Detection of Cigarette Smoke Using a Surface-Acoustic-Wave Gas Sensor with Non-Polymer-Based Oxidized Hollow Mesoporous Carbon Nanospheres
Abstract
1. Introduction
2. Materials and Methods
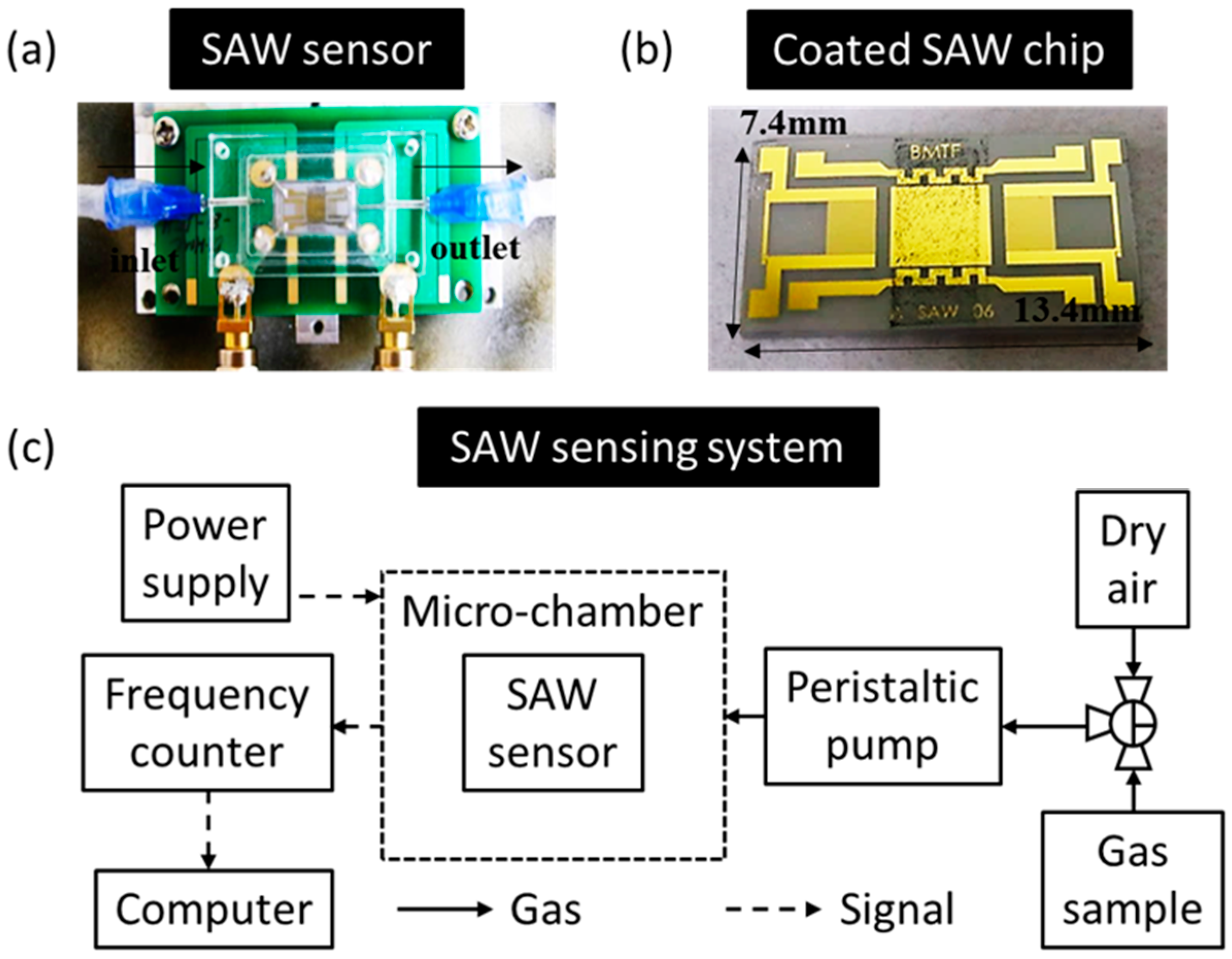
2.1. Preparation of a Surface-Acoustic-Wave (SAW) Sensor
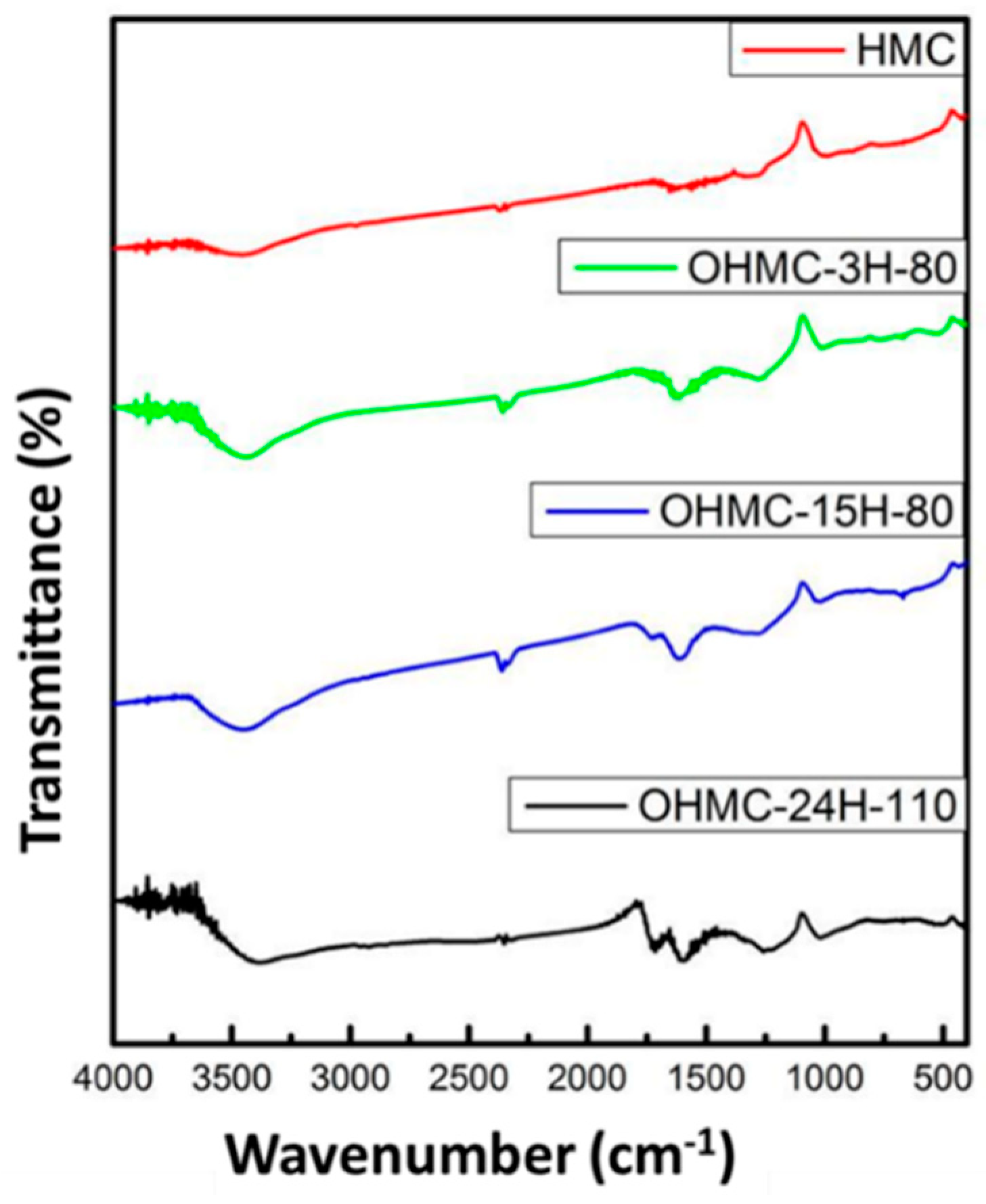
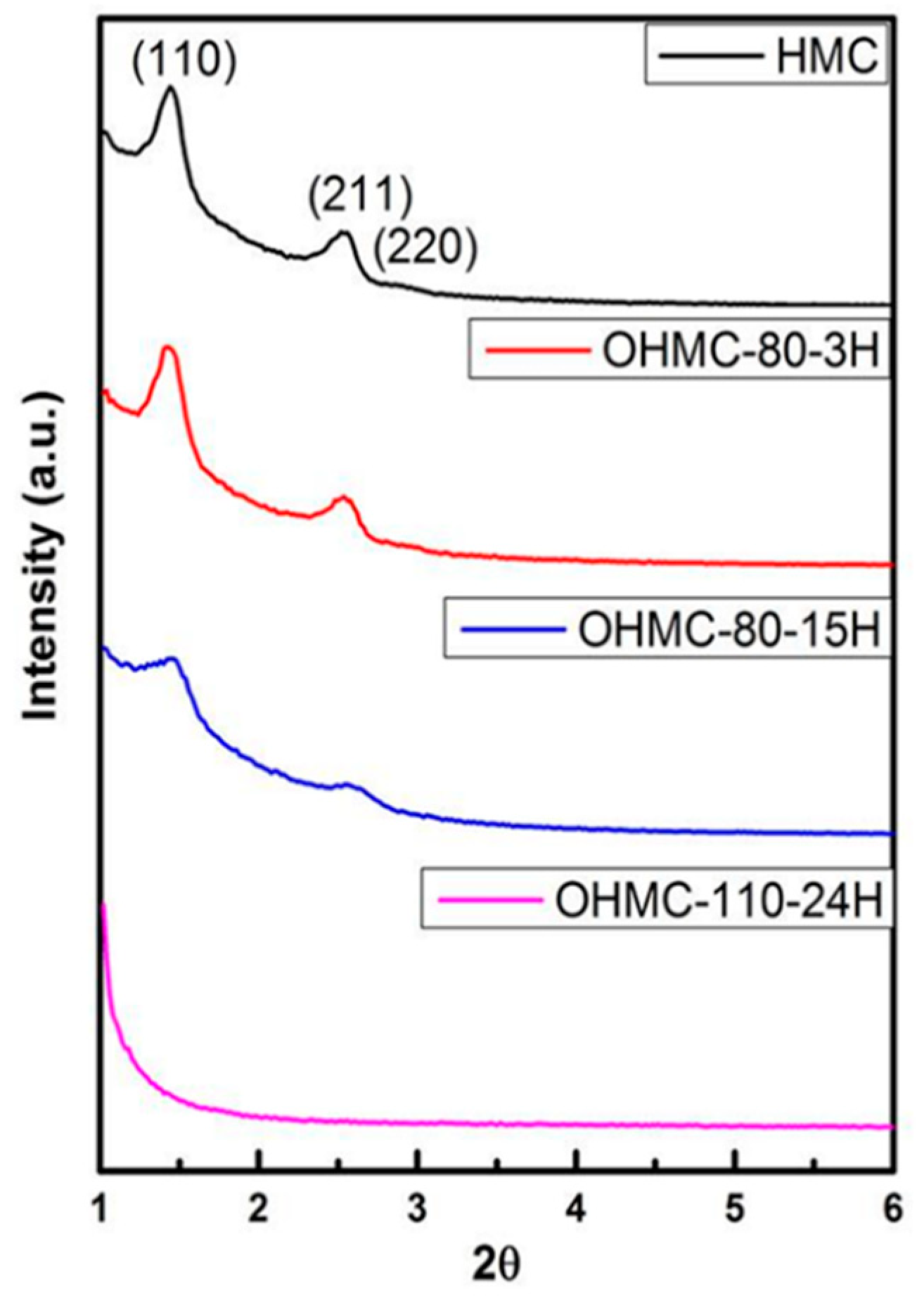
2.2. Sensitive Materials
2.3. Sensing System
3. Results
3.1. Sensitive Analysis of a Material
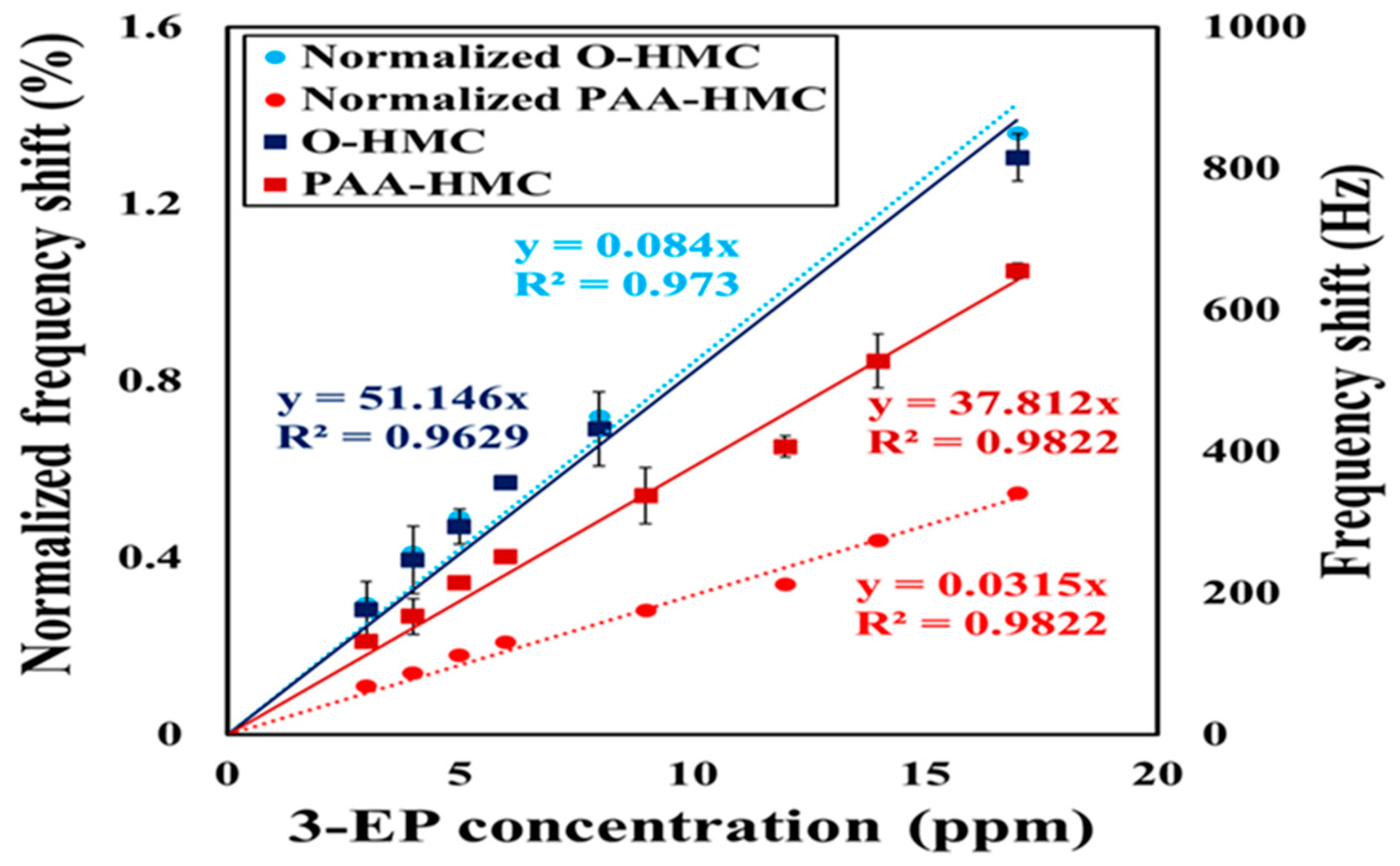
3.2. Detection of the Standard Cigarette Marker
3.3. Normalization
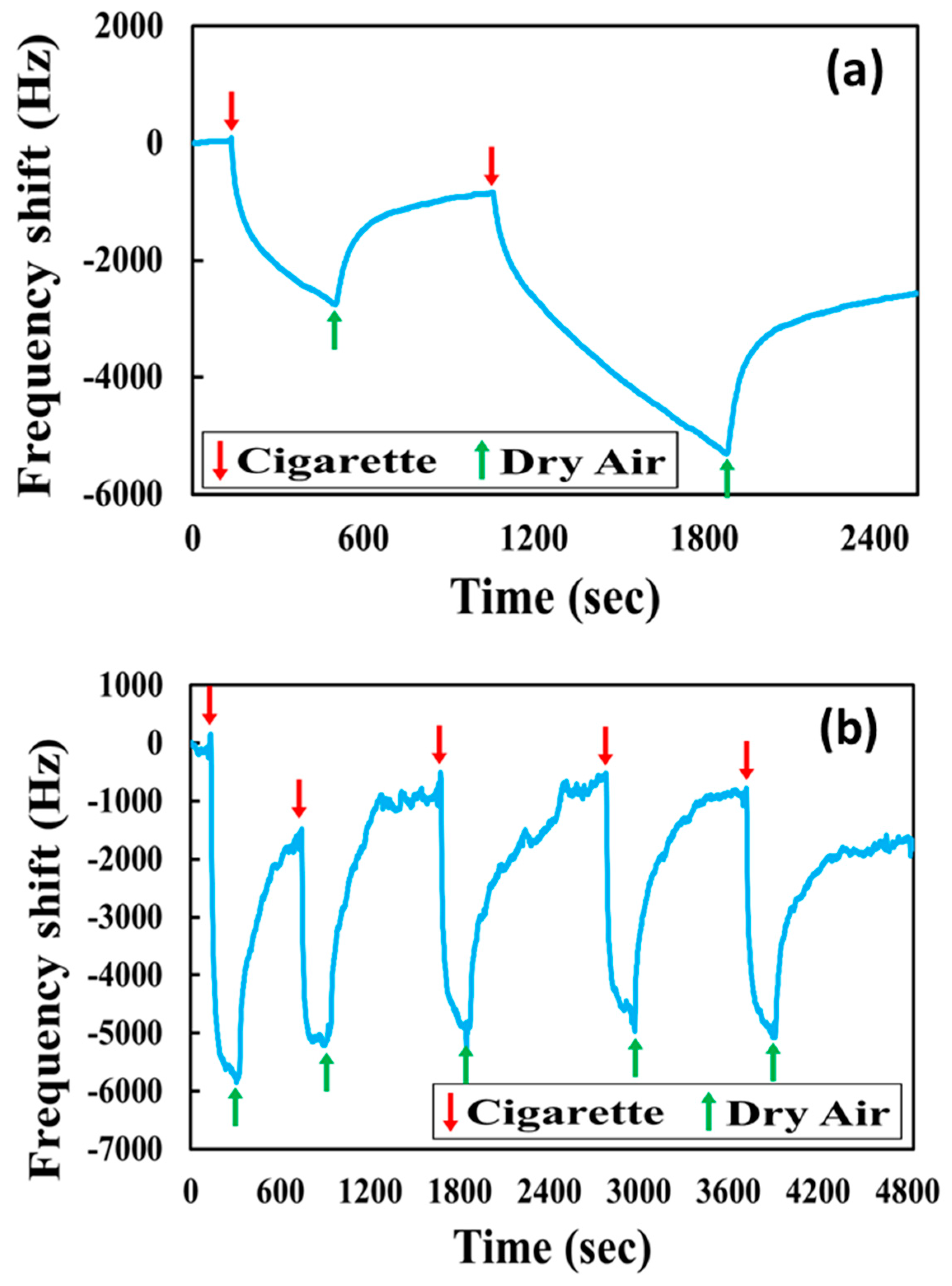
3.4. Detection of Cigarette Smoke
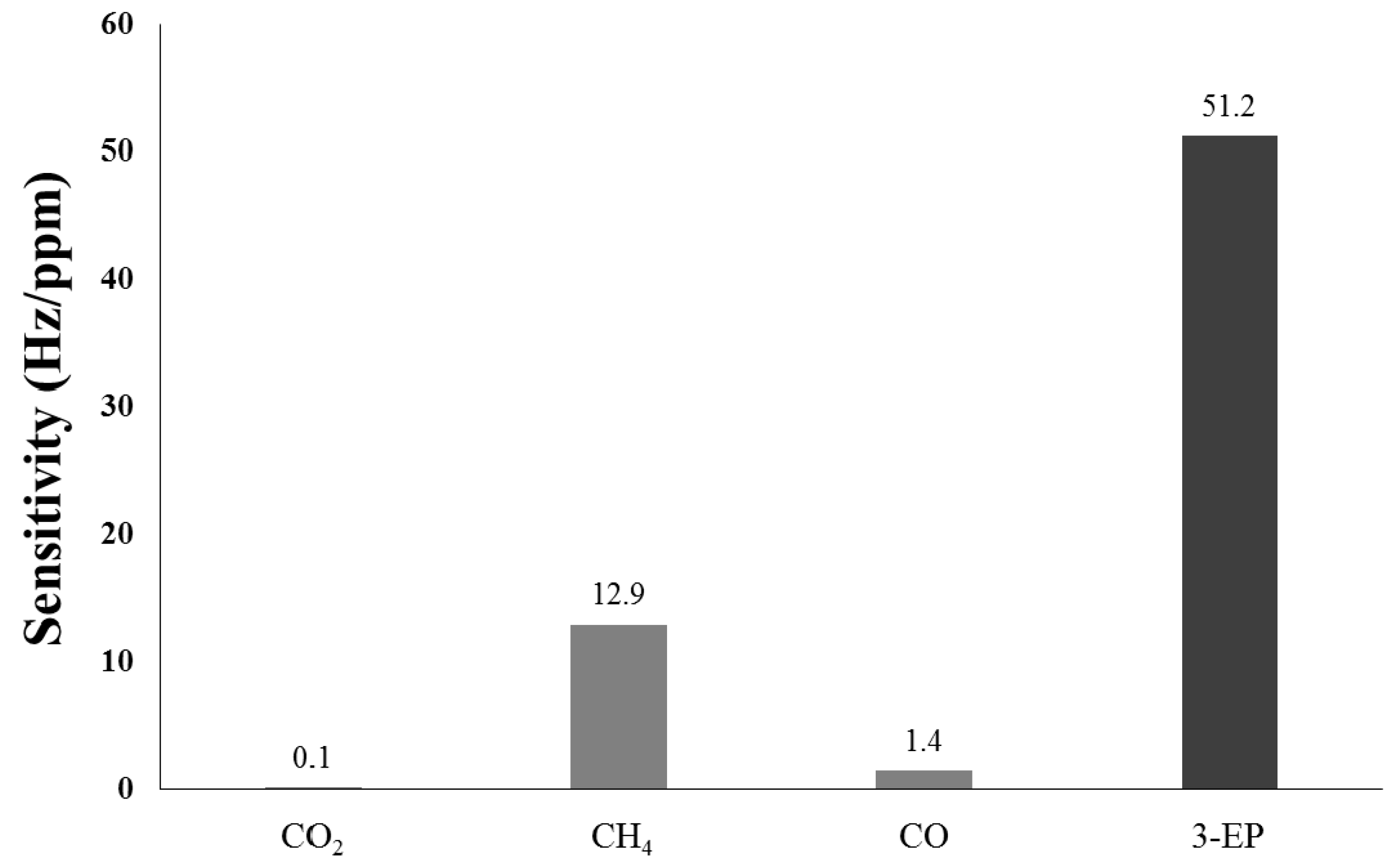
3.5. Selectivity of an Oxidized Hollow Mesoporous Carbon Nanospheres (O-HMC)-Coated SAW Sensor
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eriksen, M.P.; LeMaistre, C.A.; Newell, G.R. Health hazards of passive smoking. Ann. Rev. Public Health 1988, 9, 47–70. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.R.; Pereira da Silva, J.R.; Smith, G. The effect of tobacco ingredients on smoke chemistry. Part I: Flavourings and additives. Food Chem. Toxicol. 2004, 42, 3–37. [Google Scholar] [CrossRef]
- Baker, R.R.; Massey, E.D.; Smith, G. An overview of the effects of tobacco ingredients on smoke chemistry and toxicity. Food Chem. Toxicol. 2004, 42, S53–S83. [Google Scholar] [CrossRef]
- Apelberg, B.J.; Hepp, L.M.; Avila-Tang, E.; Gundel, L.; Hammond, S.K.; Hovell, M.F.; Hyland, A.; Klepeis, N.E.; Madsen, C.C.; Navas-Acien, A.; et al. Environmental monitoring of secondhand smoke exposure. Tob. Control 2013, 22, 147–155. [Google Scholar] [CrossRef]
- Hecht, S.S. Cigarette smoking: cancer risks, carcinogens, and mechanisms. Langenbeck’s Arch. Surg. 2006, 391, 603–613. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research; U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research; U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: Patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual. Life Outcomes 2006, 4, 79. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress; Report of the Surgeon General; U.S. Department of Health and Human Services: Rockville, MD, USA, 2014.
- Vainiotalo, S.; Vaaranrinta, R.; Tornaeus, J.; Aremo, N.; Hase, T.; Peltonen, K. Passive monitoring method for 3-ethenylpyridine: a marker for environmental tobacco smoke. Environ. Sci. Technol. 2001, 35, 1818–1822. [Google Scholar] [CrossRef] [PubMed]
- Koszowski, B.; Goniewicz, M.L.; Czogala, J.; Zymelka, A.; Sobczak, A. Simultaneous determination of nicotine and 3-vinylpyridine in single cigarette tobacco smoke and in indoor air using direct extraction to solid phase. Int. J. Environ. Anal. Chem. 2009, 89, 105–117. [Google Scholar] [CrossRef]
- Hammond, S.K.; Leaderer, B.P. A diffusion monitor to measure exposure to passive smoking. Environ. Sci. Technol. 1987, 21, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.K.; Kim, K.-H. A review of environmental tobacco smoke and its determination in air. TrAC Trends Anal. Chem. 2010, 29, 804–819. [Google Scholar] [CrossRef]
- Liu, Y.; Antwi-Boampong, S.; BelBruno, J.J.; Crane, M.A.; Tanski, S.E. Detection of secondhand cigarette smoke via nicotine using conductive polymer films. Nicotine Tob. Res. 2013, 15, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, D. Ni-coated polyaniline nanowire as chemical sensing material for cigarette smoke. J. Phys. Chem. C 2011, 115, 13554–13559. [Google Scholar] [CrossRef]
- Chen, C.C.; Chen, Y.A.; Liu, Y.J.; Yao, D.J. A multilayer concentric filter device to diminish clogging for separation of particles and microalgae based on size. Lab. Chip. 2014, 14, 1459–1468. [Google Scholar] [CrossRef]
- Hao, H.; Tang, K.T.; Ku, P.H.; Chao, J.S.; Li, C.H.; Yang, C.M.; Yao, D.J. Development of a portable electronic nose based on chemical surface acoustic wave array with multiplexed oscillator and readout electronics. Sens. Actuators B Chem. 2010, 146, 545–553. [Google Scholar] [CrossRef]
- Ku, P.-H.; Hsiao, C.Y.; Chen, M.J.; Lin, T.H.; Li, Y.T.; Liu, S.C.; Tang, K.T.; Yao, D.J.; Yang, C.M. Polymer/ordered mesoporous carbon nanocomposite platelets as superior sensing materials for gas detection with surface acoustic wave devices. Langmuir 2012, 28, 11639–11645. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Lin, C.T.; Hsu, W.L.; Chang, Y.C.; Yeh, S.R.; Li, L.J.; Yao, D.J. A flexible hydrophilic-modified graphene microprobe for neural and cardiac recording. Nanomedicine 2013, 9, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-Y.; Huang, P.-W.; Yao, D.-J. Enhanced efficiency of sorting sperm motility utilizing a microfluidic chip. Microsyst. Technol. 2015, 23. [Google Scholar] [CrossRef]
- Huang, H.Y.; Shen, H.H.; Tien, C.H.; Li, C.J.; Fan, S.K.; Liu, C.H.; Hsu, W.S.; Yao, D.J. Digital microfluidic dynamic culture of mammalian embryos on an electrowetting on dielectric (EWOD) chip. PLoS ONE 2015, 10, e0124196. [Google Scholar] [CrossRef] [PubMed]
- Drafts, B. Acoustic wave technology sensors. Microw. Theory Tech. IEEE Trans. 2001, 49, 795–802. [Google Scholar] [CrossRef]
- Sivaramakrishnan, S.; Rajamani, R.; Smith, C.S.; McGee, K.A.; Mann, K.R.; Yamashita, N. Carbon nanotube-coated surface acoustic wave sensor for carbon dioxide sensing. Sens. Actuators B Chem. 2008, 132, 296–304. [Google Scholar] [CrossRef]
- Sayago, I.; Fernández, M.J.; Fontecha, J.L.; Horrillo, M.C.; Vera, C.; Obieta, I.; Bustero, I. New sensitive layers for surface acoustic wave gas sensors based on polymer and carbon nanotube composites. Sens. Actuators B Chem. 2012, 175, 67–72. [Google Scholar] [CrossRef]
- Liu, S.; Sun, H.; Nagarajan, R.; Kumar, J.; Gu, Z.; Hwan Cho, J.; Kurup, P. Dynamic chemical vapor sensing with nanofibrous film based surface acoustic wave sensors. Sens. Actuators A Phys. 2011, 167, 8–13. [Google Scholar] [CrossRef]
- Grate, J.W.; McGill, R.A. Dewetting effects on polymer-coated surface acoustic wave vapor sensors. Anal. Chem. 1995, 67, 4015–4019. [Google Scholar] [CrossRef]
- Kao, K.; Cheng, C.; Chen, Y.; Lee, Y.-H. The characteristics of surface acoustic waves on AlN/LiNbO3 substrates. Appl. Phys. A 2003, 76, 1125–1127. [Google Scholar] [CrossRef]
- Levit, N.; Pestov, D.; Tepper, G. High surface area polymer coatings for SAW-based chemical sensor applications. Sens. Actuators B Chem. 2002, 82, 241–249. [Google Scholar] [CrossRef]
- Bazuła, P.A.; Lu, A.-H.; Nitz, J.-J.; Schüth, F. Surface and pore structure modification of ordered mesoporous carbons via a chemical oxidation approach. Microporous Mesoporous Mater. 2008, 108, 266–275. [Google Scholar] [CrossRef]
- Cheng, C.-Y.; Huang, S.-S.; Yang, C.-M.; Tang, K.-T.; Yao, D.-J. Detection of third-hand smoke on clothing fibers with a surface acoustic wave gas sensor. Biomicrofluidics 2016, 10, 011907. [Google Scholar] [CrossRef]
- Chiang, M.-C.; Hao, H.-C.; Yang, C.-M.; Yao, D.-J. Detection of hazardous vapors including mixtures in varied conditions using a surface-acoustic-wave device. ECS J. Solid State Sci. Technol. 2018, 7, Q3120–Q3125. [Google Scholar] [CrossRef]
- Lai, N.; Chih, Y.L.; Pei, H.K.; Li, L.C.; Liao, K.W.; Lin, W.T.; Chia, M.Y. Hollow mesoporous Ia3d silica nanospheres with singleunit-cell-thick shell: Spontaneous formation and drug delivery application. Nano Res. 2014, 7, 1439–1448. [Google Scholar] [CrossRef]
- Ryoo, R.; Joo, S.H.; Jun, S. Synthesis of highly ordered carbon molecular sieves via template-mediated structural transformation. J. Phys. Chem. B 1999, 103, 7743–7746. [Google Scholar] [CrossRef]
- Wohltjen, H.; Dessy, R. Surface acoustic wave probe for chemical analysis. I. Introduction and instrument description. Anal. Chem. 1979, 51, 1458–1464. [Google Scholar] [CrossRef]
- Tan, T.L.; Lebron, G.B. Determination of carbon dioxide, carbon monoxide, and methane concentrations in cigarette smoke by fourier transform infrared spectroscopy. J. Chem. Educ. 2012, 89, 383–386. [Google Scholar] [CrossRef]
- Mosher, K.; He, J.; Liu, Y.; Rupp, E.; Wilcox, J. Molecular simulation of methane adsorption in micro- and mesoporous carbons with applications to coal and gas shale systems. Int. J. Coal Geol. 2013, 109–110, 36–44. [Google Scholar] [CrossRef]


| Sensing Materials | Before Coated (MHz) | After Coated (MHz) | Coated Frequency Shift (Hz) |
|---|---|---|---|
| Polymer based | |||
| PAA-HMC | 114.71 | 114.59 | 120,000 |
| Non-polymer based | |||
| O-HMC-110 °C-24 h | 114.98 | 114.88 | 100,000 |
| O-HMC-80 °C-15 h | 114.12 | 114.07 | 50,000 |
| O-HMC-80 °C-3 h | 114.16 | 114.11 | 50,000 |
| HMC | 114.16 | 114.13 | 30,000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.-Y.; Huang, S.-S.; Yang, C.-M.; Tang, K.-T.; Yao, D.-J. Detection of Cigarette Smoke Using a Surface-Acoustic-Wave Gas Sensor with Non-Polymer-Based Oxidized Hollow Mesoporous Carbon Nanospheres. Micromachines 2019, 10, 276. https://doi.org/10.3390/mi10040276
Cheng C-Y, Huang S-S, Yang C-M, Tang K-T, Yao D-J. Detection of Cigarette Smoke Using a Surface-Acoustic-Wave Gas Sensor with Non-Polymer-Based Oxidized Hollow Mesoporous Carbon Nanospheres. Micromachines. 2019; 10(4):276. https://doi.org/10.3390/mi10040276
Chicago/Turabian StyleCheng, Chi-Yung, Shih-Shien Huang, Chia-Min Yang, Kea-Tiong Tang, and Da-Jeng Yao. 2019. "Detection of Cigarette Smoke Using a Surface-Acoustic-Wave Gas Sensor with Non-Polymer-Based Oxidized Hollow Mesoporous Carbon Nanospheres" Micromachines 10, no. 4: 276. https://doi.org/10.3390/mi10040276
APA StyleCheng, C.-Y., Huang, S.-S., Yang, C.-M., Tang, K.-T., & Yao, D.-J. (2019). Detection of Cigarette Smoke Using a Surface-Acoustic-Wave Gas Sensor with Non-Polymer-Based Oxidized Hollow Mesoporous Carbon Nanospheres. Micromachines, 10(4), 276. https://doi.org/10.3390/mi10040276






