Development of the Troponin Detection System Based on the Nanostructure
Abstract
1. Introduction
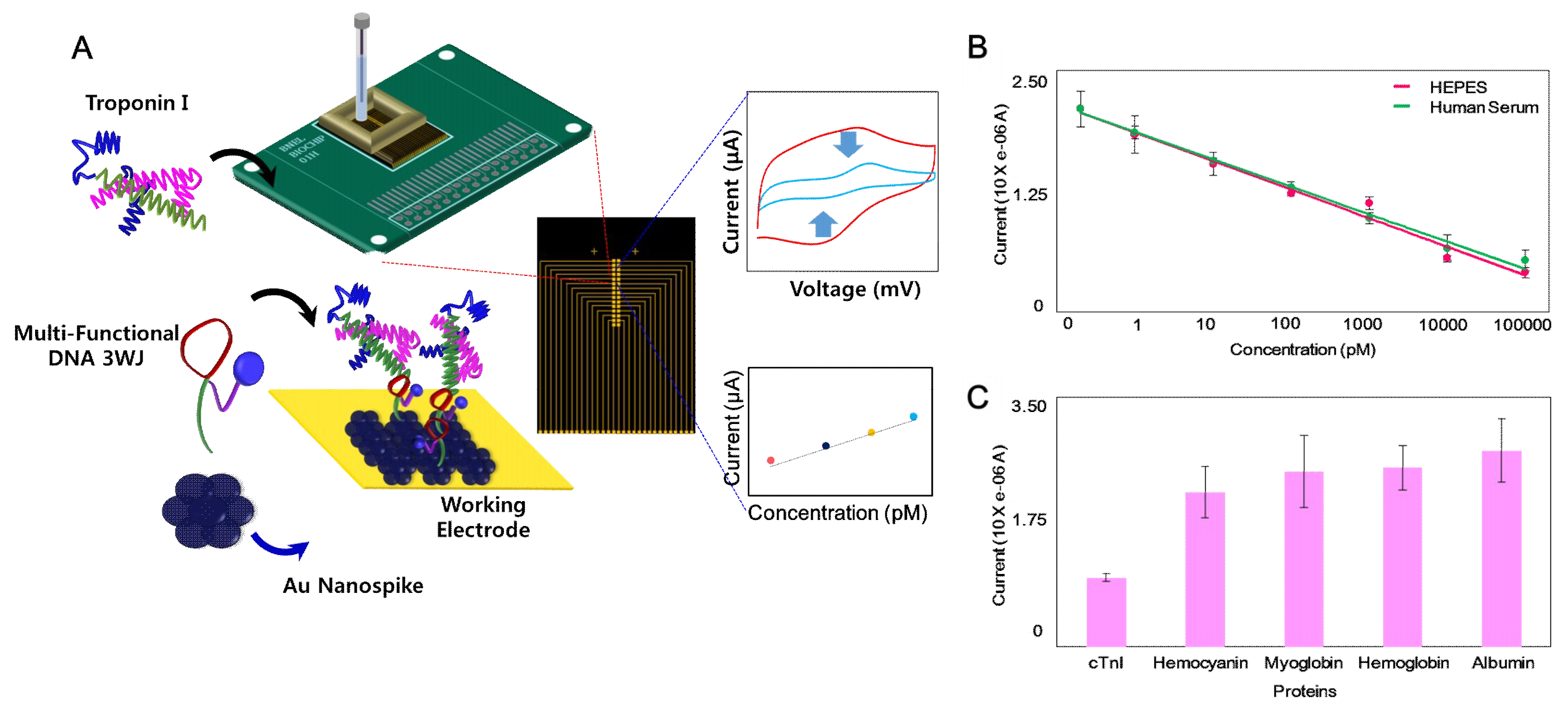
2. EC-Based TN Detection System with Nanostructure
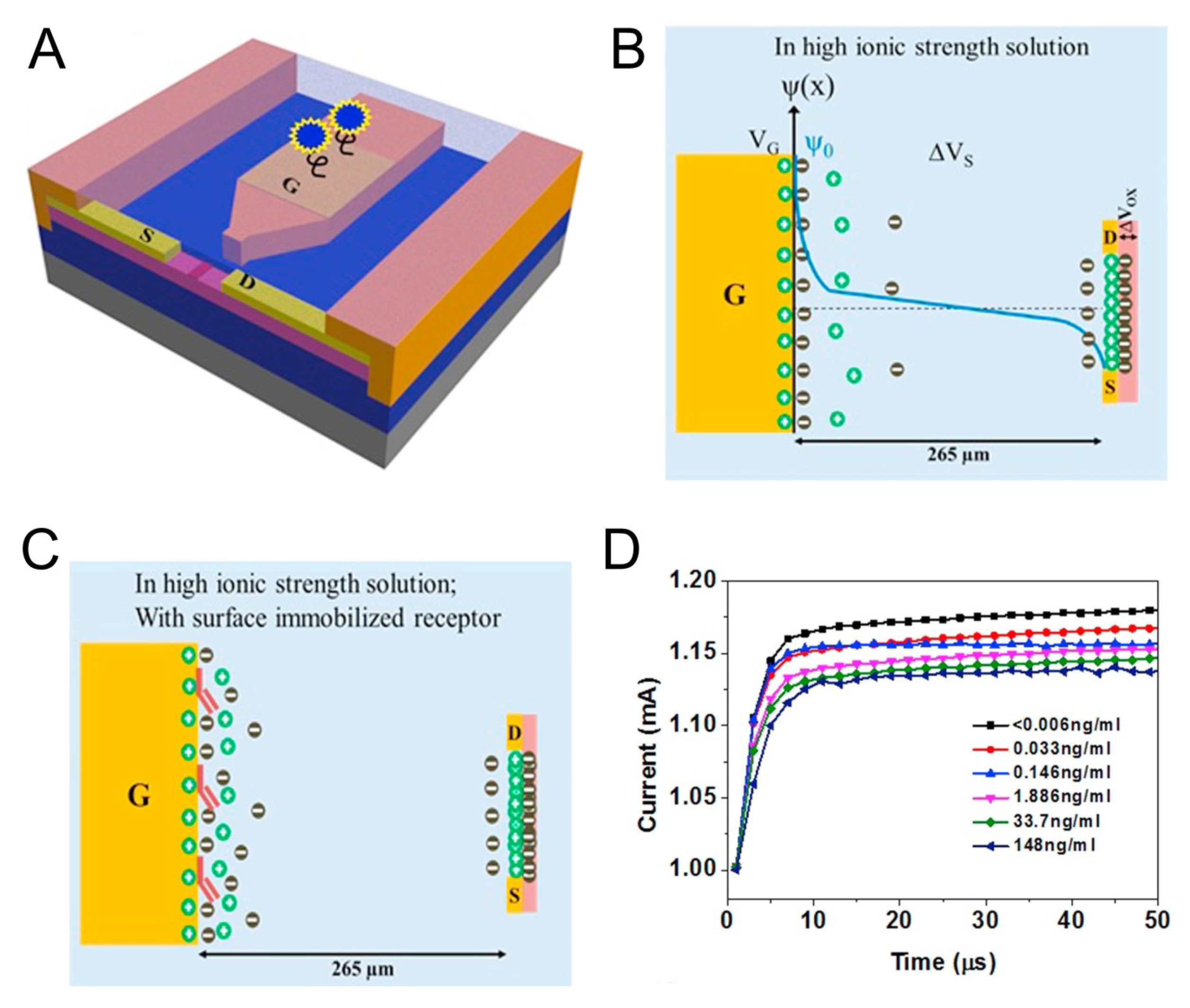
3. FET-Based TN Detection System with Nanostructure
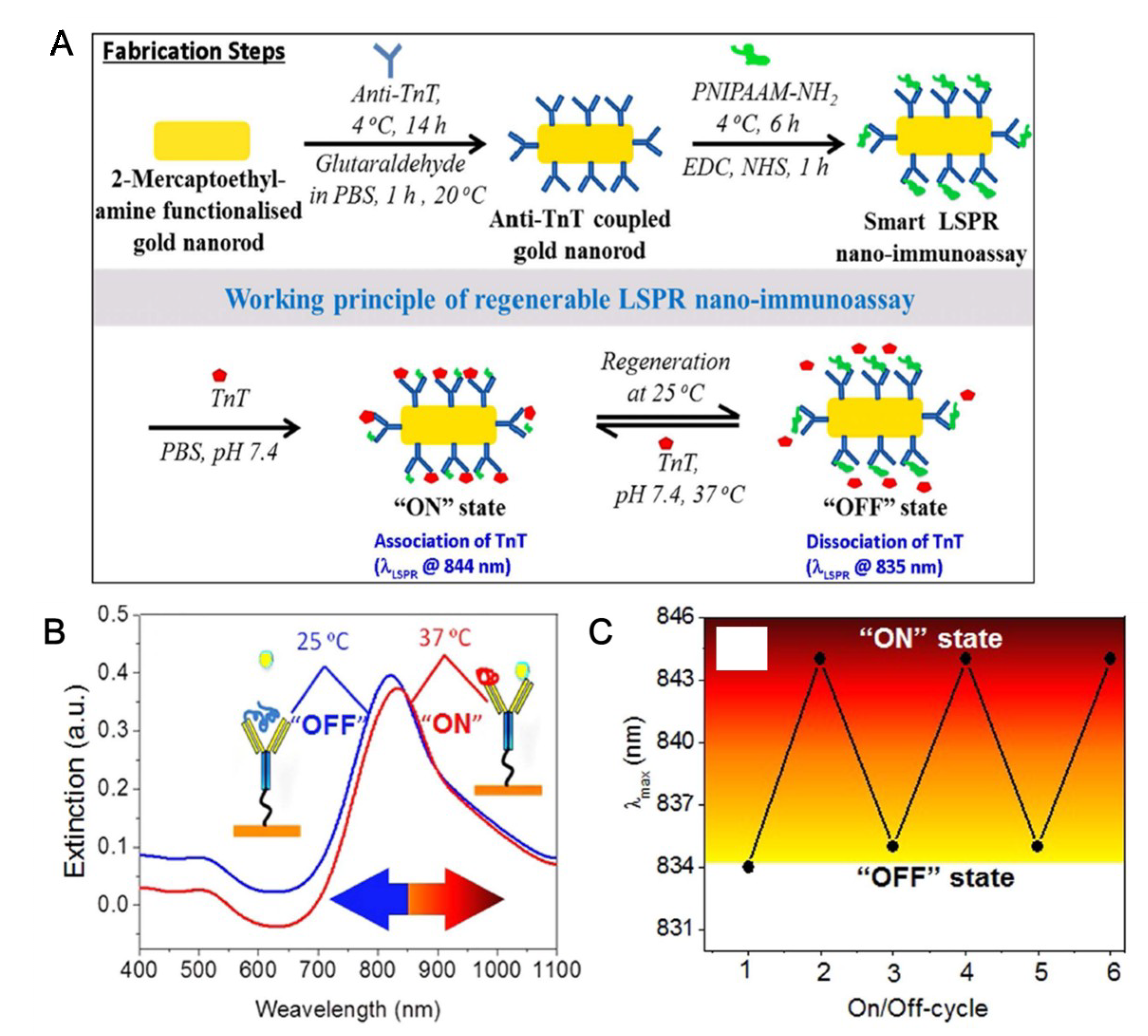
4. SPR-Based TN Detection System with Nanostructure
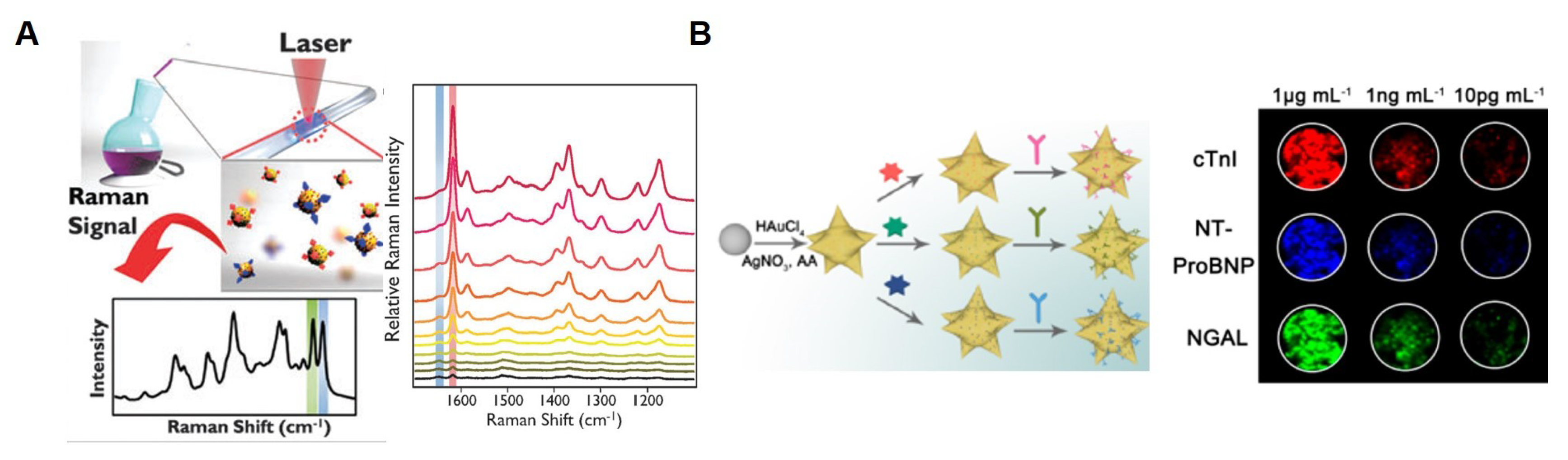
5. SERS-Based TN Detection System with Nanostructure
6. Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Brestoff, J.R.; Artis, D. Immune Regulation of Metabolic Homeostasis in Health and Disease. Cell 2015, 161, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Alwan, A. Global Status Report on Noncommunicable Diseases 2010; World Health Organization: Geneva, Switzerland, 2011; p. 176. [Google Scholar]
- Valensi, P.; Lorgis, L.; Cottin, Y. Prevalence, incidence, predictive factors and prognosis of silent myocardial infarction: A review of the literature. Arch. Cardiovasc. Dis. 2011, 104, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Wettersten, N.; Maisel, A.S. Biomarkers for heart failure: An update for practitioners of internal medicine. Am. J. Med. 2016, 129, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.K.; Wei, J.; Wenger, N.K. Ischemic heart disease in women: A focus on risk factors. Trends Cardiovasc. Med. 2015, 25, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.D. Acute myocardial infarction in developing countries: The importance of large national registries. Int. J. Cardiol. Heart Vasc. 2007, 122, 158–159. [Google Scholar]
- Threapleton, D.E.; Greenwood, D.C.; Evans, C.E.L.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Cade, J.E.; Gale, C.P.; Burley, V.J. Dietary fibre intake and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2013, 347, f6879. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, A.S.; Babuin, A.S.; Apple, F.S. Biomarkers in acute cardiac disease: The present and the future. J. Am. Coll. Cardiol. 2006, 48, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Altintas, Z.; Fakanya, W.M.; Tothill, I.E. Cardiovascular disease detection using biosensing techniques. Talanta 2014, 128, 177–186. [Google Scholar] [CrossRef]
- McDonnell, B.; Hearty, S.; Leonard, P.; O’Kennedy, R. Cardiac Biomarkers and the Case for Point-Of-Care Testing. Clin. Biochem. 2009, 42, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Suprun, E.; Bulko, T.; Lisitsa, A.; Gnedenko, O.; Ivanov, A.; Shumyantseva, V.; Archakov, A. Electrochemical Nanobiosensor for Express Diagnosis of Acute Myocardial Infarction in Undiluted Plasma. Biosens. Bioelectron. 2010, 25, 1694–1698. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.; Ng, L.L. Biomarkers in acute myocardial infarction. BMC Med. 2010, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Li, S.; Peng, Z.; Othman, A.M.; Leblanc, R. Recent Development of Cardiac Troponin I Detection. ACS Sens. 2016, 1, 106–114. [Google Scholar] [CrossRef]
- Svart, K.; Lehtinen, R.; Nieminen, T.; Nikus, K.; Lehtimaki, T.; Koobi, T.; Niemela, K.; Niemi, M.; Turjanmaa, V.; Kahonen, M.; et al. Exercise electrocardiography detection of coronary artery disease by ST-segment depression/heart rate hysteresis in women: The Finnish Cardiovascular Study. Int. J. Cardiol. Heart Vasc. 2010, 140, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Rajiah, P.; Desai, M.Y.; Kwon, D.; Flamm, S.D. MR Imaging of Myocardial Infarction. Radiographics 2013, 33, 1383–1412. [Google Scholar] [CrossRef]
- Gosalia1, A.; Haramati, L.B.; Sheth, M.P.; Spindola-Franco, H. CT Detection of Acute Myocardial Infarction. AJR Am. J. Roentgenol. 2004, 182, 1563–1566. [Google Scholar] [CrossRef] [PubMed]
- Cummins, B.; Cummins, P. Cardiac specific troponin-I release in canine experimental myocardial infarction: Development of a sensitive enzyme-linked immunoassay. J. Mol. Cell. Cardiol. 1987, 19, 999–1010. [Google Scholar] [CrossRef]
- Masson, J.F.; Battaglia, T.M.; Khairallah, P.; Beaudoin, S.; Booksh, K.S. Quantitative measurement of cardiac markers in undiluted serum. Anal. Chem. 2007, 79, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Cummins, B.; Auckland, M.L.; Cummins, P. Cardiac-specific troponin-l radioimmunoassay in the diagnosis of acute myocardial infarction. Am. Heart J. 1987, 113, 1333–1344. [Google Scholar] [CrossRef]
- Chen, Y.X.; Chen, M.W.; Lin, J.Y.; Lai, W.Q.; Huang, W.; Chen, H.Y.; Weng, G.X. Label-free optical detection of acute myocardial infarction based on blood plasma surface-enhanced raman spectroscopy. J. Appl. Spectrosc. 2016, 83, 798–804. [Google Scholar] [CrossRef]
- Cai, Y.; Kang, K.; Li, Q.; Wang, Y.; He, X. Rapid and Sensitive Detection of Cardiac Troponin I for Point-of-Care Tests Based on Red Fluorescent Microspheres. Molecules 2018, 23, 1102. [Google Scholar] [CrossRef]
- Pedrero, M.; Campuzano, S.; Pingarrón, J.M. Electrochemical Biosensors for the Determination of Cardiovascular Markers: A Review. Electroanalysis 2014, 26, 1132–1153. [Google Scholar] [CrossRef]
- Shanmugam, N.R.; Selvam, A.P.; Barrett, T.W.; Kazmierczak, S.C.; Rana, M.N.; Prasad, S. Portable nanoporous electrical biosensor for ultrasensitive detection of Troponin-T. Future Sci. OA 2015, 1, FSO24. [Google Scholar] [CrossRef] [PubMed]
- Sarangadharan, I.; Wang, S.L.; Sukesan, R.; Chen, P.C.; Dai, T.Y.; Pulikkathodi, A.K.; Hsu, C.P.; Chinang, H.H.K.; Liu, L.Y.M.; Wang, Y.L. Single Drop Whole Blood Diagnostics: Portable Biomedical Sensor for Cardiac Troponin I Detection. Anal. Chem. 2018, 90, 2867–2874. [Google Scholar] [CrossRef] [PubMed]
- Straface, A.L.; Myers, J.H.; Kirchick, H.J.; Blick, K.E. A Rapid Point-of-Care Cardiac Marker Testing Strategy Facilitates the Rapid Diagnosis and Management of Chest Pain Patients in the Emergency Department. Am. J. Clin. Pathol. 2008, 129, 788–795. [Google Scholar] [CrossRef]
- Pickering, J.W.; Young, J.M.; George, P.M.; Watson, A.S.; Aldous, S.J.; Troughton, R.W.; Pemberton, C.J.; Richards, A.M.; Cullen, L.A.; Than, M.P. Validity of a Novel Point-of-Care Troponin Assay for Single-Test Rule-Out of Acute Myocardial Infarction. JAMA Cardiol. 2018, 3, 1108–1112. [Google Scholar] [CrossRef]
- Periyakaruppan, A.; Gandhiraman, R.P.; Meyyappan, M.; Koehne, J.E. Label-Free Detection of Cardiac Troponin-I Using Carbon Nanofiber Based Nanoelectrode Arrays. Anal. Chem. 2013, 85, 3858–3863. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.; Kim, B.; Jo, S.S.; Oh, S.Y.; Park, J.K. Electrochemical detection of cardiac troponin I using a microchip with the surface-functionalized poly(dimethylsiloxane) channel. Biosens. Bioelectron. 2007, 23, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Gu, H.; Jeon, W.; Youn, H.; Her, J.; Kim, S.K.; Lee, J.; Shin, J.H.; Ban, C.I. Electrochemical Aptasensor of Cardiac Troponin I for the Early Diagnosis of Acute Myocardial Infarction. Anal. Chem. 2015, 87, 9869–9875. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, N.R.; Muthukumar, S.; Tanak, A.S.; Prasad, S. Multiplexed electrochemical detection of three cardiac biomarkers cTnI, cTnT and BNP using nanostructured ZnO-sensing platform. Future Cardiol. 2018, 14, 131–141. [Google Scholar] [CrossRef]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarron, J.M. Electrochemical biosensing approaches for diagnosis of viral infections and tropical diseases. Chemelectrochem 2017, 4, 753–777. [Google Scholar] [CrossRef]
- Lee, H.Y.; Choi, J.S.; Guruprasath, P.; Lee, B.H.; Cho, Y.W. An Electrochemical Biosensor Based on a Myoglobin-specific Binding Peptide for Early Diagnosis of Acute Myocardial Infarction. Anal. Sci. 2015, 31, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Tepeli, Y.; Ülkü, A. Electrochemical biosensors for influenza virus a detection: The potential of adaptation of these devices to POC systems. Sens. Actuators B Chem. 2018, 254, 377–384. [Google Scholar] [CrossRef]
- Jo, H.; Her, J.; Lee, H.; Shim, Y.B.; Ban, C. Highly sensitive amperometric detection of cardiac troponin I using sandwich aptamers and screen-printed carbon electrodes. Talanta 2017, 165, 442–448. [Google Scholar] [CrossRef]
- Xiong, M.; Wang, X.; Kong, Y.; Han, B. Development of Cardiac Troponin I Electrochemical Impedance Immunosensor. Int. J. Electrochem. Sci. 2017, 12, 4204–4214. [Google Scholar] [CrossRef]
- Sheng, Q.; Qiao, X.; Zhou, M.; Zheng, J. Recent progress in electrochemical sensing of cardiac troponin by using nanomaterial-induced signal amplification. Microchim. Acta 2017, 184, 1573–1585. [Google Scholar] [CrossRef]
- Qureshi, A.; Gurbuz, Y.; Niazi, J.H. Biosensors for cardiac biomarkers detection: A review. Sens. Actuators B Chem. 2012, 171, 62–76. [Google Scholar] [CrossRef]
- Lee, T.; Lee, Y.; Park, S.Y.; Hong, K.Y.; Kim, Y.; Park, C.; Chung, Y.H.; Lee, M.H.; Min, J. Fabrication of electrochemical biosensor composed of multi-functional DNA structure/Au nanospike on micro-gap/PCB system for detecting troponin I in human serum. Colloids Surf. B Biointerfaces 2019, 175, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Filho, S.L.R.; Dias, A.C.M.S.; Silva, M.M.S.; Silva, B.V.M.; Dutra, R.F. A carbon nanotube-based electrochemical immunosensor for cardiac troponin T. Microchem. J. 2013, 109, 10–15. [Google Scholar] [CrossRef]
- Ahammad, A.J.S.; Choi, Y.H.; Koh, K.; Kim, J.H.; Lee, J.J.; Lee, M. Electrochemical detection of cardiac biomarker troponin I at gold nanoparticle-modified ITO electrode by using open circuit potential. Int. J. Electrochem. Sci. 2011, 6, 1906–1916. [Google Scholar]
- Negahdary, M.; Behjati-Ardakani, M.; Sattarahmady, N.; Yadegari, H.; Heli, H. Electrochemical aptasensing of human cardiac troponin I based on anarray of gold nanodumbbells-Applied to early detection of myocardial infarction. Sens. Actuators B Chem. 2017, 252, 62–71. [Google Scholar] [CrossRef]
- Shanmugam, N.R.; Muthukumar, S.; Selvam1, A.P.; Prasad, S. Electrochemical nanostructured ZnO biosensor for ultrasensitive detection of cardiac troponin-T. Nanomedicine 2016, 11, 1345–1358. [Google Scholar] [CrossRef]
- Silva, B.V.; Cavalcanti, I.T.; Silva, M.M.; Dutra, R.F. A carbon nanotube screen-printed electrode for label-free detection of the human cardiac troponin T. Talanta 2013, 117, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, Y.; Li, P.; Lin, H.; Jin, H.; Gao, L.; Ge, S.; Zhang, Y. Evaluation of a newly developed chemiluminescence immunoassay for detecting cardiac troponin T. J. Clin. Lab. Anal. 2018, 32, e22311. [Google Scholar] [CrossRef]
- Cui, Y.; Wei, Q.; Park, H.; Lieber, C.M. Nanowire Nanosensors for Highly Sensitive and Selective Detection of Biological and Chemical Species. Science 2001, 293, 1289. [Google Scholar] [CrossRef] [PubMed]
- Stern, E.; Klemic, J.F.; Routenberg, D.A.; Wyrembak, P.N.; Turner-Evans, D.B.; Hamilton, A.D.; LaVan, D.A.; Fahmy, T.M.; Reed, M.A. Label-free immunodetection with CMOS-compatible semiconducting nanowires. Nature 2007, 445, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Choi, S.J.; Han, J.W.; Park, T.J.; Lee, S.Y.; Choi, Y.K. Double-Gate Nanowire Field Effect Transistor for a Biosensor. Nano Lett. 2010, 10, 2934–2938. [Google Scholar] [CrossRef] [PubMed]
- Rani, D.; Pachauri, V.; Mueller, A.; Vu, X.T.; Nguyen, T.C.; Ingebrandt, S. On the Use of Scalable NanoISFET Arrays of Silicon with Highly Reproducible Sensor Performance for Biosensor Applications. ACS Omega 2016, 1, 84–92. [Google Scholar] [CrossRef]
- Lee, J.O.; Wiertz, F.G.M.; Heering, H.A.; Dekker, C.; Besteman, K. Enzyme-Coated Carbon Nanotubes as Single-Molecule Biosensors. Nano Lett. 2003, 3, 727–730. [Google Scholar]
- Allen, B.L.; Kichambare, P.D.; Star, A. Carbon Nanotube Field-Effect-Transistor-Based Biosensors. Adv. Mater. 2007, 19, 1439–1451. [Google Scholar] [CrossRef]
- Ohno, Y.; Maehashi, K.; Matsumoto, K. Label-Free Biosensors Based on Aptamer-Modified Graphene Field-Effect Transistors. J. Am. Chem. Soc. 2010, 132, 18012–18013. [Google Scholar] [CrossRef]
- Soikkeli, M.; Kurppa, K.; Kainlauri, M.; Arpiainen, S.; Paananen, A.; Gunnarsson, D.; Joensuu, J.J.; Laaksonen, P.; Prunnila, M.; Linder, M.B.; et al. Graphene Biosensor Programming with Genetically Engineered Fusion Protein Monolayers. ACS Appl. Mater. Interfaces 2016, 8, 8257–8264. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Liu, W.; Xie, X.; Anselmo, A.C.; Mitragotri, S.; Banerjee, K. MoS2 Field-Effect Transistor for Next-Generation Label-Free Biosensors. ACS Nano 2014, 8, 3992–4003. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, C.; Kwon, D.; Kim, D.; Meyyappan, M.; Jeon, S.; Lee, J.S. Silicon nanowire biosensors for detection of cardiac troponin I (cTnI) with high sensitivity. Biosens. Bioelectron. 2016, 77, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Aroonyadet, N.; Song, Y.; Wang, X.; Cao, X.; Liu, Y.; Cong, S.; Wu, F.; Thompson, M.E.; Zhou, C. Highly Sensitive and Quick Detection of Acute Myocardial Infarction Biomarkers Using In2O3 Nanoribbon Biosensors Fabricated Using Shadow Masks. ACS Nano 2016, 10, 10117–10125. [Google Scholar] [CrossRef]
- Livi, P.; Kwiat, M.; Shadmani, A.; Pevzner, A.; Navarra, G.; Rothe, G.; Stettler, A.; Chen, Y.; Patolsky, F.; Hierlemann, A. Monolithic Integration of a Silicon Nanowire Field-Effect Transistors Array on a Complementary Metal-Oxide Semiconductor Chip for Biochemical Sensor Applications. Anal Chem. 2015, 87, 9982–9990. [Google Scholar] [CrossRef]
- Stern, E.; Wagner, R.; Sigworth, F.J.; Breaker, R.; Fahmy, T.M.; Reed, M.A. Importance of the Debye Screening Length on Nanowire Field Effect Transistor Sensors. Nano Lett. 2007, 7, 3405–3409. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, D.K.; Kandpal, M.; Surya, S.G. Characterization and detection of cardiac Troponin-T protein by using ‘aptamer’ mediated biofunctionalization of ZnO thin-film transistor. Appl. Surf. Sci. 2019, 466, 874–881. [Google Scholar] [CrossRef]
- Nakatsuka, N.; Yang, K.; Abendroth, J.M.; Cheung, K.M.; Xu, X.; Yang, H.; Zhao, C.; Zhu, B.; Rim, Y.S.; Yang, Y.; et al. Aptamer–field-effect transistors overcome Debye length limitations for small-molecule sensing. Science 2018, 362, 319–324. [Google Scholar] [CrossRef]
- Chu, C.H.; Sarangadharan, I.; Regmi, A.; Chen, Y.W.; Hsu, C.P.; Chang, W.H.; Lee, G.Y.; Chyi, J.I.; Chen, C.C.; Shiesh, S.C.; et al. Beyond the Debye length in high ionic strength solution: Direct protein detection with field-effect transistors (FETs) in human serum. Sci. Rep. 2017, 7, 5256. [Google Scholar] [CrossRef]
- Sarangadharan, I.; Regmi, A.; Chen, Y.W.; Hsu, C.P.; Chen, P.; Chang, W.H.; Lee, G.Y.; Chyi, J.; Shiesh, S.C.; Lee, G.B.; et al. High sensitivity cardiac troponin I detection in physiological environment using AlGaN/GaN High Electron Mobility Transistor (HEMT) Biosensors. Biosens. Bioelectron 2018, 100, 282–289. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Wu, F.; Cao, X.; Li, Z.; Alharbi, M.; Abbas, A.N.; Amer, M.R.; Zhou, C. Highly Sensitive and Wearable In2O3 Nanoribbon Transistor Biosensors with Integrated On-Chip Gate for Glucose Monitoring in Body Fluids. ACS Nano 2018, 12, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Heo, N.S.; Shukla, S.; Cho, H.J.; Vilian, A.E.; Kim, J.; Huh, Y.S. Development of gold nanoparticle-aptamer-based LSPR sensing chips for the rapid detection of Salmonella typhimurium in pork meat. Sci. Rep. 2017, 7, 10130. [Google Scholar] [CrossRef]
- Hassanpour, S.; Baradaran, B.; Hejazi, M.; Hasanzadeh, M.; Mokhtarzadeh, A.; de la Guardia, M. Recent trends in rapid detection of influenza infections by bio and nanobiosensor. Trends Anal. Chem. 2018, 98, 201–215. [Google Scholar] [CrossRef]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nanosci. Technol. A Collect. Rev. Nat. J. 2008, 7, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Baillargeat, D.; Ho, H.P.; Yong, K.T. Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications. Chem. Soc. Rev. 2014, 43, 3426–3452. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.K.; Kim, Y.K.; Park, K.W.; Lee, W.H.; Choi, J.W. Surface plasmon resonance immunosensor for the detection of Salmonella typhimurium. Biosens. Bioelectron. 2004, 19, 1497–1504. [Google Scholar] [CrossRef]
- Poltronieri, P.; Mezzolla, V.; Primiceri, E.; Maruccio, G. Biosensors for the detection of food pathogens. Foods 2014, 3, 511–526. [Google Scholar] [CrossRef]
- Ahn, H.; Song, H.; Choi, J.R.; Kim, K. A localized surface plasmon resonance sensor using double-metal-complex nanostructures and a review of recent approaches. Sensors 2018, 18, 98. [Google Scholar] [CrossRef]
- Campbell, C.T.; Kim, G. SPR microscopy and its applications to high-throughput analyses of biomolecular binding events and their kinetics. Biomaterials 2007, 28, 2380–2392. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.S.; Fan, S.K. Microfluidic surface plasmon resonance sensors: From principles to point-of-care applications. Sensors 2016, 16, 1175. [Google Scholar] [CrossRef]
- Tokel, O.; Inci, F.; Demirci, U. Advances in plasmonic technologies for point of care applications. Chem. Rev. 2014, 114, 5728–5752. [Google Scholar] [CrossRef] [PubMed]
- Masson, J.F. Surface plasmon resonance clinical biosensors for medical diagnostics. ACS Sens. 2017, 2, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Abdolrahim, M.; Rabiee, M.; Alhosseini, S.N.; Tahriri, M.; Yazdanpanah, S.; Tayebi, L. Development of optical biosensor technologies for cardiac troponin recognition. Anal. Biochem. 2015, 485, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Sun, Y.; Zhang, D.; Li, S.; Zhang, Y.; Ma, P.; Song, D. Ultrasensitive magnetic field-assisted surface plasmon resonance immunoassay for human cardiac troponin I. Biosens. Bioelectron. 2017, 96, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.C.; Kim, M.G.; Kim, E.M.; Shin, Y.B.; Lee, S.K.; Lee, S.D.; Cho, M.J.; Ro, H.S. Development of a surface plasmon resonance-based immunosensor for the rapid detection of cardiac troponin I. Biotechnol. Lett. 2011, 33, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Ashaduzzaman, M.; Deshpande, S.R.; Murugan, N.A.; Mishra, Y.K.; Turner, A.P.; Tiwari, A. On/off-switchable LSPR nano-immunoassay for troponin-T. Sci. Rep. 2017, 7, 44027. [Google Scholar] [CrossRef]
- Pawula, M.; Altintas, Z.; Tothill, I.E. SPR detection of cardiac troponin T for acute myocardial infarction. Talanta 2016, 146, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Dutra, R.F.; Mendes, R.K.; da Silva, V.L.; Kubota, L.T. Surface plasmon resonance immunosensor for human cardiac troponin T based on self-assembled monolayer. J. Pharm. Biomed. Anal. 2007, 43, 1744–1750. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.R.; Gu, C.R.; Fan, X.; Bian, Z.P.; Wu, H.F.; Yang, D.; Gu, N.; Zhang, J.N. Fabrication of Anti-human Cardiac Troponin I Immunogold Nanorods for Sensing Acute Myocardial Damage. Nanoscale Res. Lett. 2009, 4, 1428–1433. [Google Scholar] [CrossRef]
- Liu, J.T.; Chen, C.J.; Ikoma, T.; Yoshioka, T.; Cross, J.S.; Chang, S.J.; Tanaka, J. Surface plasmon resonance biosensor with high anti-fouling ability for the detection of cardiac marker troponin T. Anal. Chim. Acta 2011, 703, 80–86. [Google Scholar] [CrossRef]
- Masson, J.F.; Obando, L.; Beaudoin, S.; Booksh, K. Sensitive and real-time fiber-optic-based surface plasmon resonance sensors for myoglobin and cardiac troponin I. Talanta 2004, 62, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Chen, P.; McCadden, A.; Palaniappan, A.; Zhang, J.; Liedberg, B. Peptide functionalized gold nanoparticles with optimized particle size and concentration for colorimetric assay development: Detection of cardiac troponin I. ACS Sens. 2016, 1, 1416–1422. [Google Scholar] [CrossRef]
- Yang, C.T.; Wu, L.; Liu, X.; Tran, N.T.; Bai, P.; Liedberg, B.; Wang, Y.; Thierry, B. exploiting surface-plasmon-enhanced light scattering for the design of ultrasensitive biosensing modality. Anal. Chem. 2016, 88, 11924–11930. [Google Scholar] [CrossRef] [PubMed]
- Colthup, N. Introduction to Infrared and Raman Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Long, D.A.; Long, D.A. Raman Spectroscopy; Mcgraw-Hill: New York, NY, USA, 1977; Volume 276. [Google Scholar]
- Kneipp, K.; Kneipp, H.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Ultrasensitive chemical analysis by Raman spectroscopy. Chem. Rev. 1999, 99, 2957–2976. [Google Scholar] [CrossRef] [PubMed]
- Moskovits, M. Surface-enhanced Raman spectroscopy: A brief retrospective. J. Raman Spectrosc. 2005, 36, 485–496. [Google Scholar] [CrossRef]
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; Van Duyne, R.P. Surface-enhanced Raman spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626. [Google Scholar] [CrossRef]
- Ding, S.Y.; Yi, J.; Li, J.F.; Ren, B.; Wu, D.Y.; Panneerselvam, R.; Tian, Z.Q. Nanostructure-based plasmon-enhanced Raman spectroscopy for surface analysis of materials. Nat. Rev. Mater. 2016, 1, 16021. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, Y.; Liu, X.; Lu, W.; Dai, J.; Lei, D.Y.; MacFarlane, D.R. Hierarchical Porous Plasmonic Metamaterials for Reproducible Ultrasensitive Surface-Enhanced Raman Spectroscopy. Adv. Mater. 2015, 27, 1090–1096. [Google Scholar] [CrossRef]
- Matricardi, C.; Hanske, C.; Garcia-Pomar, J.L.; Langer, J.; Mihi, A.; Liz-Marzán, L.M. Gold Nanoparticle Plasmonic Superlattices as Surface-Enhanced Raman Spectroscopy Substrates. ACS Nano 2018, 12, 8531–8539. [Google Scholar] [CrossRef]
- Lane, L.A.; Qian, X.; Nie, S. SERS nanoparticles in medicine: From label-free detection to spectroscopic tagging. Chem. Rev. 2015, 115, 10489–10529. [Google Scholar] [CrossRef]
- Laing, S.; Gracie, K.; Faulds, K. Multiplex in vitro detection using SERS. Chem. Soc. Rev. 2016, 45, 1901–1918. [Google Scholar] [CrossRef] [PubMed]
- Aioub, M.; El-Sayed, M.A. A real-time surface enhanced raman spectroscopy study of plasmonic photothermal cell death using targeted gold nanoparticles. J. Am. Chem. Soc. 2016, 138, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Chon, H.; Lee, S.; Yoon, S.Y.; Lee, E.K.; Chang, S.I.; Choo, J. SERS-based competitive immunoassay of troponin I and CK-MB markers for early diagnosis of acute myocardial infarction. Chem. Commun. 2014, 50, 1058–1060. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Xu, S.; Zhang, J.; Chen, X.J.; Jiang, L.P.; Zheng, T.; Zhu, J.J. Plasmon Near-Field Coupling of Bimetallic Nanostars and Hierarchical Bimetallic SERS “Hot Field”: Toward Ultrasensitive Simultaneous Detection of Multiple Cardiorenal Syndrome Biomarkers. Anal. Chem. 2018, 91, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Wang, Y.; Liu, Y.; Liu, H.; Fu, L.; Wen, J.; Li, J.; Wei, P.; Chen, L. Graphene oxide/gold nanoparticles-based amplification method for SERS immunoassay of cardiac troponin I. Analyst 2019, 144, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Garza, J.T.; Cote, G.L. Collection method of SERS active nanoparticles for sensitive and precise measurements. Anal. Chem. 2017, 89, 13120–13127. [Google Scholar] [CrossRef]
- Noble, J.; Attree, S.; Horgan, A.; Knight, A.; Kumarswami, N.; Porter, R.; Worsley, G. Optical scattering artifacts observed in the development of multiplexed surface enhanced Raman spectroscopy nanotag immunoassays. Anal. Chem. 2012, 84, 8246–8252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Huang, L.; Liu, B.; Ni, H.; Sun, L.; Su, E.; Zhao, X. Quantitative and ultrasensitive detection of multiplex cardiac biomarkers in lateral flow assay with core-shell SERS nanotags. Biosens. Bioelectron. 2018, 106, 204–211. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, L.; Liu, B.; Su, E.; Chen, H.Y.; Gu, Z.; Zhao, X. Quantitative detection of multiplex cardiac biomarkers with encoded SERS nanotags on a single T line in lateral flow assay. Sens. Actuators B Chem. 2018, 277, 502–509. [Google Scholar] [CrossRef]
| Bioprobe | Detection Method | Detection Limit | Nanostructure | Ref |
|---|---|---|---|---|
| Antibody | CV/EIS | 0.2 ng/mL | Carbon nanofiber | [27] |
| Antibody | CV/EIS | 24 pg/mL | Gold nanoparticle | [34] |
| Aptamer | CA | 24 pg/mL (1 pM) | Fc-modified silica nanoparticle | [29] |
| Aptamer | DPV | 8 pg/mL | Au nanodumbbells | [41] |
| Aptamer | CV | 24 pg/mL (1 pM) (in a dilluted serum) | Au nanospike | [38] |
| Antibody | Direct electrical detection | 5 pg/mL (cTnI) | Silicon nanowires | [54] |
| Antibody | Sandwich immunoassay, Electrical detection | 1 pg/mL (cTnI) | Indium oxide (In2O3) Nanoribbons | [55] |
| Antibody | Direct electrical detection | 1 nM (cTnT) | Silicon nanowires | [56] |
| Aptamer | Direct electrical detection | 10 μg/mL (cTnT) | Zinc oxide (ZnO) thin film | [58] |
| Antibody, Aptamer | Electric-double layer, Direct electrical detection | 6 pg/mL (cTnI) | AlGaN/GaN nanoribbons | [61] |
| Bioprobe | Detection Method | Detection Limit | Nanostructure | Ref |
|---|---|---|---|---|
| Antibody | SPR | 1.25 ng/mL | Magnetic multi-walled carbon nano-tubes(MMWCNTs)/Hollow gold nanoparticles(HGNPs) | [75] |
| Antibody | SPR | 68 ng/L | - | [76] |
| Antibody | LSPR | 7.6 fg/mL | Gold nanorod | [77] |
| Antibody | SPR | 0.5 ng/mL | Gold nanoparticle | [78] |
| Antibody | SPR | 0.05 ng/mL | - | [79] |
| Antibody | SPR | 100 ng/mL | Gold nanorod | [80] |
| Antibody | SERS | 33.7 pg/mL | Magnetic microparticle/Gold nanoparticle | [94] |
| Antibody | SERS | 0.76 pg/Ml | Bimetallic nanostar (gold-silver)/Gold-silver nanoarray | [95] |
| Antibody | SERS | 5 pg/mL | Graphene oxide/Gold nanoparticle/Magnetic microparticle | [96] |
| Antibody | SERS | 12.9 fM | Magnetic microparticle/Silver nanoparticle | [97] |
| Antibody | Lateral immunoassay, SERS | 1 ng/mL | Gold nanoparticle | [98] |
| Antibody | Lateral immunoassay, SERS | 0.44 pg/mL | Silver-gold core-shell nanoparticle | [99] |
| Antibody | Lateral immunoassay, SERS | 0.89 pg/mL | Silver-gold core-shell nanoparticle | [100] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.; Ahn, J.-H.; Choi, J.; Lee, Y.; Kim, J.-M.; Park, C.; Jang, H.; Kim, T.-H.; Lee, M.-H. Development of the Troponin Detection System Based on the Nanostructure. Micromachines 2019, 10, 203. https://doi.org/10.3390/mi10030203
Lee T, Ahn J-H, Choi J, Lee Y, Kim J-M, Park C, Jang H, Kim T-H, Lee M-H. Development of the Troponin Detection System Based on the Nanostructure. Micromachines. 2019; 10(3):203. https://doi.org/10.3390/mi10030203
Chicago/Turabian StyleLee, Taek, Jae-Hyuk Ahn, Jinha Choi, Yeonju Lee, Jin-Myung Kim, Chulhwan Park, Hongje Jang, Tae-Hyung Kim, and Min-Ho Lee. 2019. "Development of the Troponin Detection System Based on the Nanostructure" Micromachines 10, no. 3: 203. https://doi.org/10.3390/mi10030203
APA StyleLee, T., Ahn, J.-H., Choi, J., Lee, Y., Kim, J.-M., Park, C., Jang, H., Kim, T.-H., & Lee, M.-H. (2019). Development of the Troponin Detection System Based on the Nanostructure. Micromachines, 10(3), 203. https://doi.org/10.3390/mi10030203







