Development of a Spherical Model with a 3D Microchannel: An Application to Glaucoma Surgery
Abstract
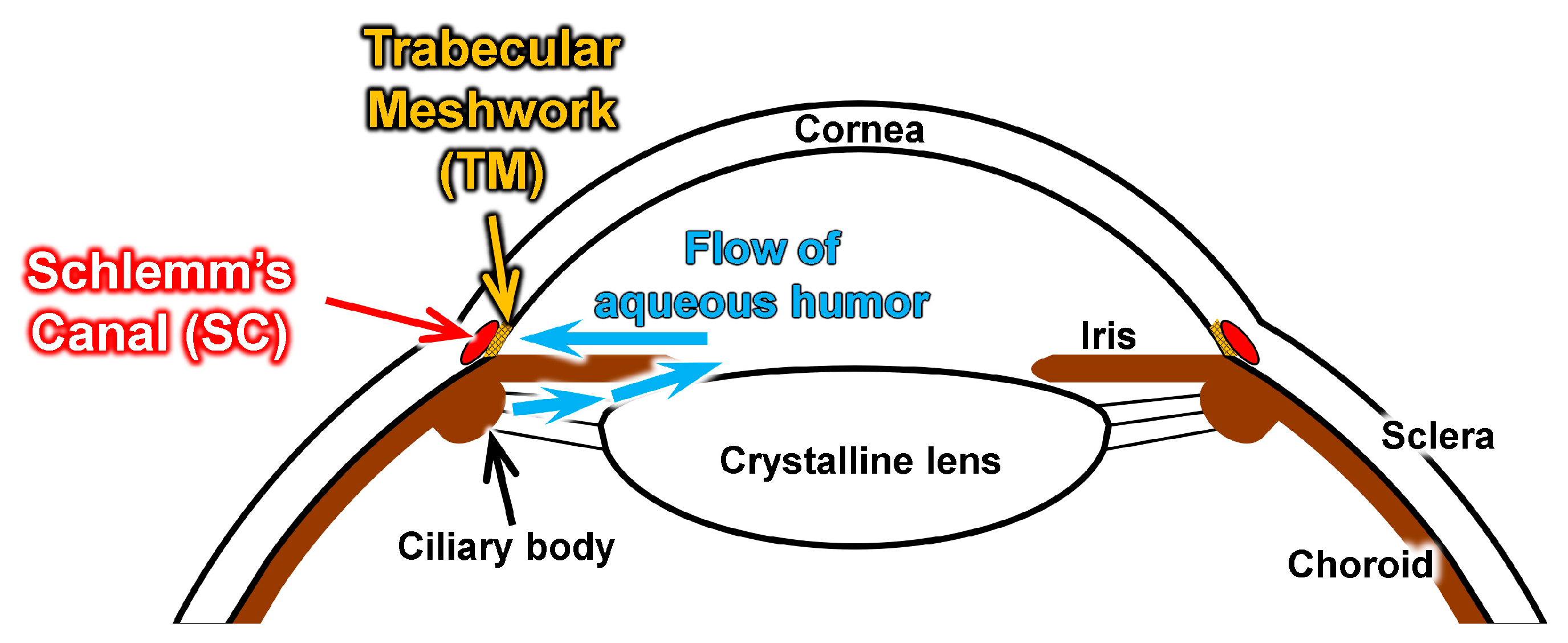
:1. Introduction
2. Materials and Methods
2.1. Design Concept
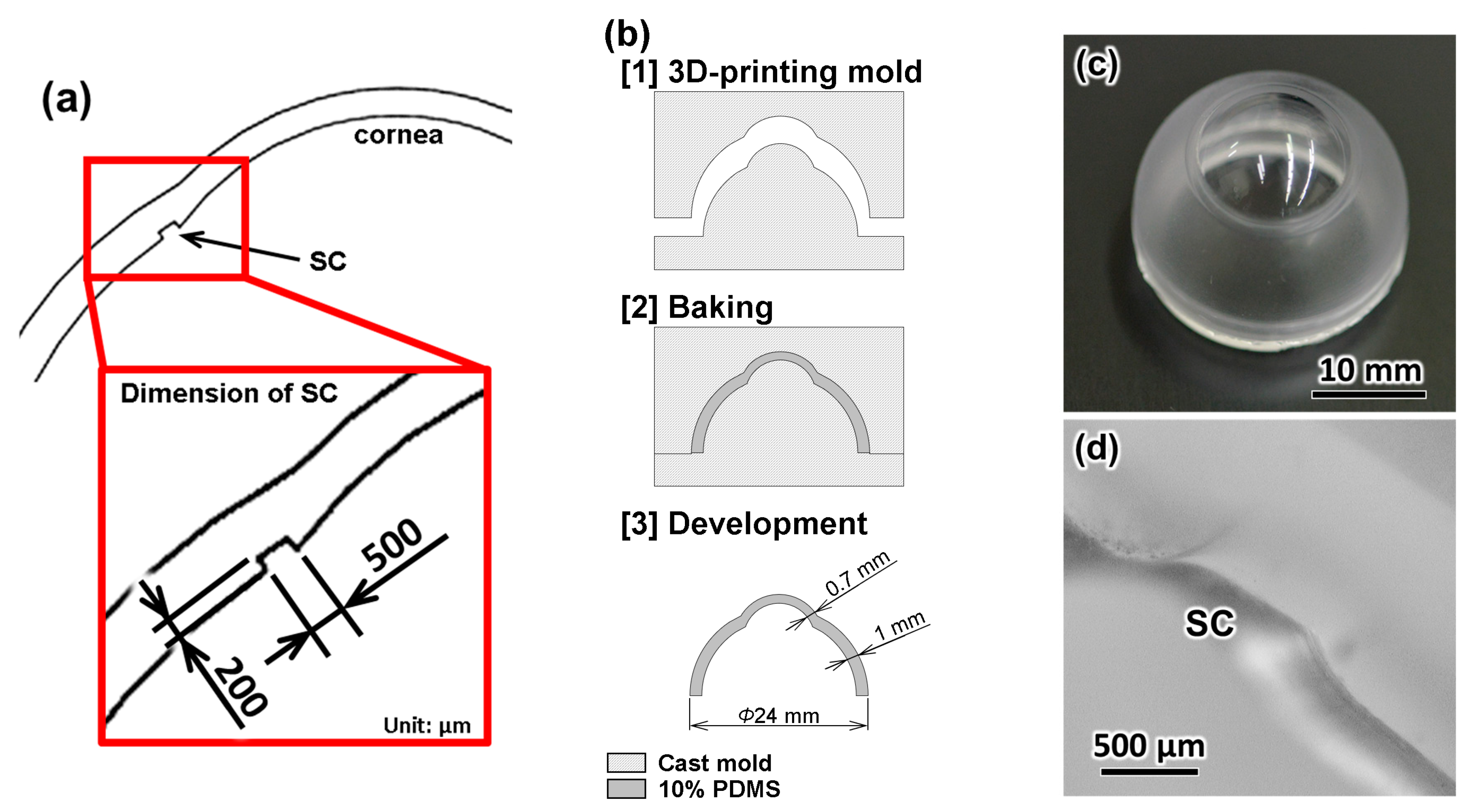
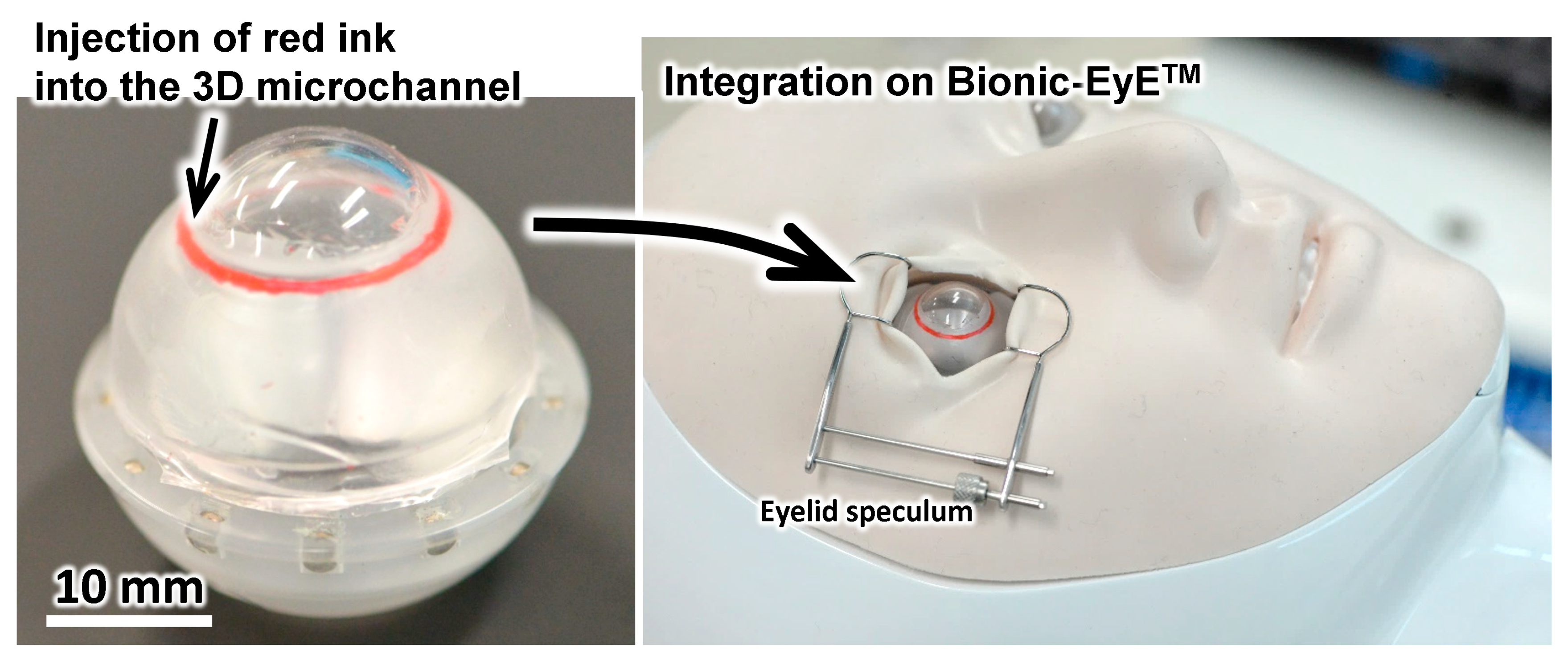
2.2. Fabrication of the Eyeball Model
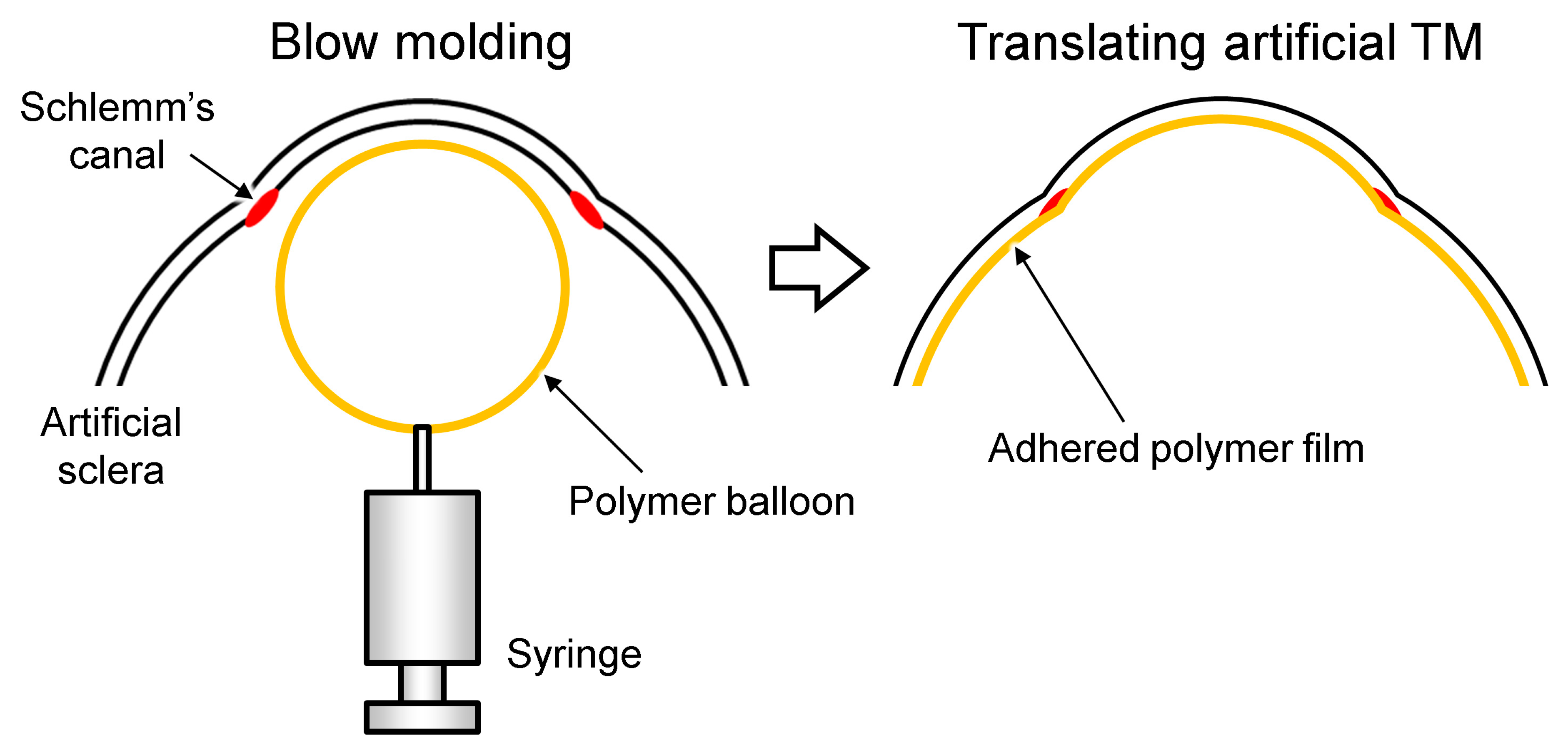
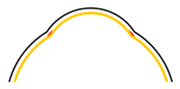
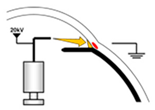
2.3. Fabrication of the TM
2.4. Thickness Measurement of Artificial TM
2.5. Measurement of the Mechanical Properties of TM
3. Results
3.1. Eyeball Model
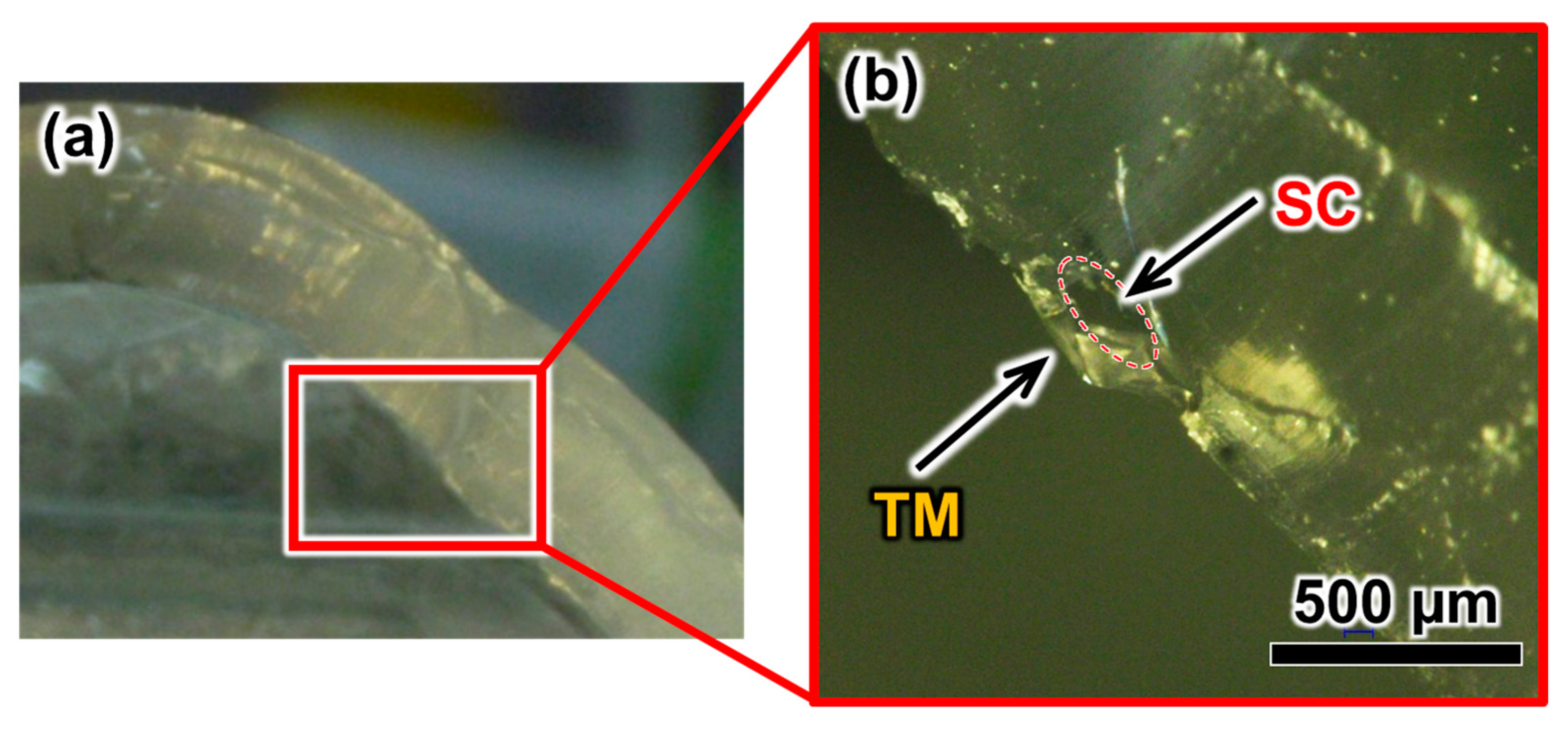
3.2. Fabrication Results of the TM
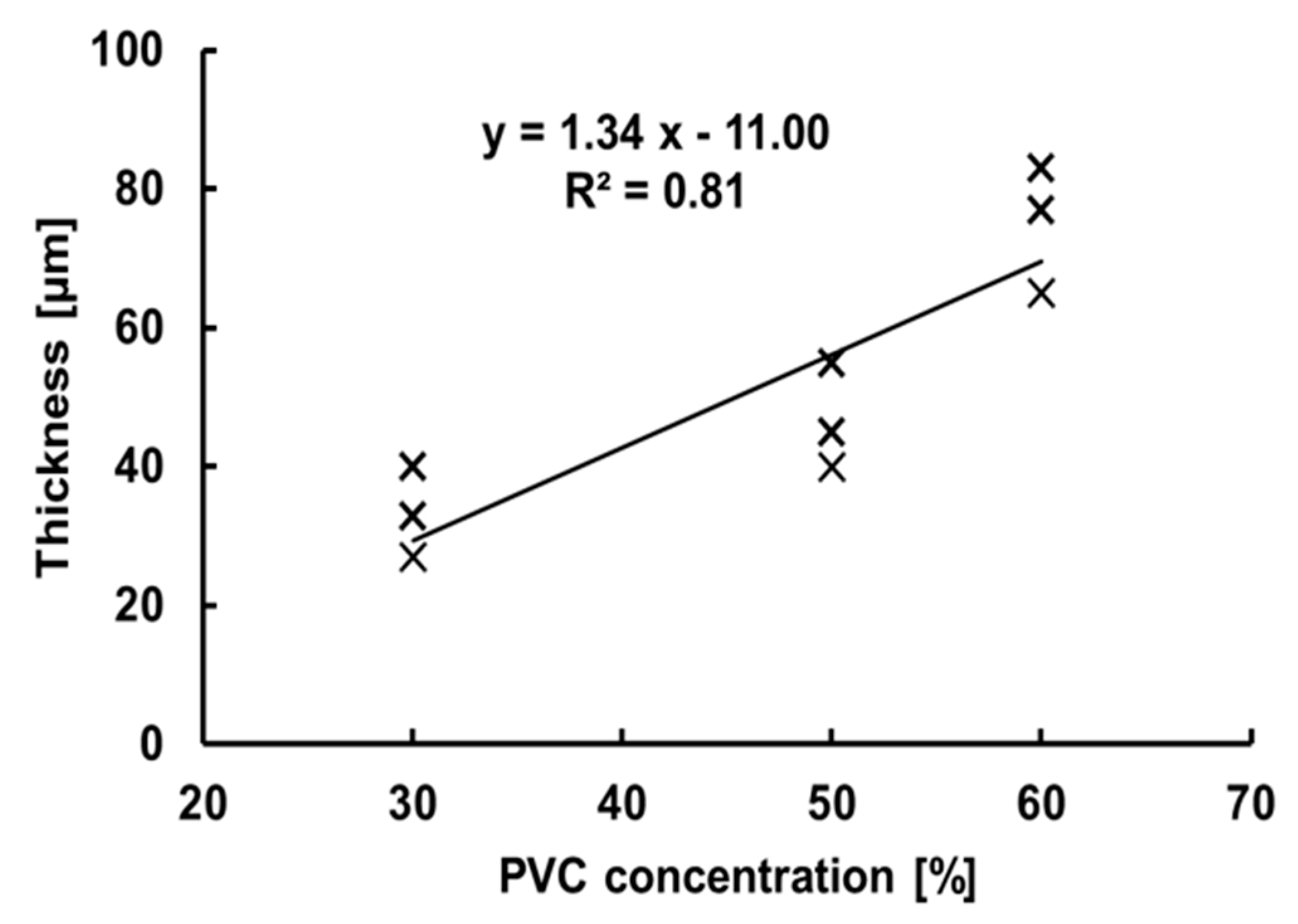
3.3. Evaluation of the TM Thickness
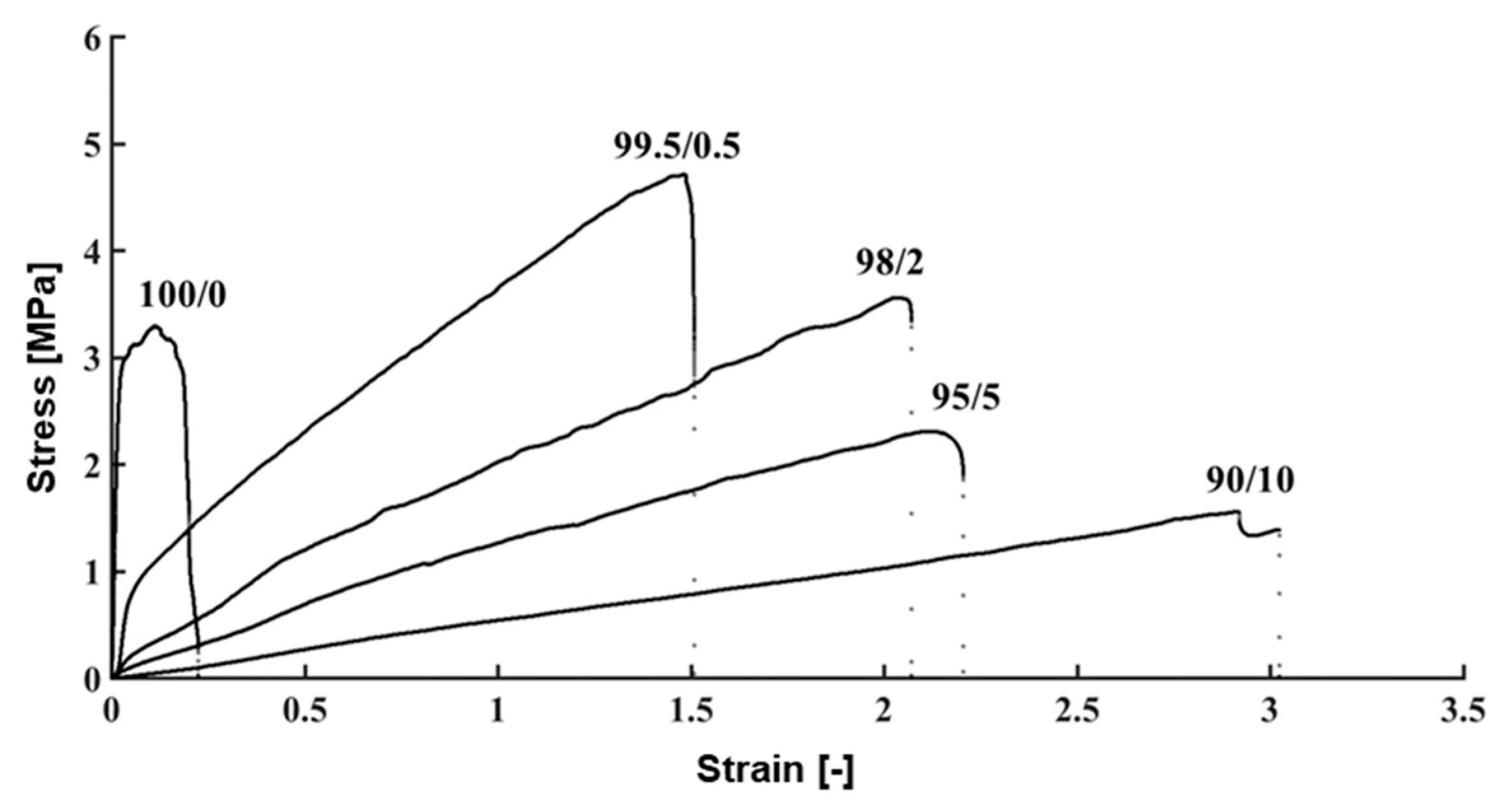
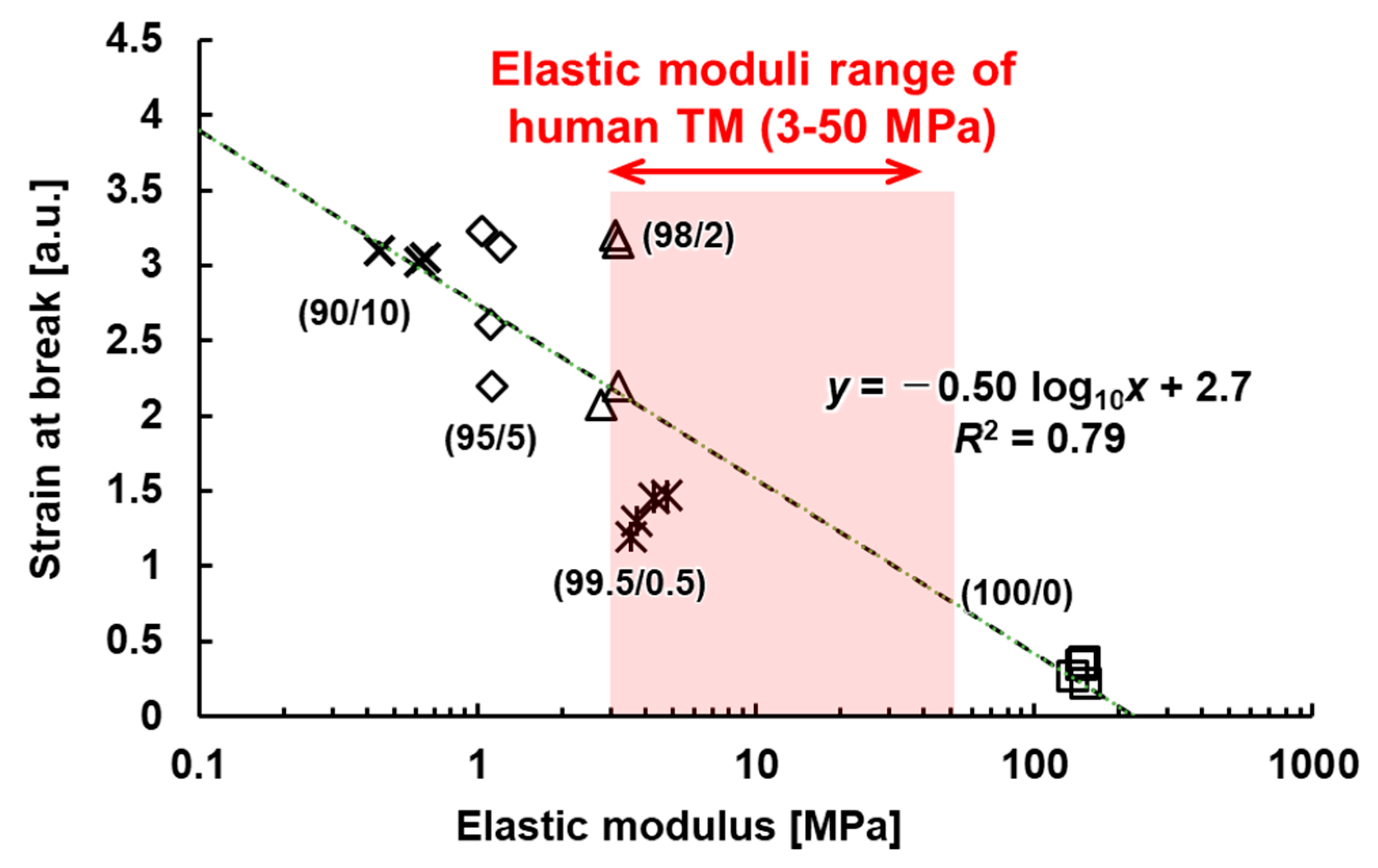
3.4. Evaluation of Mechanical Properties of the TM
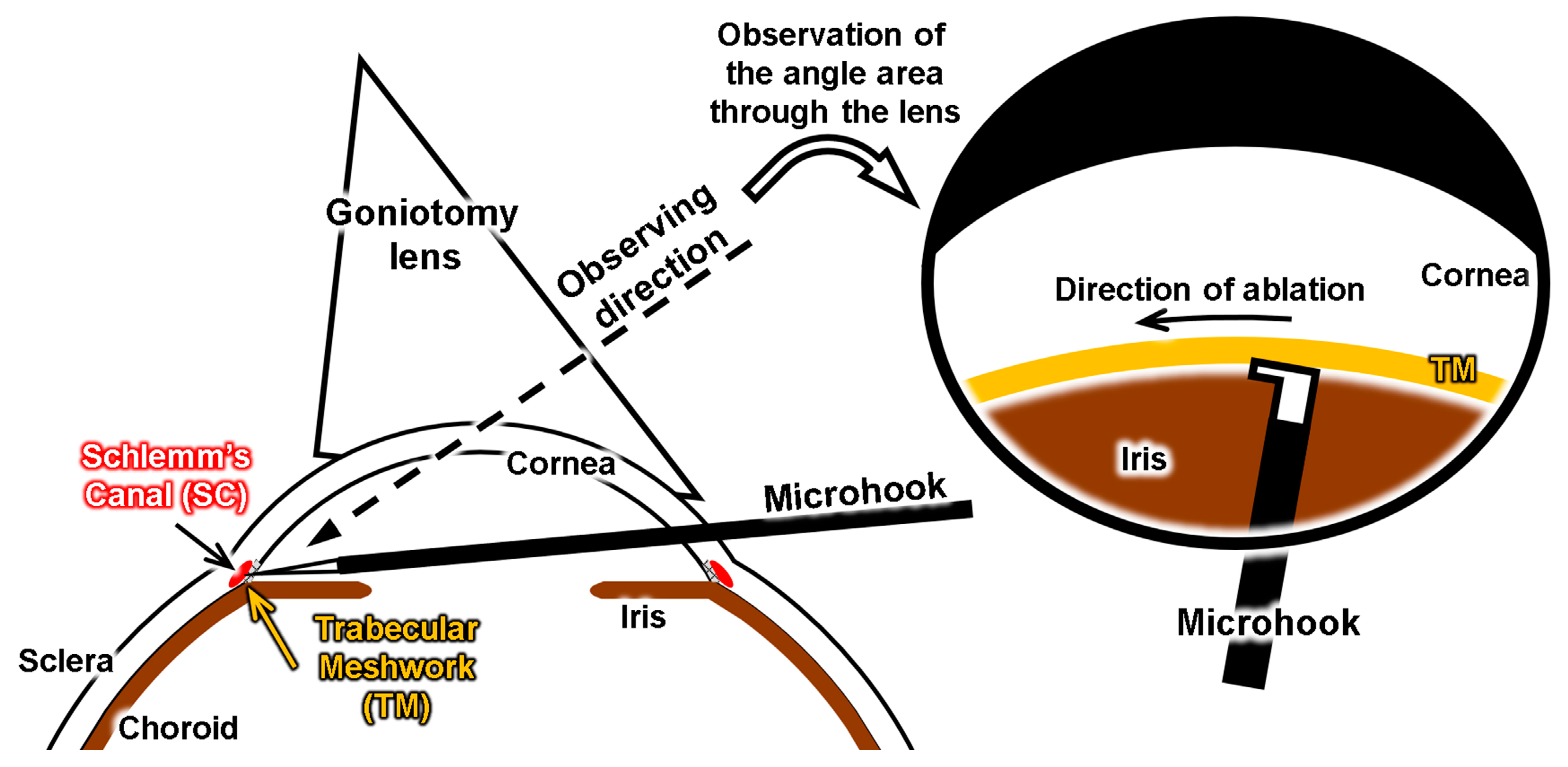
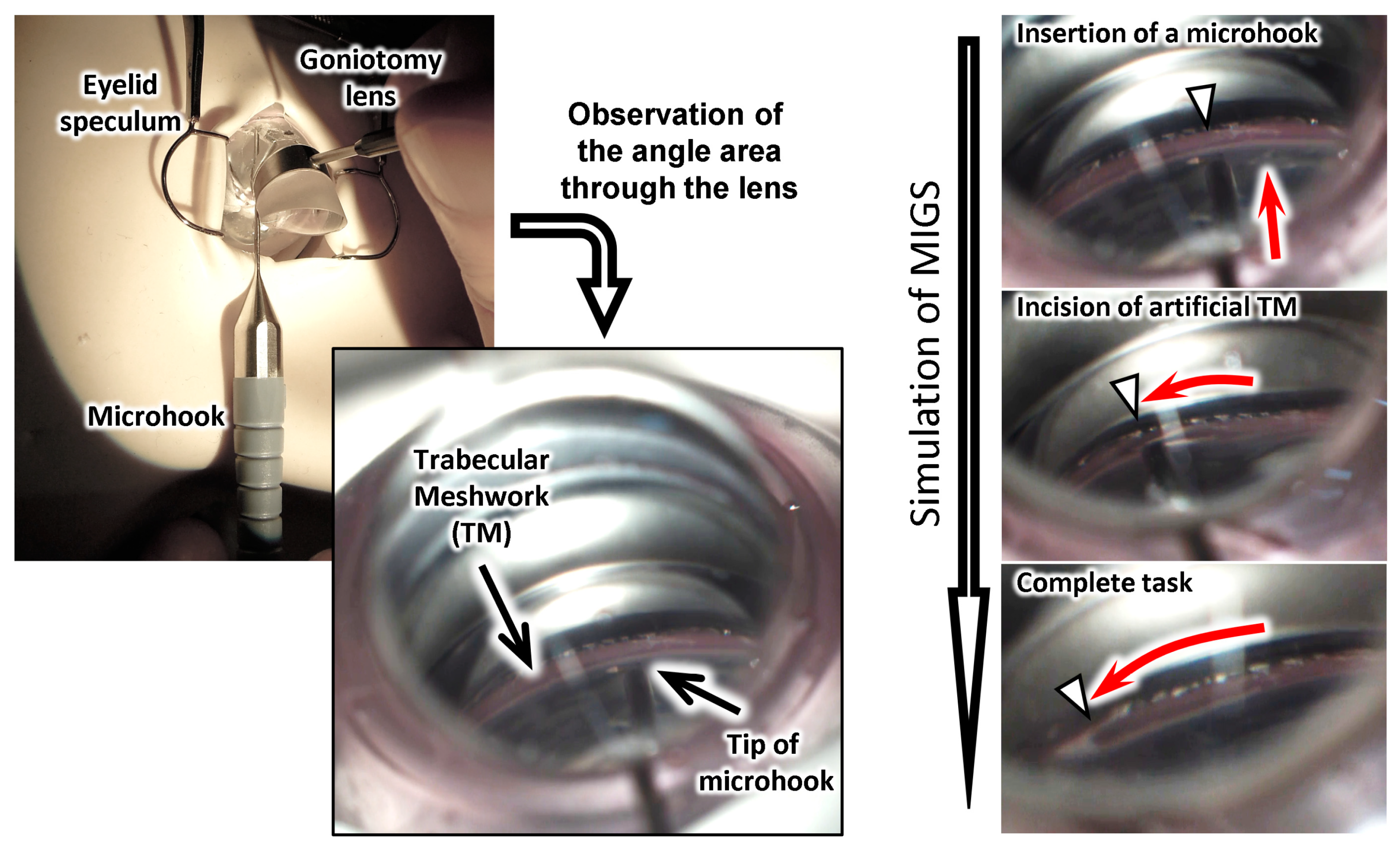
3.5. Simulation of Surgery with the Eye Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rengier, F.; Mehndiratta, A.; Von Tengg-Kobligk, H.; Zechmann, C.M.; Unterhinninghofen, R.; Kauczor, H.U.; Giesel, F.L. 3D printing based on imaging data: Review of medical applications. Int. J. Comput. Assist. Radiol. Surg. 2010, 5, 335–341. [Google Scholar] [CrossRef]
- Kneebone, R. Simulation in surgical training: educational issues and practical implications. Med. Educ. 2003, 37, 267–277. [Google Scholar] [CrossRef]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Broman, A.T. Number of people with glaucoma worldwide. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef]
- Goel, M.; Picciani, R.G.; Lee, R.K.; Bhattacharya, S.K. Aqueous humor dynamics: A review. Open Ophthalmol. J. 2010, 4, 52. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.R. The pathology of the out flow system in primary and secondary glaucoma. Eye 1995, 9, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Spileers, W.; Goethals, M. Structural changes of the lamina cribrosa and of the trabeculum in primary open angle glaucoma (POAG). Bull. Soc. Belge Ophthalmol. 1992, 244, 27. [Google Scholar]
- Tamm, E.R. The trabecular meshwork outflow pathways: structural and functional aspects. Exp. Eye Res. 2009, 88, 648–655. [Google Scholar] [CrossRef]
- Grant, W.M. Clinical measurements of aqueous outflow. AMA Arch. Ophthalmol. 1951, 46, 113–131. [Google Scholar] [CrossRef]
- Grant, W.M. Experimental Aqueous Perfusion in Enucleated Human Eyes. Arch. Ophthalmol. 1963, 69, 783–801. [Google Scholar] [CrossRef]
- Brubaker, R.F. Targeting outflow facility in glaucoma management. Surv. Ophthalmol. 2003, 48. [Google Scholar] [CrossRef]
- Akura, J.; Pokharel, K. Artificial Lens for Cataract Surgery Practice. U.S. Patent No. 8,821,166, 2 September 2014. [Google Scholar]
- Van Dalen, J.T.W.; Carda, D.D. Model Human Eye and Face Manikin for Use therewith. U.S. Patent No. 8,684,743, 1 April 2014. [Google Scholar]
- Bernal, A. Ophthalmic Surgical Simulation System. U.S. Patent Application No. 14,468,769, 3 March 2016. [Google Scholar]
- Borenstein, J.T.; Tupper, M.M.; Mack, P.J.; Weinberg, E.J.; Khalil, A.S.; Hsiao, J.; García-Cardeña, G. Functional endothelialized microvascular networks with circular cross-sections in a tissue culture substrate. Biomed. Microdev. 2010, 12, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Kamei, K.I.; Mashimo, Y.; Koyama, Y.; Fockenberg, C.; Nakashima, M.; Nakajima, M.; Li, J.; Chen, Y. 3D printing of soft lithography mold for rapid production of polydimethylsiloxane-based microfluidic devices for cell stimulation with concentration gradients. Biomed. Microdev. 2015, 17, 17–36. [Google Scholar] [CrossRef]
- Gallab, M.; Tomita, K.; Omata, S.; Arai, F. Fabrication of 3D Capillary Vessel Models with Circulatory Connection Ports. Micromachines 2018, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Beebe, D.J.; Mensing, G.A.; Walker, G.M. Physics and Applications of Microfluidics in Biology. Annu. Rev. Biomed. Eng. 2002, 4, 261–286. [Google Scholar] [CrossRef]
- Omata, S.; Someya, Y.; Adachi, S.; Masuda, T.; Hayakawa, T.; Harada, K.; Mitsuishi, M.; Totsuka, K.; Araki, F.; Takao, M.; et al. A surgical simulator for peeling the inner limiting membrane during wet conditions. PLoS ONE 2018, 13, e0196131. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M. Microhook ab interno trabeculotomy, a novel minimally invasive glaucoma surgery. Clin. Ophthalmol. (Auckland, NZ) 2018, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Yamagishi, Y.; Takei, N.; Owaki, H.; Matsusaki, M.; Akashi, M.; Arai, F. Three-Dimensional Assembly of Multilayered Tissues Using Water Transfer Printing. JRM 2013, 25, 690–697. [Google Scholar] [CrossRef]
- Dietlein, T.S.; Jacobi, P.C.; Luke, C.; Krieglstein, G.K. Morphological variability of the trabecular meshwork in glaucoma patients: Implications for non-perforating glaucoma surgery. Br. J. Ophthalmol. 2000, 84, 1354–1359. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; Da Silva, M.A.; Dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Strobl, G.R. Non-Linear Mechanics. In The Physics of Polymers; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Khetani, S.R.; Bhatia, S.N. Microscale culture of human liver cells for drug development. Nat. Biotechnol. 2008, 26, 120–126. [Google Scholar] [CrossRef]
- Stevens, K.R.; Ungrin, M.D.; Schwartz, R.E.; Ng, S.; Carvalho, B.; Christine, K.S.; Chaturvedi, R.R.; Li, C.Y.; Zandstra, P.W.; Chen, C.S.; et al. InVERT molding for scalable control of tissue microarchitecture. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Johnston, I.D.; McCluskey, D.K.; Tan, C.K.L.; Tracey, M.C. Mechanical characterization of bulk Sylgard 184 for microfluidics and microengineering. J. Micromech. Microeng. 2014, 24, 035017. [Google Scholar] [CrossRef]
- Friberg, T.R.; Lace, J.W. A comparison of the elastic properties of human choroid and sclera. Exp. Eye Res. 1988, 47, 429–436. [Google Scholar] [CrossRef]
- Camras, L.J.; Stamer, W.D.; Epstein, D.; Gonzalez, P.; Yuan, F. Differential effects of trabecular meshwork stiffness on outflow facility in normal human and porcine eyes. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5242–5250. [Google Scholar] [CrossRef]
- Camras, L.J.; Stamer, W.D.; Epstein, D.; Gonzalez, P.; Yuan, F. Circumferential tensile stiffness of glaucomatous trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 2014, 55, 814–823. [Google Scholar] [CrossRef] [PubMed]
| Method Property | Dip Coating 1 | Electrospinning 2 | Hydraulic Transfer Printing 3 | Blow Molding 4 |
|---|---|---|---|---|
 |  |  |  | |
| Uniformity of Thickness | - | ✓ | ✓ | ✓ |
| Reproducibility of Mechanical Properties | - | ✓ | ✓ | ✓ |
| Ease of Maintenance of SC Tube Structure | - | - | ✓ | ✓ |
| Adequateness of Thin Film Forming | - | - | - | ✓ |
| Tissues and Materials | Elastic Modulus (MPa) | Tensile Strength (MPa) | Strain at Breaking (mm/mm) |
|---|---|---|---|
| Human Tissues | |||
| Sclera | 2.0 [28] | - | 0.2 [28] |
| TM Tissue (Normal Eye) | 0.2–1.1 [29] | 2.0 [29] | 0.06 [29] |
| TM Tissue (Glaucomatous Eye) | 3.0–52.6 [30] | - | - |
| Artificial Materials | |||
| PDMS | 1.3–2.9 [27] | 3.5–7.6 [27] | - |
| PVC/DEHP (100/0) | 147 ± 6 | 3.0 ± 0.3 | 0.30 ± 0.06 |
| PVC/DEHP (99.5/0.5) | 4.1 ± 0.5 | 4.0 ± 0.5 | 1.4 ± 0.2 |
| PVC/DEHP (98/2) | 3.1 ± 0.2 | 3.6 ± 0.9 | 2.7 ± 0.6 |
| PVC/DEHP (95/5) | 1.12 ± 0.06 | 2.3 ± 0.6 | 2.8 ± 0.5 |
| PVC/DEHP (90/10) | 0.6 ± 0.1 | 1.6 ± 0.2 | 3.1 ± 0.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallab, M.; Omata, S.; Harada, K.; Mitsuishi, M.; Sugimoto, K.; Ueta, T.; Totsuka, K.; Araki, F.; Takao, M.; Aihara, M.; et al. Development of a Spherical Model with a 3D Microchannel: An Application to Glaucoma Surgery. Micromachines 2019, 10, 297. https://doi.org/10.3390/mi10050297
Gallab M, Omata S, Harada K, Mitsuishi M, Sugimoto K, Ueta T, Totsuka K, Araki F, Takao M, Aihara M, et al. Development of a Spherical Model with a 3D Microchannel: An Application to Glaucoma Surgery. Micromachines. 2019; 10(5):297. https://doi.org/10.3390/mi10050297
Chicago/Turabian StyleGallab, Mahmoud, Seiji Omata, Kanako Harada, Mamoru Mitsuishi, Koichiro Sugimoto, Takashi Ueta, Kiyohito Totsuka, Fumiyuki Araki, Muneyuki Takao, Makoto Aihara, and et al. 2019. "Development of a Spherical Model with a 3D Microchannel: An Application to Glaucoma Surgery" Micromachines 10, no. 5: 297. https://doi.org/10.3390/mi10050297
APA StyleGallab, M., Omata, S., Harada, K., Mitsuishi, M., Sugimoto, K., Ueta, T., Totsuka, K., Araki, F., Takao, M., Aihara, M., & Arai, F. (2019). Development of a Spherical Model with a 3D Microchannel: An Application to Glaucoma Surgery. Micromachines, 10(5), 297. https://doi.org/10.3390/mi10050297





