Effect of ZnO Nanoparticles Coating Layers on Top of ZnO Nanowires for Morphological, Optical, and Photovoltaic Properties of Dye-Sensitized Solar Cells
Abstract
:1. Introduction
2. Experimental
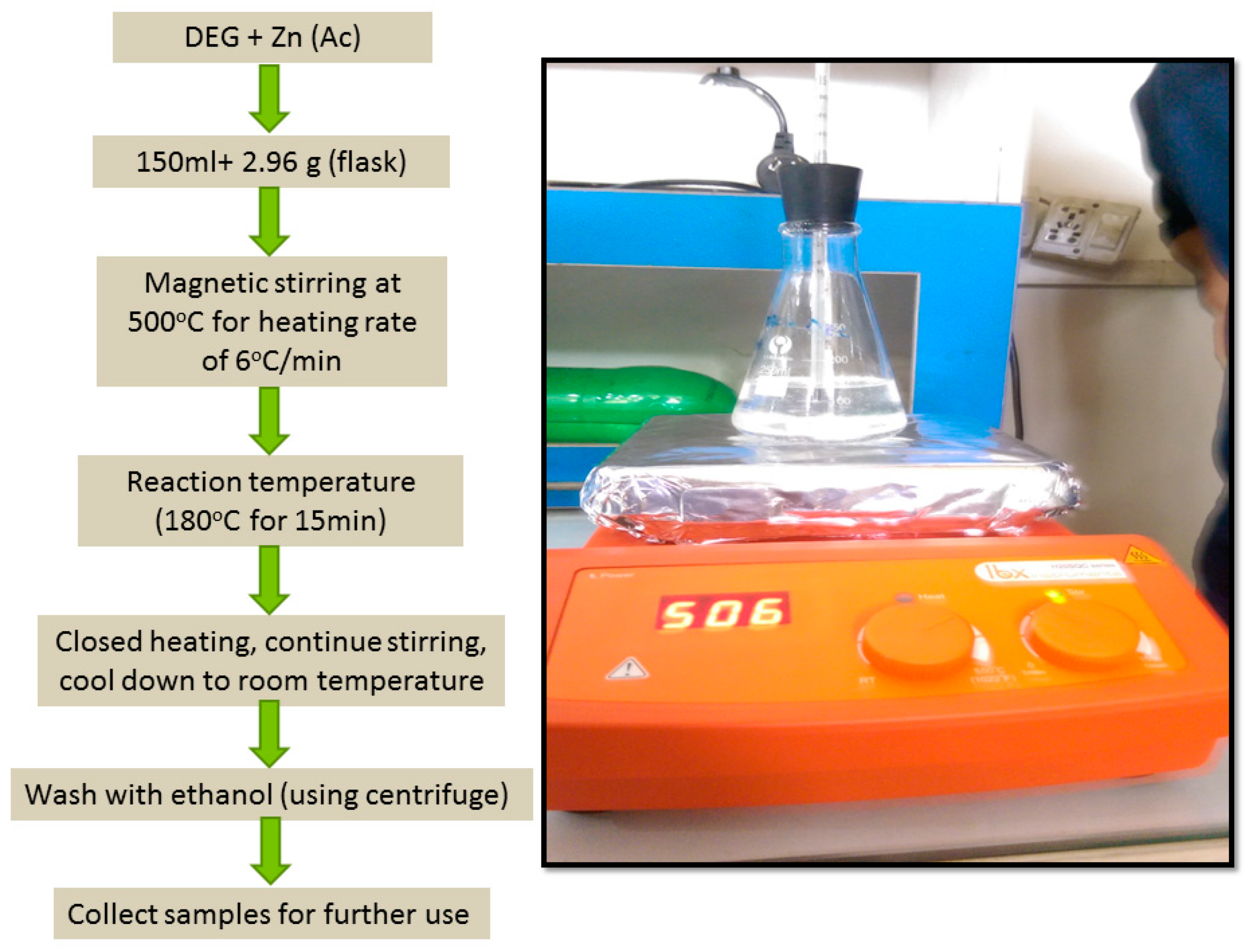
2.1. Synthesis of ZnO Nanoparticles
2.2. ZnO Nanowire Arraysand Nanoparticle Compound Structures Synthesis
2.3. Measurements and Characterizations
2.4. Cell Assembly
3. Results and Discussion
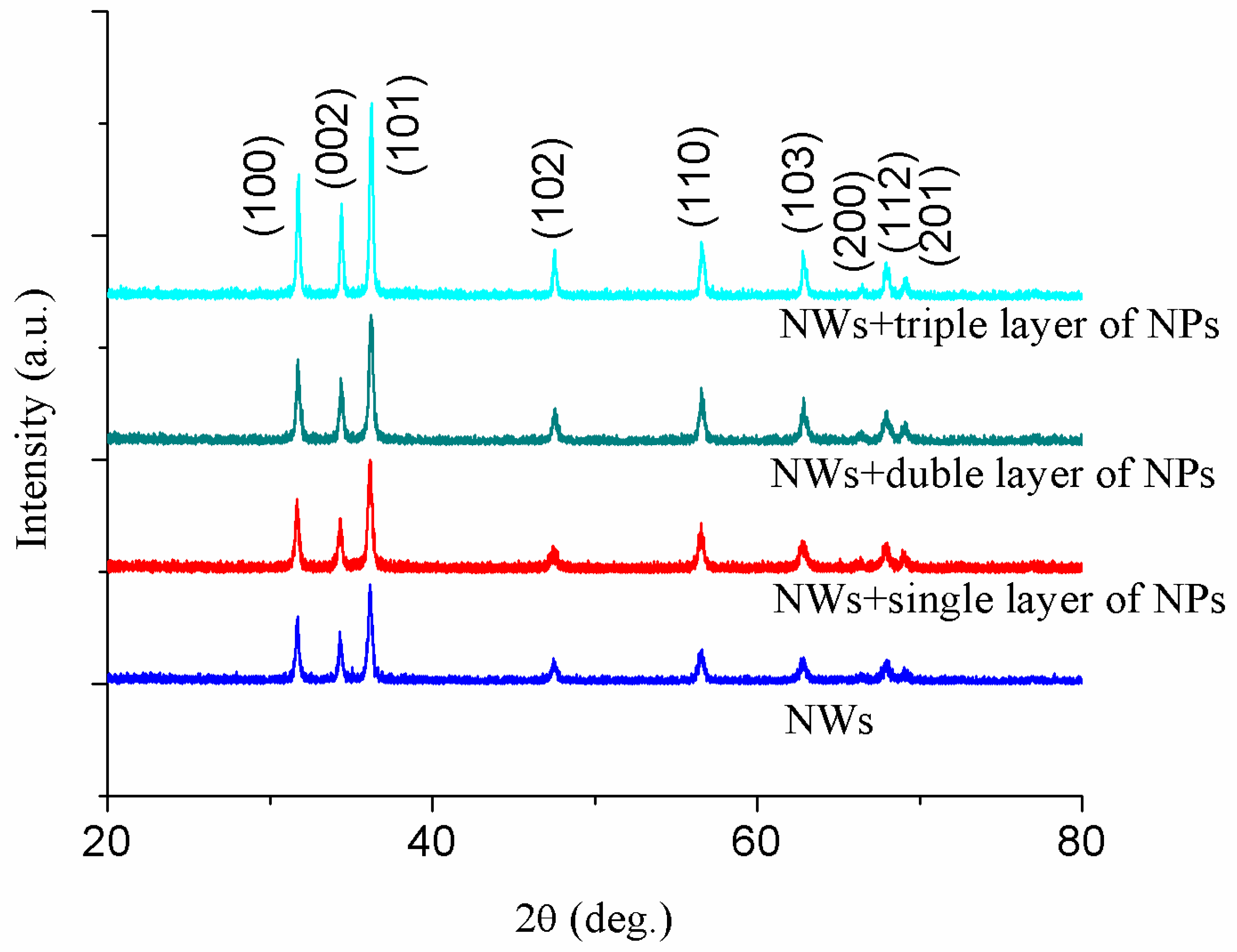
3.1. Structural Analysis
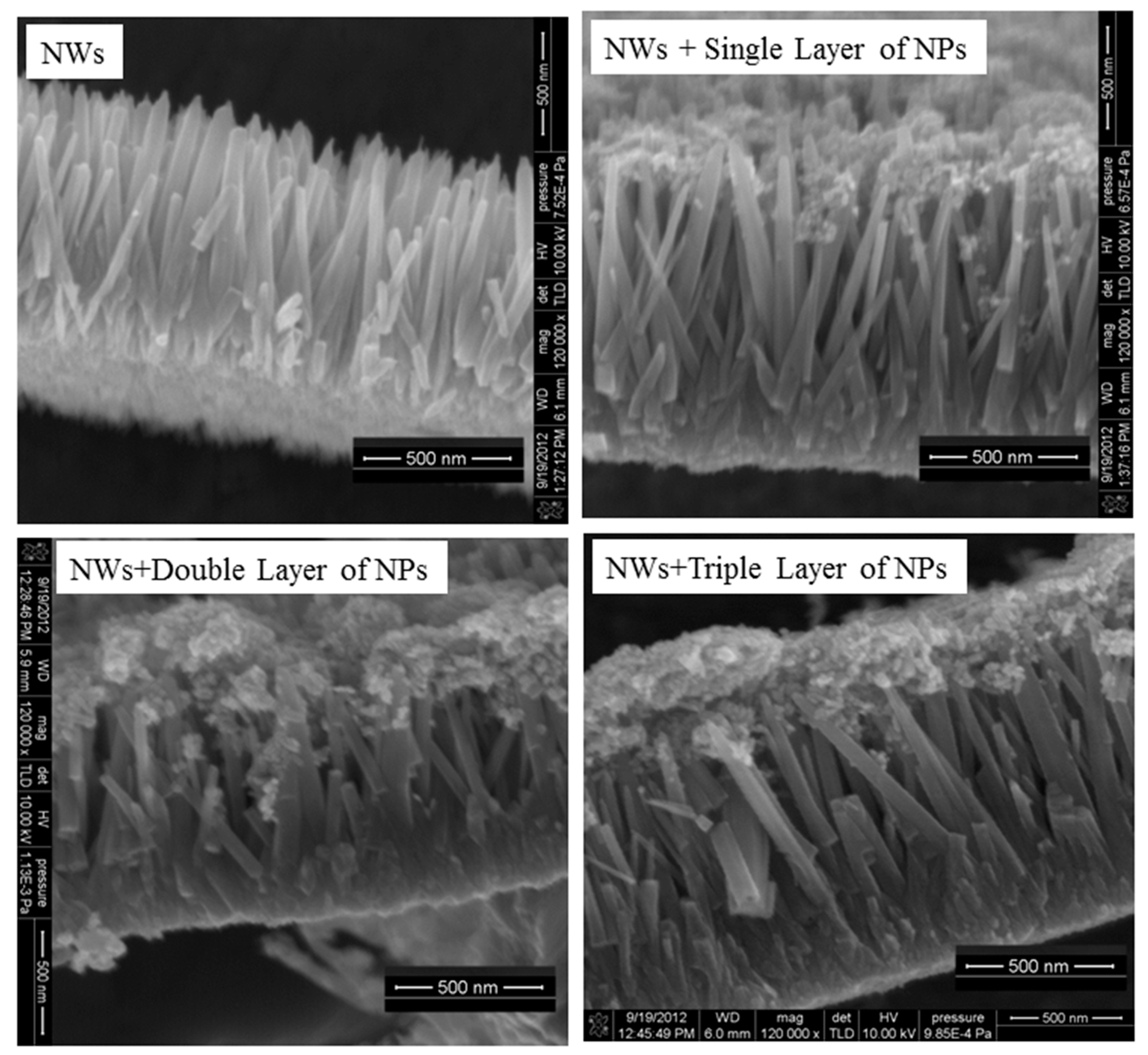
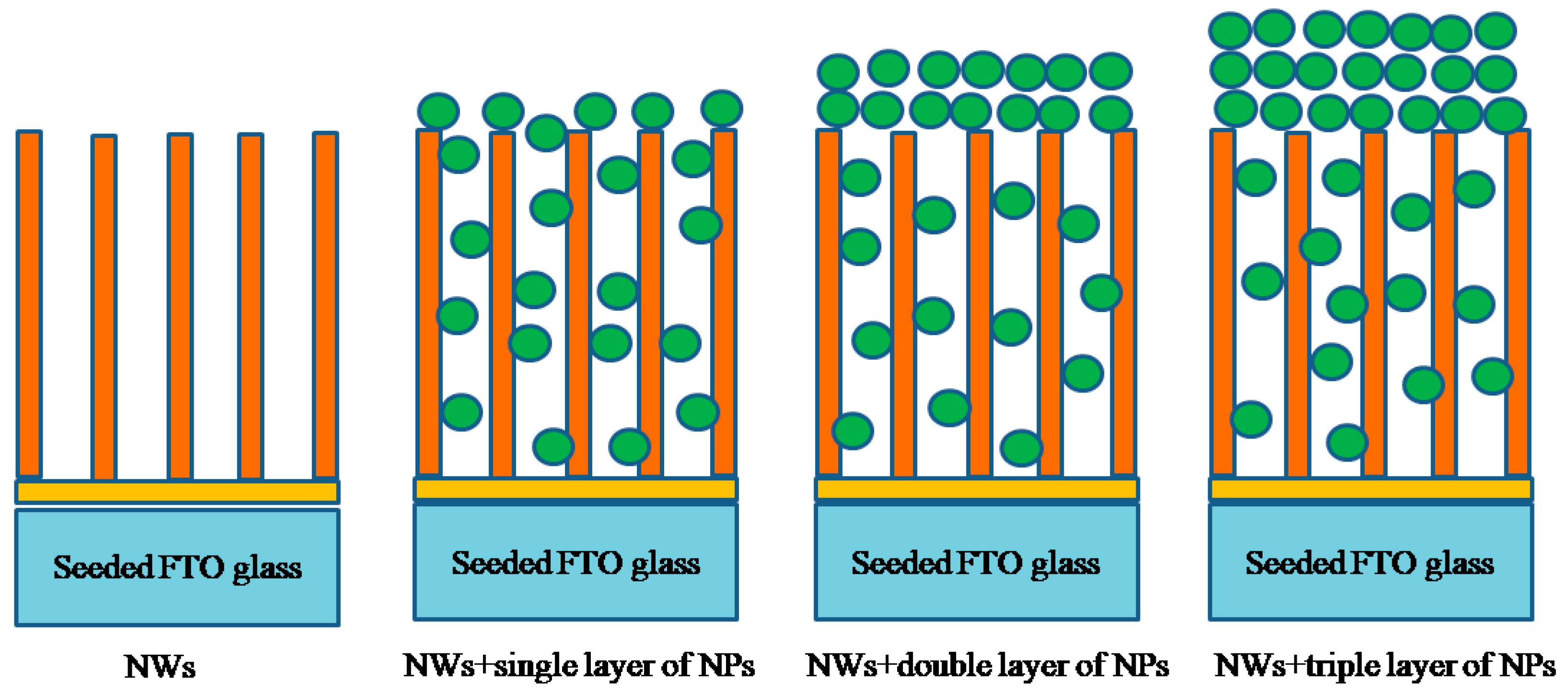
3.2. Morphological Analysis
3.3. Optical Analysis of Dye/ZnO Anodes
3.4. Current Voltage (I–V) Analysis of DSSCs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 353–737. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cao, G.Z.; Yang, Z.; Wang, D.; Dubois, D.; Zhou, X.; Graff, G.L.; Pederson, L.R.; Zhang, J.-G. Oriented nanostructures for energy conversion and storage. Chem. Sus. Chem. 2008, 1, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Goetzberger, A.; Hebling, C. Photovoltaic materials, past, present, future. Sol. Energy Mater. Sol. Cells 2000, 62, 13546–13551. [Google Scholar] [CrossRef]
- Goetzberger, A.; Hebling, C.; Schock, H.W. Photovoltaic materials, history, status and outlook. Mater. Sci. Eng. R Rep. 2003, 1, 40–46. [Google Scholar] [CrossRef]
- Bagnall, D.M.; Boreland, M. Photovoltaic technologies. Energy Pol. 2008, 36, 4390–4396. [Google Scholar] [CrossRef]
- Green, M.A. Recent developments in photovoltaics. Sol. Energy 2004, 76, 3–8. [Google Scholar] [CrossRef]
- Oliver, M.; Jackson, T. The market for solar photovoltaics. Energy Pol. 1999, 27, 371–385. [Google Scholar] [CrossRef]
- Lieber, C.M.; Wang, Z.L. Functional nanowires. MRS Bull. 2007, 32, 99–108. [Google Scholar] [CrossRef]
- Sattler, K. Handbook of Nanophysics: Nanotubes and Nanowires; CRC: Boca Raton, FL, USA, 2011. [Google Scholar]
- Wang, Z. Nanowires and Nanobelts: Materials Properties and Devices: Volume 2: Nanowires and Nanobelts of Functional Materials; Springer: Berlin, Germany, 2005. [Google Scholar]
- Pan, Z.W.; Dai, Z.R.; Wang, Z.L. Nanobelts of semiconducting oxides. Science 2001, 291, 1947–1949. [Google Scholar] [CrossRef]
- Wang, Z. Oxide nanobelts and nanowires—growth, properties and applications. J. Nanosci. Nanotechnol. 2008, 8, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Feldheim, D.; Foss, C. Metal Nanoparticles: Synthesis, Characterization and Applications; CRC: Boca Raton, FL, USA, 2002. [Google Scholar]
- Sugimoto, T. Fine Particles: Synthesis, Characterization and Mechanisms of Growth; CRC: Boca Raton, FL, USA, 2000. [Google Scholar]
- Grätzel, M. Sol-gel processed TiO2 films for photovoltaic applications. J. Sol–Gel Sci. Technol. 2001, 22, 7–13. [Google Scholar] [CrossRef]
- Saito, R.; Dresselhaus, G.; Dresselhaus, M. Physical Properties of Carbon Nanotubes; Imperial College Press: London, UK, 1999. [Google Scholar]
- Baughman, R.H.; Zakhidov, A.A.; de Heer, W.A. Carbon nanotubes–the route toward applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Grätzel, M. Solar energy conversion by dye-sensitized photovoltaic cells. Inorg. Chem. 2005, 44, 6841–6851. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Greene, L.E.; Johnson, J.C.; Saykally, R.; Yang, P.D. Nanowire dye-sensitized solar cells. Nat. Mater. 2005, 4, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Baxter, J.B.; Aydil, E.S. Nanowire-based dye-sensitized solar cells. Appl. Phys. Lett. 2005, 86, 053114–053117. [Google Scholar] [CrossRef]
- Paulose, M.; Shankar, K.; Varghese, O.K.; Mor, G.K.; Grimes, C.A. Application of highly-ordered TiO2 nanotube-arrays in heterojunction dye-sensitized solar cells. J. Phys. D 2006, 39, 2498–2503. [Google Scholar] [CrossRef]
- Wang, H.; Yip, C.T.; Cheung, K.Y.; Djurisic, A.B.; Xie, M.H.; Leung, Y.H.; Chan, W.K. Titania-nanotube-array-based photovoltaic cells. Appl. Phys. Lett. 2006, 89, 023508–023510. [Google Scholar] [CrossRef]
- Jiang, C.Y.; Sun, X.W.; Lo, G.Q.; Kwong, D.L.; Wang, J.X. Improved dye-sensitized solar cells with a ZnO-nanoflower photoanode. Appl. Phys. Lett. 2007, 90, 263501–263503. [Google Scholar] [CrossRef]
- Cheng, H.M.; Chiu, W.H.; Lee, C.H.; Tsai, S.Y.; Hsieh, W.F. Formation of branched ZnO nanowires from solvothermal method and dye-sensitized solar cells applications. J. Phys. Chem. C 2008, 112, 16359–16364. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, H.; Hsing, I.M.; Yang, S. A new photoanode architecture of dye sensitized solar cell based on ZnO nanotetrapods with no need for calcination. Electrochem. Commun. 2009, 11, 1057–1060. [Google Scholar] [CrossRef]
- Baxter, J.B.; Aydil, E.S. Dye-sensitized solar cells based on semiconductor morphologies with ZnO nanowires. Energy Mater. Sol. Cells 2006, 90, 607–622. [Google Scholar] [CrossRef]
- Ku, C.H.; Wu, J.J. Electron transport properties in ZnO nanowire array/noparticle composite dye-sensitized solar cells. Appl. Phys. Lett. 2007, 91, 093117. [Google Scholar] [CrossRef]
- Ku, C.H.; Wu, J.J. Dye-sensitized solar cells based on nanoparticle-decorated ZnO/TiO2 core/shell nanorod arrays. Appl. Phys. Lett. 2007, 91, 093117–093123. [Google Scholar]
- Law, M.; Greene, L.E.; Radenovic, A.; Kuykendall TLiphardt, J.; Yang, P.D. ZnO−Al2O3 and ZnO−TiO2 Core−Shell Nanowire Dye-Sensitized Solar Cell. J. Phys. Chem. B 2006, 110, 22652. [Google Scholar] [CrossRef]
- Saleem, M.; Fang, L.; Ahmad, M.A.; Raza, R.; Wu, F.; Li, W.J.; Xu, C.L.; Hu, L.; Xue, S.J. Synthesis, Characteristic and Appliction of ZnO Nanowires in dye-sensitized solar cells via Water Bath method. Nano 2014, 9, 1450061–1450068. [Google Scholar] [CrossRef]
- Saleem, M.; Fang, L.; Shaukat, S.F.; Ahmad, M.A.; Raza, R.; Akhtar, M.N.; Jamil, A.; Aslam, S.; Abbas, G. Structural and photovoltaic characteristics of hierarchical ZnO nanostructures electrodes. Appl. Surf. Sci. 2015, 334, 145–150. [Google Scholar] [CrossRef]
- Wang, Z.S.; Kawauchi, H.; Kashima, T.; Arakawa, H. Significant influence of TiO2 photoelectrode morphology on the energy conversion efficiency of N719 dye–sensitized solar cell. Coord. Chem. Rev. 2004, 248, 1381–1389. [Google Scholar] [CrossRef]
- Tan, B.; Wu, Y.Y. Dye–sensitized solar cells based on anatase TiO2 nanoparticle/nanowire composites. J. Phys. Chem. B 2006, 110, 15932–15938. [Google Scholar] [CrossRef]
- Lee, B.; Hwang, D.K.; Guo, P.; Ho, S.T.; Buchholtz, D.B.; Wang, C.Y.; Chang, R.P.H. Materials, interfaces, and photon confinement in dye–sensitized solar cells. J. Phys. Chem. B 2010, 114, 14582–14591. [Google Scholar] [CrossRef]
- Hsu, C.P.; Lee, K.M.; Huang, J.T.W.; Lin, C.Y.; Lee, C.H.; Wang, L.P.; Tsai, S.Y.; Ho, K.C. EIS analysis on low temperature fabrication of TiO2 porous films for dyesensitized solar cells. Electrochim. Acta 2008, 53, 7514–7522. [Google Scholar] [CrossRef]
- Suri, P.; Mehra, R.M. Effect of electrolytes on the photovoltaic performance of a hybrid dye sensitized ZnO solar cell. Sol. Energy Mater. Sol. Cells 2007, 91, 518–524. [Google Scholar] [CrossRef]
- Sheng, X.; Zhao, Y.; Zhai, J.; Jiang, L.; Zhu, D. Electro-hydrodynamic fabrication of ZnO-based dye sensitized solar cells. Appl. Phys. A 2007, 87, 715–719. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Li, K. Dye-sensitized solar cells based on oriented ZnO nanowire-covered TiO2 nanoparticle composite film electrodes. Mater. Lett. 2009, 63, 1102–1104. [Google Scholar] [CrossRef]
- Pasquier, A.D.; Chen, H.; Lu, Y. Dye sensitized solar cells using well-aligned zinc oxide nanotip arrays. Appl. Phys. Lett. 2006, 89, 253513–253515. [Google Scholar] [CrossRef]
- Baxter, J.B.; Walker, A.M.; van Ommering, K.; Aydil, E.S. Synthesis and characterization of ZnO nanowires and their integration into dye-sensitized solar cells. Nanotechnology 2006, 17, S304–S312. [Google Scholar] [CrossRef]
- Gao, H.; Fang, G.; Wang, M.; Liu, N.; Yuan, L.; Li, C.; Ai, L.; Zhang, J.; Zhou, C.; Wu, S.; et al. The effect of growth conditions on the properties of ZnO nanorod dye-sensitized solar cells. Mater. Res. Bull. 2008, 43, 3345–3351. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Shen, W.Z. Synthesis of ZnO compound nanostructures via a chemical route for photovoltaic applications. Appl. Phys. Lett. 2010, 256, 7472–7476. [Google Scholar] [CrossRef]
- Ku, C.H.; Wu, J.J. Chemical bath deposition of ZnO nanowire–nanoparticle composite electrodes for use in dye-sensitized solar cells. Nanotechnology 2007, 18, 505706. [Google Scholar] [CrossRef]
- Yodyingyong, S.; Zhang, Q.; Park, K.; Dandeneau, C.S.; Zhou, X.; Triampo, D.; Cao, G. ZnO nanoparticles and nanowire array hybrid photoanodes for dye-sensitized solar cells. Appl. Phys. Lett. 2010, 96, 073115. [Google Scholar] [CrossRef]
- Victoire–Marie, G.; Thierry, P. From nanowires to hierarchical structures of template-free electrodeposited ZnO for efficient dye-sensitized solar cells. Energy Environ. Sci. 2011, 4, 297. [Google Scholar]
- Haller, S.; Suguira, T.; Lincot, D.; Yoshida, T. Design of a hierarchical structure of ZnO by electrochemistry for ZnO-based dye-sensitized solar cells. Phys. Status Solidi A 2010, 207, 2252–2257. [Google Scholar] [CrossRef]
- Urgessa, Z.N.; Ruessa, R.; Djiokap, S.R.T.; Botha, J.R.; Schlettwein, D. Effect of morphology and surface treatment on the performance of ZnO nanorod-based dye-sensitized solar cells. J. Alloys Compd. 2019, 798, 249–256. [Google Scholar] [CrossRef]
- Kopidakis, N.; Schiff, E.A.; Park, N.G.; van de Lagemaat, J.; Frank, A.J. Ambipolar Diffusion of Photocarriers in Electrolyte-Filled, Nanoporous TiO2. J. Phys. Chem. B 2000, 104, 3930–3936. [Google Scholar] [CrossRef]

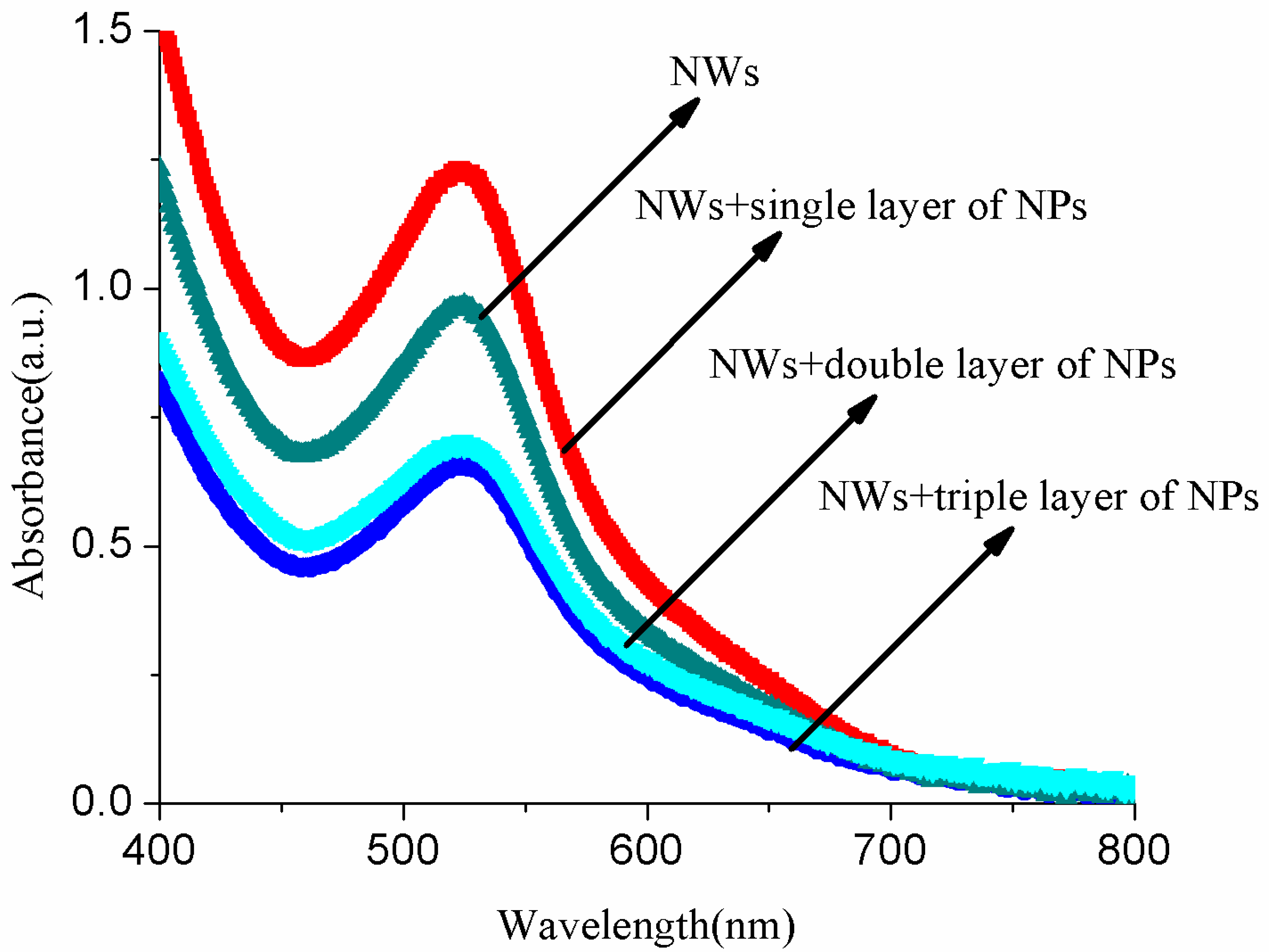
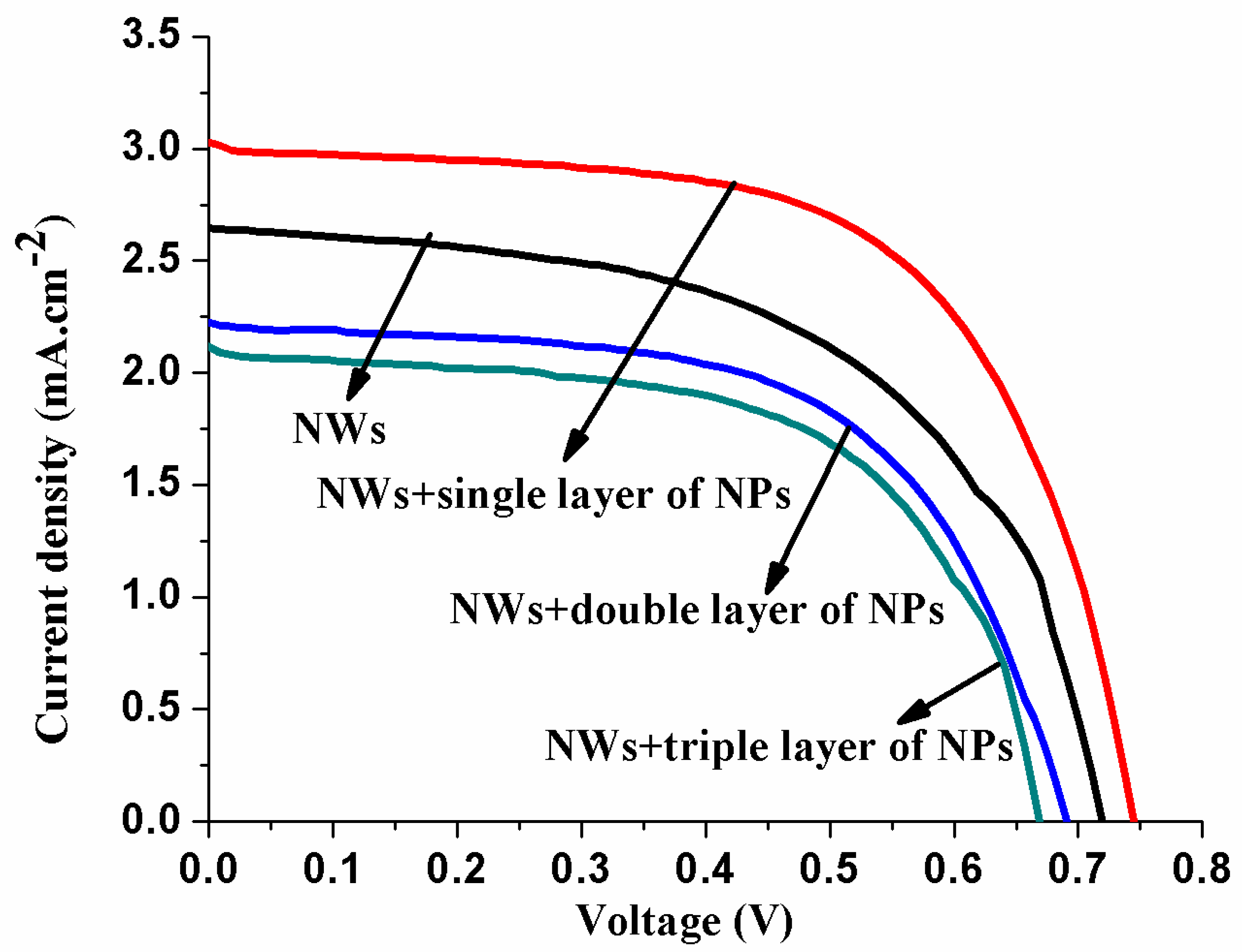
| Samples | Short-Circuit Photocurrent Density Jsc (mA/cm2) | Open-Circuit Voltage Voc (V) | Fill Factor (FF) | η (%) |
|---|---|---|---|---|
| NWs | 2.64 ± 0.021 | 0.72 ± 0.020 | 0.74 ± 0.025 | 1.43 ± 0.015 |
| NWs + single of NPs | 3.02 ± 0.010 | 0.74 ± 0.015 | 0.76 ± 0.020 | 2.28 ± 0.011 |
| NWs + double of NPs | 2.22 ± 0.005 | 0.69 ± 0.012 | 0.62 ± 0.005 | 0.93 ± 0.017 |
| NWs + triple of NPs | 2.11 ± 0.011 | 0.68 ± 0.010 | 0.58 ± 0.005 | 0.86 ± 0.001 |
| Samples | Short-Circuit Photocurrent Density Jsc (mA/cm2) | Open-Circuit Voltage Voc (V) | Fill Factor (FF) | η (%) |
|---|---|---|---|---|
| ZnO NW–NP hybrid cell [27] | 3.00 | 0.77 | 0.65 | 1.3 |
| ZnO NW [44] | 2.5 | 0.44 | 0.47 | 0.45 |
| ZnO NW–NP composite [44] | 8.33 | 0.58 | 0.58 | 2.77 |
| ZnO NW [45] | 4.55 | 0.60 | 0.41 | 1.16 |
| ZnO NW–NP hybrid cell [45] | 15.16 | 0.61 | 0.46 | 4.24 |
| ZnO NW [46] | 2.52 | 0.53 | 0.37 | 0.49 |
| ZnO NW–NP hierarchical [46] | 5.4 | 0.62 | 0.60 | 2.03 |
| ZnO NR–NP hybrid cell [47] | 5.39 | 0.52 | 0.57 | 1.6 |
| ZnO NR–NP hybrid cell [48] | 4.57 | 0.45 | 0.34 | 0.69 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, M.; Farooq, W.A.; Khan, M.I.; Akhtar, M.N.; Rehman, S.U.; Ahmad, N.; Khalid, M.; Atif, M.; AlMutairi, M.A.; Irfan, M. Effect of ZnO Nanoparticles Coating Layers on Top of ZnO Nanowires for Morphological, Optical, and Photovoltaic Properties of Dye-Sensitized Solar Cells. Micromachines 2019, 10, 819. https://doi.org/10.3390/mi10120819
Saleem M, Farooq WA, Khan MI, Akhtar MN, Rehman SU, Ahmad N, Khalid M, Atif M, AlMutairi MA, Irfan M. Effect of ZnO Nanoparticles Coating Layers on Top of ZnO Nanowires for Morphological, Optical, and Photovoltaic Properties of Dye-Sensitized Solar Cells. Micromachines. 2019; 10(12):819. https://doi.org/10.3390/mi10120819
Chicago/Turabian StyleSaleem, Muhammad, W. A. Farooq, M. I. Khan, Majid. Niaz. Akhtar, Saif Ur Rehman, Naseeb Ahmad, Muhammad Khalid, M. Atif, Mona A. AlMutairi, and Muhammad Irfan. 2019. "Effect of ZnO Nanoparticles Coating Layers on Top of ZnO Nanowires for Morphological, Optical, and Photovoltaic Properties of Dye-Sensitized Solar Cells" Micromachines 10, no. 12: 819. https://doi.org/10.3390/mi10120819
APA StyleSaleem, M., Farooq, W. A., Khan, M. I., Akhtar, M. N., Rehman, S. U., Ahmad, N., Khalid, M., Atif, M., AlMutairi, M. A., & Irfan, M. (2019). Effect of ZnO Nanoparticles Coating Layers on Top of ZnO Nanowires for Morphological, Optical, and Photovoltaic Properties of Dye-Sensitized Solar Cells. Micromachines, 10(12), 819. https://doi.org/10.3390/mi10120819







