A Capillary-Evaporation Micropump for Real-Time Sweat Rate Monitoring with an Electrochemical Sensor
Abstract
1. Introduction
2. Materials and Methods
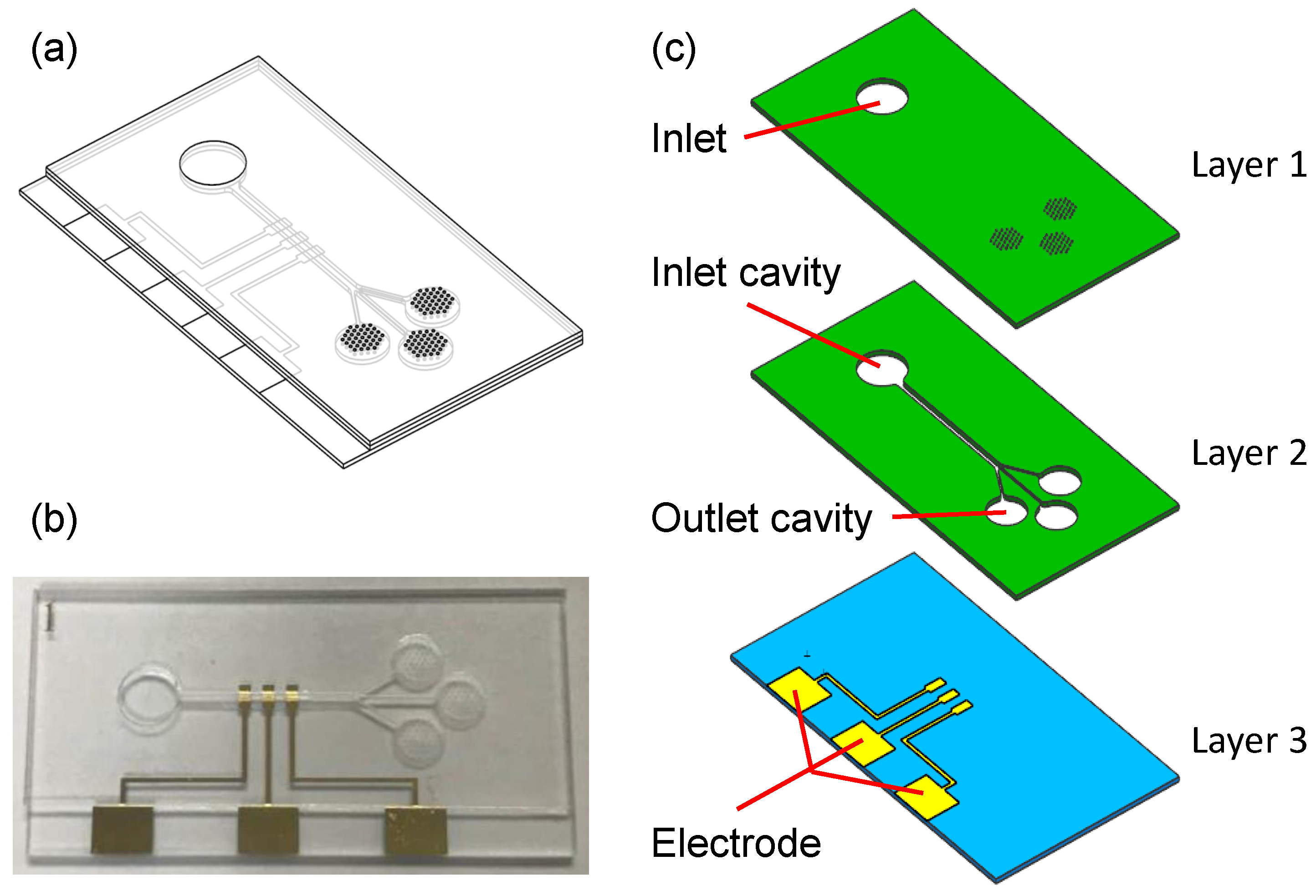
2.1. Microfluidic Device Fabrication
2.2. Theoretical and Numerical Analysis of Evaporation Rate
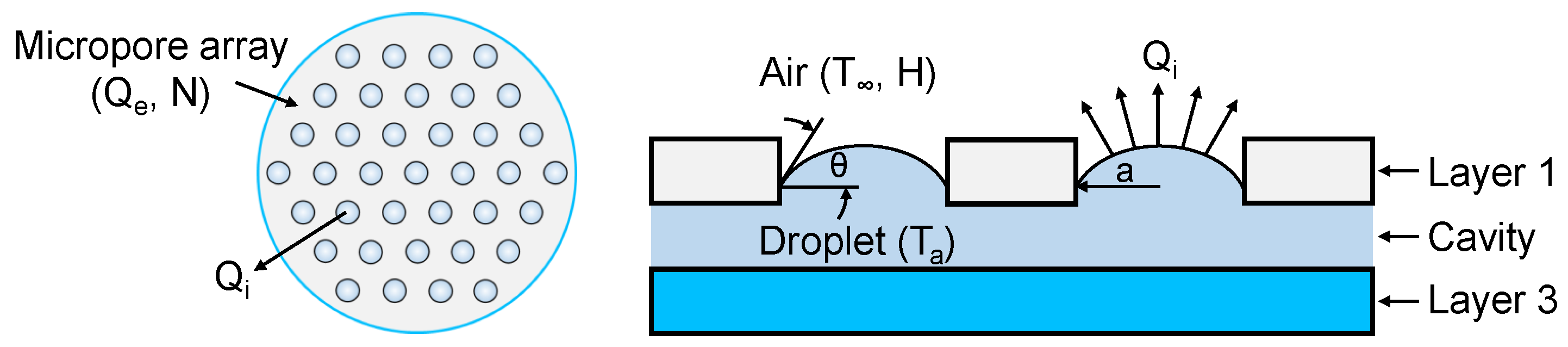
2.2.1. Evaporation Theory
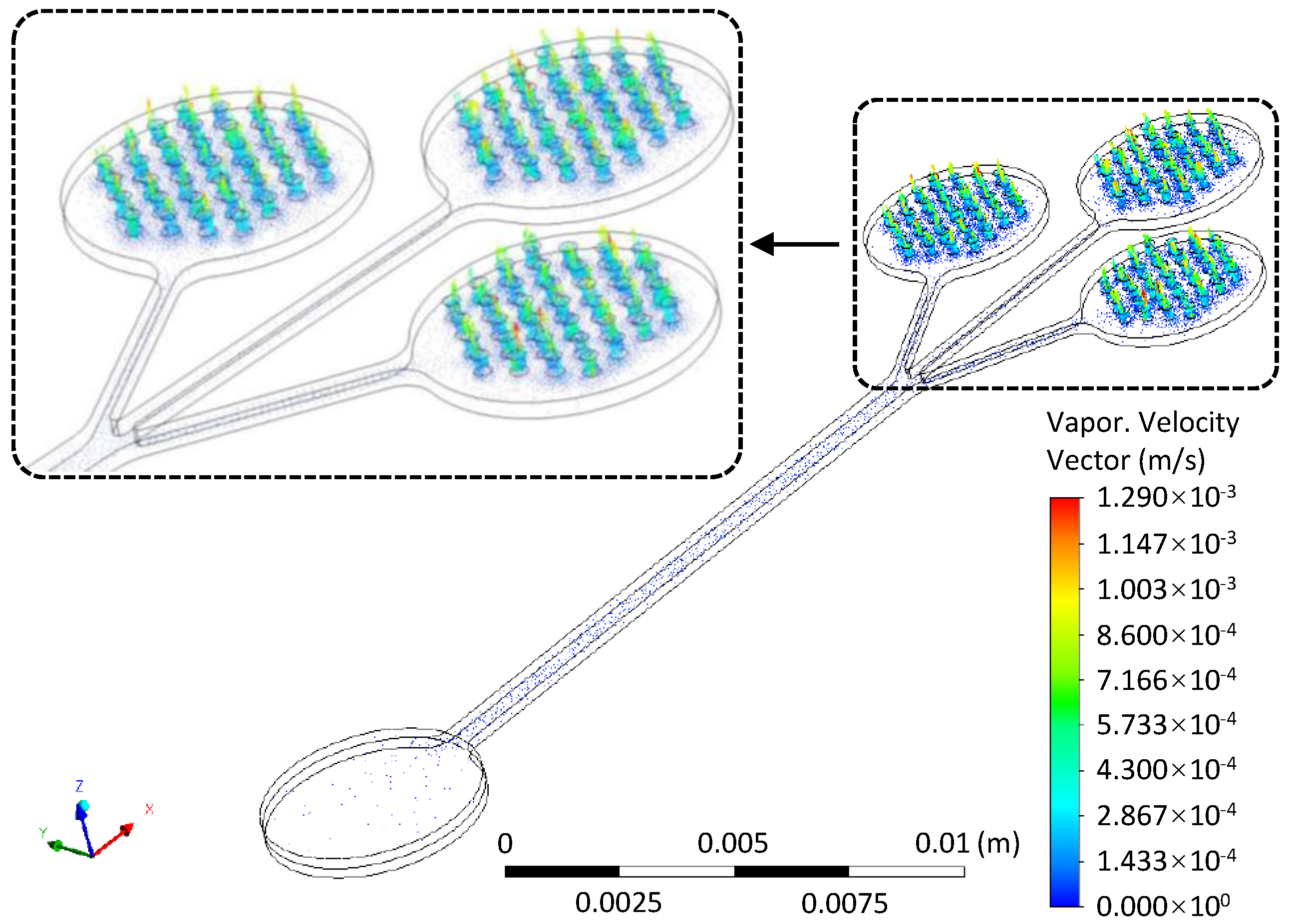
2.2.2. ANSYS Numerical Simulation
2.3. Characterization of Electrochemical Sensor
2.3.1. Electrochemical Principle for Flow Rate Monitoring
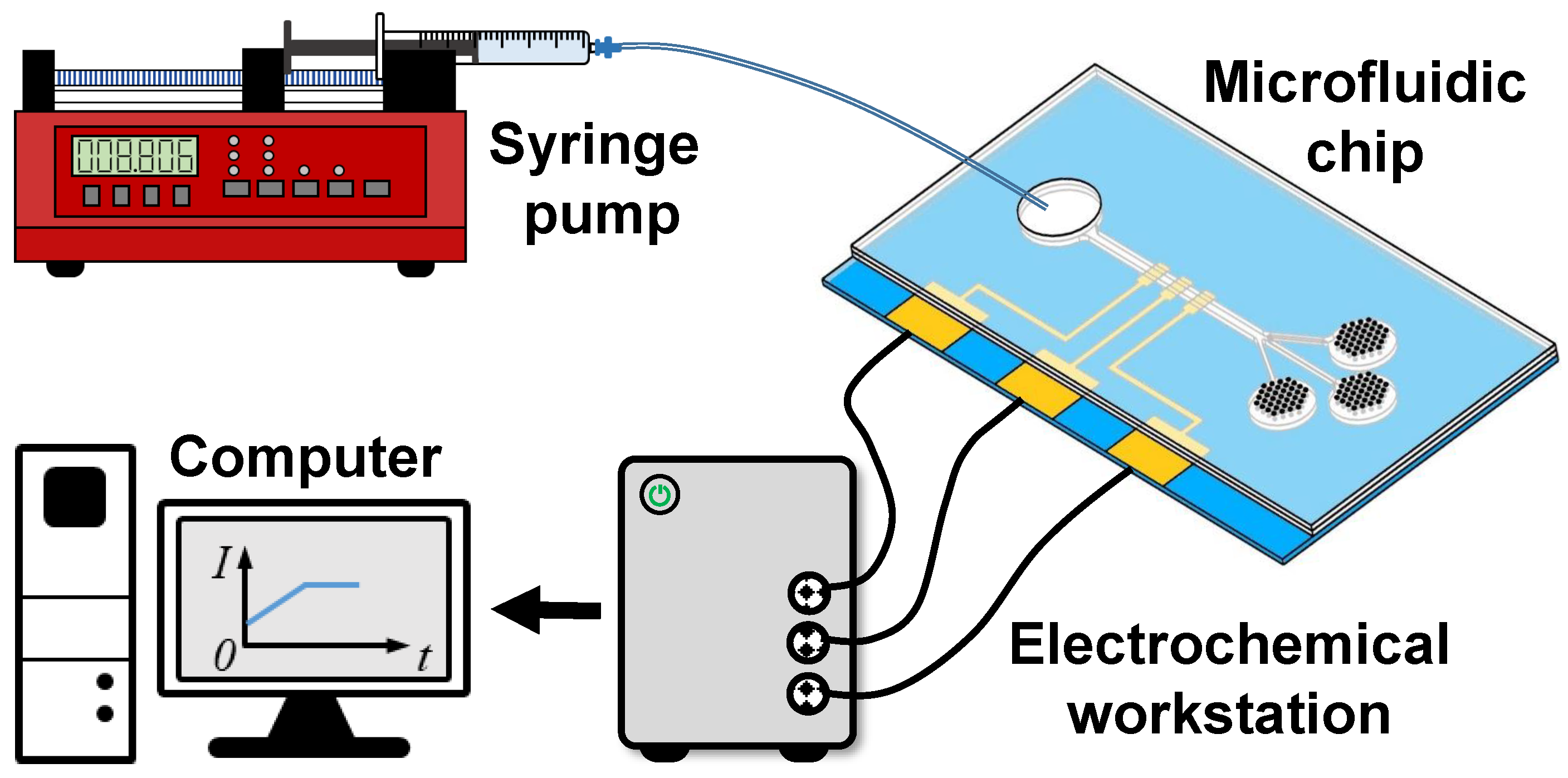
2.3.2. Experimental Validation
3. Results
3.1. Maximum Evaporation Rate
3.2. The Capacity of Sweat Collection
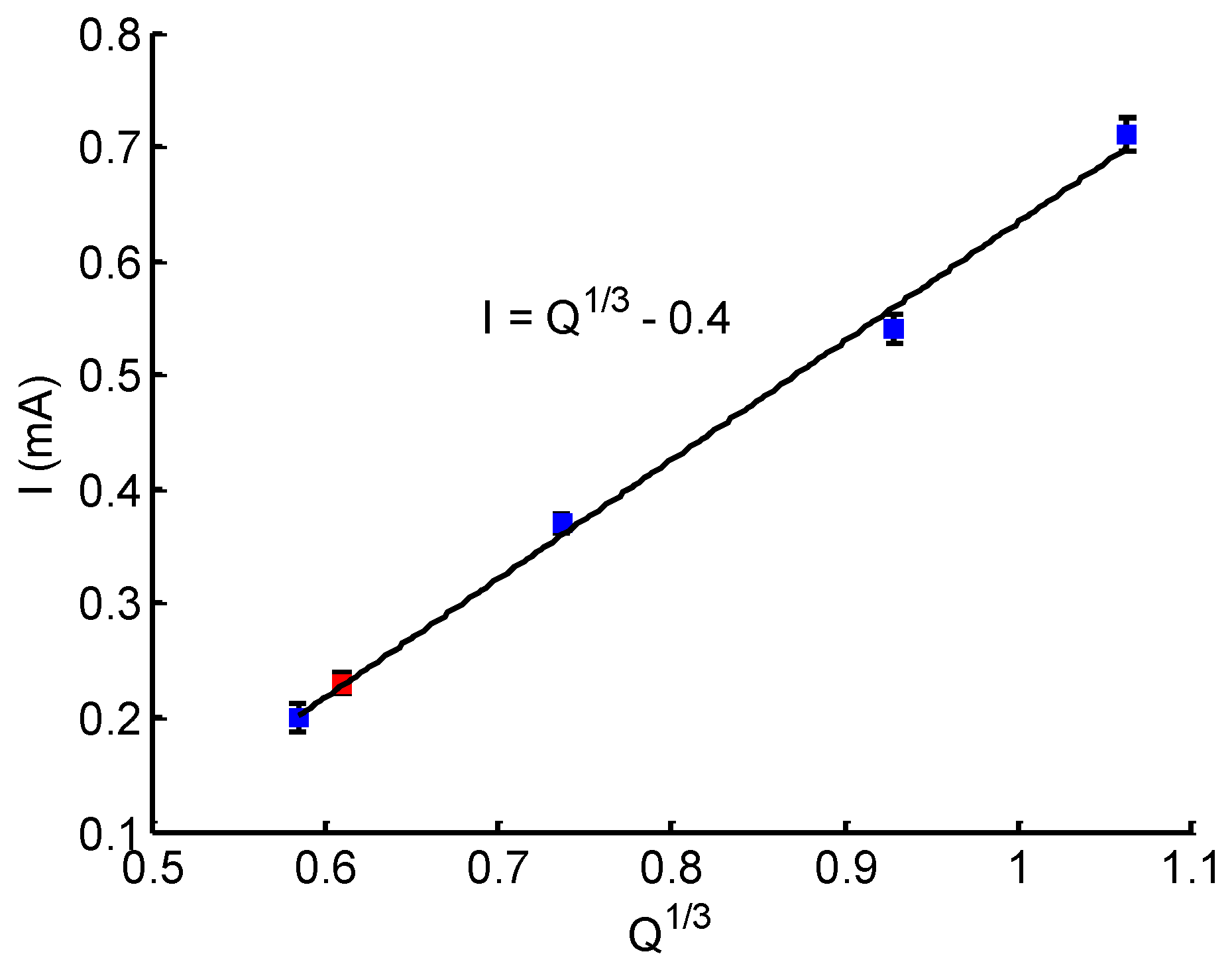
3.3. Calibration of the Flow Rate Sensor
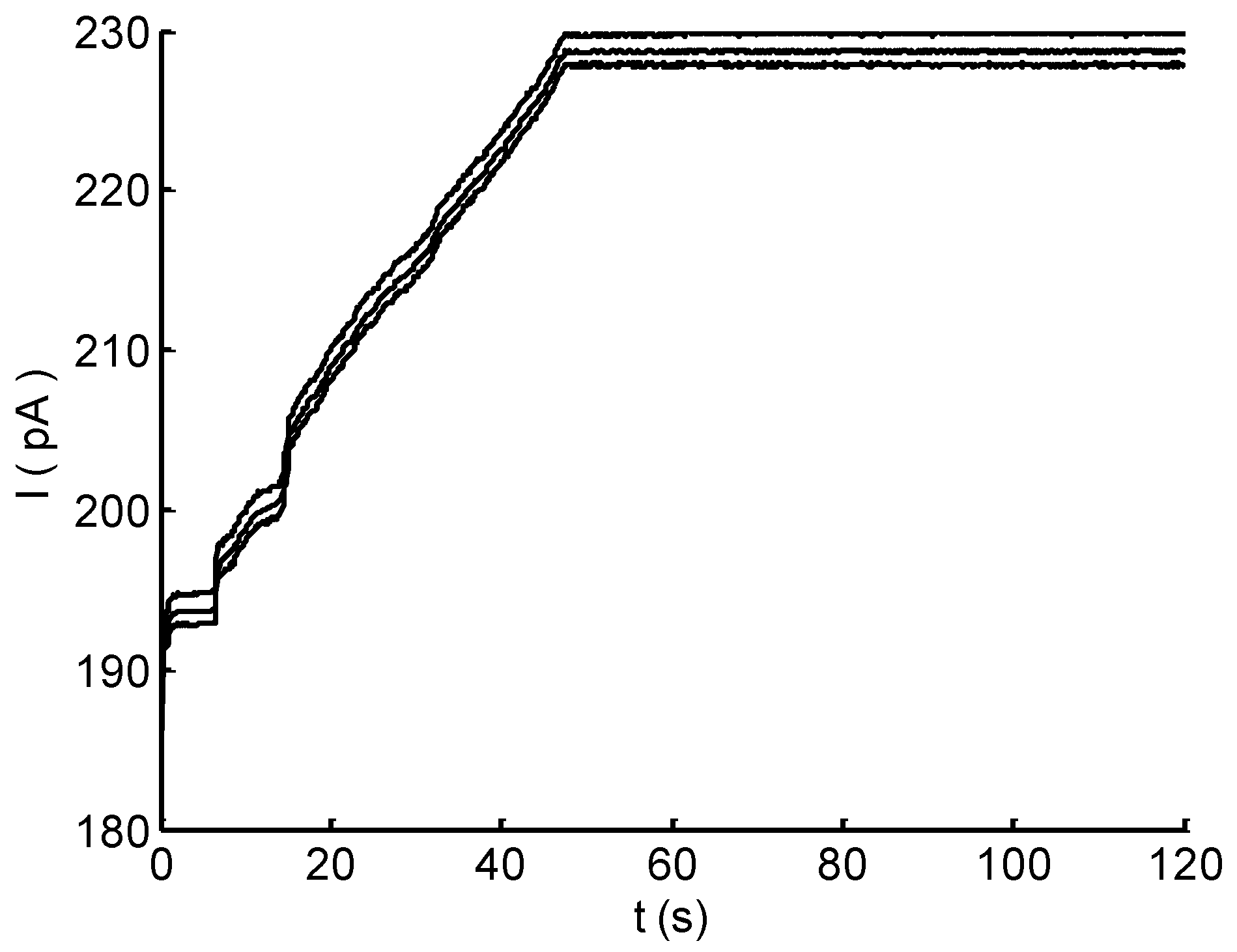
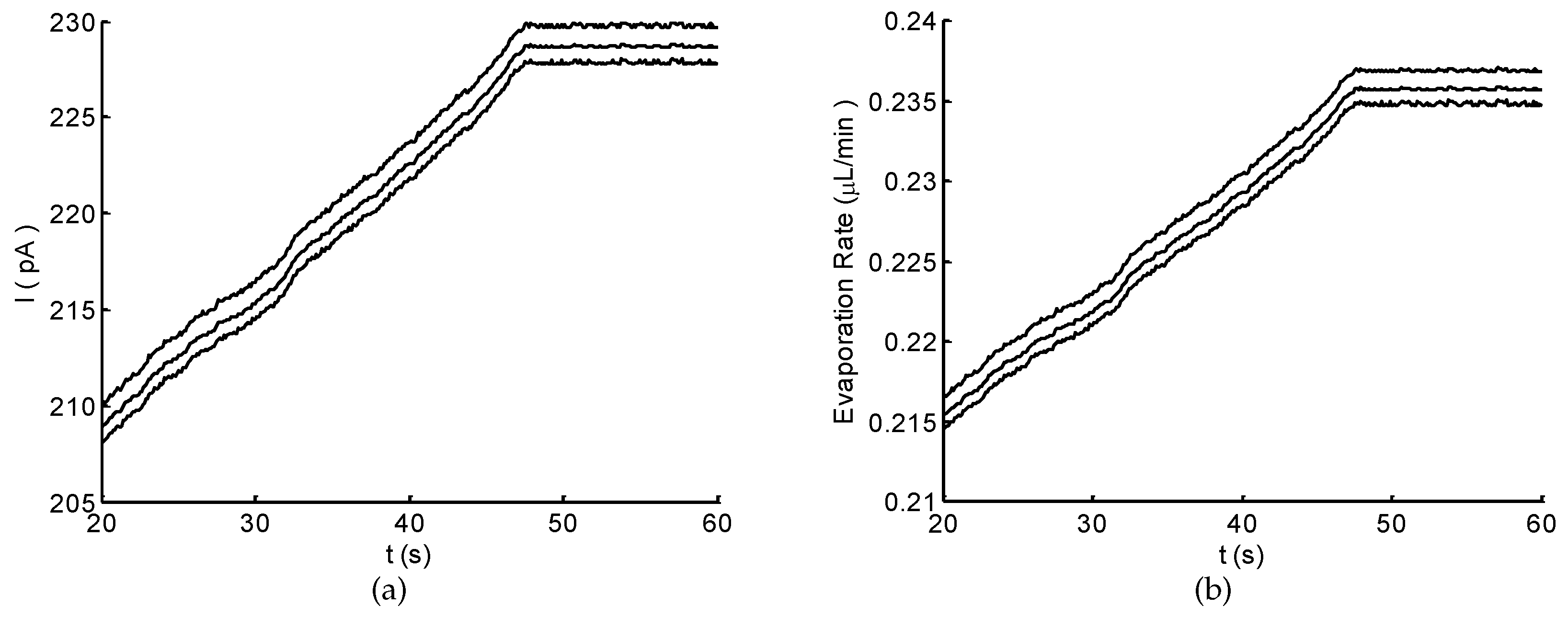
3.4. Real-Time Monitoring of Flow Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jia, W.; Bandodkar, A.J.; Valdés-Ramírez, G.; Windmiller, J.R.; Yang, Z.; Ramírez, J.; Chan, G.; Wang, J. Electrochemical tattoo biosensors for real-Time noninvasive lactate monitoring in human perspiration. Anal. Chem. 2013, 85, 6553–6560. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Gutruf, P.; Choi, J.; Lee, K.; Sekine, Y.; Reeder, J.T.; Jeang, W.J.; Aranyosi, A.J.; Lee, S.P.; Model, J.B.; et al. Battery-free, skin-interfaced microfluidic/electronic systems for simultaneous electrochemical, colorimetric, and volumetric analysis of sweat. Sci. Adv. 2019, 5, eaav3294. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Hong, S.Y.; Jeong, Y.R.; Yun, J.; Park, H.; Jin, S.W.; Lee, G.; Oh, J.H.; Lee, H.; Lee, S.S.; et al. A Skin-Attachable, Stretchable Electrochemical Sweat Sensor for Glucose and pH Detection. ACS Appl. Mater. Interfaces 2018, 10, 13729–13740. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Jeang, W.J.; Ghaffari, R.; Rogers, J.A. Wearable sensors for biochemical sweat analysis. Annu. Rev. Anal. Chem. 2019, 12, 8.1–8.22. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Mohammadifar, M.; Choi, S. A single-use, self-powered, paper-based sensor patch for detection of exercise-induced hypoglycemia. Micromachines 2017, 8, 265. [Google Scholar]
- Curto, V.F.; Coyle, S.; Byrne, R.; Angelov, N.; Diamond, D.; Benito-Lopez, F. Concept and development of an autonomous wearable micro-fluidic platform for real time pH sweat analysis. Sens. Actuators B Chem. 2012, 175, 263–270. [Google Scholar] [CrossRef]
- Godek, S.F. Sweat rate and fluid turnover in American football players compared with runners in a hot and humid environment. Br. J. Sports Med. 2005, 39, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Schazmann, B.; Morris, D.; Slater, C.; Beirne, S.; Fay, C.; Reuveny, R.; Moyna, N.; Diamond, D. A wearable electrochemical sensor for the real-time measurement of sweat sodium concentration. Anal. Methods 2010, 2, 342–348. [Google Scholar] [CrossRef]
- Shirreffs, S.M.; Maughan, R.J. Whole body sweat collection in humans: An improved method with preliminary data on electrolyte content. J. Appl. Physiol. 1997, 82, 336–341. [Google Scholar] [CrossRef]
- Francis, J.; Stamper, I.; Heikenfeld, J.; Gomez, E.F. Digital nanoliter to milliliter flow rate sensor with in vivo demonstration for continuous sweat rate measurement. Lab Chip 2019, 19, 178–185. [Google Scholar] [CrossRef]
- Buono, M.J.; Claros, R.; DeBoer, T.; Wong, J. Na+ secretion rate increases proportionally more than the Na+ reabsorption rate with increases in sweat rate. J. Appl. Physiol. 2008, 105, 1044–1048. [Google Scholar] [CrossRef]
- Kaya, T.; Liu, G.; Ho, J.; Yelamarthi, K.; Miller, K.; Edwards, J.; Stannard, A. Wearable sweat sensors: Background and current trends. Electroanalysis 2019, 31, 411–421. [Google Scholar] [CrossRef]
- Berthier, E.; Beebe, D.J. Flow rate analysis of a surface tension driven passive micropump. Lab Chip. 2007, 7, 1475–1478. [Google Scholar] [CrossRef]
- Guan, Y.X.; Xu, Z.R.; Dai, J.; Fang, Z.L. The use of a micropump based on capillary and evaporation effects in a microfluidic flow injection chemiluminescence system. Talanta 2006, 68, 1384–1389. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Bentley, S.; Schmid, H.; Hunziker, P.; Delamarche, E. Continuous flow in open microfluidics using controlled evaporation. Lab Chip 2005, 5, 1355. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Frijns, A.J.H.; Mandamparambil, R.; den Toonder, J.M.J. A microfluidic device based on an evaporation-driven micropump. Biomed. Microdevices 2015, 17, 47. [Google Scholar] [CrossRef]
- Li, J.M.; Liu, C.; Xu, Z.; Zhang, K.P.; Ke, X.; Li, C.Y.; Wang, L.D. A bio-inspired micropump based on stomatal transpiration in plants. Lab Chip. 2011, 11, 2785. [Google Scholar] [CrossRef]
- Xu, Z.R.; Zhong, C.H.; Guan, Y.X.; Chen, X.W.; Wang, J.H.; Fang, Z.L. A microfluidic flow injection system for DNA assay with fluids driven by an on-chip integrated pump based on capillary and evaporation effects. Lab Chip 2008, 8, 1658–1663. [Google Scholar] [CrossRef]
- Temiz, Y.; Delamarche, E. Sub-nanoliter, real-time flow monitoring in microfluidic chips using a portable device and smartphone. Sci. Rep. 2018, 8, 10603. [Google Scholar] [CrossRef]
- Yang, Y.; Xing, S.; Fang, Z.; Li, R.; Koo, H.; Pan, T. Wearable microfluidics: fabric-based digital droplet flowmetry for perspiration analysis. Lab Chip 2017, 17, 926. [Google Scholar] [CrossRef]
- Lien, V.; Vollmer, F. Microfluidic flow rate detection based on integrated optical fiber cantilever. Lab Chip 2007, 7, 1352–1356. [Google Scholar] [CrossRef] [PubMed]
- Matzeu, G.; Fay, C.; Vaillant, A.; Coyle, S.; Diamond, D. A wearable device for monitoring sweat rates via image analysis. IEEE Trans. Biomed. Eng. 2016, 63, 1672–1680. [Google Scholar] [CrossRef] [PubMed]
- Salvo, P.; Di Francesco, F.; Costanzo, D.; Ferrari, C.; Trivella, M.G.; De Rossi, D. A Wearable Sensor for Measuring Sweat Rate. IEEE Sens. J. 2010, 10, 1557–1558. [Google Scholar] [CrossRef]
- Morris, N.B.; Cramer, M.N.; Hodder, S.G.; Havenith, G.; Jay, O. A comparison between the technical absorbent and ventilated capsule methods for measuring local sweat rate. J. Appl. Physiol. 2013, 114, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; Kang, D.; Xue, Y.; Lee, S.; Pielak, R.M.; Kim, J.; Hwang, T.; Min, S.; Banks, A.; Bastien, P.; et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 2016, 8, 366ra165. [Google Scholar] [CrossRef] [PubMed]
- Iftekhar, A.T.; Ho, J.C.T.; Mellinger, A.; Kaya, T. A Real-Time Wireless Sweat Rate Measurement System for Physical Activity Monitoring. J. Appl. Phys. 2017, 121, 094505. [Google Scholar] [CrossRef]
- Brueck, A.; Iftekhar, T.; Stannard, A.; Yelamarthi, K.; Kaya, T. 3D modeling and characterization of a calorimetric flow rate sensor for sweat rate sensing applications. Sensors 2018, 18, 533. [Google Scholar] [CrossRef]
- Semenov, S.; Starov, V.M.; Velarde, M.G.; Rubio, R.G. Droplets evaporation: Problems and solutions. Eur. Phys. J. Spec. Top. 2011, 197, 265–278. [Google Scholar] [CrossRef]
- Schönfel, F.; Graf, K.H.; Hardt, S.; Butt, H.J. Evaporation dynamics of sessile liquid drops in still air with constant contact radius. Int. J. Heat Mass Transf. 2008, 51, 3696–3699. [Google Scholar] [CrossRef]
- Kokalj, T.; Cho, H.; Jenko, M.; Lee, L.P. Biologically inspired porous cooling membrane using arrayed-droplets evaporation. Appl. Phys. Lett. 2010, 96, 163703. [Google Scholar] [CrossRef]
- Wang, J. Mass transport and current response. In Analytical Electrochemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 103–106. [Google Scholar]
- Walker, G.M.; Beebe, D.J. An evaporation-based microfluidic sample concentration method. Lab Chip 2002, 2, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Nilson, S.E.; Assmann, S.M. The control of transpiration. Insights from arabidopsis. Plant Physiol. 2007, 143, 19–27. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.-M.; Li, Y.-J.; Han, D.; Zhu, H.-C.; Xue, C.-D.; Chui, H.-C.; Cao, T.; Qin, K.-R. A Capillary-Evaporation Micropump for Real-Time Sweat Rate Monitoring with an Electrochemical Sensor. Micromachines 2019, 10, 457. https://doi.org/10.3390/mi10070457
Chen X-M, Li Y-J, Han D, Zhu H-C, Xue C-D, Chui H-C, Cao T, Qin K-R. A Capillary-Evaporation Micropump for Real-Time Sweat Rate Monitoring with an Electrochemical Sensor. Micromachines. 2019; 10(7):457. https://doi.org/10.3390/mi10070457
Chicago/Turabian StyleChen, Xiao-Ming, Yong-Jiang Li, Dan Han, Hui-Chao Zhu, Chun-Dong Xue, Hsiang-Chen Chui, Tun Cao, and Kai-Rong Qin. 2019. "A Capillary-Evaporation Micropump for Real-Time Sweat Rate Monitoring with an Electrochemical Sensor" Micromachines 10, no. 7: 457. https://doi.org/10.3390/mi10070457
APA StyleChen, X.-M., Li, Y.-J., Han, D., Zhu, H.-C., Xue, C.-D., Chui, H.-C., Cao, T., & Qin, K.-R. (2019). A Capillary-Evaporation Micropump for Real-Time Sweat Rate Monitoring with an Electrochemical Sensor. Micromachines, 10(7), 457. https://doi.org/10.3390/mi10070457






