15 | 51 | |||
| SMA-driven | [16] | Yes | Power line | - | 40 | 15 | 3 | |
| Micropump-driven | [17] | No | WPT | Wireless | 16.5 | 73 | 20 | 1.6 |
| Micromotor-driven | [18] | No | WPT | Wireless | 44 | 128 | 17 | 3 |
| [10] | No | WPT | Wireless | 10.5 | 27 | 14 | 4.2 | |
| This work | No | WPT | Wireless | 38 | 33 | 24 | ≥7.4 | |
| Overall size | Diameter | 24 mm |
| Body length | 33 mm | |
| Two EMs | Diameter range | 24–61 mm |
| Length | 3.5 mm | |
| LM | Diameter | 16 mm |
| Length | 26 mm | |
| Periodic stroke | 38 mm | |
| Contact device | Length × Width × Thickness | 15 mm × 7 mm × 0.5 mm |
| Power source | Diameter range | 16–23.5 mm |
| Length | 25 mm | |
| Camera [22] | Diameter | 12 mm |
| Length | 8 mm | |
| Telemetry circuit [10] (housed in a copper shell) | Diameter | 14 mm |
| Length | 4 mm |
| PRM (waterproof) | Size | 6.3 mm × 24.5 mm |
| Output torque | 452 gf·cm | |
| Working voltage | 3.3 V | |
| Rated/Stall current | 40/190 mA | |
| AG I/II | Teeth number (incomplete) | 55 (20) |
| Modulus | 0.2 mm | |
| Gear I/II/III/IV | Teeth number | 26/26/12/12 |
| Modulus | 0.2 mm | |
| Arc-shaped leg | Arc-diameter | 22 mm |
| Radian | 0.594π |
| PRM | Size | 6.3 mm × 19 mm |
| Output torque | 280 gf·cm | |
| Working voltage | 3.3 V | |
| Rated/Stall current | 40/190 mA | |
| Gear V/VI/VII | Teeth number | 26/26/12/12 |
| Modulus | 0.2 mm | |
| Lead-screw I/II | Thread direction | Right-hand/left-hand |
| Nominal diameter | 2 mm | |
| Thread angle | 60° | |
| Thread pitch | 0.4 mm | |
| Nut I/II | Axial length | 2.5 mm |
| Ferrite core | Overall size | (16~23) mm × 20 mm |
| Lateral groove size | 20 mm × 4 mm × 2 mm | |
| Material/Permeability | Mn-Zn/R6K | |
| Winding a | Single-turn mutual inductance | 1.37 × H |
| Number of turns | 68 | |
| Wire diameter | 0.15 mm | |
| ESR | 16.34 | |
| Winding b | Single-turn mutual inductance | 1.54 × H |
| Number of turns | 60 | |
| Wire diameter | 0.15 mm | |
| ESR | 13.56 | |
| Winding c | Single-turn mutual inductance | 1.82 × H |
| Number of turns | 52 | |
| Wire diameter | 0.2 mm | |
| ESR | 9.73 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Zhang, Z.; Yan, G. Development of a Capsule Robot for Exploring the Colon. Micromachines 2019, 10, 456. https://doi.org/10.3390/mi10070456
Gao J, Zhang Z, Yan G. Development of a Capsule Robot for Exploring the Colon. Micromachines. 2019; 10(7):456. https://doi.org/10.3390/mi10070456
Chicago/Turabian StyleGao, Jinyang, Zenglei Zhang, and Guozheng Yan. 2019. "Development of a Capsule Robot for Exploring the Colon" Micromachines 10, no. 7: 456. https://doi.org/10.3390/mi10070456
APA StyleGao, J., Zhang, Z., & Yan, G. (2019). Development of a Capsule Robot for Exploring the Colon. Micromachines, 10(7), 456. https://doi.org/10.3390/mi10070456




