Study of Hole-Transporter-Free Perovskite Solar Cells based on Fully Printable Components
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Pastes for Screen Printing
2.2. Device Construction
2.3. Characterization
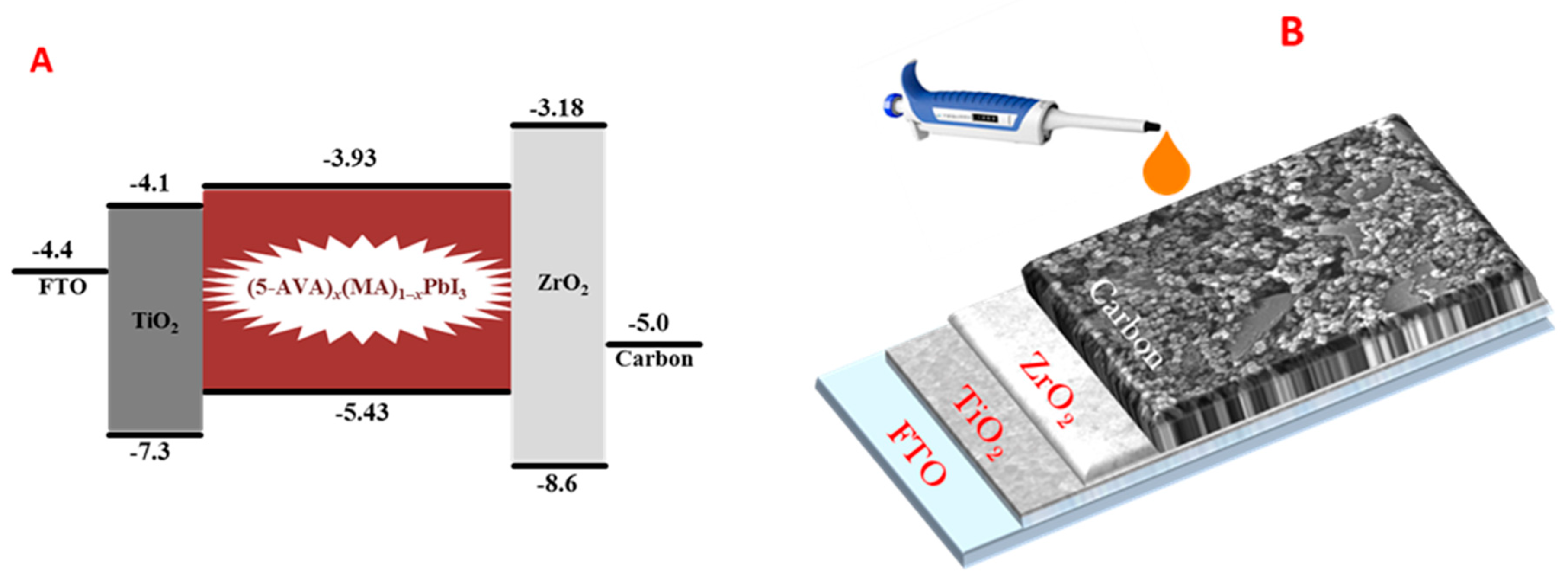
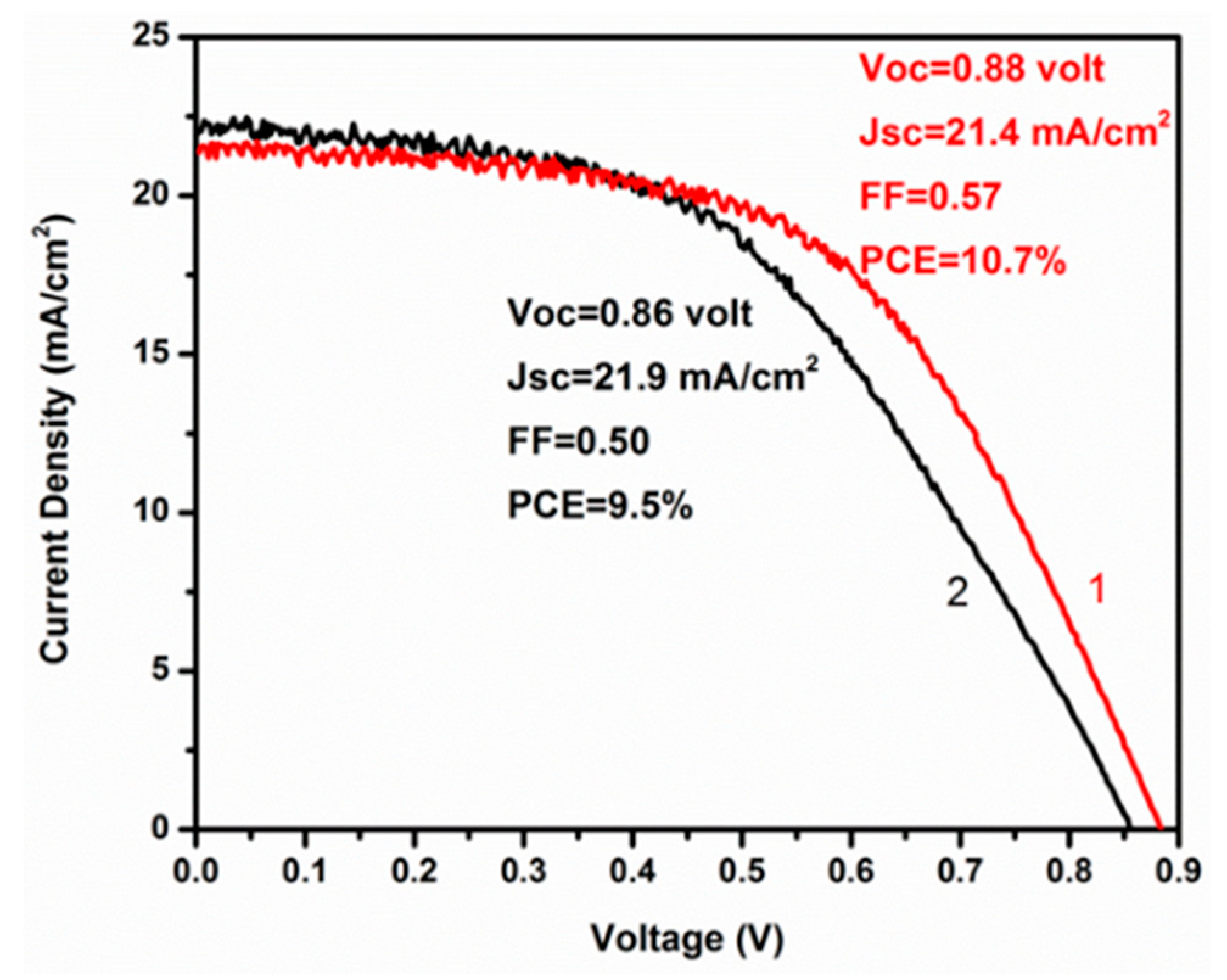
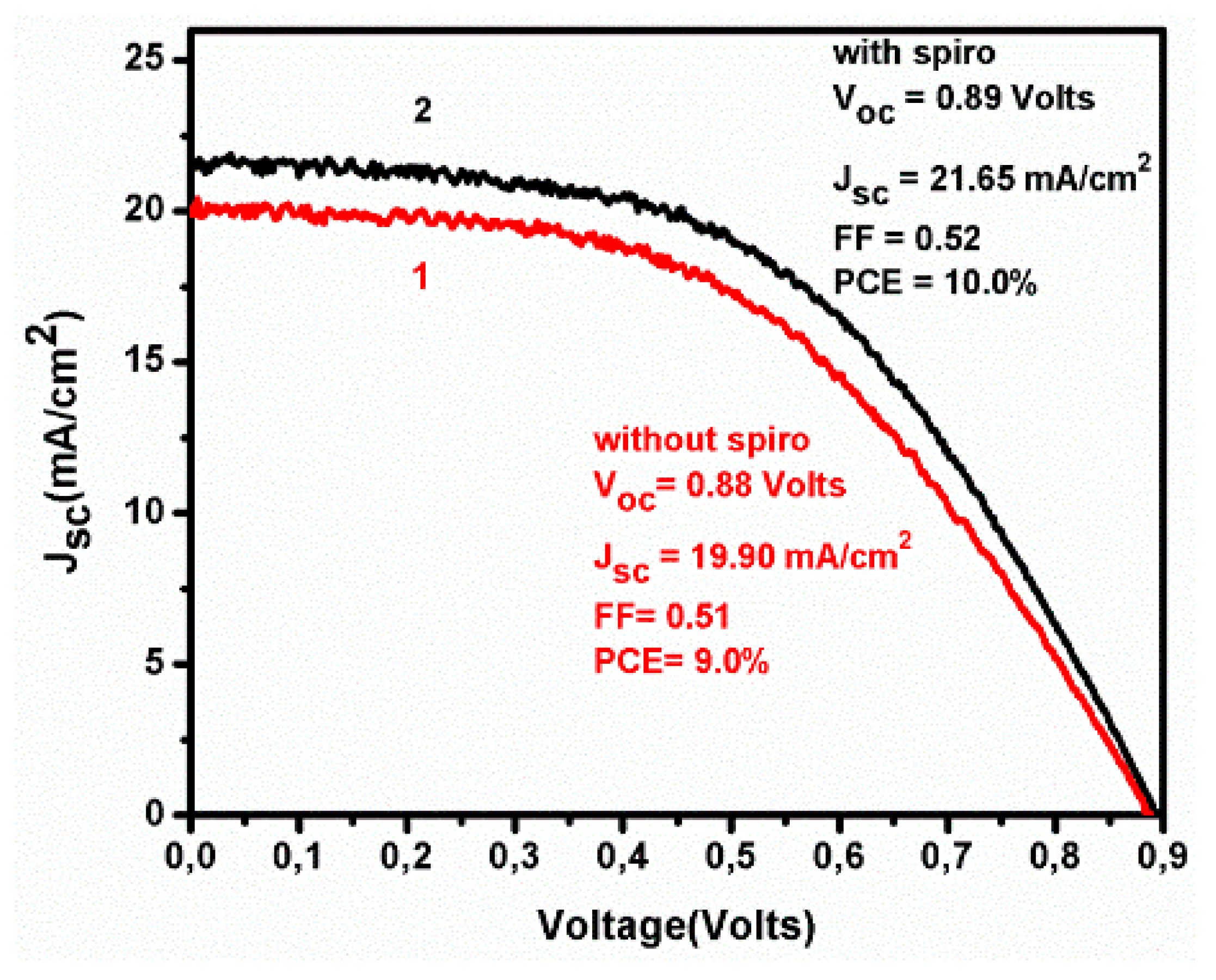
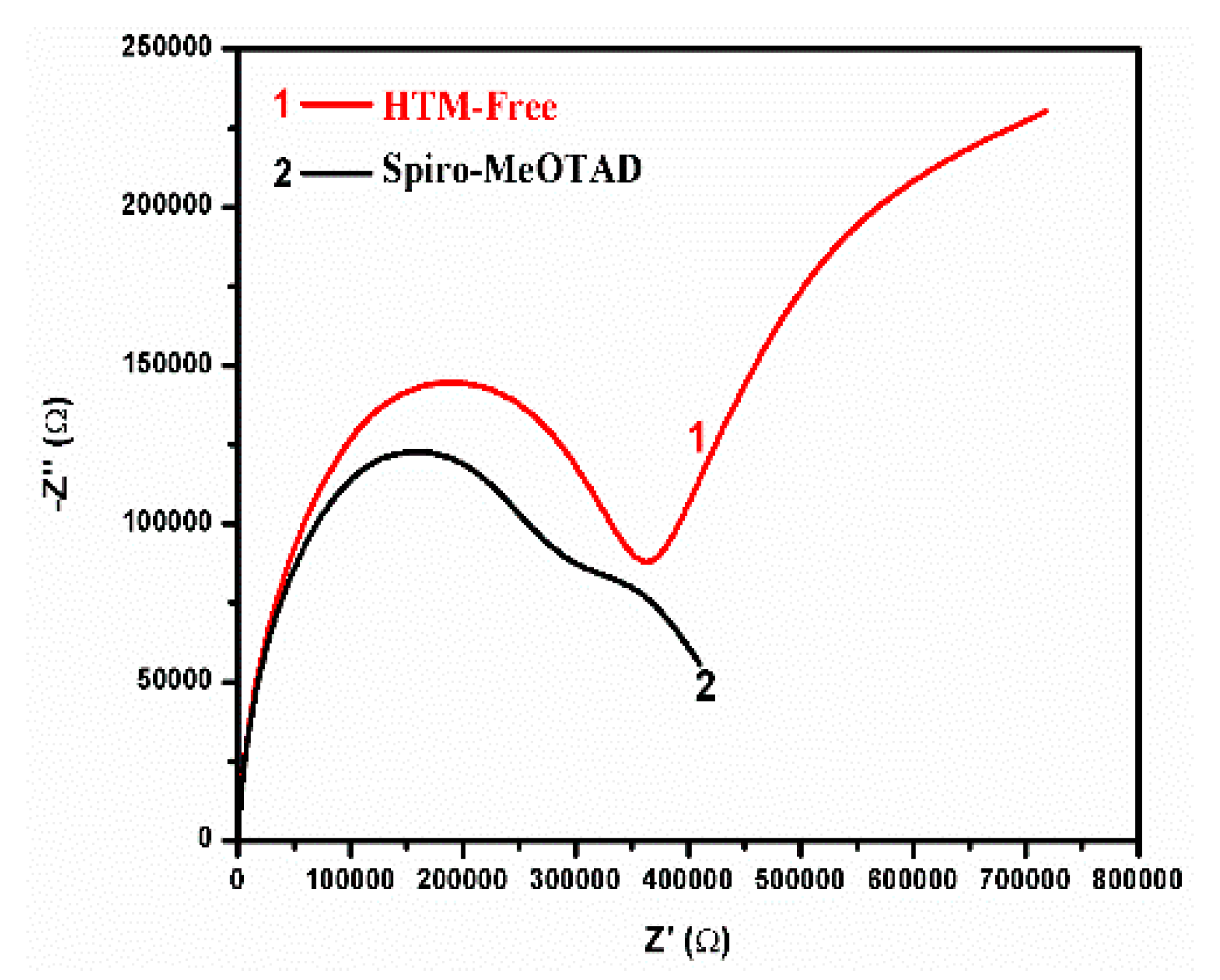
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 31, 6050–6051. [Google Scholar] [CrossRef]
- Correa-Baena, J.; Saliba, M.; Buonassisi, T.; Grätzel, M.; Abate, A.; Tress, W.; Hagfeldt, A. Promises and challenges of perovskite solar cells. Science 2017, 358, 739–744. [Google Scholar] [CrossRef]
- Kovalenko, M.V.; Protesescu, L.; Bodnarchuk, M.I. Properties and potential optoelectronic applications of lead halide perovskite nanocrystals. Science 2017, 358, 745–750. [Google Scholar] [CrossRef]
- Peng, J.; Chen, Y.; Zheng, K.; Pullerits, T.; Liang, Z. Insights into charge carrier dynamics in organo-metal halide perovskites: from neat films to solar cells. Chem. Soc. Rev. 2017, 46, 5714–5729. [Google Scholar] [CrossRef] [PubMed]
- NREL Chart Homepage. Available online: https://www.nrel.gov/pv/assets/images/efficiency-chart.png (accessed on 1 October 2017).
- Nia, N.Y.; Matteocci, F.; Cina, L.; Carlo, A.D. High-Efficiency Perovskite Solar Cell Based on Poly(3-Hexylthiophene): Influence of Molecular Weight and Mesoscopic Scaffold Layer. ChemSusChem. 2017, 10, 3854–3860. [Google Scholar] [CrossRef] [PubMed]
- Nouri, E.; Wang, Y.L.; Chen, Q.; Xu, J.J.; Paterakis, G.; Dracopoulos, V.; Xu, Z.-X.; Tasis, D.; Mohammadi, M.R.; Lianos, P. Introduction of Graphene Oxide as Buffer Layer in Perovskite Solar Cells and the Promotion of Soluble n-Butyl-substituted Copper Phthalocyanine as Efficient Hole Transporting Material. Electrochim. Acta 2017, 233, 36–43. [Google Scholar] [CrossRef]
- Huang, F.; Jin, S.; Wei, Y.; Gu, L.; Guo, Q.; Xu, H.; Yang, X.; Wu, T.; He, X.; Huang, Y.; et al. Fast fabricated high performance antisolvent-free perovskite solar cells via dual-flash process. Electrochim. Acta 2018, 259, 402–409. [Google Scholar] [CrossRef]
- Liang, C.; Li, P.; Zhang, Y.; Gu, H.; Cai, Q.; Liu, X.; Wang, J.; Wen, H.; Shao, G. Mild solution-processed metal-doped TiO2 compact layers for hysteresis-less and performance-enhanced perovskite solar cells. J. Power Sources 2017, 372, 235–244. [Google Scholar] [CrossRef]
- Shaikh, J.S.; Shaikh, N.S.; Sheikh, A.D.; Mali, S.S.; Kale, A.J.; Kanjanaboos, P.; Hong, C.K.; Kim, J.H.; Patil, P.S. Perovskite solar cells: In pursuit of efficiency and stability. Mater. Des. 2017, 136, 54–80. [Google Scholar] [CrossRef]
- Gopi, C.V.V.M.; Haritha, M.V.; Prabakar, K.; Kim, H.J. Low-temperature easy-processed carbon nanotube contact for high-performance metal- and hole-transporting layer-free perovskite solar cells. J. Photochem. Photobiol. 2017, 332, 265–272. [Google Scholar] [CrossRef]
- Yue, G.; Chen, D.; Wang, P.; Zhang, J.; Hu, Z.; Zhu, Y. Low-temperature prepared carbon electrodes for hole-conductor-free mesoscopic perovskite solar cells. Electrochim. Acta 2016, 218, 84–90. [Google Scholar] [CrossRef]
- Xiao, M.; Zhao, L.; Wei, S.; Li, Y.; Dong, B.; Xu, Z.; Wan, L.; Wang, S. Application of mixed-organic-cation for high performance hole-conductor-free perovskite solar cells. J. Colloid Interface Sci. 2018, 510, 118–126. [Google Scholar] [CrossRef]
- Maniarasua, S.; Korukonda, T.B.; Manjunath, V.; Ramasamy, E.; Ramesh, M.; Veerappan, G. Recent advancement in metal cathode and hole-conductor-free perovskite solar cells for low-cost and high stability: A route towards commercialization. Renew. Sustain. Energy Rev. 2018, 82, 845–857. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, T.; Tang, Z.; Liao, G. A large-area hole-conductor-free perovskite solar cell based on a low-temperature carbon counter electrode. Mater. Res. Bull. 2017, 96, 196–200. [Google Scholar] [CrossRef]
- Shiraishi, M.; Ata, M. Work function of carbon nanotubes. Carbon 2001, 39, 1913–1917. [Google Scholar] [CrossRef]
- Ku, Z.; Rong, Y.; Xu, M.; Liu, T.; Han, H. Full Printable Processed Mesoscopic CH3NH3PbI3/TiO2 Heterojunction Solar Cells with Carbon Counter Electrode. Sci Rep. 2013, 3, 3132–3137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, T.; Liu, L.; Hu, M.; Yang, Y.; Mei, A.; Han, H. The effect of carbon counters electrodes on fully printable mesoscopic perovskite solar cells. J. Mater. Chem. A 2015, 3, 9165–9170. [Google Scholar] [CrossRef]
- Rong, Y.; Han, H. Monolithic quasi-solid-state dye-sensitized solar cells based on graphene-modified mesoscopic carbon-counter electrodes. J. Nanophoton. 2013, 7, 073090. [Google Scholar] [CrossRef]
- Raminafshar, C.; Dracopoulos, V.; Mohammadi, M.R.; Lianos, P. Carbon based perovskite solar cells constructed by screen-printed components. Electrochim. Acta 2018, 276, 261–267. [Google Scholar] [CrossRef]
- Hashmi, S.G.; Martineau, D.; Dar, M.I.; Myllymäki, T.T.T.; Sarikka, T.; Ulla, V.; Zakeeruddin, S.M.; Grätzel, M. High performance carbon-based printed perovskite solar cells with humidity assisted thermal treatment. J. Mater. Chem. A 2017, 5, 12060–12067. [Google Scholar] [CrossRef]
- Grancini, G.; Roldán-Carmona, C.; Zimmermann, I.; Mosconi, E.; Lee, X.; Martineau, D.; Narbey, S.; Oswald, F.; De Angelis, F.; Graetzel, M.; Nazeeruddin, M.K. One-Year stable perovskite solar cells by 2D/3D interface engineering. Nat. Commun. 2017, 8, 5684–5692. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yang, S. Stabilizing and scaling up carbon-based perovskite solar cells. J. Mater. Res. 2017, 32, 3011–3020. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuang, X.; Zhou, K.; Cai, C.; Hu, Z.; Zhang, J.; Zhu, Y. Vibration treated carbon electrode for highly efficient hole-conductor-free perovskite solar cells. Org. Electron. 2018, 52, 159–164. [Google Scholar] [CrossRef]
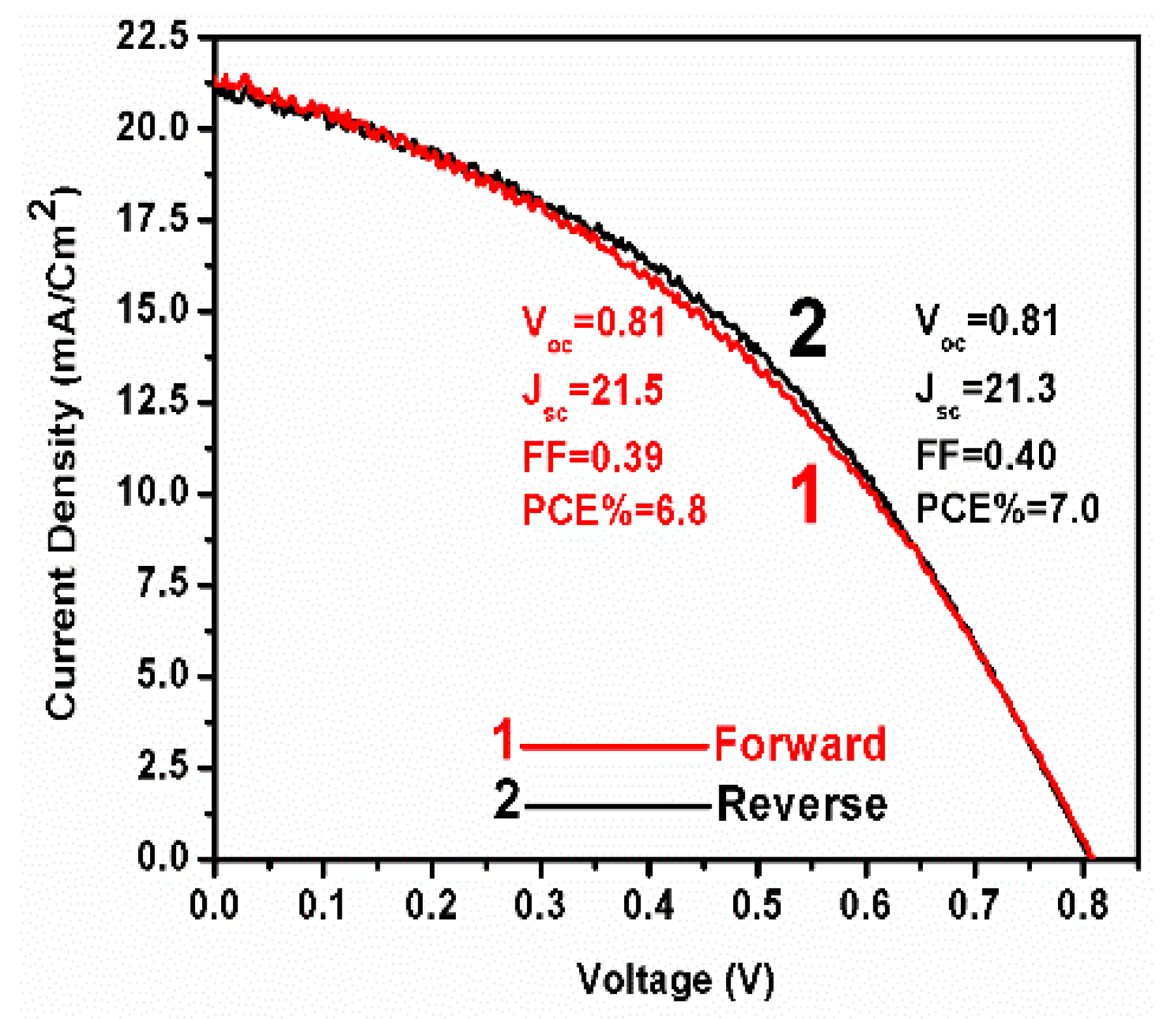
| Addition of spiro-OMeTAD | Bias (V) | Light | Fitted circuit | Rs1 (Ω) | R12 (kΩ) | R23 (kΩ) | R34 (kΩ) | R44 (kΩ) | CPE1-T5 (μF) | n16 | CPE2-T7 (nF) | n28 | C29 (nF) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | 0.0 | No | 1 | 46 | 660 | 353 | - | - | 2.8 | 0.77 | 28.9 | 0.91 | - |
| No | 0.0 | No | 1 | 41 | 166 | 277 | - | - | 1 | 0.86 | 28.1 | 0.92 | - |
| Yes | 0.0 | Yes | 1 | 56 | 0.08 | 0.15 | - | - | 1.3 × 104 | 0.38 | 1400 | 0.71 | - |
| No | 0.0 | Yes | 1 | 41 | 0.08 | 0.06 | - | - | 1.5 × 104 | 0.51 | 3200 | 0.64 | - |
| Yes | 0.8 | No | 2 | 60 | 2.0 | - | 6.1 | 31 | 0.09 | 0.92 | - | - | 16 |
| No | 0.8 | No | 2 | 56 | 1.2 | - | 12.7 | 51 | 0.12 | 0.92 | - | - | 13 |
| Yes | 0.8 | Yes | 2 | ~0 | 0.09 | - | ~0 | 0.02 | 0.004 | ~1 | - | - | 6.8 |
| No | 0.8 | Yes | 2 | ~0 | 0.09 | - | ~0 | 0.02 | 0.007 | ~1 | - | - | 33 |
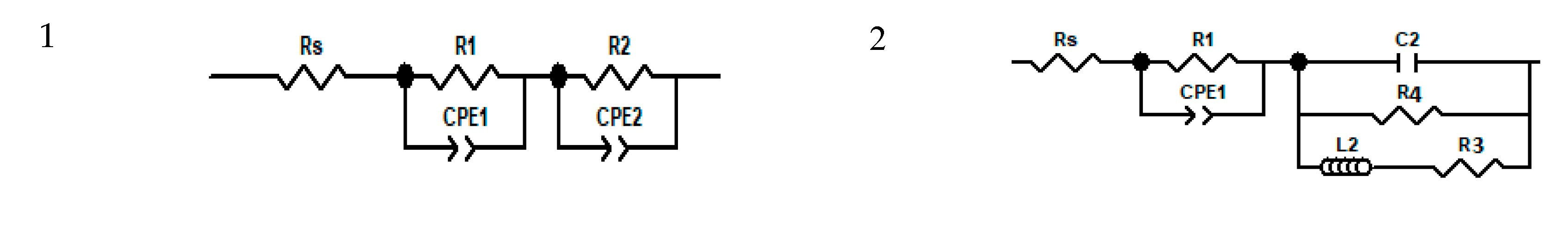
- Rs—Series Resistance
- R1—Charge accumulation and recombination resistance at the perovskite/charge collector interfaces
- R2—Charge transfer Resistance at Carbon/Perovskite interface
- R3 and R4—Charge accumulation resistances at the interfaces
- CPE1-T—Constant Phase Element formed at the perovskite charge collector interfaces
- n1—Power of CPE1
- CPE2-T—Constant Phase Element formed at the Carbon/Perovskite interface
- n2—Power of CPE2
- C2—Capacitor formed at the Carbon/Perovskite interface 3.3. Formatting of Mathematical Components.
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raminafshar, C.; Raptis, D.; Mohammadi, M.R.; Lianos, P. Study of Hole-Transporter-Free Perovskite Solar Cells based on Fully Printable Components. Micromachines 2019, 10, 266. https://doi.org/10.3390/mi10040266
Raminafshar C, Raptis D, Mohammadi MR, Lianos P. Study of Hole-Transporter-Free Perovskite Solar Cells based on Fully Printable Components. Micromachines. 2019; 10(4):266. https://doi.org/10.3390/mi10040266
Chicago/Turabian StyleRaminafshar, Camellia, Dimitrios Raptis, Mohammad Reza Mohammadi, and Panagiotis Lianos. 2019. "Study of Hole-Transporter-Free Perovskite Solar Cells based on Fully Printable Components" Micromachines 10, no. 4: 266. https://doi.org/10.3390/mi10040266
APA StyleRaminafshar, C., Raptis, D., Mohammadi, M. R., & Lianos, P. (2019). Study of Hole-Transporter-Free Perovskite Solar Cells based on Fully Printable Components. Micromachines, 10(4), 266. https://doi.org/10.3390/mi10040266





