Structuring of Bioceramics by Micro-Grinding for Dental Implant Applications
Abstract
1. Introduction
2. Materials and Methods
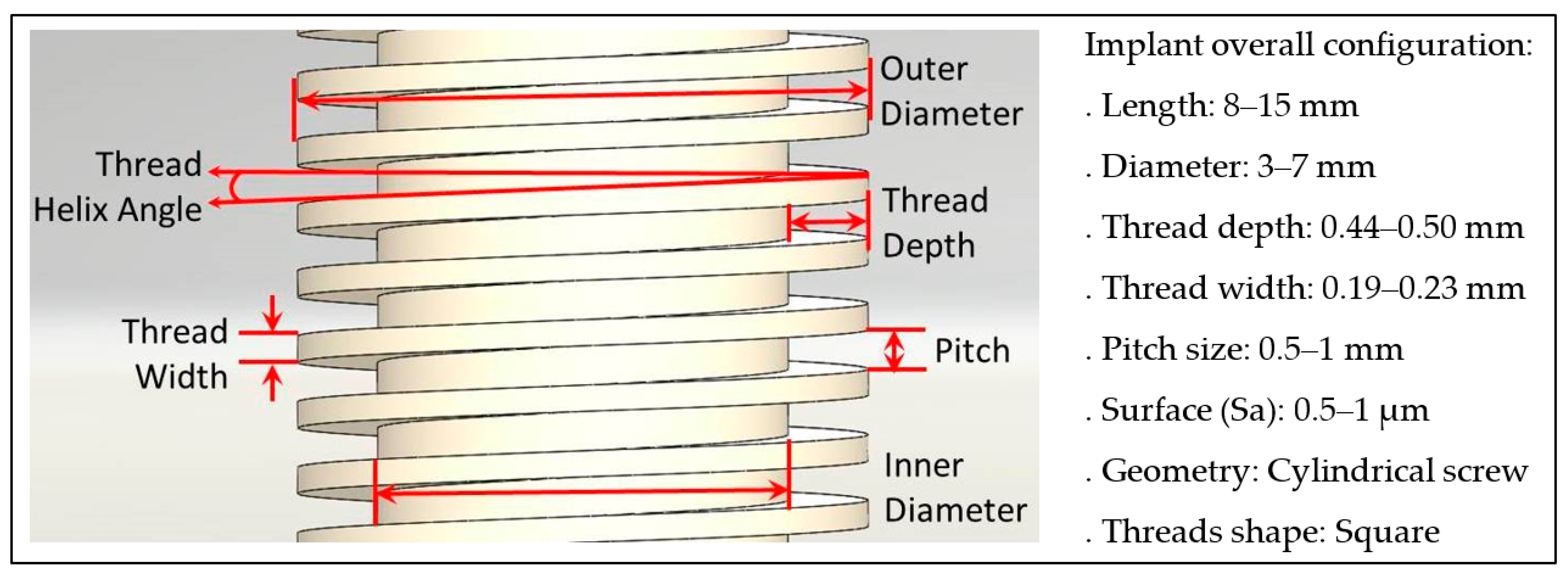
2.1. Case Study
2.2. Material Selection
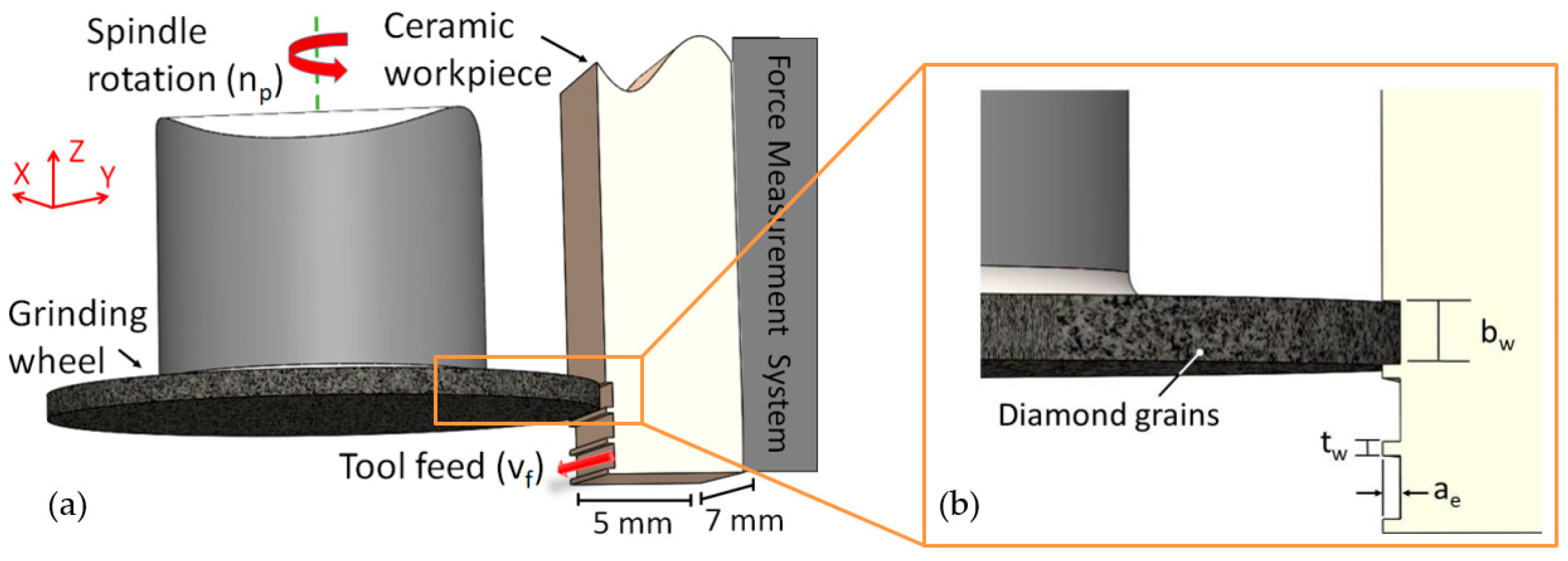
2.3. Process Kinematics and Experimental Conditions
2.4. Workpiece Characterisation and Process Monitoring
3. Results
3.1. Surface and Subsurface Damage (SSD) Evaluation
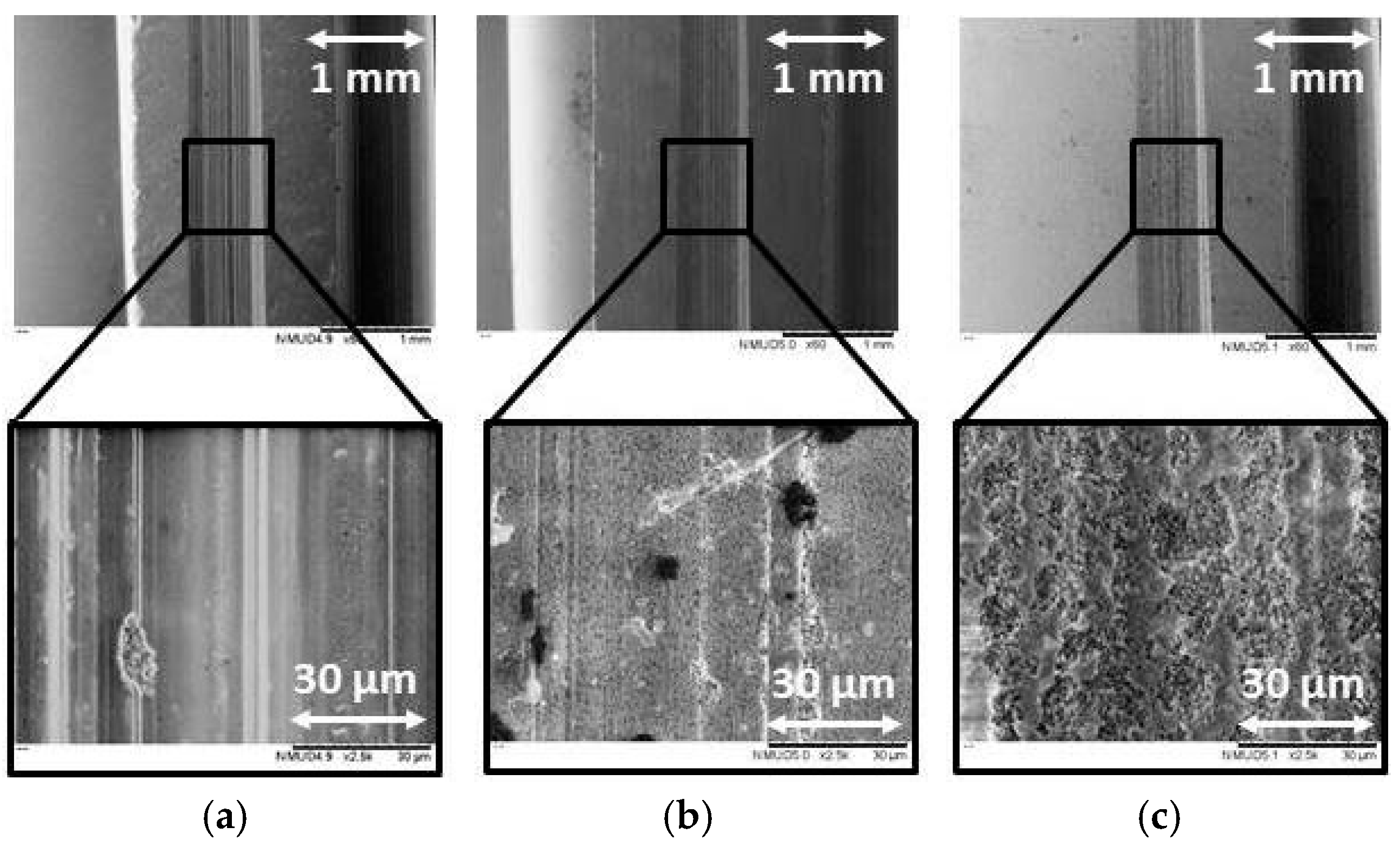
3.1.1. Microstructure
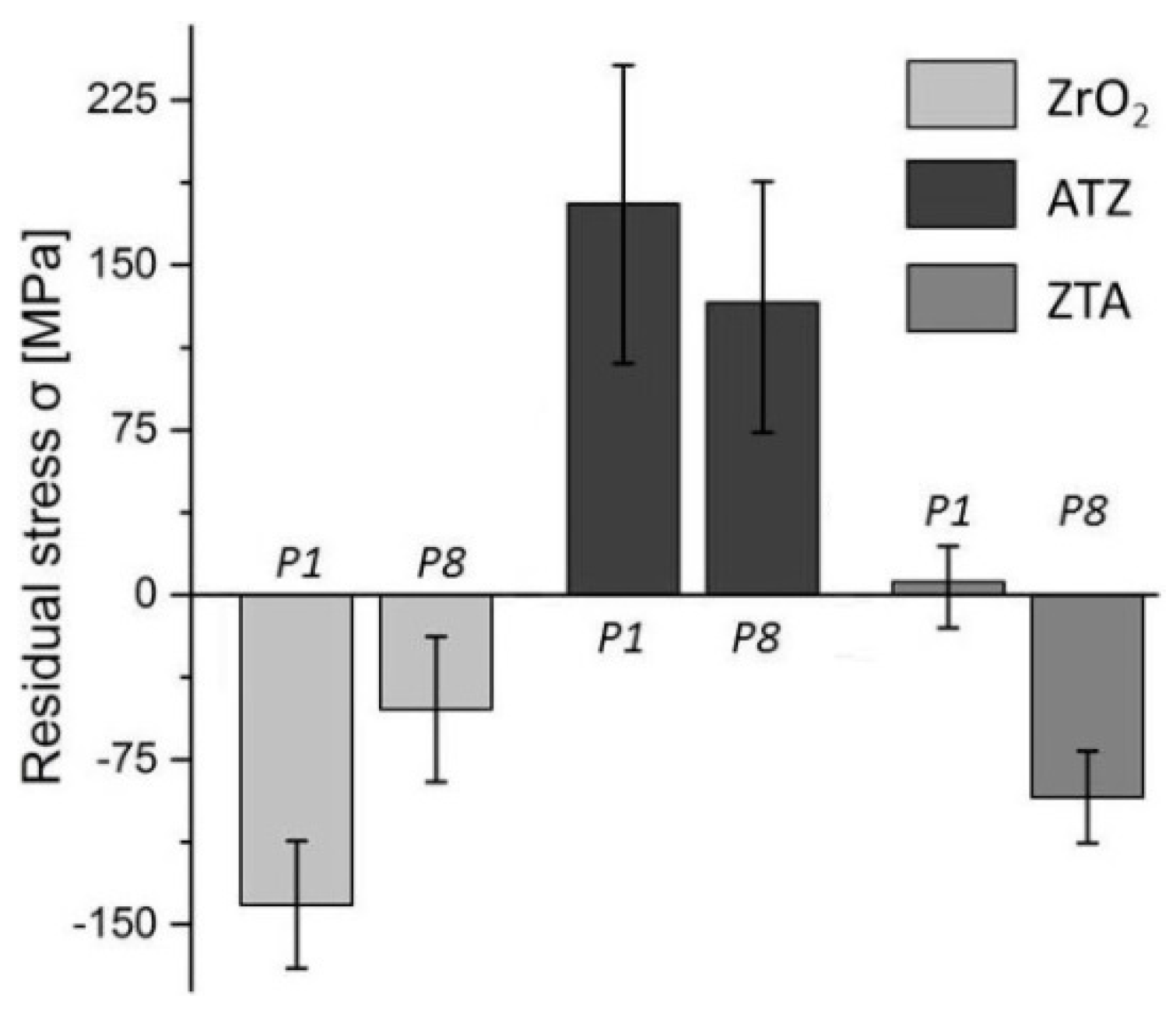
3.1.2. Residual Stress
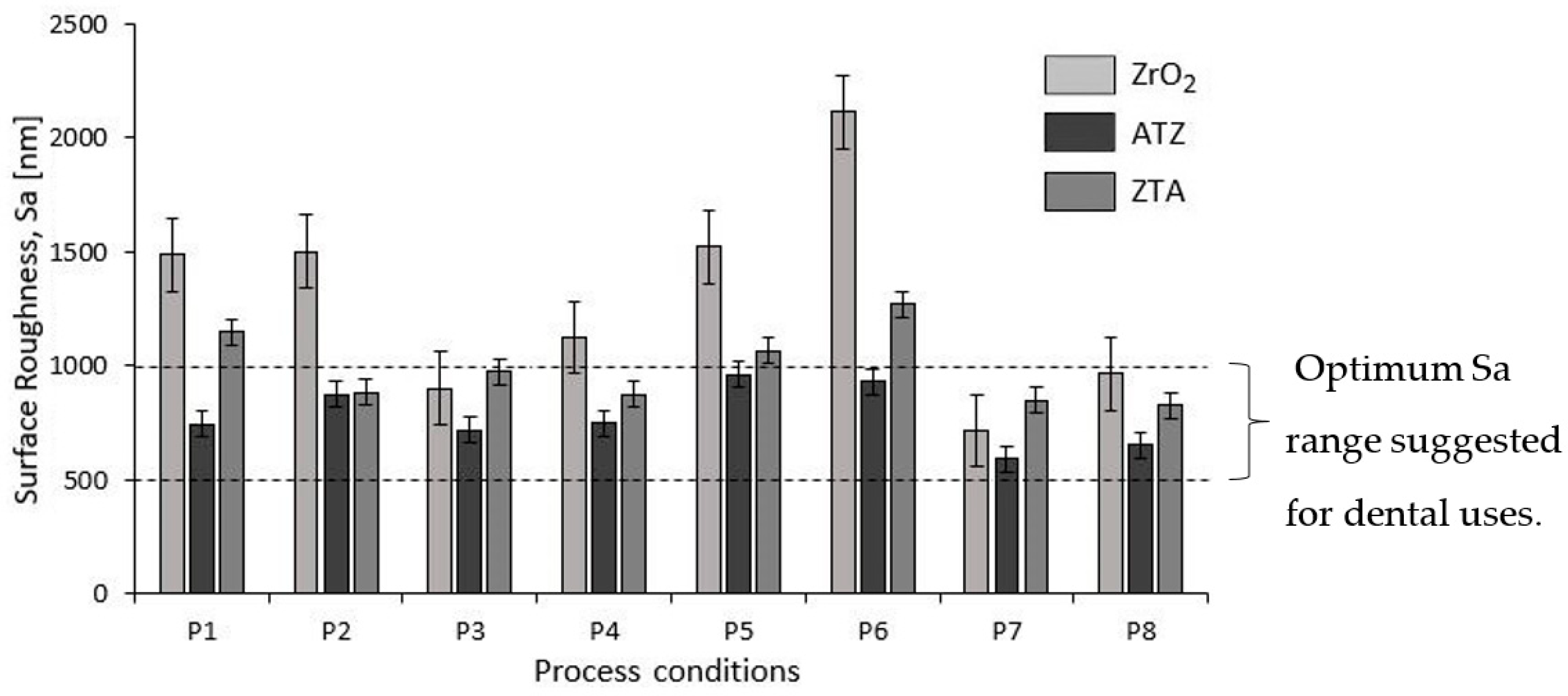
3.1.3. Surface Roughness
3.2. Process Monitoring
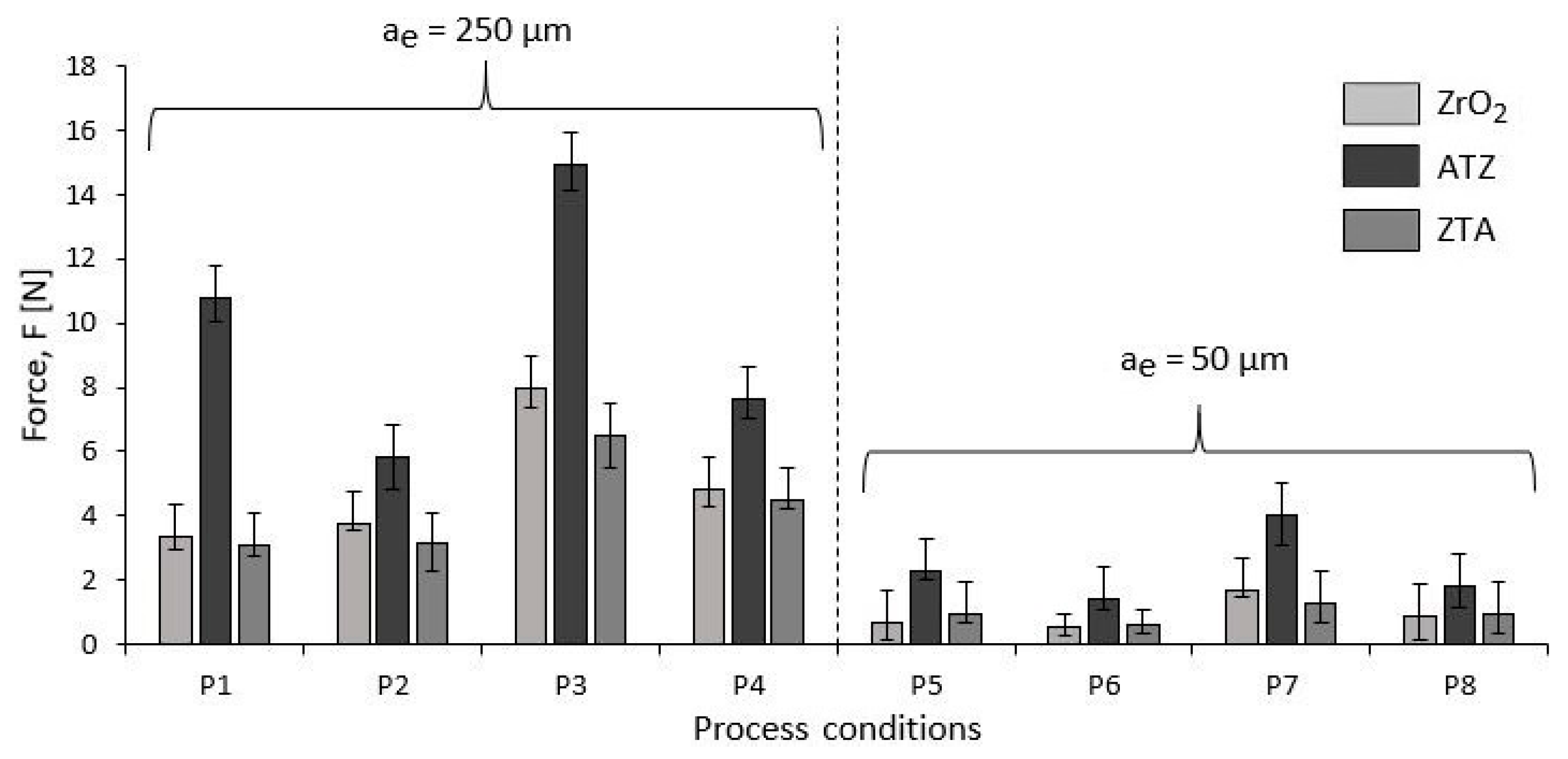
Grinding Forces
4. Discussion
4.1. Microstructure
4.2. Residual Stress
4.3. Influence of Processing Parameters on Surface Roughness and Grinding Forces
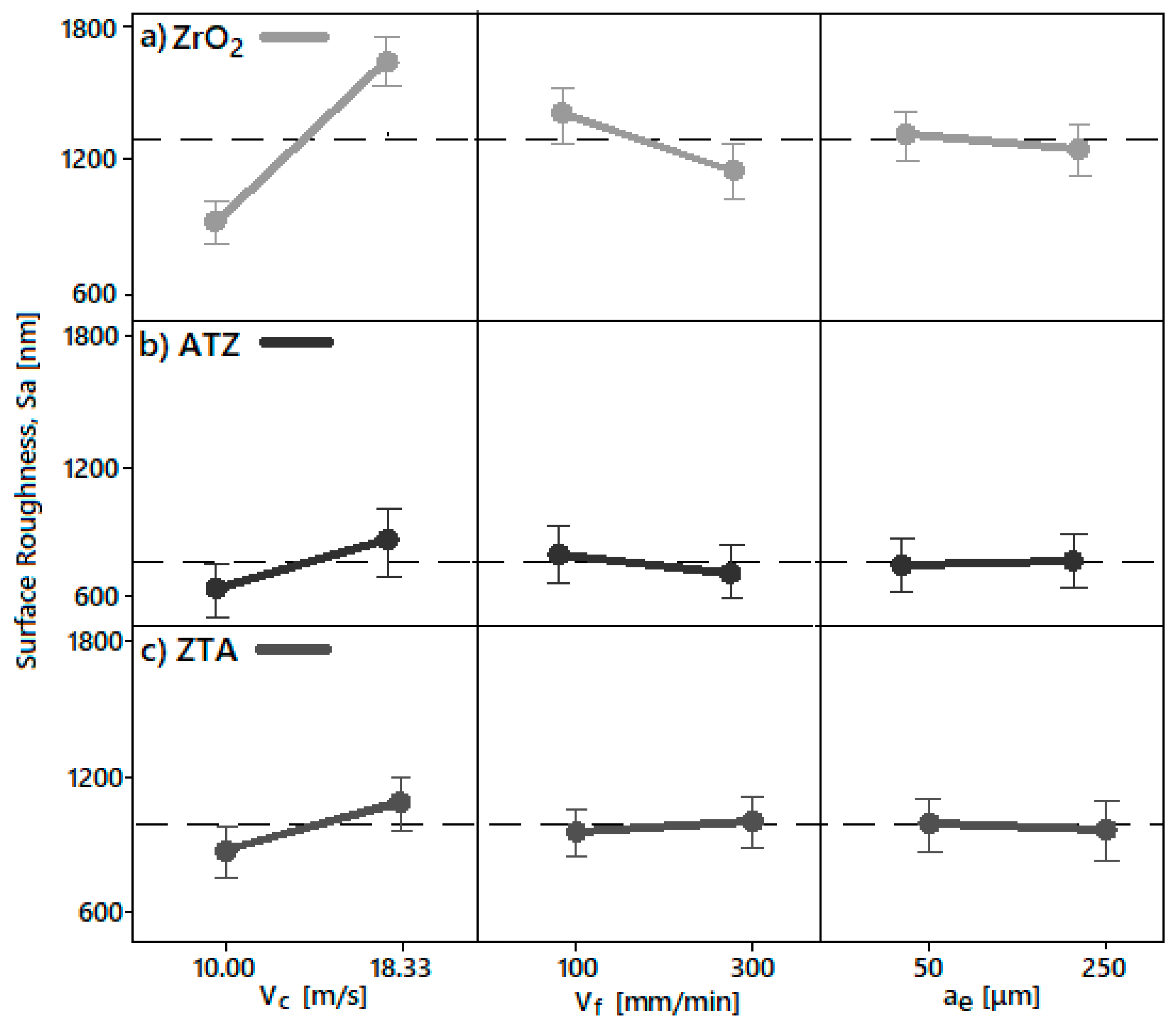
4.3.1. Surface Roughness
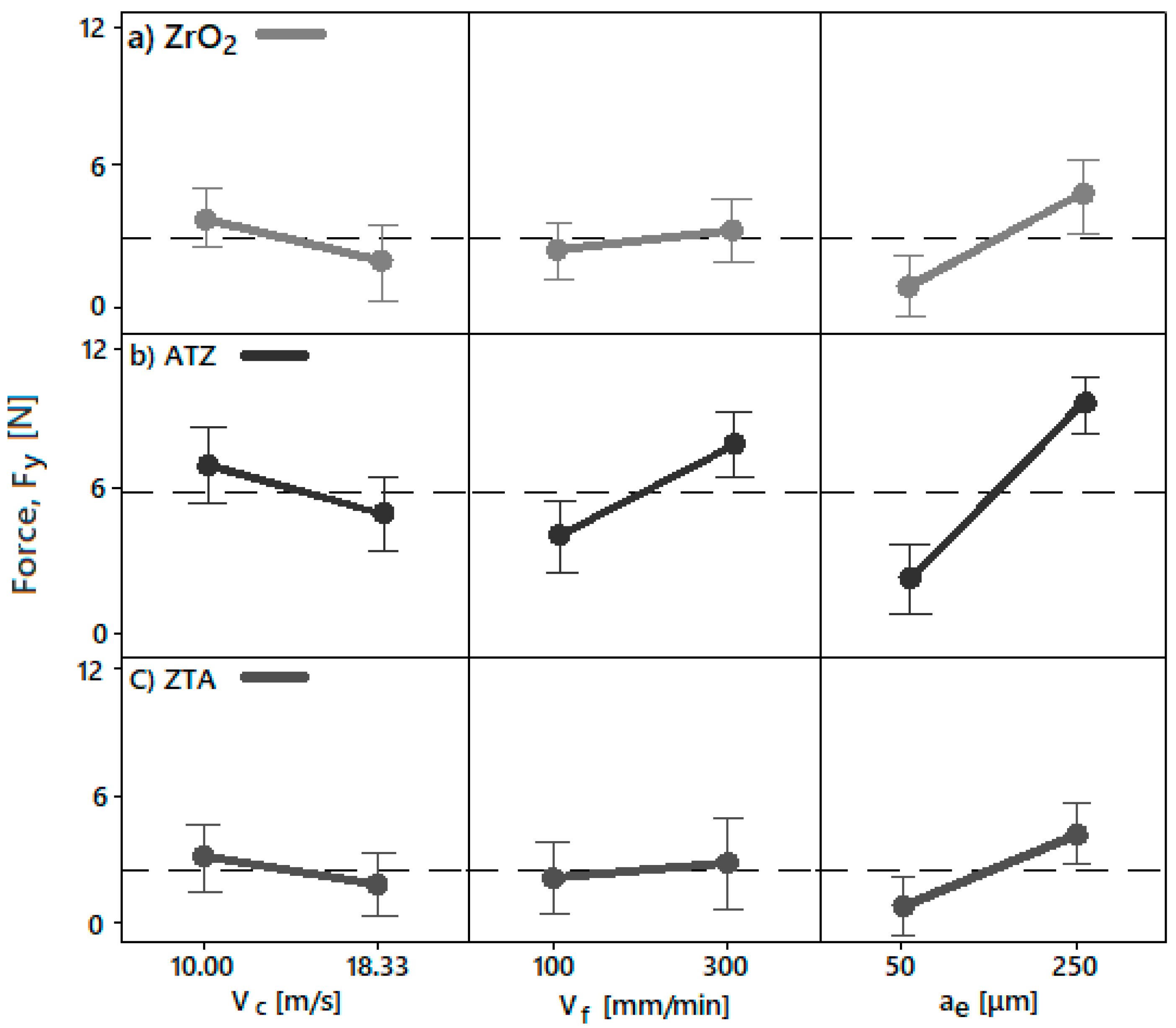
4.3.2. Grinding Forces
5. Conclusions
- The microstructures of the ground ATZ and ZTA workpieces showed brittle intercrystalline breakouts, high roughness, and bulging at the scratch edges. Although the ground ZrO2 surfaces had parallel grinding marks with micro-ploughing deformation, their microstructure had a larger amount of ductile areas than the other specimens.
- For a successful implant and mechanical stability in the jaw, compressive residual stresses on the material surface are recommended. ZrO2 ceramics had shown the best response concerning the residual stresses among the ceramics tested for dental application. Herein, higher compressive stresses after grinding were observed due to the toughening mechanics of the zirconia phase.
- The different surface roughness (Sa) and force (F) responses due to the different grinding parameters were directly correlated to the intrinsic physical properties and chemical composition of the bioceramics investigated. Basically, the machining conditions P3, P4, P7, and P8 generated the optimal surface roughness suggested for dental implants, i.e., between 500 and 1000 nm, on all machined bioceramic materials. This was due to the highest peripheral speed, vc, response on the surface roughness, which indicated that the low level tested (10.00 m/s) was beneficial to achieve an optimal Sa for dental uses. Regarding the process monitoring, the depth of cut (ae) had the highest influence on the grinding forces (Fy) response when it was larger—herein, the machining process with an ae of 50 μm are indicated for less tool wear and best implant integrity.
- ZrO2 ceramics machined with the grinding conditions P7 (vc = 10.00 m/s, vf = 300 mm/min, and ae = 50 μm) and P8 (vc = 10.00 m/s, vf = 100 mm/min, and ae = 50 μm) are suggested for further dental applications due to their optimal Sa range, smoother microstructures, compressive residual stress, as well as low forces generated during machining.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Process | ZrO2 | ATZ | ZTA |
|---|---|---|---|
| P1 | 1488.30 ± 152.46 | 744.74 ± 10.83 | 1148.46 ± 17.25 |
| P2 | 1502.08 ± 41.03 | 875.75 ± 51.44 | 884.17 ± 45.23 |
| P3 | 901.90 ± 57.05 | 720.76 ± 17.91 | 982.88 ± 10.78 |
| P4 | 1125.77 ± 2.46 | 749.89 ± 51.89 | 874.31 ± 13.80 |
| P5 | 1522.83 ± 89.90 | 961.05 ± 60.98 | 1065.36 ± 62.40 |
| P6 | 2113.38 ± 54.18 | 932.95 ± 17.07 | 1272.16 ± 47.94 |
| P7 | 716.62 ± 38.73 | 509.51 ± 27.51 | 850.40 ± 88.68 |
| P8 | 965.73 ± 19.05 | 652.46 ± 33.69 | 826.89 ± 13.89 |
| Process | ZrO2 | ATZ | ZTA |
|---|---|---|---|
| P1 | 3.37 ± 0.45 | 10.76 ± 0.72 | 3.07 ± 0.33 |
| P2 | 3.77 ± 0.22 | 5.80 ± 1.01 | 3.12 ± 1.14 |
| P3 | 7.95 ± 0.58 | 14.91 ± 1.12 | 6.50 ± 0.98 |
| P4 | 4.85 ± 0.54 | 7.64 ± 0.59 | 4.50 ± 0.30 |
| P5 | 0.65 ± 0.52 | 2.29 ± 0.25 | 0.94 ± 0.28 |
| P6 | 0.56 ± 0.18 | 1.42 ± 0.33 | 0.58 ± 0.22 |
| P7 | 1.67 ± 0.71 | 4.03 ± 0.94 | 1.31 ± 0.66 |
| P8 | 0.86 ± 0.28 | 1.80 ± 0.67 | 0.95 ± 0.61 |
References
- Gaviria, L.; Salcido, J.P.; Guda, T.; Ong, J.L. Current trends in dental implants. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 50–60. [Google Scholar] [CrossRef]
- Shemtov-Yona, K.; Rittel, D. On the mechanical integrity of retrieved dental implants. J. Mech. Behav. Biomed. Mater. 2015, 49, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Özkurt, Z.; Kazazoğlu, E. Zirconia dental implants: a literature review. J. Oral Implantol. 2011, 37, 367–376. [Google Scholar] [CrossRef]
- Muris, J.; Kleverlaan, C.J. Hypersensitivity to Dental Alloys. In Metal Allergy: From Dermatitis to Implant and Device Failure, 1st ed.; Chen, J.K., Thyssen, J.P., Eds.; Springer: Berlin, Germany, 2018; pp. 285–300. [Google Scholar]
- Richerson, D.W.; Lee, W.E. Modern Ceramic Engineering: Properties, Processing, and Use in Design; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Brinksmeier, E.; Mutlugünes, Y.; Klocke, F.; Aurich, J.C.; Shore, P.; Ohmori, H. Ultra-precision grinding. CIRP Ann. 2010, 59, 652–671. [Google Scholar] [CrossRef]
- Bianchi, E.C.; de Aguiar, P.R.; Diniz, A.E.; Canarim, R.C. Optimization of ceramics grinding. In Advances in Ceramics - Synthesis and Characterization, Processing and Specific Applications, 1st ed.; IntechOpen: London, UK, 2011. [Google Scholar]
- González, H.; Calleja, A.; Pereira, O.; Ortega, N.; López de Lacalle, L.; Barton, M. Super abrasive machining of integral rotary components using grinding flank tools. Metals 2018, 8, 24. [Google Scholar] [CrossRef]
- Zhang, Y.; Lawn, B.R. Novel zirconia materials in dentistry. J. Dent. Res. 2018, 97, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Denkena, B.; Breidenstein, B.; Busemann, S.; Lehr, C.M. Impact of hard machining on zirconia based ceramics for dental applications. Procedia CIRP 2017, 65, 248–252. [Google Scholar] [CrossRef]
- Allahkarami, M.; Hanan, J.C. Residual stress and phase transformation in Zirconia restoration ceramics. Adv. Bioceram. Porous. Ceram. V Ceram. Eng. Sci. Proc. 2012, 574, 37–47. [Google Scholar]
- Branemark, P.I. Osseointegration and its experimental background. J. Prosthet. Dent. 1983, 50, 399–410. [Google Scholar] [CrossRef]
- Manikyamba, Y.J.; Sajjan, S.; AV, R.R.; Rao, B.; Nair, C.K. Implant thread designs: An overview. Trends Prosthodont. Dent. Implantol. 2018, 8, 11–20. [Google Scholar]
- Strickstrock, M.; Rothe, H.; Grohmann, S.; Hildebrand, G.; Zylla, I.M.; Liefeith, K. Influence of surface roughness of dental zirconia implants on their mechanical stability, cell behavior and osseointegration. BioNanoMaterials 2017, 18, 1–10. [Google Scholar] [CrossRef]
- Secatto, F.B.S.; Elias, C.N.; Segundo, A.S.; Cosenza, H.B.; Cosenza, F.R.; Guerra, F.L.B. The morphology of collected dental implant prosthesis screws surface after six months to twenty years in chewing. Dent. Oral Craniofac. Res. 2017, 3, 7. [Google Scholar]
- Zhong, Z.W. Ductile or partial ductile mode machining of brittle materials. Int. J. Adv. Manuf. Technol. 2013, 21, 579–585. [Google Scholar] [CrossRef]
- Bifano, T.G.; Dow, T.A.; Scattergood, R.O. Ductile-regime grinding: A new technology for machining brittle materials. J. Eng. Ind. 1991, 113, 184–189. [Google Scholar] [CrossRef]
- Yang, M.; Li, C.; Zhang, Y.; Jia, D.; Zhang, X.; Hou, Y.; Li, L.; Wang, J. Maximum undeformed equivalent chip thickness for ductile-brittle transition of zirconia ceramics under different lubrication conditions. Int. J. Mach. Tools Manuf. 2017, 122, 55–65. [Google Scholar] [CrossRef]
- Fook, P.; Riemer, O. Characterisation of zirconia-based ceramics after micro-grinding. ASME J. Micro. Nano-Manuf. 2019. [Google Scholar] [CrossRef]
- Ryu, H.S.; Namgung, C.; Lee, J.H.; Lim, Y.J. The influence of thread geometry on implant osseointegration under immediate loading: a literature review. J. Adv. Prosthodont. 2014, 6, 547–554. [Google Scholar] [CrossRef]
- Javed, F.; Ahmed, H.B.; Crespi, R.; Romanos, G.E. Role of primary stability for successful osseointegration of dental implants: Factors of influence and evaluation. Interv. Med. Appl. Sci. 2013, 5, 162–167. [Google Scholar] [CrossRef]
- Dahiya, V.; Shukla, P.; Gupta, S. Surface topography of dental implants: A review. J. Dent. Implants 2014, 4, 66–71. [Google Scholar]
- Werkstoffe TKC - Technische Keramik GmbH. Available online: https://tkc-keramik.de/ (accessed on 25 April 2019).
- Egea, S.A.; Martynenko, V.; Krahmer, M.D.; López de Lacalle, L.; Benítez, A.; Genovese, G. On the cutting performance of segmented diamond blades when dry-cutting concrete. Materials 2018, 11, 264. [Google Scholar] [CrossRef]
- Galvanic tools for hard and brittle material processing - Schott Diamantwerkzeuge GmbH. Available online: https://schott-diamantwerkzeuge.com (accessed on 25 April 2019).
- İşerı, U.; Özkurt, Z.; Kazazoğlu, E.; Küçükoğlu, D. Influence of grinding procedures on the flexural strength of zirconia ceramics. Braz. Dent. J. 2010, 21, 528–532. [Google Scholar] [CrossRef]
- Emami, M.; Sadeghi, M.H.; Sarhan, A.A.D.; Hasani, F. Investigating the Minimum Quantity Lubrication in grinding of Al2O3 engineering ceramic. J. Clean. Prod. 2014, 66, 632–643. [Google Scholar] [CrossRef]
- Chang, C.W.; Kuo, C.P. Evaluation of surface roughness in laser-assisted machining of aluminum oxide ceramics with Taguchi method. Int. J. Mach. Tools Manuf. 2017, 47, 141–147. [Google Scholar] [CrossRef]
- Singh, B.; Singh, G. Experimental investigation of cutting parameters influence on surface finish during turning of steel using Taguchi approach. Int. J. Prod. Res. 2018, 13, 49–67. [Google Scholar] [CrossRef]
- Abdo, B.; Darwish, S.M.; El-Tamimi, A.M. Parameters optimization of rotary ultrasonic machining of zirconia ceramic for surface roughness using statistical Taguchi’s experimental design. Appl. Mech. Mater. 2012, 184, 11–17. [Google Scholar] [CrossRef]
- Zeng, W.M.; Li, Z.C.; Pei, Z.J.; Treadwell, C. Experimental observation of tool wear in rotary ultrasonic machining of advanced ceramics. Int. J. Mach. Tools Manuf. 2005, 45, 1468–1473. [Google Scholar] [CrossRef]
- Lemons, J.E. Biomaterials, biomechanics, tissue healing, and immediate-function dental implants. J. Oral Implantol. 2004, 30, 318–324. [Google Scholar] [CrossRef]
- Brinksmeier, E.; Cammett, J.T.; König, W.; Leskovar, P.; Peters, J.; Tönshoff, H.K. Residual stresses—measurement and causes in machining processes. Cirp Ann. 1982, 31, 491–510. [Google Scholar] [CrossRef]
- Pradell, T.; Glaude, P.; Peteves, S.D.; Bullock, E. Measurement of Residual Stress in Engineering Ceramics; Physical Sciences; European Commission: Brussels, Belgium, 1990; p. 88, EUR 12635 EN. [Google Scholar]
- Minitab, I. MINITAB Release 17: Statistical Software for Windows; Minitab Inc.: State College, PA, USA, 2014. [Google Scholar]


| Material | Density (g/cm3) | Fracture Toughness KIC (MPa m1/2) | Young’s Modulus (GPa) | Hardness HV10 (GPa) | Flexural Strength (MPa) | Fraction of ZrO2 (%) |
|---|---|---|---|---|---|---|
| ZrO2-TZP | 6.03 | 4.8 | 200 | 11 | 1000 | > 95 |
| ATZ | 5.50 | 7 | 220 | 14 | 820 | 76 |
| ZTA | 4.10 | 8 | 380 | 16 | 440 | 14 |
| Peripheral Speed, vc (m/s) | Feed Speed, vf (mm/min) | Depth of Cut, ae (μm) | Material |
|---|---|---|---|
| 10.00, 18.33 | 100, 300 | 50, 250 | ZrO2, ATZ, ZTA |
| Process Condition | Peripheral Speed, vc (m/s) | Feed Speed, vf (mm/min) | Depth of Cut, ae (μm) |
|---|---|---|---|
| P1 | 18.33 | 300 | 250 |
| P2 | 18.33 | 100 | 250 |
| P3 | 10.00 | 300 | 250 |
| P4 | 10.00 | 100 | 250 |
| P5 | 18.33 | 300 | 50 |
| P6 | 18.33 | 100 | 50 |
| P7 | 10.00 | 300 | 50 |
| P8 | 10.00 | 100 | 50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fook, P.; Berger, D.; Riemer, O.; Karpuschewski, B. Structuring of Bioceramics by Micro-Grinding for Dental Implant Applications. Micromachines 2019, 10, 312. https://doi.org/10.3390/mi10050312
Fook P, Berger D, Riemer O, Karpuschewski B. Structuring of Bioceramics by Micro-Grinding for Dental Implant Applications. Micromachines. 2019; 10(5):312. https://doi.org/10.3390/mi10050312
Chicago/Turabian StyleFook, Pablo, Daniel Berger, Oltmann Riemer, and Bernhard Karpuschewski. 2019. "Structuring of Bioceramics by Micro-Grinding for Dental Implant Applications" Micromachines 10, no. 5: 312. https://doi.org/10.3390/mi10050312
APA StyleFook, P., Berger, D., Riemer, O., & Karpuschewski, B. (2019). Structuring of Bioceramics by Micro-Grinding for Dental Implant Applications. Micromachines, 10(5), 312. https://doi.org/10.3390/mi10050312





