A Low-Noise-Level Heart Sound System Based on Novel Thorax-Integration Head Design and Wavelet Denoising Algorithm
Abstract
1. Introduction
2. Methods
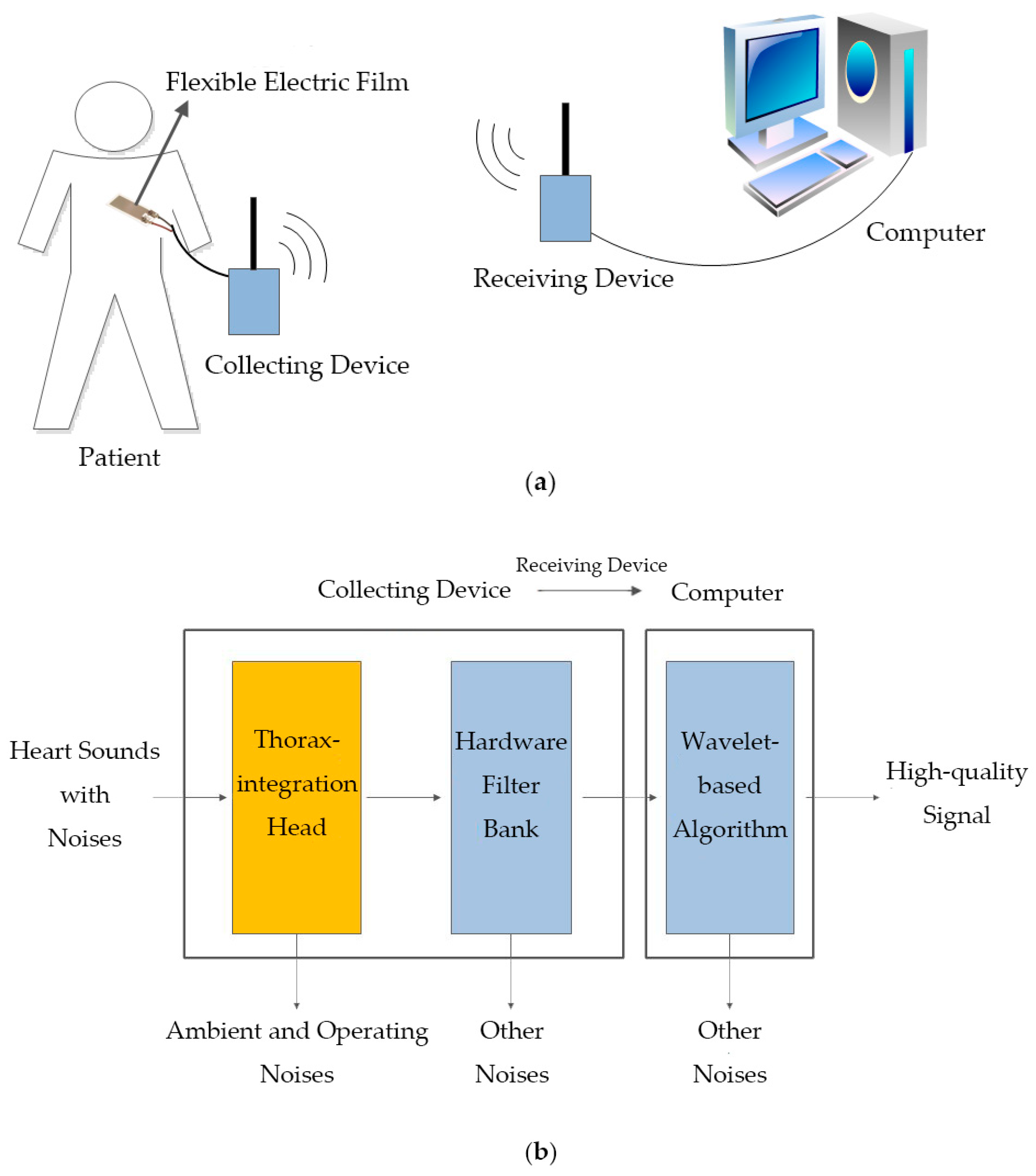
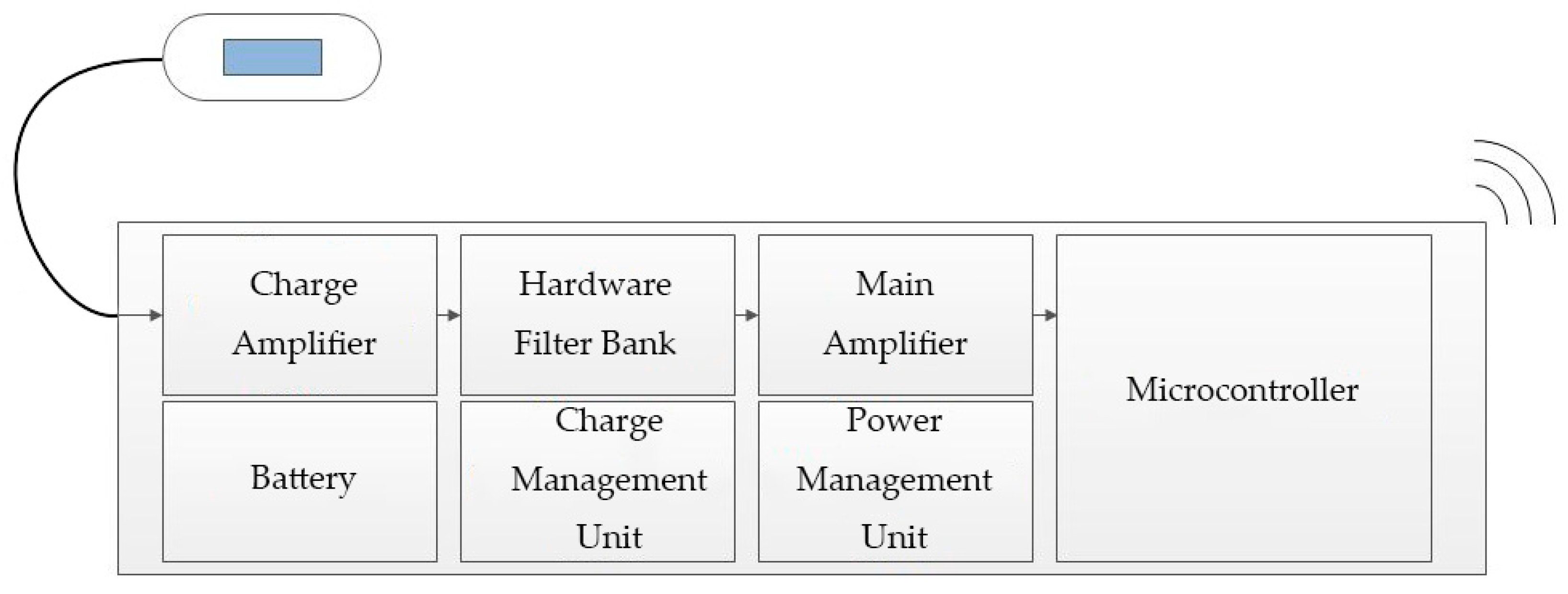
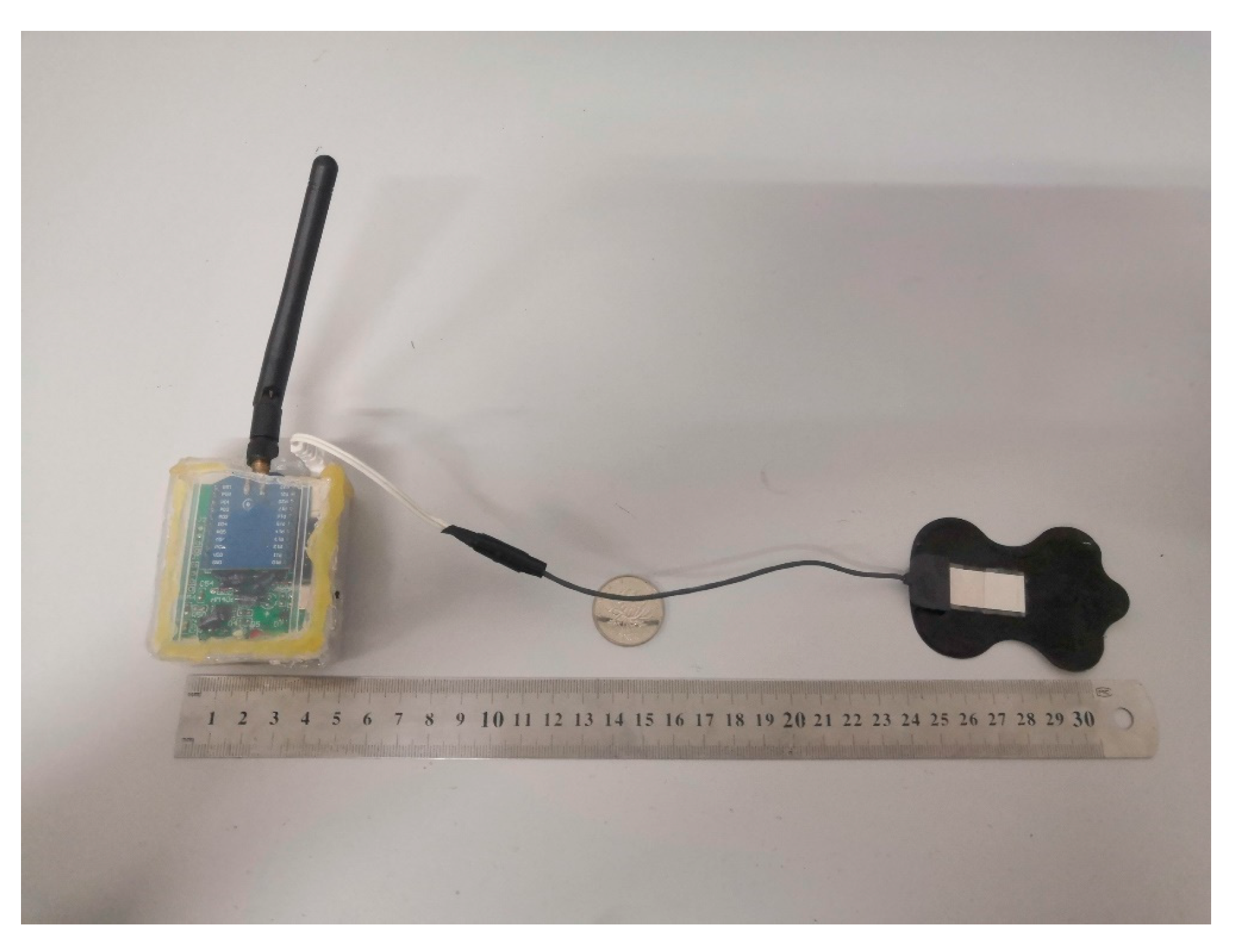
2.1. Collecting and Receiving Devices
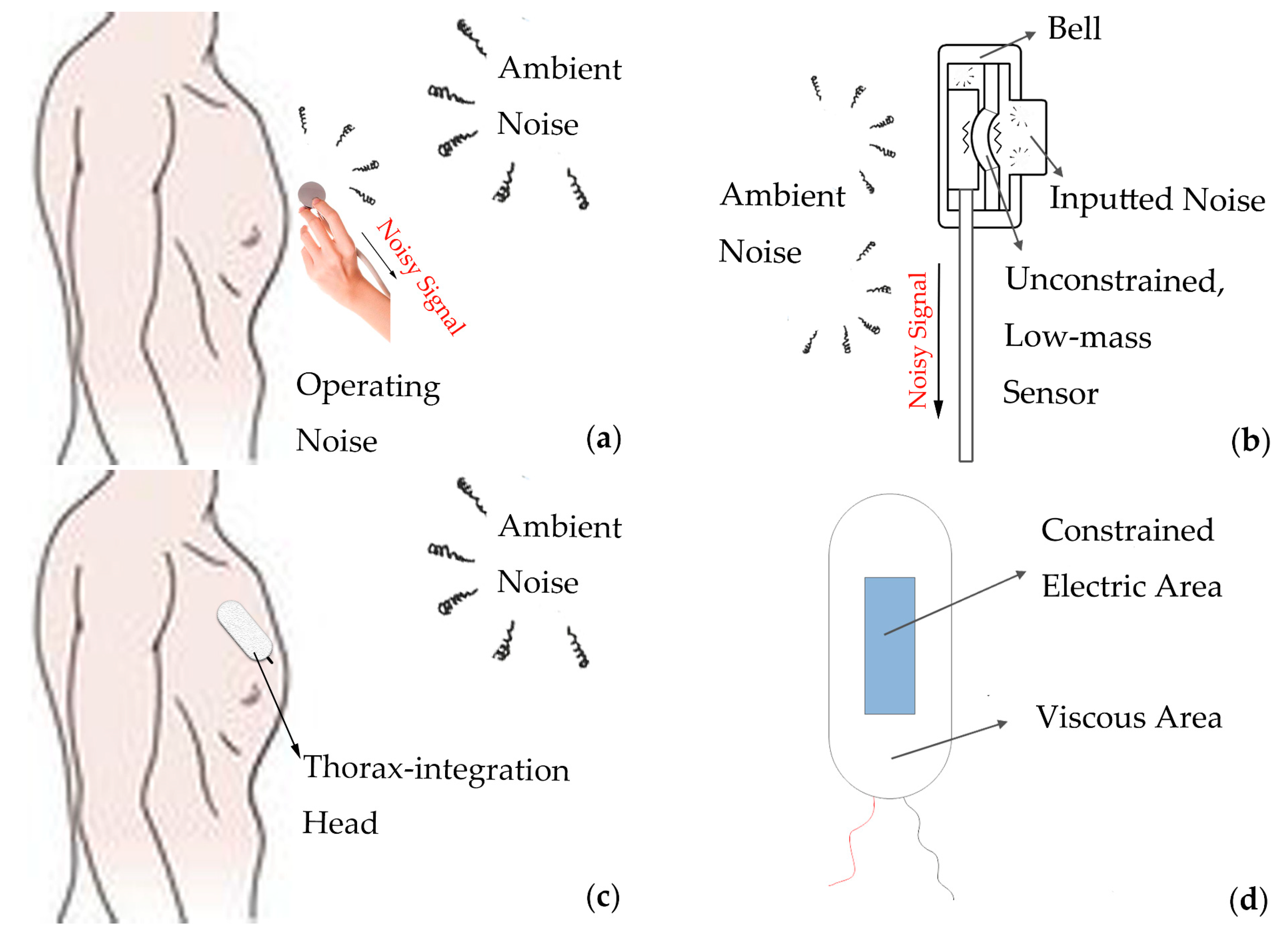
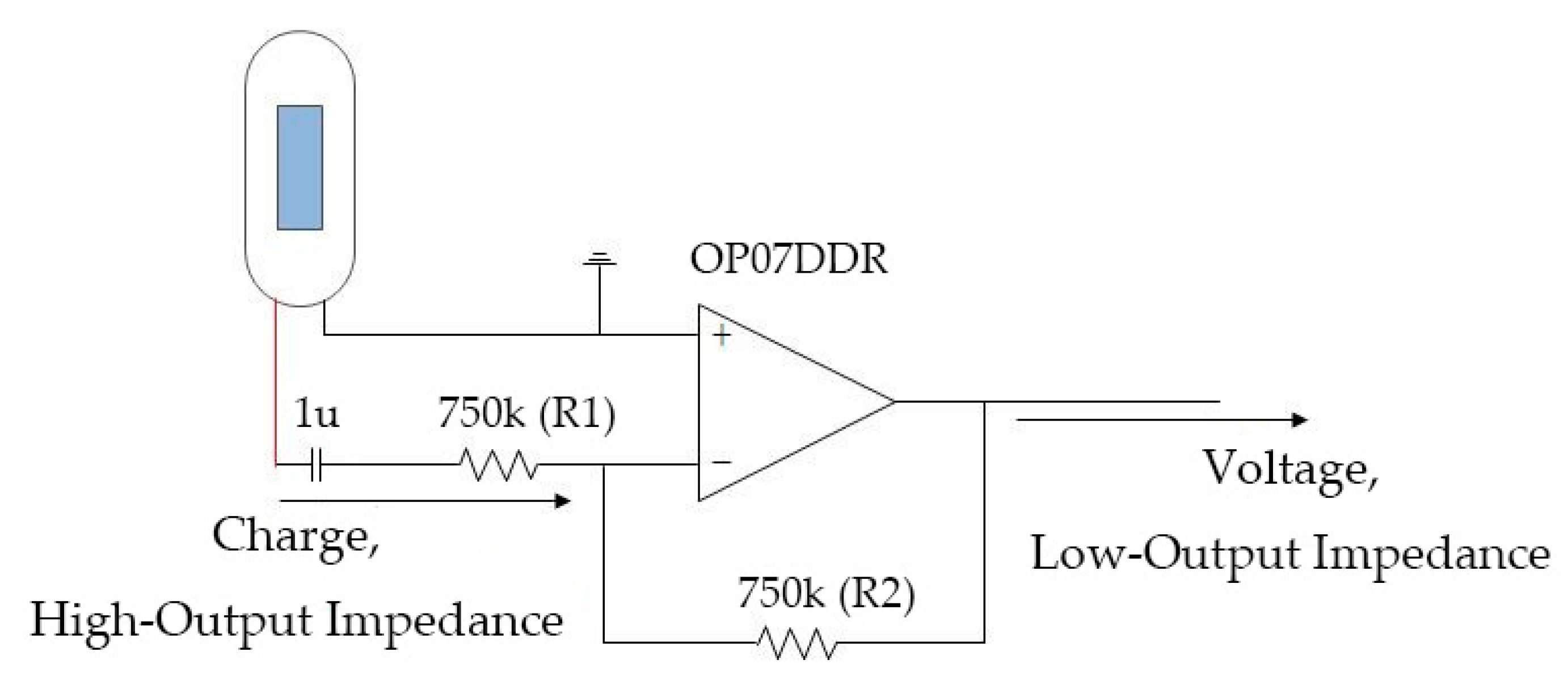
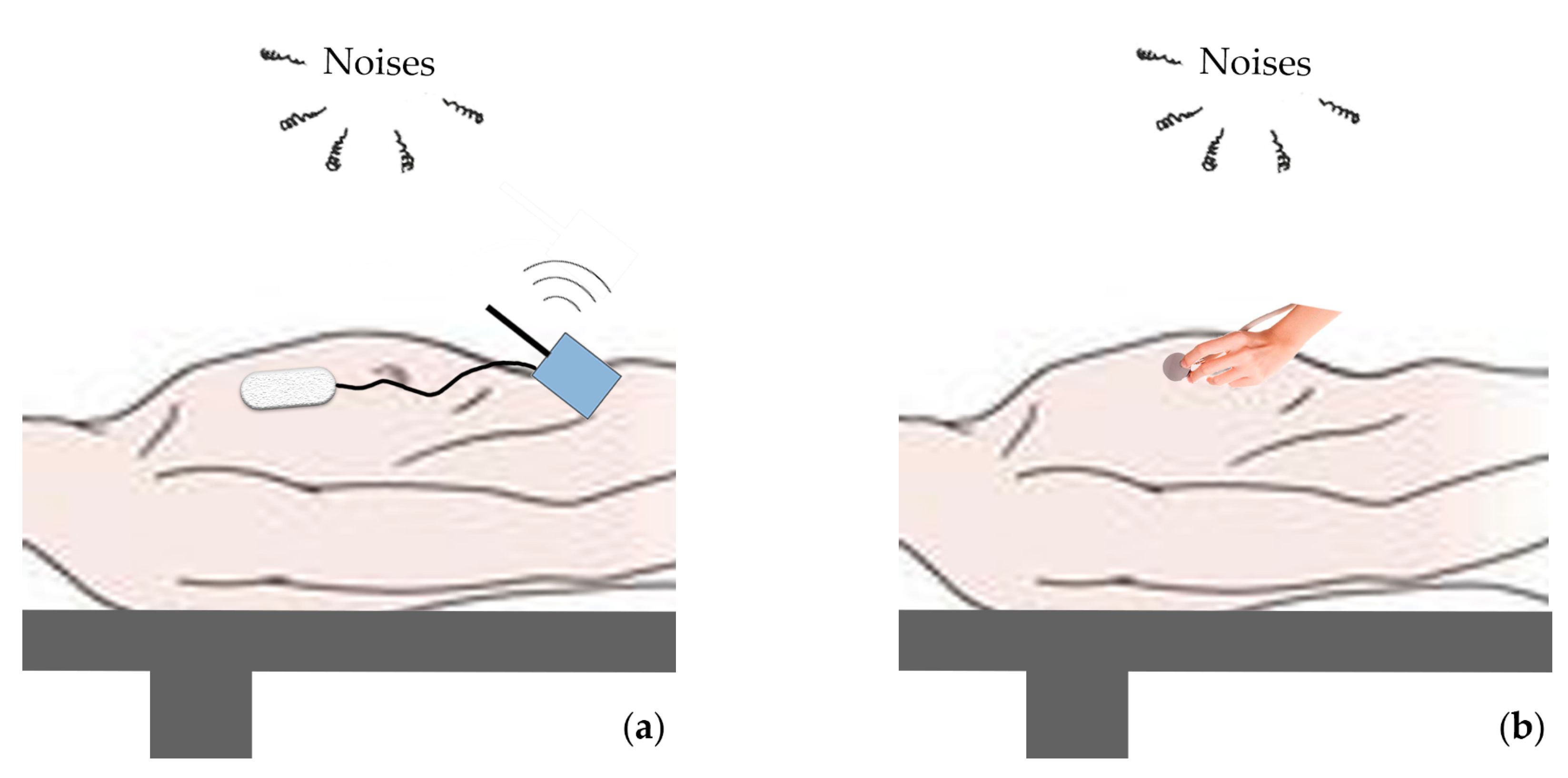
2.1.1. Novel Thorax-Integration Head
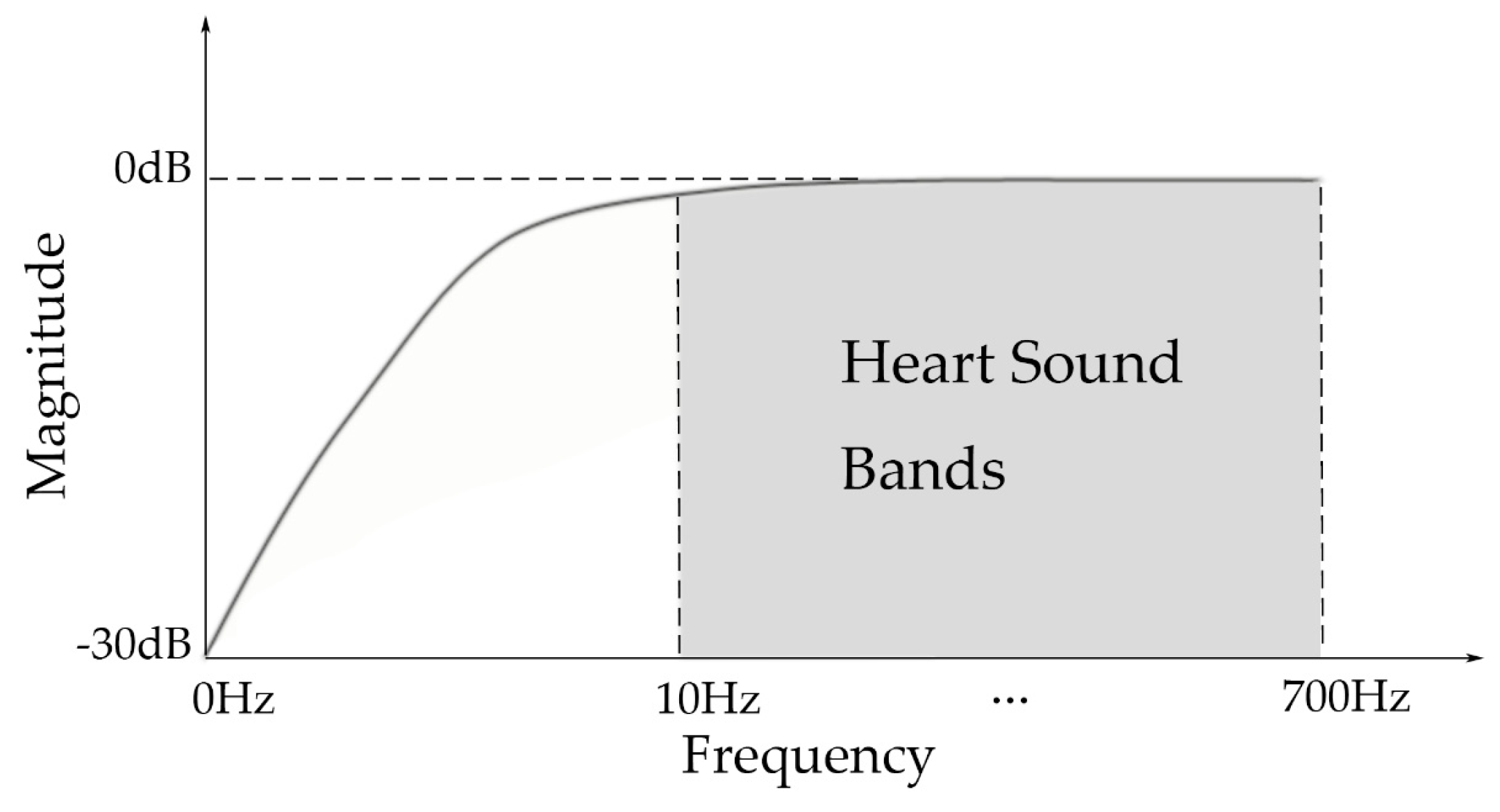
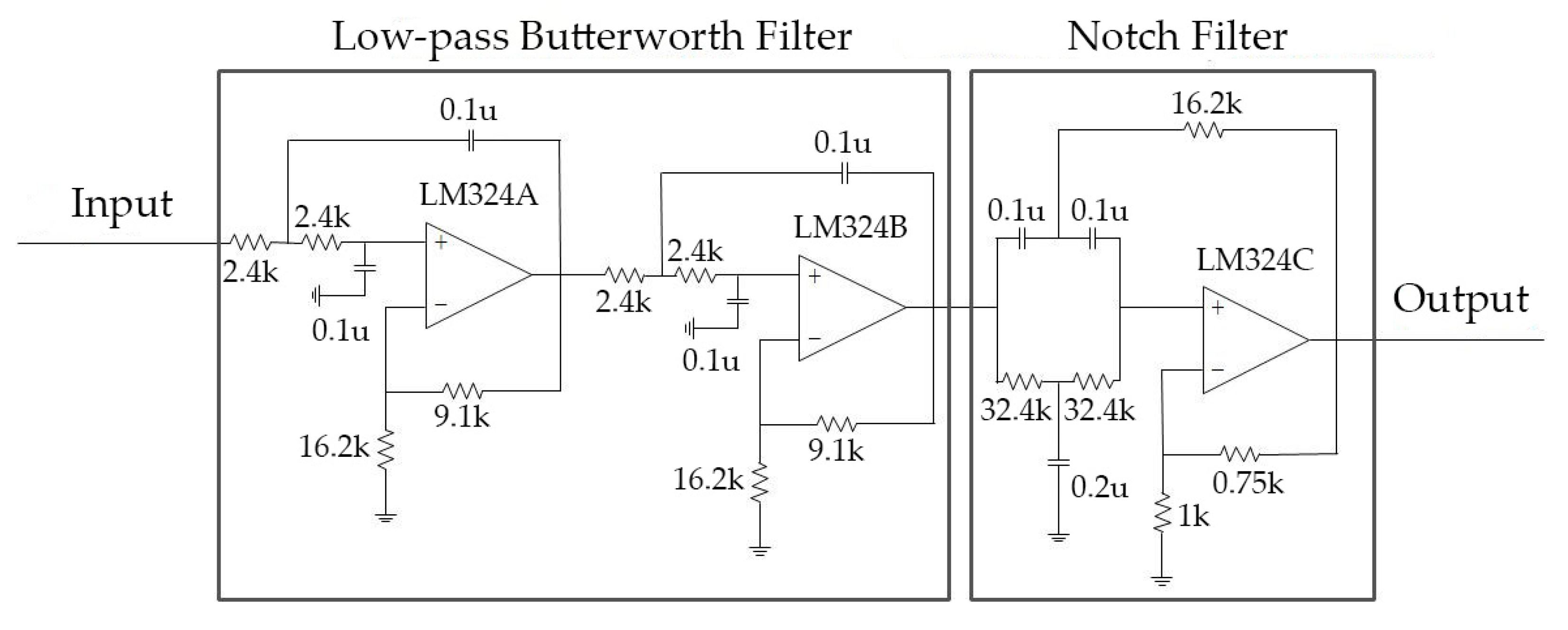
2.1.2. Hardware Filter Bank
2.1.3. Other Hardware Design
2.2. Wavelet-Based Denoising Algorithm
2.2.1. Discrete Wavelet Transform
2.2.2. Hard Thresholding
2.2.3. Soft Thresholding
2.2.4. Minimax Thresholding Techniques
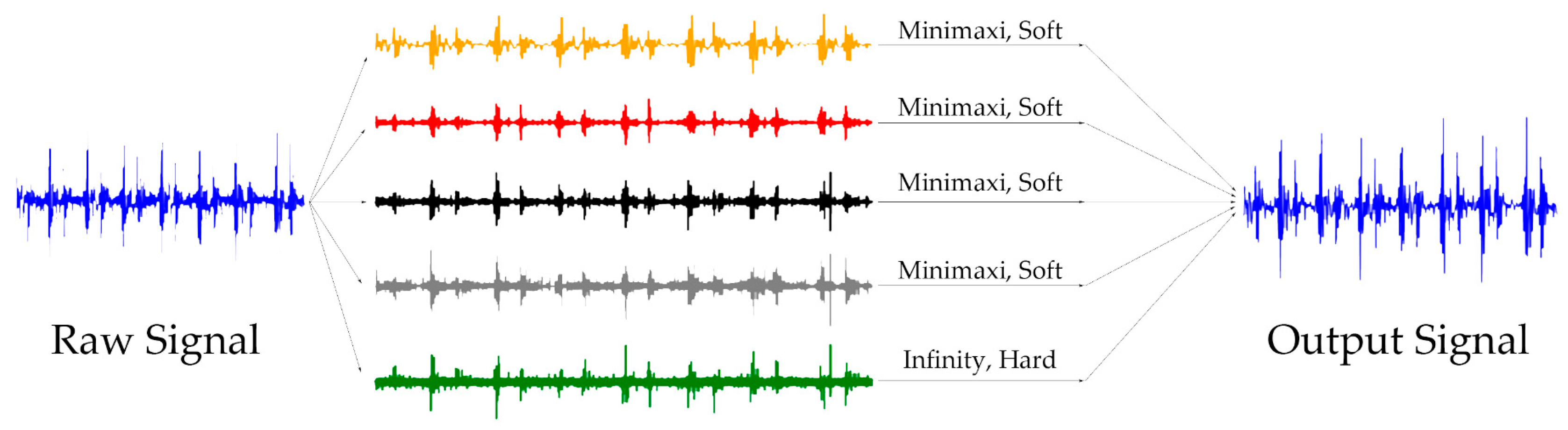
2.2.5. Denoising Procedure
3. Experiment
3.1. Denoising Experiment
3.2. Reserve Pathological Information Experiment
4. Results
4.1. The Established Prototype
4.2. Results of Experiments
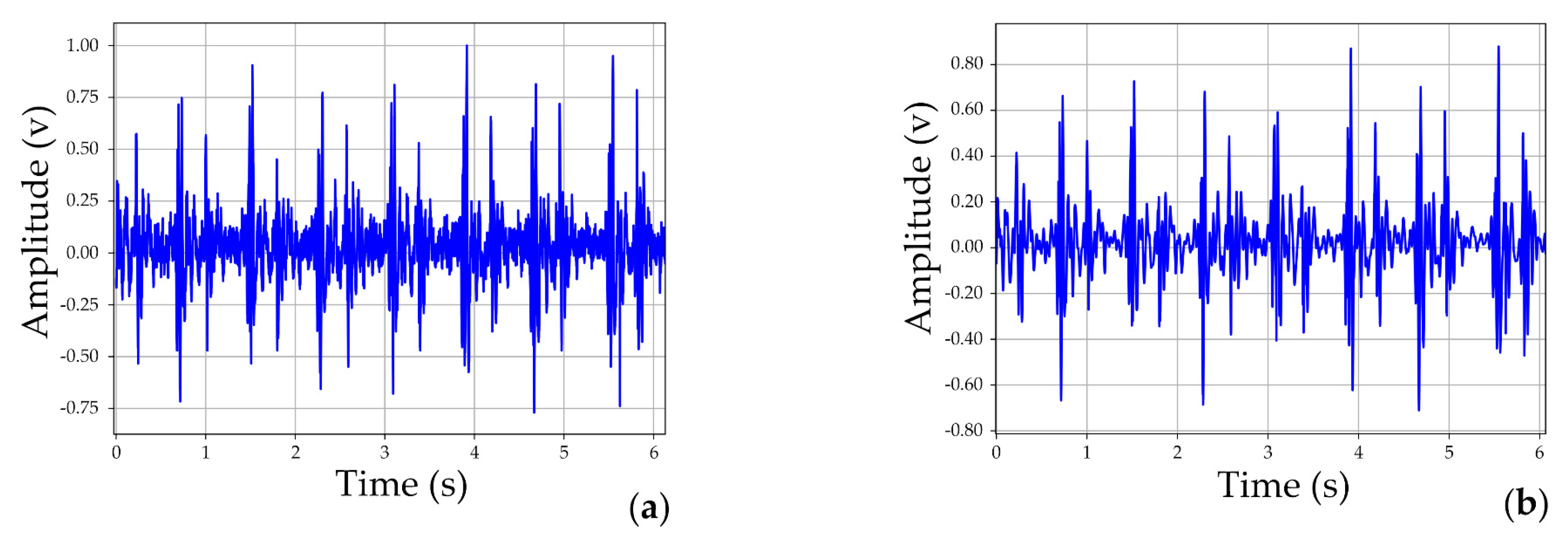
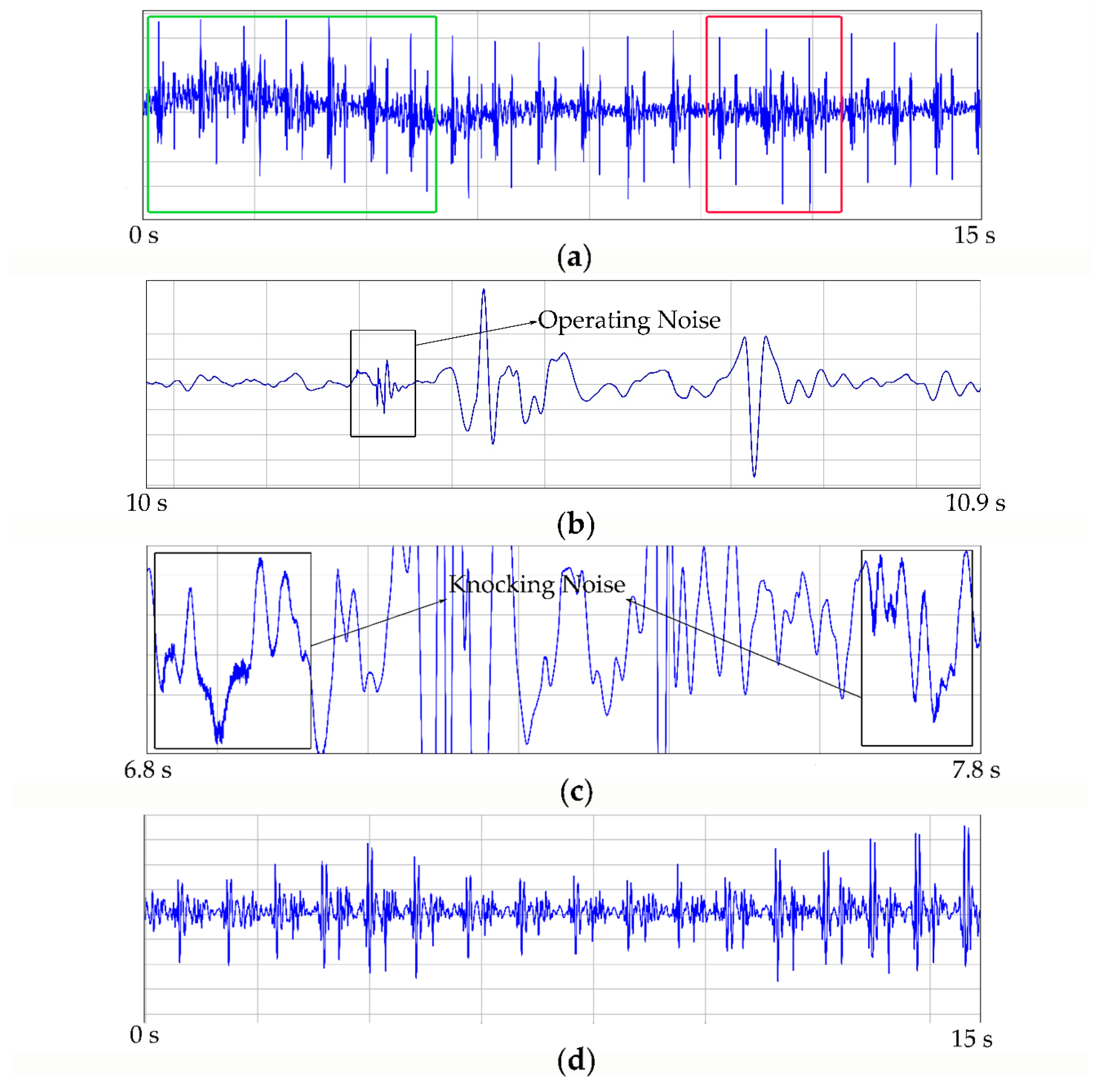
4.2.1. Results of Denoising Experiment
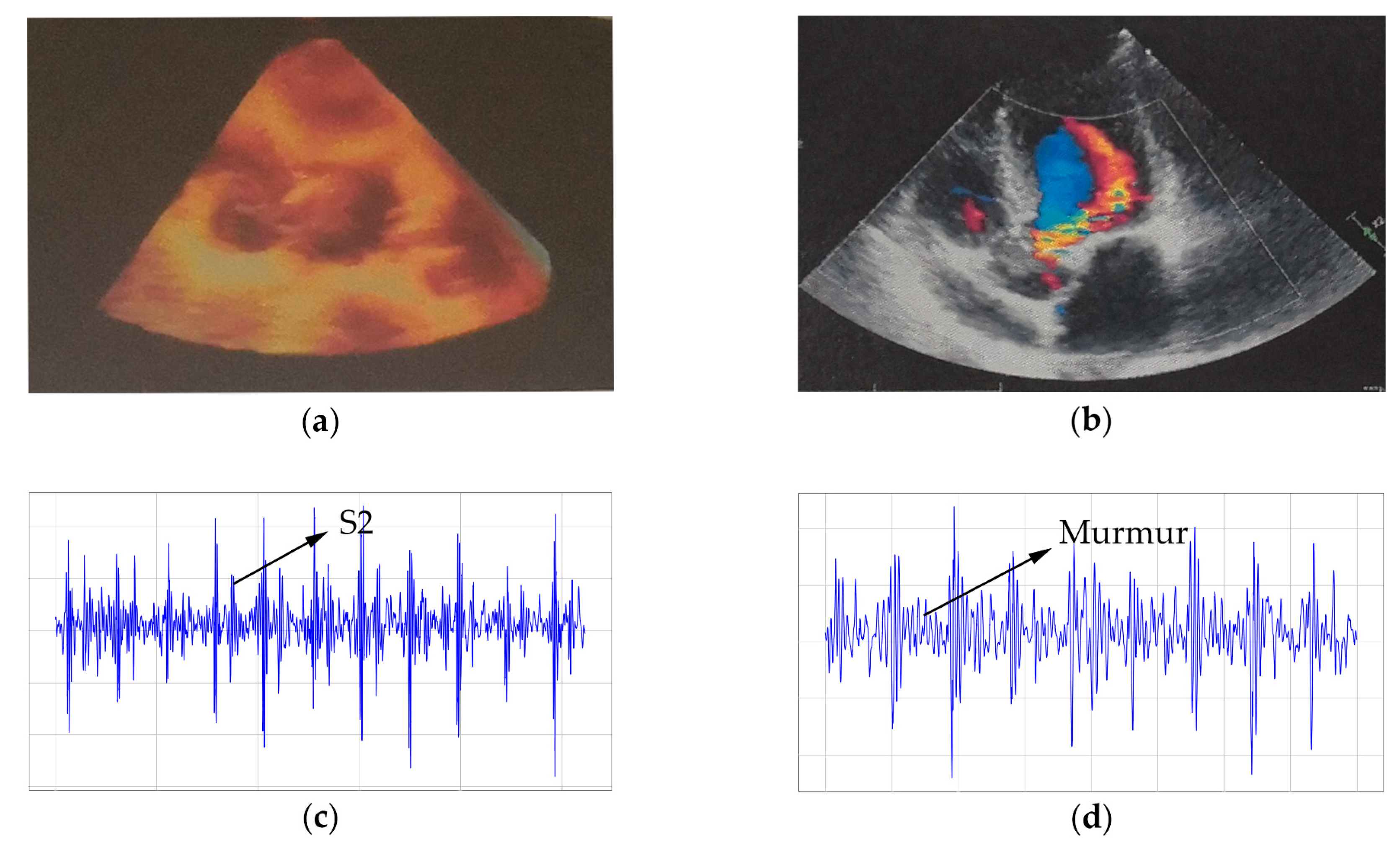
4.2.2. Results of Reserve Pathological Information Experiment
5. Discussion
5.1. The Excellent Capability of Thorax-Integration Head in Denoising
5.2. Noise Components in Raw Signal
5.3. Complete Pathological Information in Its Recordings
5.4. Limitations and Prospects
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- 2015 World Statistics on Cardiovascular Disease. Available online: www.who.int/mediacentre/factsheets/fs317/en/ (accessed on 19 October 2019).
- Clifford, G.D.; Liu, C.Y.; Moody, B.; Springer, D.; Silva, I.; Li, Q.; Mark, R.G. Classification of nomal/abnormal heart sound recordings. In Proceedings of the 2016 Computing in Cardiology Conference (CinC), Vancouver, BC, Canada, 11–14 September 2016; pp. 309–612. [Google Scholar]
- Wang, P.; Lim, C.S.; Chauhan, S.; Foo, J.Y.A.; Anantharaman, V. Phonocardiographic signal analysis method using a modified hidden Markov model. Ann. Biomed. Eng. 2007, 35, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Fye, W.B. An ear to the chest: An illustrated history of the evolution of the stethoscope. Bull. Hist. Med. 2003, 77, 198–200. [Google Scholar] [CrossRef]
- Syed, Z.; Leeds, D.; Curtis, D.; Nesta, F.; Levine, R.A.; Guttag, J. A framework for the analysis of acoustical cardiac signals. IEEE Trans. Biomed. Eng. 2007, 54, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Silverman, B.; Balk, M. Digital stethoscope—Improved auscultation at the bedside. Am. J. Cardiol. 2019, 123, 984–985. [Google Scholar] [CrossRef]
- Welsby, P.D.; Parry, G.; Smith, D. The stethoscope: some preliminary investigations. Postgrad. Med. J. 2003, 79, 695–698. [Google Scholar]
- Murphy, R.L.H. In defense of the stethoscope. Resp. Care 2008, 53, 355–369. [Google Scholar]
- Weitz, H.H.; Mangione, S. In defense of the stethoscope and the bedside. Am. J. Med. 2000, 108, 669–671. [Google Scholar] [CrossRef]
- Belloni, F.; Della, G.D.; Riva, M.; Malcangi, M. A new digital stethoscope with environmental noise cancellation. In Proceedings of the 12th WSEAS International Conference on Mathematical and Computational Methods in Science and Engineering, Faro, Portugal, 3–5 November 2010; pp. 3–5. [Google Scholar]
- Jatupaiboon, N.; Pan-ngum, S.; Israsena, P. Electronic stethoscope prototype with adaptive noise cancellation. In Proceedings of the 2010 Eighth International Conference on ICT and Knowledge Engineering, Bangkok, Thailand, 24–25 November 2010; pp. 32–36. [Google Scholar]
- Ghavami, M. Adaptive line enhancement using parallel IIR filter with a step by step algorithm. Int. J. Eng. 1998, 11, 73–81. [Google Scholar]
- Gnitecki, J.; Moussavi, Z.M.K. Separating heart sounds from lung sounds. IEEE Eng. Med. Biol. Mag. 2007, 26, 20–29. [Google Scholar] [CrossRef]
- Nelson, G.; Rajamani, R.; Erdman, A. Vibro-acoustic model of a piezoelectric-based stethoscope for chest sound measurements. Meas. Sci. Technol. 2015, 26, 095701. [Google Scholar] [CrossRef]
- Zun, L.S.; Downey, L. The effect of noise in the emergency department. Acad. Emerg. Med. 2005, 12, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Hall, L.T.; Maple, J.L.; Agzarian, J.; Abbott, D. Sensor system for heart sound biomonitor. Microelectron. J. 2000, 31, 583–592. [Google Scholar] [CrossRef]
- Choi, S.; Jiang, Z.W. Cardiac sound murmurs classification with autoregressive spectral analysis and multi-support vector machine technique. Comput. Biol. Med. 2010, 40, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.P. An innovative intelligent system based on automatic diagnostic feature extraction for diagnosing heart diseases. Knowledge-Based Syst. 2015, 75, 224–238. [Google Scholar] [CrossRef]
- Cheng, X.F.; Zhang, Z. Denoising method of heart sound signals based on self-construct heart sound wavelet. Aip Adv. 2014, 4, 1203–1209. [Google Scholar] [CrossRef]
- Wang, F.; Ji, Z. Application of the dual-tree complex wavelet transform in biomedical signal denoising. Biomed. Mater. Eng. 2014, 24, 109–115. [Google Scholar] [CrossRef]
- Verma, N.; Verma, A.K. Performance analysis of wavelet thresholding methods in denoising of audio signals of some Indian Musical Instruments. Int. J. Eng. Sci. Technol. 2012, 4, 2040–2045. [Google Scholar]
- Daubechies, I.; Bates, B.J. Ten lectures on wavelets. J. Acoust. Soc. Am. 1993, 93, 1671. [Google Scholar] [CrossRef]
- Sudha, S.; Suresh, G.R.; Sukanesh, R. Wavelet based image denoising using adaptive thresholding. Proc. Int. Conf. Comput. Intell. Multimed. Appl. India 2007, 3, 296–300. [Google Scholar]
- Karthikeyan, P.; Murugappan, M.; Yaacob, S. ECG signal denoising using wavelet thresholding techniques in human stress assessment. Int. J. Electr. Eng. Inform. 2012, 4, 306–319. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, S.H. Noise estimation based on standard deviation and sigmoid function using a posteriori signal to noise ratio in nonstationary noisy environments. Int. J. Control Autom. Syst. 2008, 6, 818–827. [Google Scholar]
- Chen, L.; Tang, X.Y.; Sanyal, A.; Yoon, Y.; Cong, J.; Sun, N. A 0.7-V 0.6-mu W 100-kS/s low-power SAR ADC with statistical estimation-based noise reduction. IEEE J. Solid-State Circuit 2017, 52, 1388–1398. [Google Scholar] [CrossRef]
- Liu, C.Y.; Springer, D.; Clifford, G.D. Performance of an open-source heart sound segmentation algorithm on eight independent databases. Physiol. Meas. 2017, 38, 1730–1745. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.D.; Liu, C.Y.; Moody, B.; Millet, J.; Schmidt, S.; Li, Q.; Silva, I.; Mark, R.G. Recent advances in heart sound analysis. Physiol. Meas. 2017, 38, E10–E25. [Google Scholar] [CrossRef]
- Liu, C.Y.; Springer, D.; Li, Q.; Moody, B.; Juan, R.A.; Chorro, F.J.; Castells, F.; Roig, J.M.; Silva, I.; Johnson, A.E.W.; et al. An open access database for the evaluation of heart sound algorithms. Physiol. Meas. 2016, 37, 2181–2213. [Google Scholar] [CrossRef]
| Item | Manufacturer | Chip |
|---|---|---|
| Preamplifier | Texas Instrument | OP07DDR |
| Filter bank | Texas Instrument | LM324DR |
| Main amplifier | Texas Instrument | OP07DDR |
| Charge management unit | H&Msemi | HM4061 |
| Power management unit | Suosemi Techology, Maxim Intergrated | SX1308, MAX660 |
| Microcontroller | Texas Instrument | CC2530 |
| Size (mm) | Weight (g) | Sampling Rate (Hz) | Battery Life (h) | Charging Time (h) |
|---|---|---|---|---|
| 65 × 50 × 25 | 73.4 | 2000 | 18–20 | 3–4 |
| Noises | Knocking | Cheering | Typing | Clapping | Striking | Mean |
|---|---|---|---|---|---|---|
| ENR | 59.05% | 17.43% | 12.47% | 41.94% | 17.44% | 21.26% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Zhang, R.; Chang, S.; Liu, C.; Sha, X. A Low-Noise-Level Heart Sound System Based on Novel Thorax-Integration Head Design and Wavelet Denoising Algorithm. Micromachines 2019, 10, 885. https://doi.org/10.3390/mi10120885
Zhang S, Zhang R, Chang S, Liu C, Sha X. A Low-Noise-Level Heart Sound System Based on Novel Thorax-Integration Head Design and Wavelet Denoising Algorithm. Micromachines. 2019; 10(12):885. https://doi.org/10.3390/mi10120885
Chicago/Turabian StyleZhang, Shuo, Ruiqing Zhang, Shijie Chang, Chengyu Liu, and Xianzheng Sha. 2019. "A Low-Noise-Level Heart Sound System Based on Novel Thorax-Integration Head Design and Wavelet Denoising Algorithm" Micromachines 10, no. 12: 885. https://doi.org/10.3390/mi10120885
APA StyleZhang, S., Zhang, R., Chang, S., Liu, C., & Sha, X. (2019). A Low-Noise-Level Heart Sound System Based on Novel Thorax-Integration Head Design and Wavelet Denoising Algorithm. Micromachines, 10(12), 885. https://doi.org/10.3390/mi10120885






