Automatic Morphology Control of Liquid Metal using a Combined Electrochemical and Feedback Control Approach
Abstract
1. Introduction
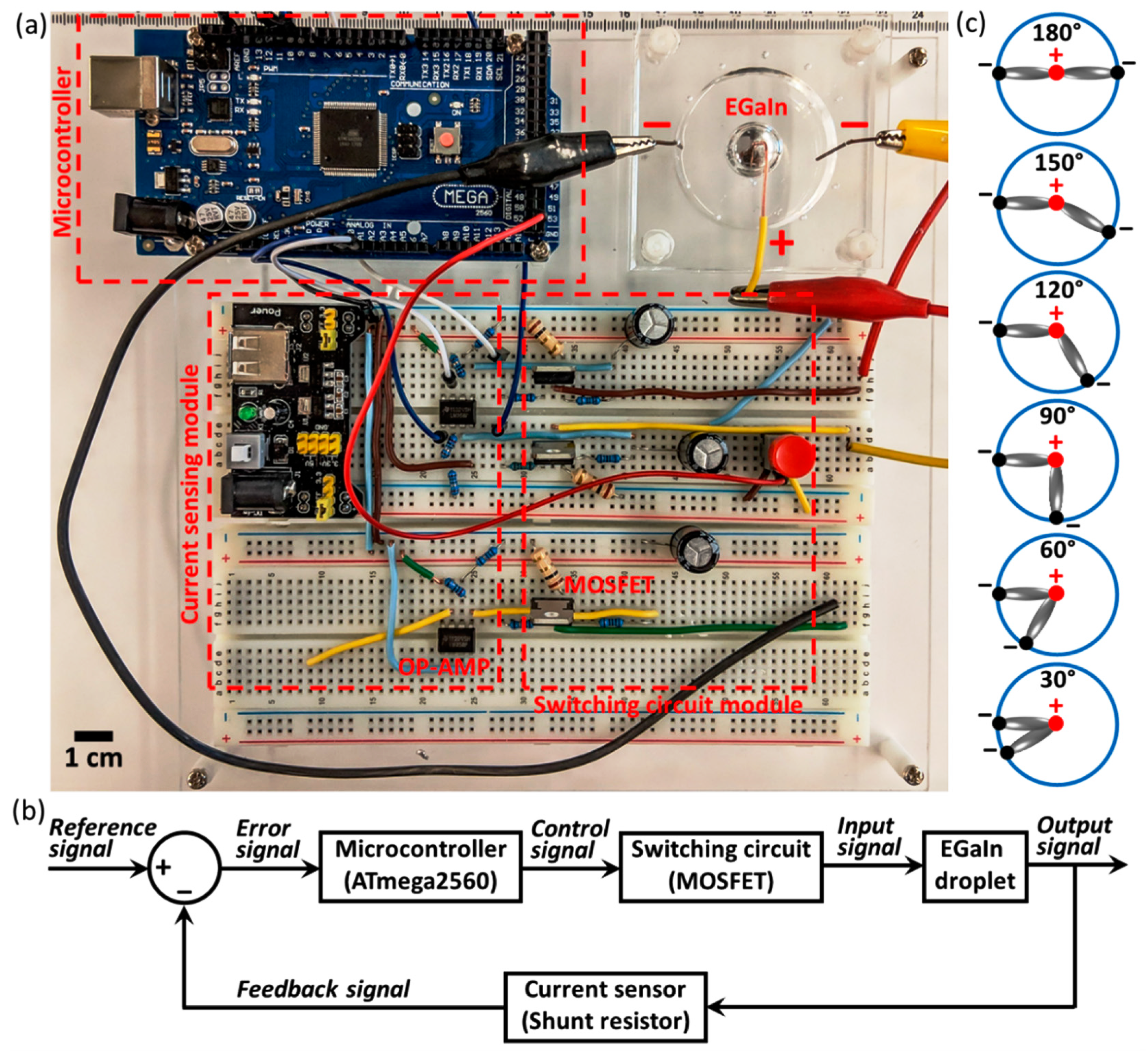
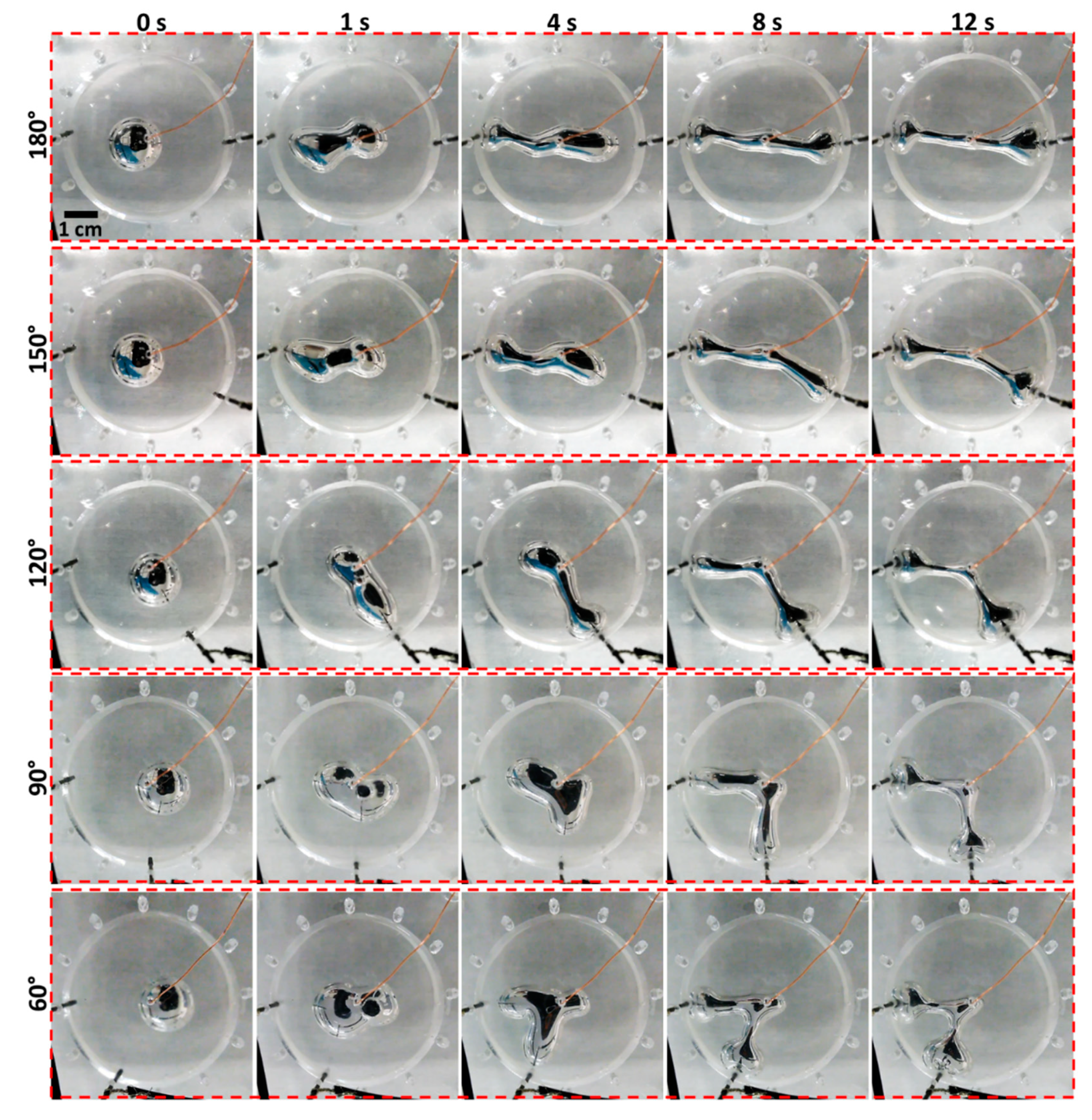
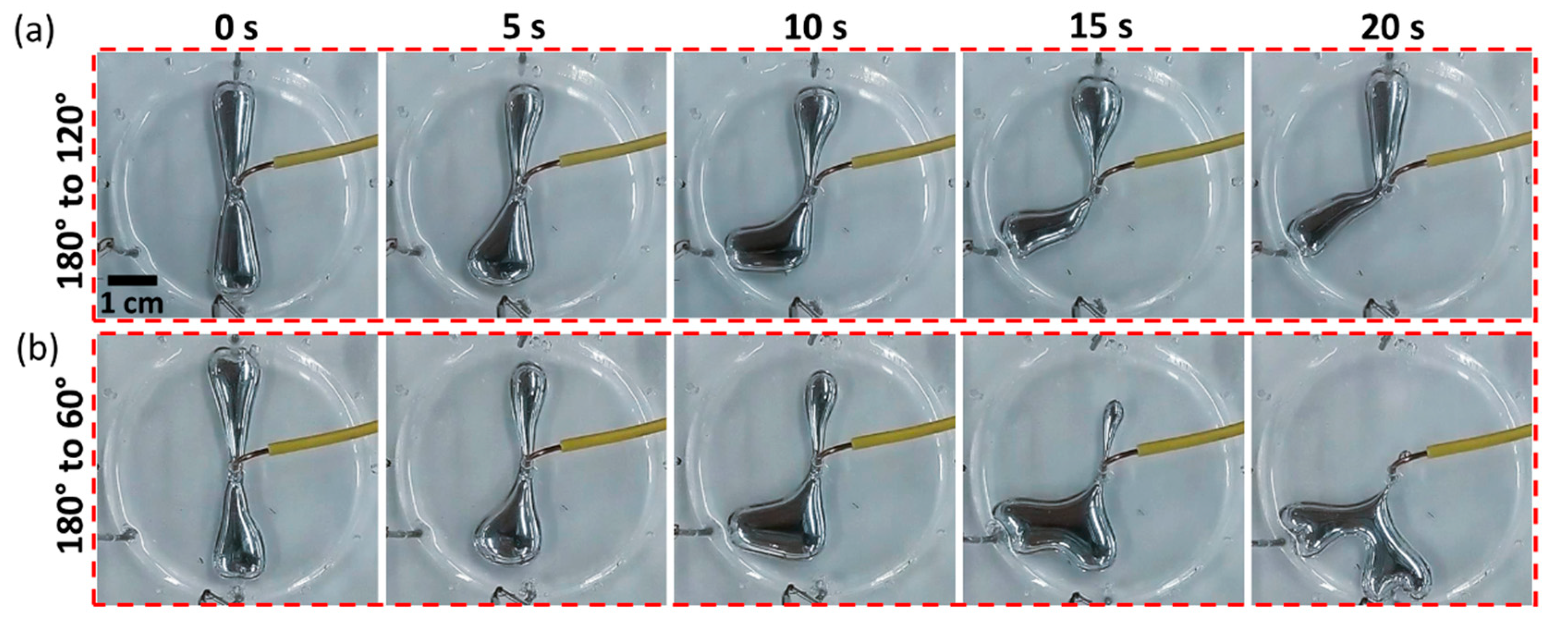
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Dickey, M.D.; Chiechi, R.C.; Larsen, R.J.; Weiss, E.A.; Weitz, D.A.; Whitesides, G.M. Eutectic Gallium-Indium (EGaIn): A Liquid Metal Alloy for the Formation of Stable Structures in Microchannels at Room Temperature. Adv. Funct. Mater. 2008, 18, 1097–1104. [Google Scholar] [CrossRef]
- Liu, T.; Sen, P.; Kim, C. Characterization of Nontoxic Liquid-Metal Alloy Galinstan for Applications in Microdevices. J. Microelectromech. Syst. 2012, 21, 443–450. [Google Scholar] [CrossRef]
- Dickey, M.D. Stretchable and Soft Electronics using Liquid Metals. Adv. Mater. 2017, 29, 1606425. [Google Scholar] [CrossRef]
- Carey, B.J.; Ou, J.Z.; Clark, R.M.; Berean, K.J.; Zavabeti, A.; Chesman, A.S.R.; Russo, S.P.; Lau, D.W.M.; Xu, Z.Q.; Bao, Q.; et al. Wafer-scale two-dimensional semiconductors from printed oxide skin of liquid metals. Nat. Commun. 2017, 8, 14482. [Google Scholar] [CrossRef] [PubMed]
- Esrafilzadeh, D.; Zavabeti, A.; Jalili, R.; Atkin, P.; Choi, J.; Carey, B.J.; Brkljača, R.; O’Mullane, A.P.; Dickey, M.D.; Officer, D.L.; et al. Room temperature CO2 reduction to solid carbon species on liquid metals featuring atomically thin ceria interfaces. Nat. Commun. 2019, 10, 865. [Google Scholar] [CrossRef] [PubMed]
- Daeneke, T.; Khoshmanesh, K.; Mahmood, N.; de Castro, I.A.; Esrafilzadeh, D.; Barrow, S.J.; Dickey, M.D.; Kalantar-zadeh, K. Liquid metals: Fundamentals and applications in chemistry. Chem. Soc. Rev. 2018, 47, 4073–4111. [Google Scholar] [CrossRef]
- Khoshmanesh, K.; Tang, S.-Y.; Zhu, J.Y.; Schaefer, S.; Mitchell, A.; Kalantar-zadeh, K.; Dickey, M.D. Liquid metal enabled microfluidics. Lab Chip 2017, 17, 974–993. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Hu, Q.; Lin, Y.; Pacardo, D.B.; Wang, C.; Sun, W.; Ligler, F.S.; Dickey, M.D.; Gu, Z. Transformable liquid-metal nanomedicine. Nat. Commun. 2015, 6, 10066. [Google Scholar] [CrossRef] [PubMed]
- Dickey, M.D. Emerging Applications of Liquid Metals Featuring Surface Oxides. ACS Appl. Mater. Interfaces 2014, 6, 18369–18379. [Google Scholar] [CrossRef]
- Lin, Y.; Genzer, J.; Li, W.; Qiao, R.; Dickey, M.D.; Tang, S.-Y. Sonication-enabled rapid production of stable liquid metal nanoparticles grafted with poly(1-octadecene-alt-maleic anhydride) in aqueous solutions. Nanoscale 2018, 10, 19871–19878. [Google Scholar] [CrossRef]
- Tang, S.-Y.; Qiao, R.; Lin, Y.; Li, Y.; Zhao, Q.; Yuan, D.; Yun, G.; Guo, J.; Dickey, M.D.; Huang, T.J.; et al. Functional Liquid Metal Nanoparticles Produced by Liquid-Based Nebulization. Adv. Mater. Technol. 2019, 4, 1800420. [Google Scholar] [CrossRef]
- Tang, S.-Y.; Joshipura, I.D.; Lin, Y.; Kalantar-Zadeh, K.; Mitchell, A.; Khoshmanesh, K.; Mitchell, A.; Khoshmanesh, K.; Dickey, M.D. Liquid-Metal Microdroplets Formed Dynamically with Electrical Control of Size and Rate. Adv. Mater. 2016, 28, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Gol, B.; Tovar-Lopez, F.J.; Kurdzinski, M.E.; Tang, S.-Y.; Petersen, P.; Mitchell, A.; Khoshmanesha, K. Continuous transfer of liquid metal droplets across a fluid–fluid interface within an integrated microfluidic chip. Lab Chip 2015, 15, 2476–2485. [Google Scholar] [CrossRef]
- Lin, Y.; Cooper, C.; Wang, M.; Adams, J.J.; Genzer, J.; Dickey, M.D. Handwritten, Soft Circuit Boards and Antennas Using Liquid Metal Nanoparticles. Small 2015, 11, 6397–6403. [Google Scholar] [CrossRef] [PubMed]
- Kazem, N.; Hellebrekers, T.; Majidi, C. Soft Multifunctional Composites and Emulsions with Liquid Metals. Adv. Mater. 2017, 29, 1605985. [Google Scholar] [CrossRef]
- Hussain, N.; Liang, T.; Zhang, Q.; Anwar, T.; Huang, Y.; Lang, J.; Huang, K.; Wu, H. Ultrathin Bi Nanosheets with Superior Photoluminescence. Small 2017, 13, 1701349. [Google Scholar] [CrossRef]
- Cumby, B.L.; Hayes, G.J.; Dickey, M.D.; Justice, R.S.; Tabor, C.E.; Heikenfeld, J.C. Reconfigurable liquid metal circuits by Laplace pressure shaping. Appl. Phys. Lett. 2012, 101, 174102. [Google Scholar] [CrossRef]
- Ni, J.; Zhong, C.-J.; Coldiron, S.J.; Porter, M.D. Electrochemically Actuated Mercury Pump for Fluid Flow and Delivery. Anal. Chem. 2001, 73, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Trlica, C.; Dickey, M.D. Recapillarity: Electrochemically Controlled Capillary Withdrawal of a Liquid Metal Alloy from Microchannels. Adv. Funct. Mater. 2015, 25, 671–678. [Google Scholar] [CrossRef]
- Gough, R.C.; Morishita, A.M.; Dang, J.H.; Hu, W.; Shiroma, W.A.; Ohta, A.T. Continuous Electrowetting of Non-toxic Liquid Metal for RF Applications. IEEE Access 2014, 2, 874–882. [Google Scholar] [CrossRef]
- Tang, S.-Y.; Sivan, V.; Khoshmanesh, K.; O’Mullane, A.P.; Tang, X.; Gol, B.; Eshtiaghi, N.; Lieder, F.; Petersen, P.; Mitchell, A.; et al. Electrochemically induced actuation of liquid metal marbles. Nanoscale 2013, 5, 5949–5957. [Google Scholar] [CrossRef] [PubMed]
- Chrimes, A.F.; Berean, K.J.; Mitchell, A.; Rosengarten, G.; Kalantar-zadeh, K. Controlled Electrochemical Deformation of Liquid-Phase Gallium. ACS Appl. Mater. Interface 2016, 8, 3833–3839. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-Y.; Khoshmanesh, K.; Sivan, V.; Petersen, P.; O’Mullane, A.P.; Abbott, D.; Mitchell, A.; Kalantar-zadeh, K. Liquid metal enabled pump. Proc. Natl. Acad. Sci. USA 2014, 111, 3304–3309. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Eaker, C.B.; Bowden, E.F.; Dickey, M.D. Giant and switchable surface activity of liquid metal via surface oxidation. Proc. Natl. Acad. Sci. USA 2014, 111, 14047–14051. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, Y.; Moya, J.L.B.; Memoli, G.; Neate, T.; Sahoo, D.R.; Robinson, S.; Pearson, J.; Jones, M.; Subramanian, S. Programmable Liquid Matter: 2D Shape Deformation of Highly Conductive Liquid Metals in a Dynamic Electric Field. In Proceedings of the 2017 ACM International Conference on Interactive Surfaces and Spaces, Brighton, UK, 17–20 October 2017; pp. 142–150. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Mohamed Cassim Mohamed Anver, H.; Zhang, Y.; Tang, S.-Y.; Li, W. Automatic Morphology Control of Liquid Metal using a Combined Electrochemical and Feedback Control Approach. Micromachines 2019, 10, 209. https://doi.org/10.3390/mi10030209
Li M, Mohamed Cassim Mohamed Anver H, Zhang Y, Tang S-Y, Li W. Automatic Morphology Control of Liquid Metal using a Combined Electrochemical and Feedback Control Approach. Micromachines. 2019; 10(3):209. https://doi.org/10.3390/mi10030209
Chicago/Turabian StyleLi, Ming, Hisham Mohamed Cassim Mohamed Anver, Yuxin Zhang, Shi-Yang Tang, and Weihua Li. 2019. "Automatic Morphology Control of Liquid Metal using a Combined Electrochemical and Feedback Control Approach" Micromachines 10, no. 3: 209. https://doi.org/10.3390/mi10030209
APA StyleLi, M., Mohamed Cassim Mohamed Anver, H., Zhang, Y., Tang, S.-Y., & Li, W. (2019). Automatic Morphology Control of Liquid Metal using a Combined Electrochemical and Feedback Control Approach. Micromachines, 10(3), 209. https://doi.org/10.3390/mi10030209






