Nanostructured Fe,Co-Codoped MoO3 Thin Films
Abstract
1. Introduction
2. Experimental Method
2.1. Fe-Co Codoped MoO3 Thin Films Deposition
2.2. Techniques Used for the Fe-Co Codoped MoO3 Thin Film Characterization
3. Structural Investigation
3.1. X-ray Diffraction Analyses
3.2. SEM-EDAX Characterization
3.3. SEM and TEM Observations
4. Optical Investigations
5. Electropyroelectric (EPE) Investigation
6. Photoluminescence
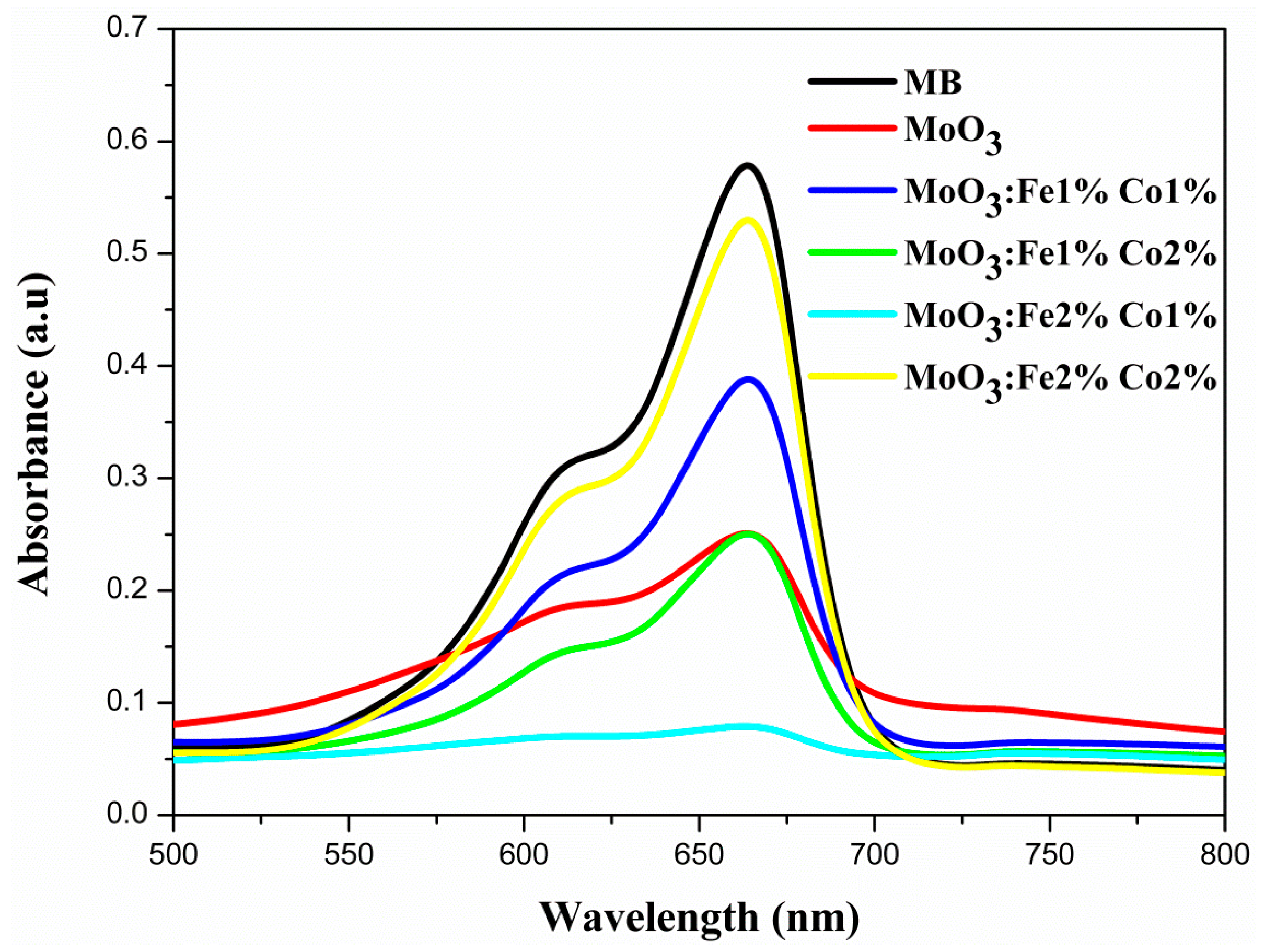
7. Photocatalytic Performance
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- He, S.; Li, W.; Feng, L.; Yang, W. Rational interaction between the aimed gas and oxide surfaces enabling high-performance sensor: The case of acidic α-MoO3 nanorods for selective detection of triethylamine. J. Alloys Compd. 2019, 783, 574–582. [Google Scholar] [CrossRef]
- Chan, X.; Akter, N.; Yang, P.; Ooi, C.; James, A.; Boscoboinik, J.A.; Parise, J.B.; Kim, T. Fundamental study of furfuryl alcohol dehydration reaction over molybdenum oxide catalyst. Mol. Catal. 2019, 466, 19–25. [Google Scholar] [CrossRef]
- Han, Q.; Wang, X.; Liu, X.; Zhang, Y.; Cai, S.; Qi, C.; Wang, C.; Yang, R. MoO3−x nanodots with dual enzyme mimic activities as multifunctional modulators for amyloid assembly and neurotoxicity. J. Colloid Interface Sci. 2019, 539, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Tamboli, P.S.; Jagtap, C.V.; Kadam, V.S.; Ingle, R.V.; Vhatkar, R.S.; Mahajan, S.S.; Pathan, H.M. Spray pyrolytic deposition of α-MoO3 film and its use in dye-sensitized solar cell. Appl. Phys. A 2018. [Google Scholar] [CrossRef]
- Manivel, A.; Lee, G.-J.; Chen, C.-Y.; Chen, J.-H.; Ma, S.-H.; Horng, T.-L.; Wu, J.J. Synthesis of MoO3 nanoparticles for azo dye degradation by catalytic ozonation. Mater. Res. Bull. 2015, 62, 184–191. [Google Scholar] [CrossRef]
- Santos-Beltrán, M.; Paraguay-Delgado, F.; García, R.; Antúnez-Flores, W.; Ornelas-Gutiérrez, C.; Santos-Beltrán, A. Fast methylene blue removal by MoO3 nanoparticles. J. Mater. Sci. Mater. Electron. 2016, 28, 2935–2948. [Google Scholar] [CrossRef]
- Unnarkat, A.P.; Sridhar, T.; Wang, H.; Mahajani, S.M.; Suresh, A.K. Study of cobalt molybdenum oxide supported on mesoporous silica for liquid phase cyclohexane oxidation. Catal. Today 2018, 310, 116–129. [Google Scholar] [CrossRef]
- Dhara, A.; Hodes, G.; Sarkar, S.K. Two stage chemical bath deposition of MoO3 nanorod films. RSC Adv. 2014, 4, 53694–53700. [Google Scholar] [CrossRef]
- Ponce-Mosso, M.; Pérez-González, M.; García-Tinoco, P.E.; Crotte-Ledesma, H.; Morales-Luna, M.; Tomás, S.A. Enhanced photocatalytic activity of amorphous MoO3 thin films deposited by rf reactive magnetron sputtering. Catal. Today 2018. [Google Scholar] [CrossRef]
- Di, L.; Yang, H.; Xian, T.; Chen, X. Construction of Z-Scheme g-C3N4/CNT/Bi2Fe4O9 Composites with Improved Simulated-Sunlight Photocatalytic Activity for the Dye Degradation. Micromachines 2018, 9, 613. [Google Scholar] [CrossRef]
- Fox, M.A.; Dulay, M.T. Heterogeneous photocatalysis. Chem. Rev. 1993, 93, 341–357. [Google Scholar] [CrossRef]
- Kudo, A. Development of photocatalyst materials for water splitting. Int. J. Hydrogen Energy 2006, 31, 197–202. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Chen, D.; Qin, Q.; Wu, Y.; Huang, F.; Li, W. Preparation of FeOOH/Cu with High Catalytic Activity for Degradation of Organic Dyes. Materials 2019, 12, 338. [Google Scholar] [CrossRef] [PubMed]
- Laurenti, M.; Cauda, V. Porous Zinc Oxide Thin Films: Synthesis Approaches and Applications. Coatings 2018, 8, 67. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, C.; Xu, L.; Ma, Y.; Hou, W.; Zhu, J.-J. Single-crystalline orthorhombic molybdenum oxide nanobelts: Synthesis and photocatalytic properties. CrystEngComm 2010, 12, 3740–3747. [Google Scholar] [CrossRef]
- Qu, Q.; Zhang, W.-B.; Huang, K.; Chen, H.-M. Electronic structure, optical properties and band edges of layered MoO3: A first-principles investigation. Comput. Mater. Sci. 2017, 130, 242–248. [Google Scholar] [CrossRef]
- Zheng, Z.; Chen, J.; Wang, Y.; Wang, X.; Chen, X.; Liu, P.; Xu, J.; Xie, W.; Chen, H.; Deng, S.; et al. Highly Confined and Tunable Hyperbolic Phonon Polaritons in Van Der Waals Semiconducting Transition Metal Oxides. Adv. Mater. 2018, 30, e1705318. [Google Scholar] [CrossRef]
- De Castro, I.A.; Datta, R.S.; Ou, J.Z.; Castellanos-Gomez, A.; Sriram, S.; Daeneke, T.; Kalantar-Zadeh, K. Molybdenum Oxides—From Fundamentals to Functionality. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Boukhachem, A.; Mokhtari, M.; Benameur, N.; Ziouche, A.; Martínez, M.; Petkova, P.; Ghamnia, M.; Cobo, A.; Zergoug, M.; Amlouk, M. Structural optical magnetic properties of Co doped α-MoO3 sprayed thin films. Sens. Actuators A Phys. 2017, 253, 198–209. [Google Scholar] [CrossRef]
- Kamoun, O.; Boukhachem, A.; Amlouk, M.; Ammar, S. Physical study of Eu doped MoO3 thin films. J. Alloys Compd. 2016, 687, 595–603. [Google Scholar] [CrossRef]
- Kamoun, O.; Boukhachem, A.; Alleg, S.; Jeyadevan, B.; Amlouk, M. Physical study of nano-structured MoO3 films codoped with cobalt and nickel in which there is a ferro-diamagnetic transition. J. Alloys Compd. 2018, 741, 847–854. [Google Scholar] [CrossRef]
- Alizadeh, S.; Hassanzadeh-Tabrizi, S.A. MoO3 fibers and belts: Molten salt synthesis, characterization and optical properties. Ceram. Int. 2015, 41, 10839–10843. [Google Scholar] [CrossRef]
- Chithambararaj, A.; Bose, A.C. Investigation on structural, thermal, optical and sensing properties of meta-stable hexagonal MoO(3) nanocrystals of one dimensional structure. Beilstein J. Nanotechnol. 2011, 2, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Chithambararaj, A.; Sanjini, N.S.; Velmathi, S.; Bose, A.C. Preparation of h-MoO3 and alpha-MoO3 nanocrystals: Comparative study on photocatalytic degradation of methylene blue under visible light irradiation. Phys. Chem. Chem. Phys. 2013, 15, 14761–14769. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Yao, J. Photochromism of molybdenum oxide. J. Photochem. Photobiol. C Photochem. Rev. 2003, 4, 125–143. [Google Scholar] [CrossRef]
- Hsu, C.-S.; Chan, C.-C.; Huang, H.-T.; Peng, C.-H.; Hsu, W.-C. Electrochromic properties of nanocrystalline MoO3 thin films. Thin Solid Film. 2008, 516, 4839–4844. [Google Scholar] [CrossRef]
- Li, J.; Liu, X. Preparation and characterization of α-MoO3 nanobelt and its application in supercapacitor. Mater. Lett. 2013, 112, 39–42. [Google Scholar] [CrossRef]
- Li, T.; Zeng, W.; Zhang, Y.; Hussain, S. Nanobelt-assembled nest-like MoO3 hierarchical structure: Hydrothermal synthesis and gas-sensing properties. Mater. Lett. 2015, 160, 476–479. [Google Scholar] [CrossRef]
- Subba Reddy, C.V.; Qi, Y.Y.; Jin, W.; Zhu, Q.Y.; Deng, Z.R.; Chen, W.; Mho, S.-I. An electrochemical investigation on (MoO3+PVP+PVA) nanobelts for lithium batteries. J. Solid State Electrochem. 2007, 11, 1239–1243. [Google Scholar] [CrossRef]
- Subbiah, J.; Kim, D.Y.; Hartel, M.; So, F. MoO3/poly(9,9-dioctylfluorene-co-N-[4-(3-methylpropyl)]-diphenylamine) double-interlayer effect on polymer solar cells. Appl. Phys. Lett. 2010, 96. [Google Scholar] [CrossRef]
- Boukhachem, A.; Bouzidi, C.; Boughalmi, R.; Ouerteni, R.; Kahlaoui, M.; Ouni, B.; Elhouichet, H.; Amlouk, M. Physical investigations on MoO3 sprayed thin film for selective sensitivity applications. Ceram. Int. 2014, 40, 13427–13435. [Google Scholar] [CrossRef]
- Cao, B.; Wang, X.; Rino, L.; Wu, J.; He, Y.; Feng, Z.; Dong, B. Morphology and upconversion properties of rare-earth-doped MoO3 jellyfish-like plate microarchitecture. Mater. Lett. 2018, 213, 4–6. [Google Scholar] [CrossRef]
- Kolodziej, M.; Lalik, E.; Colmenares, J.C.; Lisowski, P.; Gurgul, J.; Duraczyńska, D.; Drelinkiewicz, A. Physicochemical and catalytic properties of Pd/MoO3 prepared by the sonophotodeposition method. Mater. Chem. Phys. 2018, 204, 361–372. [Google Scholar] [CrossRef]
- Martínez, H.M.; Torres, J.; López Carreño, L.D.; Rodríguez-García, M.E. Effect of the substrate temperature on the physical properties of molybdenum tri-oxide thin films obtained through the spray pyrolysis technique. Mater. Charact. 2013, 75, 184–193. [Google Scholar] [CrossRef]
- Arifa, H.; Boukhachem, A.; Askri, B.; Boubaker, K.; Yumak, A.; Raouadi, K. Structural, optical and conductivity investigations on κ-Al2O3 ceramics for powder metallurgical production and sensitivity applications. Ceram. Int. 2016, 42, 2147–2157. [Google Scholar] [CrossRef]
- Boukhachem, A.; Ouni, B.; Karyaoui, M.; Madani, A.; Chtourou, R.; Amlouk, M. Structural, opto-thermal and electrical properties of ZnO:Mo sprayed thin films. Mater. Sci. Semicond. Process. 2012, 15, 282–292. [Google Scholar] [CrossRef]
- Angeles-Chavez, C.; Toledo-Antonio, J.A.; Cortes-Jacome, M.A. Chemical quantification of Mo-S, W-Si and Ti-V. energy dispersive X-ray spectroscopy. In X-ray Spectroscopy; Sharma, S.K., Ed.; IntechOpen: London, UK, 2012; pp. 119–136. [Google Scholar] [CrossRef]
- Kamoun, O.; Boukhachem, A.; Mrabet, C.; Yumak, A.; Petkova, P.; Boubaker, K.; Amlouk, M. Effect of europium content on physical properties of In2O3 thin films for sensitivity and optoelectronic applications. Bull. Mater. Sci. 2016, 39, 777–788. [Google Scholar] [CrossRef]
- Li, X.-L.; Liu, J.-F.; Li, Y.-D. Low-temperature synthesis of large-scale single-crystal molybdenum trioxide (MoO3) nanobelts. Appl. Phys. Lett. 2002, 81, 4832–4834. [Google Scholar] [CrossRef]
- Bouzidi, C.; Sdiri, N.; Boukhachem, A.; Elhouichet, H.; Férid, M. Impedance analysis of BaMo1−xWxO4 ceramics. Superlattices Microstruct. 2015, 82, 559–573. [Google Scholar] [CrossRef]
- Tauc, J. The Optical Properties of Solids; Academic: Waltham, MA, USA, 1966. [Google Scholar]
- Urbach, F. The Long-Wavelength Edge of Photographic Sensitivity and of the Electronic Absorption of Solids. Phys. Rev. 1953, 92, 1324. [Google Scholar] [CrossRef]
- Mott, N.F.; Davis, E.A. Electronic Processes in Non-Crystalline Materials; Clarendon Press: Oxford, UK, 1979. [Google Scholar]
- Mami, A.; Mellouki, I.; Ben Mbarek, M.; Amlouk, M.; Yacoubi, N. Deep Thermal Investigations on Ag2S Thin Film Along with Electropyroelectric and Photothermal Deflection Techniques. IEEE Sens. J. 2016. [Google Scholar] [CrossRef]
- Borca-Tasciuc, D.-A.; Chen, G.; Prieto, A.; Martín-González, M.S.; Stacy, A.; Sands, T.; Ryan, M.; Fleurial, J. Thermal properties of electrodeposited bismuth telluride nanowires embedded in amorphous alumina. Appl. Phys. Lett. 2004, 85, 6001–6003. [Google Scholar] [CrossRef]
- Lee, S.; Kang, S. Effect of a ZnO addition on the thermal properties of diopside-based glass ceramics for LED packages. Contemp. Eng. Sci. 2016, 9, 1425–1436. [Google Scholar] [CrossRef]
- Minoru, I.; Kousuke, H.; Shuji, O. Optical properties and electronic structures of layered MoO3 single crystals. J. Phys. Condens. Matter 2001, 13, 6853. [Google Scholar]
- Zhao, Y.; Liu, J.; Zhou, Y.; Zhang, Z.; Xu, Y.; Naramoto, H.; Yamamoto, S. Preparation of MoO3 nanostructures and their optical properties. J. Phys. Condens. Matter 2003, 15, L547–L552. [Google Scholar] [CrossRef]
- Rabindar, K.S.; Reddy, G.B. Synthesis and characterization of α-MoO3 microspheres packed with nanoflakes. J. Phys. D Appl. Phys. 2014, 47, 065305. [Google Scholar]
- Dieterle, M.; Mestl, G. Raman spectroscopy of molybdenum oxides. Phys. Chem. Chem. Phys. 2002, 4, 822–826. [Google Scholar] [CrossRef]
- Łabanowska, M. Paramagnetic defects in MoO3—Revisited. Phys. Chem. Chem. Phys. 1999, 1, 5385–5392. [Google Scholar] [CrossRef]
- Navas, I.; Vinodkumar, R.; Mahadevan Pillai, V.P. Self-assembly and photoluminescence of molybdenum oxide nanoparticles. Appl. Phys. A 2011, 103, 373–380. [Google Scholar] [CrossRef]
- Song, L.X.; Xia, J.; Dang, Z.; Yang, J.; Wang, L.B.; Chen, J. Formation, structure and physical properties of a series of α-MoO3 nanocrystals: From 3D to 1D and 2D. CrystEngComm 2012, 14. [Google Scholar] [CrossRef]
- Wongkrua, P.; Thongtem, T.; Thongtem, S. Synthesis of h- andα-MoO3 by Refluxing and Calcination Combination: Phase and Morphology Transformation, Photocatalysis, and Photosensitization. J. Nanomater. 2013, 2013, 79. [Google Scholar] [CrossRef]
- Krishnaprasanth, A.; Seetha, M. Solvent free synthesis of Ta2O5 nanoparticles and their photocatalytic properties. AIP Adv. 2018, 8, 055017. [Google Scholar] [CrossRef]
- Mageshwari, K.; Mali, S.S.; Sathyamoorthy, R.; Patil, P.S. Template-free synthesis of MgO nanoparticles for effective photocatalytic applications. Powder Technol. 2013, 249, 456–462. [Google Scholar] [CrossRef]
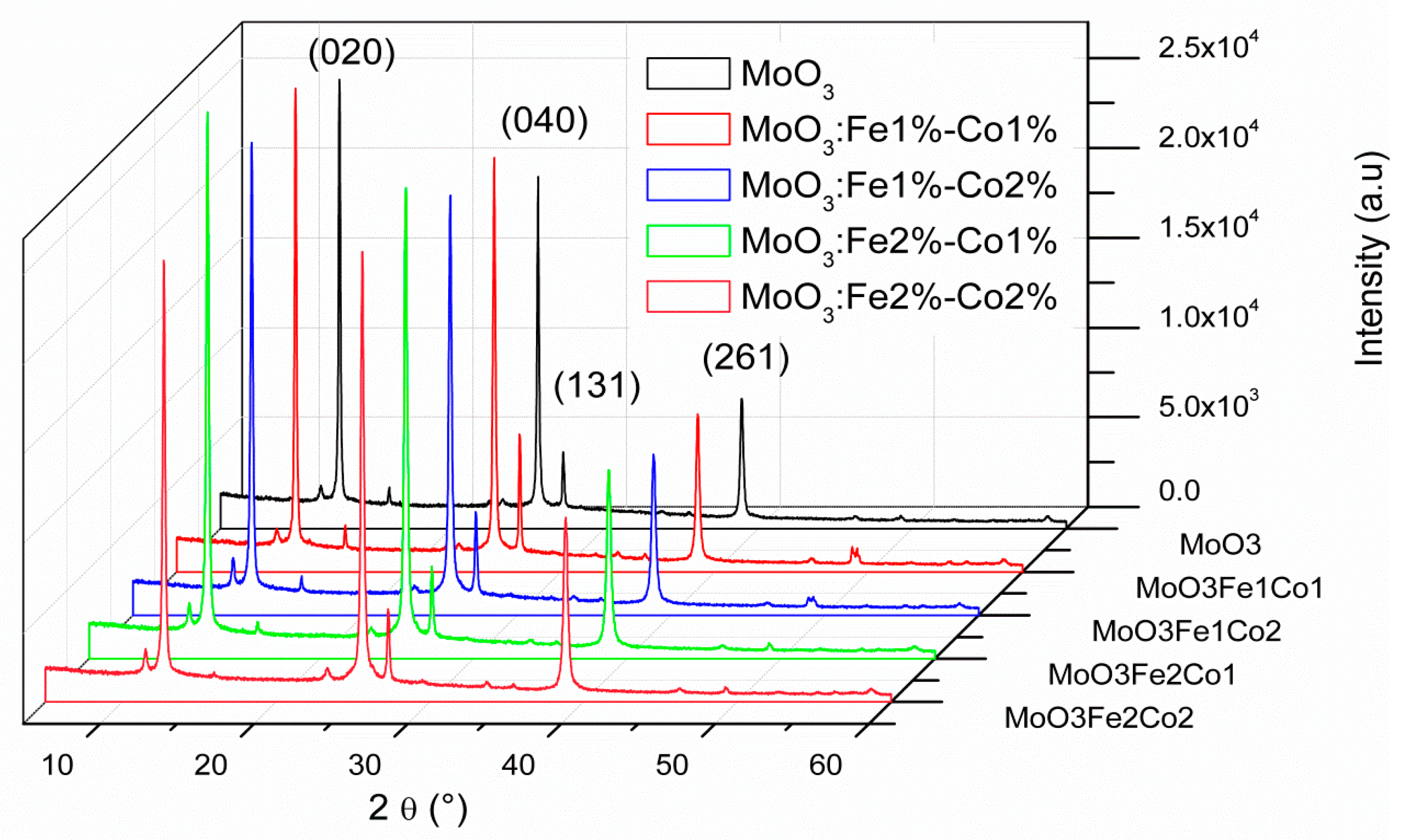











| d(hkl) (Å) | ||||||
|---|---|---|---|---|---|---|
| (hkl) | 2θhkl | MoO3 | Fe 1% Co1% | Fe 1% Co 2% | Fe 2% Co 1% | Fe 2% Co 2% |
| (020) | 12.73 | 6.95 | 6.94 | 6.94 | 6.94 | 6.94 |
| (040) | 25.58 | 3.48 | 3.47 | 3.47 | 3.47 | 3.47 |
| (131) | 27.29 | 3.26 | 3.26 | 3.26 | 3.26 | 3.26 |
| (261) | 38.88 | 2.31 | 2.31 | 2.31 | 2.31 | 2.31 |
| TC | |||||
|---|---|---|---|---|---|
| (hkl) | MoO3 | MoO3: Fe 1% Co 1% | MoO3: Fe 1% Co 2% | MoO3: Fe 2% Co 1% | MoO3: Fe 2% Co 2% |
| (020) | 1.78 | 1.63 | 1.64 | 1.68 | 1.51 |
| (040) | 1.40 | 1.38 | 1.46 | 1.46 | 1.55 |
| (131) | 0.30 | 0.46 | 0.36 | 0.28 | 0.32 |
| (261) | 0.52 | 0.53 | 0.54 | 0.58 | 0.62 |
| %Fe:%Co | ξ (10−4) | D (nm) | δdis (1013 lines/m2) |
|---|---|---|---|
| 0 | 44 | 82.7 | 15 |
| 1:1 | 48 | 77.4 | 17 |
| 1:2 | 50 | 71.1 | 20 |
| 2:1 | 55 | 65.1 | 24 |
| 2:2 | 46 | 69.2 | 21 |
| Element | O Kα | Mo Ll | Mo Lα1 | Mo Lβ1 | Mo Kα2 | Mo Kα1 | Mo Kβ1 |
|---|---|---|---|---|---|---|---|
| Energy, keV | 0.523 | 2.015 | 2.293 | 2.394 | 17.376 | 17.481 | 19.609 |
| %Fe:%Co | Eg (eV) | EU (meV) |
|---|---|---|
| MoO3 | 3.75 | 370 |
| 1:1 | 3.80 | 218 |
| 1:2 | 3.85 | 245 |
| 2:1 | 3.88 | 200 |
| 2:2 | 3.95 | 150 |
| %Fe:%Co | Thermal Conductivity K, (W/mK) | Thermal Diffusivity D, (10−6 m/s) | Heat Capacity C, (106 J/Km) | Thermal Effusivity e (103 J/(Km2s1/2)) |
|---|---|---|---|---|
| 0 | 24.10 ± 0.02 | 3.80 ± 0.05 | 6.34 | 12.36 |
| 1:1 | 25.50 ± 0.03 | 4.10 ± 0.04 | 6.21 | 12.59 |
| 1:2 | 25.62 ± 0.03 | 4.65 ± 0.05 | 5.50 | 11.88 |
| 2:1 | 25.86 ± 0.04 | 5.15 ± 0.05 | 5.02 | 11.39 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamoun, O.; Mami, A.; Amara, M.A.; Vidu, R.; Amlouk, M. Nanostructured Fe,Co-Codoped MoO3 Thin Films. Micromachines 2019, 10, 138. https://doi.org/10.3390/mi10020138
Kamoun O, Mami A, Amara MA, Vidu R, Amlouk M. Nanostructured Fe,Co-Codoped MoO3 Thin Films. Micromachines. 2019; 10(2):138. https://doi.org/10.3390/mi10020138
Chicago/Turabian StyleKamoun, Olfa, Amel Mami, Mohamed Aymen Amara, Ruxandra Vidu, and Mosbah Amlouk. 2019. "Nanostructured Fe,Co-Codoped MoO3 Thin Films" Micromachines 10, no. 2: 138. https://doi.org/10.3390/mi10020138
APA StyleKamoun, O., Mami, A., Amara, M. A., Vidu, R., & Amlouk, M. (2019). Nanostructured Fe,Co-Codoped MoO3 Thin Films. Micromachines, 10(2), 138. https://doi.org/10.3390/mi10020138





