Sheathless Shape-Based Separation of Candida Albicans Using a Viscoelastic Non-Newtonian Fluid
Abstract
1. Introduction
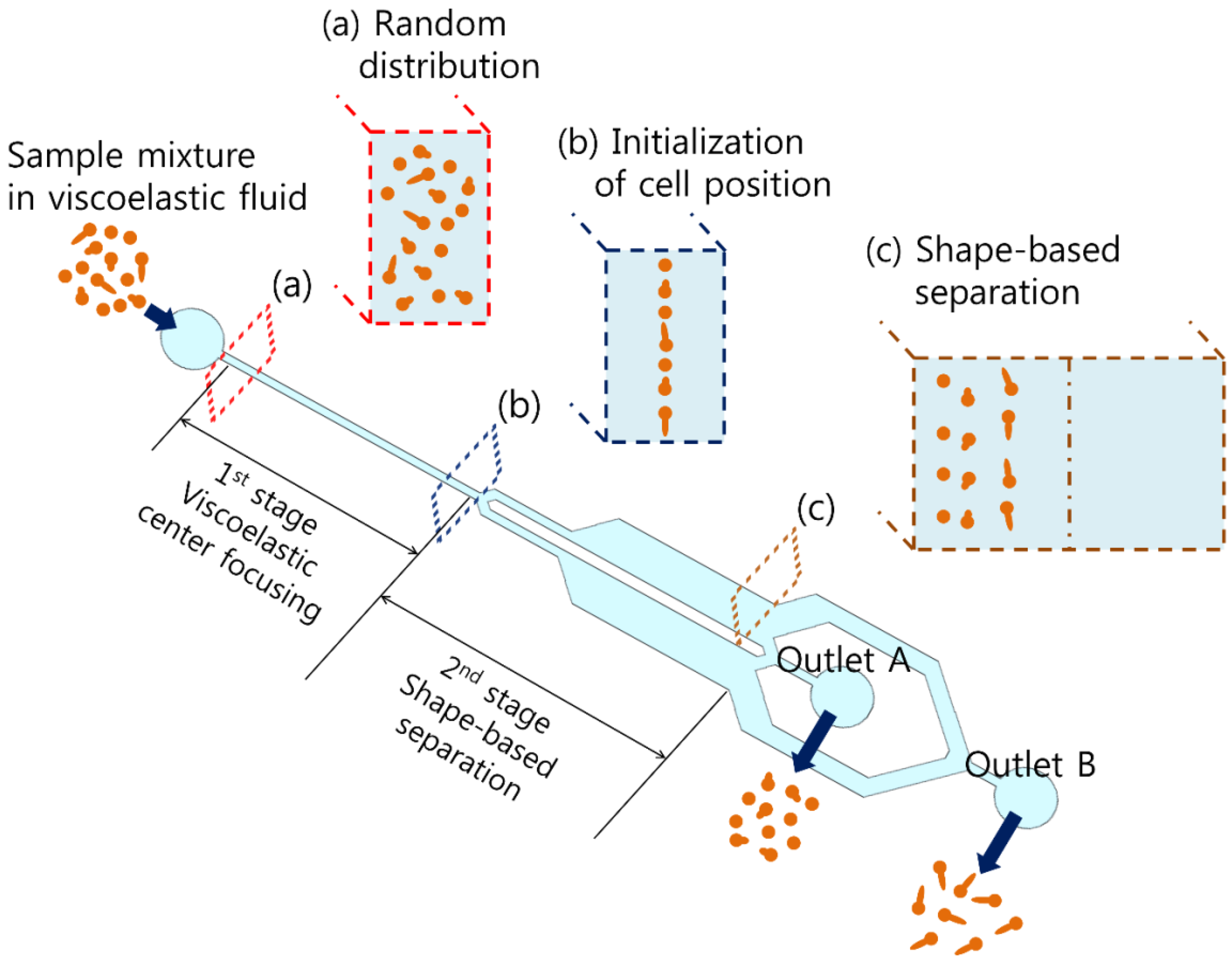
2. Working Principle
3. Materials and Methods
3.1. Device Design and Fabrication
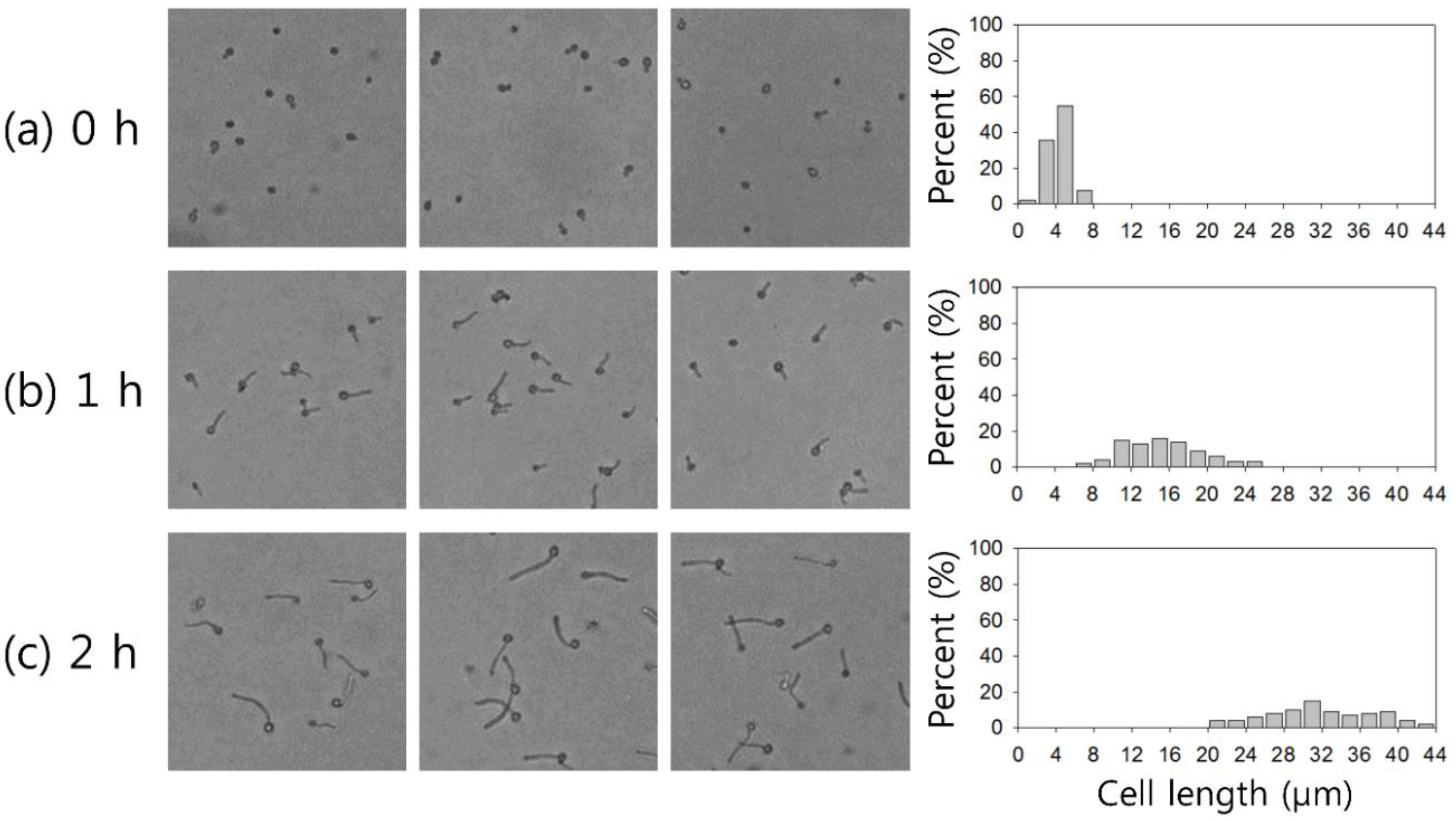
3.2. Sample Preparation
3.3. Experimental Procedure
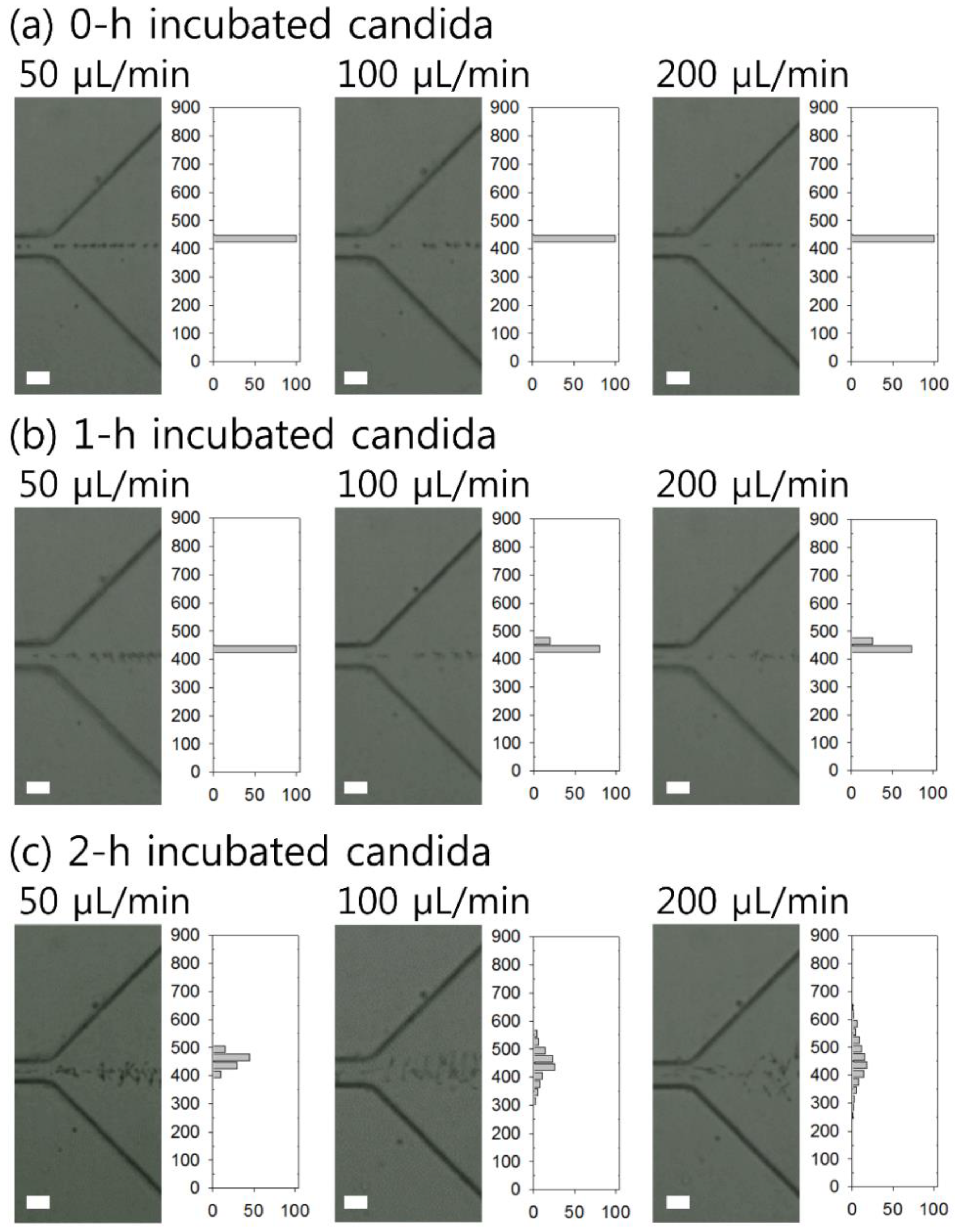
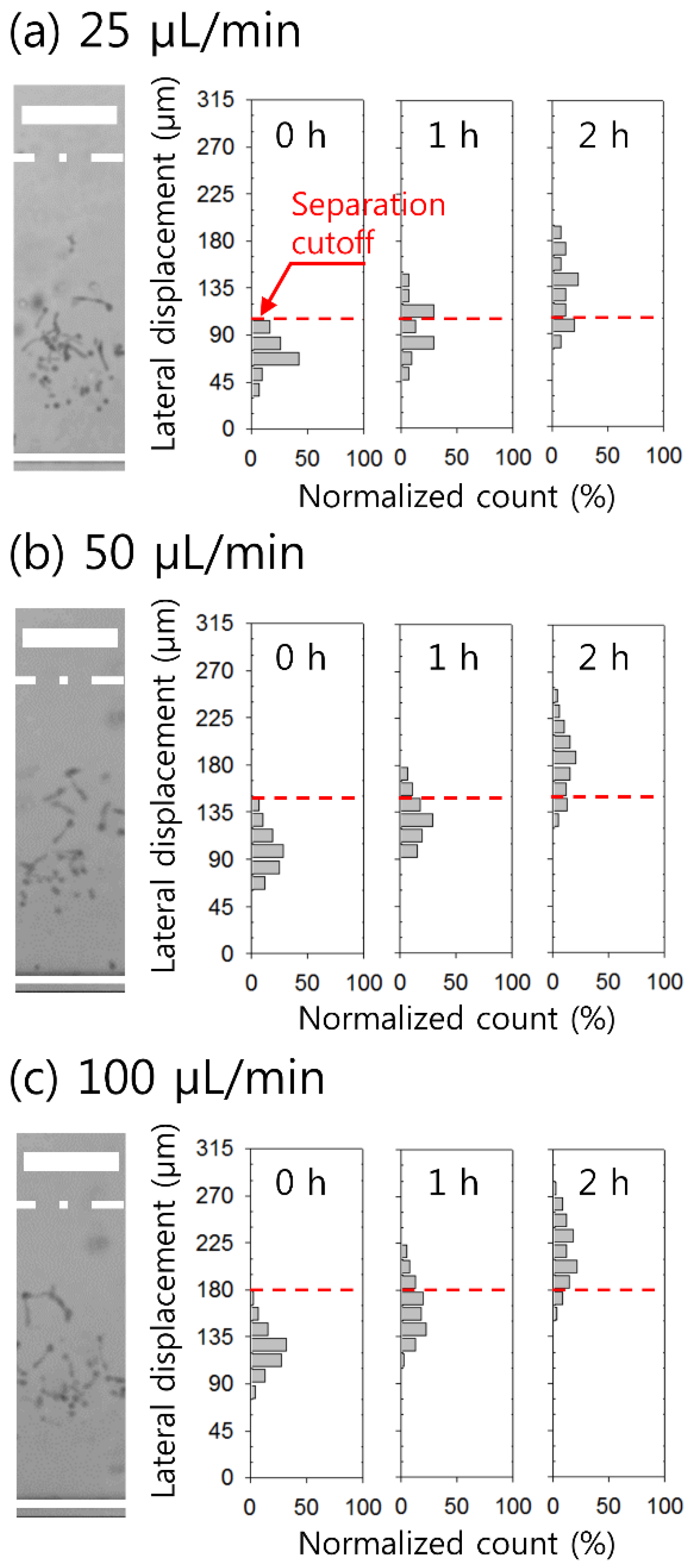
4. Results and Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pappas, P.G.; Rex, J.H.; Sobel, J.D.; Filler, S.G.; Dismukes, W.E.; Walsh, T.J.; Edwards, J.E. Guidelines for treatment of candidiasis. Clin. Infect. Dis. 2004, 38, 161–189. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, D. Serum tube identification of Candida albicans. J. Clin. Pathol. 1962, 15, 563–565. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, D.C.; Locas, M.-C.; Restieri, C.; Laverdiere, M. Utility of the germ tube test for direct identification of Candida albicans from positive blood culture bottles. J. Clin. Microbiol. 2008, 46, 3508–3509. [Google Scholar] [CrossRef] [PubMed]
- Braude, A.I.; Davis, C.E.; Fierer, J. Infectious Diseases and Medical Microbiology; Philadelphia, W.B., Ed.; Saunders Co.: Christchurch, New Zealand, 1986. [Google Scholar]
- JO, I.; Eghubare, A.; Omoregie, R. Germ Tube Formation in Candida Albicans: Evaluation of Human and Animal Sera and Incubation Atmosphere. Shiraz E-Medical J. 2005, 6, 21–25. [Google Scholar]
- Brayman, T.G.; Wilks, J.W. Sensitive assay for antifungal activity of glucan synthase inhibitors that uses germ tube formation in Candida albicans as an end point. Antimicrob. Agents Chemother. 2003, 47, 3305–3310. [Google Scholar] [CrossRef] [PubMed]
- Rusu, E.; Radu-Popescu, M.; Pelinescu, D.; Vassu, T. Treatment with some anti-inflammatory drugs reduces germ tube formation in Candida albicans strains. Braz. J. Microbiol. 2014, 45, 1379–1383. [Google Scholar] [CrossRef]
- Karimi, A.; Yazdi, S.; Ardekani, A. Hydrodynamic mechanisms of cell and particle trapping in microfluidics. Biomicrofluidics 2013, 7, 021501. [Google Scholar] [CrossRef]
- Pamme, N. Continuous flow separations in microfluidic devices. Lab A Chip 2007, 7, 1644–1659. [Google Scholar] [CrossRef]
- Sajeesh, P.; Sen, A.K. Particle separation and sorting in microfluidic devices: A review. Microfluid. Nanofluidics 2014, 17, 1–52. [Google Scholar] [CrossRef]
- Gascoyne, P.R.; Noshari, J.; Anderson, T.J.; Becker, F.F. Isolation of rare cells from cell mixtures by dielectrophoresis. Electrophoresis 2009, 30, 1388–1398. [Google Scholar] [CrossRef]
- Pethig, R. Dielectrophoresis: Status of the theory, technology, and applications. Biomicrofluidics 2010, 4, 022811. [Google Scholar] [CrossRef] [PubMed]
- Regtmeier, J.; Duong, T.T.; Eichhorn, R.; Anselmetti, D.; Ros, A. Dielectrophoretic manipulation of DNA: Separation and polarizability. Anal. Chem. 2007, 79, 3925–3932. [Google Scholar] [CrossRef] [PubMed]
- Hejazian, M.; Li, W.; Nguyen, N.-T. Lab on a chip for continuous-flow magnetic cell separation. Lab A Chip 2015, 15, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Cheng, R.; Miller, J.R.; Mao, L. Label-free microfluidic manipulation of particles and cells in magnetic liquids. Adv. Funct. Mater. 2016, 26, 3916–3932. [Google Scholar] [CrossRef] [PubMed]
- Augustsson, P.; Magnusson, C.; Nordin, M.; Lilja, H.; Laurell, T. Microfluidic, label-free enrichment of prostate cancer cells in blood based on acoustophoresis. Anal. Chem. 2012, 84, 7954–7962. [Google Scholar] [CrossRef]
- Laurell, T.; Petersson, F.; Nilsson, A. Chip integrated strategies for acoustic separation and manipulation of cells and particles. Chem. Soc. Rev. 2007, 36, 492–506. [Google Scholar] [CrossRef]
- Li, P.; Mao, Z.; Peng, Z.; Zhou, L.; Chen, Y.; Huang, P.-H.; Truica, C.I.; Drabick, J.J.; El-Deiry, W.S.; Dao, M. Acoustic separation of circulating tumor cells. Proc. Natl. Acad. Sci. USA 2015, 112, 4970–4975. [Google Scholar] [CrossRef]
- Nam, J.; Lim, H.; Kim, D.; Shin, S. Separation of platelets from whole blood using standing surface acoustic waves in a microchannel. Lab A Chip 2011, 11, 3361–3364. [Google Scholar] [CrossRef]
- Wang, Z.; Zhe, J. Recent advances in particle and droplet manipulation for lab-on-a-chip devices based on surface acoustic waves. Lab A Chip 2011, 11, 1280–1285. [Google Scholar] [CrossRef]
- Martel, J.M.; Toner, M. Inertial focusing in microfluidics. Annu. Rev. Biomed. Eng. 2014, 16, 371–396. [Google Scholar] [CrossRef]
- McGrath, J.; Jimenez, M.; Bridle, H. Deterministic lateral displacement for particle separation: A review. Lab A Chip 2014, 14, 4139–4158. [Google Scholar] [CrossRef] [PubMed]
- Warkiani, M.E.; Khoo, B.L.; Wu, L.; Tay, A.K.P.; Bhagat, A.A.S.; Han, J.; Lim, C.T. Ultra-fast, label-free isolation of circulating tumor cells from blood using spiral microfluidics. Nat. Protoc. 2016, 11, 134. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tu, C.; Liang, Y.; Huang, B.; Fang, Y.; Liang, X.; Papautsky, I.; Ye, X. Isolation of cells from whole blood using shear-induced diffusion. Sci. Rep. 2018, 8, 9411. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Papautsky, I. Size-dependent enrichment of leukocytes from undiluted whole blood using shear-induced diffusion. Lab A Chip 2019, 19, 3416–3426. [Google Scholar] [CrossRef]
- Takagi, J.; Yamada, M.; Yasuda, M.; Seki, M. Continuous particle separation in a microchannel having asymmetrically arranged multiple branches. Lab A Chip 2005, 5, 778–784. [Google Scholar] [CrossRef]
- Ebert, E.C.; Nagar, M.; Hagspiel, K.D. Gastrointestinal and hepatic complications of sickle cell disease. Clin. Gastroenterol. Hepatol. 2010, 8, 483–489. [Google Scholar] [CrossRef]
- Janča, J.; Halabalová, V.; Růžička, J. Role of the shape of various bacteria in their separation by microthermal field-flow fractionation. J. Chromatogr. A 2010, 1217, 8062–8071. [Google Scholar] [CrossRef]
- Martin, S.G. Geometric control of the cell cycle. Cell Cycle 2009, 8, 3643–3647. [Google Scholar] [CrossRef]
- Mitragotri, S.; Lahann, J. Physical approaches to biomaterial design. Nat. Mater. 2009, 8, 15. [Google Scholar] [CrossRef]
- Valero, A.; Braschler, T.; Rauch, A.; Demierre, N.; Barral, Y.; Renaud, P. Tracking and synchronization of the yeast cell cycle using dielectrophoretic opacity. Lab A Chip 2011, 11, 1754–1760. [Google Scholar] [CrossRef]
- Sugaya, S.; Yamada, M.; Seki, M. Observation of nonspherical particle behaviors for continuous shape-based separation using hydrodynamic filtration. Biomicrofluidics 2011, 5, 024103. [Google Scholar] [CrossRef] [PubMed]
- Beech, J.P.; Holm, S.H.; Adolfsson, K.; Tegenfeldt, J.O. Sorting cells by size, shape and deformability. Lab A Chip 2012, 12, 1048–1051. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Muñoz, H.E.; Goda, K.; Di Carlo, D. Shape-based separation of microalga Euglena gracilis using inertial microfluidics. Sci. Rep. 2017, 7, 10802. [Google Scholar] [CrossRef] [PubMed]
- d’Avino, G.; Maffettone, P.; Greco, F.; Hulsen, M. Viscoelasticity-induced migration of a rigid sphere in confined shear flow. J. Non-Newton. Fluid Mech. 2010, 165, 466–474. [Google Scholar]
- Leshansky, A.M.; Bransky, A.; Korin, N.; Dinnar, U. Tunable nonlinear viscoelastic “focusing” in a microfluidic device. Phys. Rev. Lett. 2007, 98, 234501. [Google Scholar] [CrossRef]
- Nam, J.; Lim, H.; Kim, D.; Jung, H.; Shin, S. Continuous separation of microparticles in a microfluidic channel via the elasto-inertial effect of non-Newtonian fluid. Lab A Chip 2012, 12, 1347–1354. [Google Scholar] [CrossRef]
- Ahn, S.W.; Lee, S.S.; Lee, S.J.; Kim, J.M. Microfluidic particle separator utilizing sheathless elasto-inertial focusing. Chem. Eng. Sci. 2015, 126, 237–243. [Google Scholar] [CrossRef]
- Nam, J.; Jang, W.S.; Lim, C.S. Non-electrical powered continuous cell concentration for enumeration of residual white blood cells in WBC-depleted blood using a viscoelastic fluid. Talanta 2019, 197, 12–19. [Google Scholar] [CrossRef]
- Liu, C.; Guo, J.; Tian, F.; Yang, N.; Yan, F.; Ding, Y.; Wei, J.; Hu, G.; Nie, G.; Sun, J. Field-free isolation of exosomes from extracellular vesicles by microfluidic viscoelastic flows. ACS Nano 2017, 11, 6968–6976. [Google Scholar] [CrossRef]
- Nam, J.; Namgung, B.; Lim, C.T.; Bae, J.-E.; Leo, H.L.; Cho, K.S.; Kim, S. Microfluidic device for sheathless particle focusing and separation using a viscoelastic fluid. J. Chromatogr. A 2015, 1406, 244–250. [Google Scholar] [CrossRef]
- Nam, J.; Shin, Y.; Tan, J.K.S.; Lim, Y.B.; Lim, C.T.; Kim, S. High-throughput malaria parasite separation using a viscoelastic fluid for ultrasensitive PCR detection. Lab A Chip 2016, 16, 2086–2092. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Tan, J.K.S.; Khoo, B.L.; Namgung, B.; Leo, H.L.; Lim, C.T.; Kim, S. Hybrid capillary-inserted microfluidic device for sheathless particle focusing and separation in viscoelastic flow. Biomicrofluidics 2015, 9, 064117. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Xuan, X. Elasto-inertial pinched flow fractionation for continuous shape-based particle separation. Anal. Chem. 2015, 87, 11523–11530. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhu, L.; Hua, R.-M.; Xuan, X. Continuous sheath-free separation of particles by shape in viscoelastic fluids. Appl. Phys. Lett. 2015, 107, 264102. [Google Scholar] [CrossRef]
- Li, D.; Zielinski, J.; Kozubowski, L.; Xuan, X. Continuous sheath-free separation of drug-treated human fungal pathogen Cryptococcus neoformans by morphology in biocompatible polymer solutions. Electrophoresis 2018, 39, 2362–2369. [Google Scholar] [CrossRef]
- Schaap, A.; Dumon, J.; Den Toonder, J. Sorting algal cells by morphology in spiral microchannels using inertial microfluidics. Microfluid. Nanofluidics 2016, 20, 125. [Google Scholar] [CrossRef]
- Barnes, H.A.; Hutton, J.F.; Walters, K. An Introduction to Rheology; Elsevier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Lim, E.J.; Ober, T.J.; Edd, J.F.; Desai, S.P.; Neal, D.; Bong, K.W.; Doyle, P.S.; McKinley, G.H.; Toner, M. Inertio-elastic focusing of bioparticles in microchannels at high throughput. Nat. Commun. 2014, 5, 4120. [Google Scholar] [CrossRef]
- Seo, K.W.; Byeon, H.J.; Huh, H.K.; Lee, S.J. Particle migration and single-line particle focusing in microscale pipe flow of viscoelastic fluids. RSC Adv. 2014, 4, 3512. [Google Scholar] [CrossRef]
- Tehrani, M. An experimental study of particle migration in pipe flow of viscoelastic fluids. J. Rheol. 1996, 40, 1057. [Google Scholar] [CrossRef]
- Zhou, Y.; Basu, S.; Wohlfahrt, K.J.; Lee, S.F.; Klenerman, D.; Laue, E.D.; Seshia, A.A. A microfluidic platform for trapping, releasing and super-resolution imaging of single cells. Sens. Actuators B Chem. 2016, 232, 680–691. [Google Scholar] [CrossRef]
- Lim, H.; Back, S.M.; Hwang, M.H.; Lee, D.-H.; Choi, H.; Nam, J. Sheathless high-throughput circulating tumor cell separation using viscoelastic non-newtonian fluid. Micromachines 2019, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Matare, T.; Nziramasanga, P.; Gwanzura, L.; Robertson, V. Experimental germ tube induction in Candida albicans: An evaluation of the effect of sodium bicarbonate on morphogenesis and comparison with pooled human serum. Biomed Res. Int. 2017, 2017, 1976273. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Giridhar, P.V.; Kasper, S.; Papautsky, I. Modulation of aspect ratio for complete separation in an inertial microfluidic channel. Lab A Chip 2013, 13, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Schuech, R.; Hoehfurtner, T.; Smith, D.J.; Humphries, S. Motile curved bacteria are Pareto-optimal. Proc. Natl. Acad. Sci. 2019, 116, 14440–14447. [Google Scholar] [CrossRef] [PubMed]
- Perumal, P.; Mekals, S.; Chaffin, W.L. Role for cell density in antifungal drug resistance in candida albicans biofilms. Antimicrob. Agents Chemother. 2007, 51, 2454–2463. [Google Scholar] [CrossRef]
- Seneviratne, C.J.; Jin, L.J.; Samaranayake, Y.H.; Samaranayake, L.P. Cell density and cell aging as factors modulating antifungal resistance of candida albicans biofilms. Antimicrob. Agents Chemother. 2008, 52, 3259–3266. [Google Scholar] [CrossRef]
- Li, Y.; Chang, W.; Zhang, M.; Li, X.; Jiao, Y.; Lou, H. Synergistic and drug-resistant reversing effects of diorcinol D combined with fluconazole against Candida albicans. FEMS Yeast Res. 2015, 15, fov001. [Google Scholar] [CrossRef]
- De Luca, C.; Guglielminetti, M.; Ferrario, A.; Calabr, M.; Casari, E. Candidemia: Species involved, virulence factors and antimycotic susceptibility. New Microbiol. 2012, 35, 459–468. [Google Scholar]
- Tu, C.; Zhou, J.; Liang, Y.; Huang, B.; Fang, Y.; Liang, X.; Ye, X. A flexible cell concentrator using inertial focusing. Biomed. Microdevices 2017, 19, 83. [Google Scholar] [CrossRef]
- Seo, K.W.; Kang, Y.J.; Lee, S.J. Lateral migration and focusing of microspheres in a microchannel flow of viscoelastic fluids. Phys. Fluids 2014, 26, 063301. [Google Scholar] [CrossRef]
- D’Avino, G.; Greco, F.; Maffettone, P.L. Particle migration due to viscoelasticity of the suspending liquid and its relevance in microfluidic devices. Annu. Rev. Fluid Mech. 2017, 49, 341–360. [Google Scholar] [CrossRef]
- D’Avino, G.; Romeo, G.; Villone, M.M.; Greco, F.; Netti, P.A.; Maffettone, P.L. Single line particle focusing induced by viscoelasticity of the suspending liquid: Theory, experiments and simulations to design a micropipe flow-focuser. Lab A Chip 2012, 12, 1638. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Lim, H.; Kim, C.; Kang, J.Y.; Shin, S. Density-dependent separation of encapsulated cells in a microfluidic channel by using a standing surface acoustic wave. Biomicrofluidics 2012, 6, 024120. [Google Scholar] [CrossRef] [PubMed]
- Murua, A.; Portero, A.; Orive, G.; Hernández, R.M.; de Castro, M.; Pedraz, J.L. Cell microencapsulation technology: Towards clinical application. J. Control. Release 2008, 132, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Warkiani, M.E.; Khoo, B.L.; Tan, D.S.-W.; Bhagat, A.A.S.; Lim, W.-T.; Yap, Y.S.; Lee, S.C.; Soo, R.A.; Han, J.; Lim, C.T. An ultra-high-throughput spiral microfluidic biochip for the enrichment of circulating tumor cells. Analyst 2014, 139, 3245–3255. [Google Scholar] [CrossRef]
- Yuan, D.; Zhao, Q.; Yan, S.; Tang, S.-Y.; Zhang, Y.; Yun, G.; Nguyen, N.-T.; Zhang, J.; Li, M.; Li, W. Sheathless separation of microalgae from bacteria using a simple straight channel based on viscoelastic microfluidics. Lab A Chip 2019, 19, 2811–2821. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, J.; Jee, H.; Jang, W.S.; Yoon, J.; Park, B.G.; Lee, S.J.; Lim, C.S. Sheathless Shape-Based Separation of Candida Albicans Using a Viscoelastic Non-Newtonian Fluid. Micromachines 2019, 10, 817. https://doi.org/10.3390/mi10120817
Nam J, Jee H, Jang WS, Yoon J, Park BG, Lee SJ, Lim CS. Sheathless Shape-Based Separation of Candida Albicans Using a Viscoelastic Non-Newtonian Fluid. Micromachines. 2019; 10(12):817. https://doi.org/10.3390/mi10120817
Chicago/Turabian StyleNam, Jeonghun, Hyunseul Jee, Woong Sik Jang, Jung Yoon, Borae G. Park, Seong Jae Lee, and Chae Seung Lim. 2019. "Sheathless Shape-Based Separation of Candida Albicans Using a Viscoelastic Non-Newtonian Fluid" Micromachines 10, no. 12: 817. https://doi.org/10.3390/mi10120817
APA StyleNam, J., Jee, H., Jang, W. S., Yoon, J., Park, B. G., Lee, S. J., & Lim, C. S. (2019). Sheathless Shape-Based Separation of Candida Albicans Using a Viscoelastic Non-Newtonian Fluid. Micromachines, 10(12), 817. https://doi.org/10.3390/mi10120817





