Different Regimes of Opto-fluidics for Biological Manipulation
Abstract
1. Introduction
2. Methods
3. Results
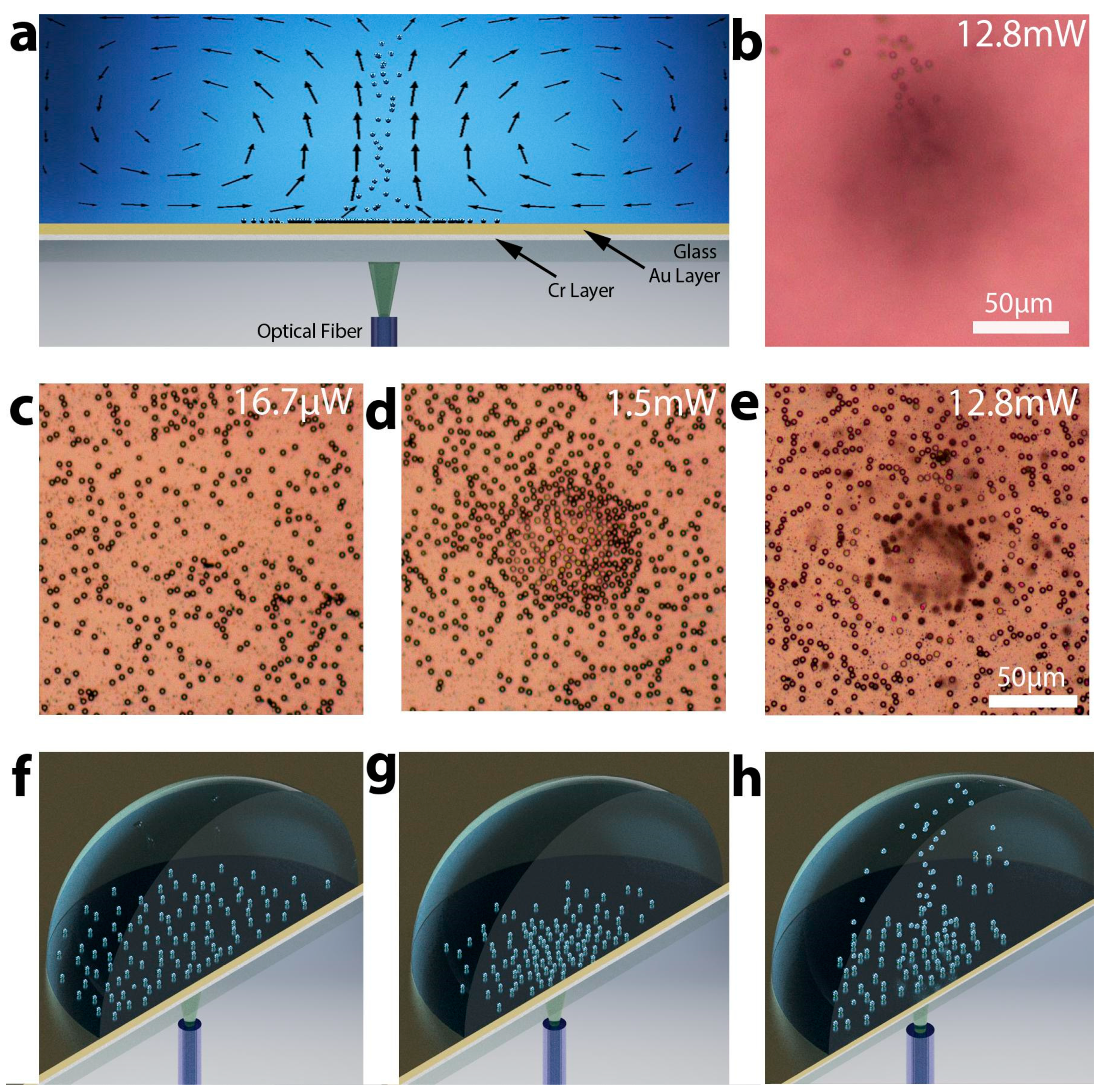
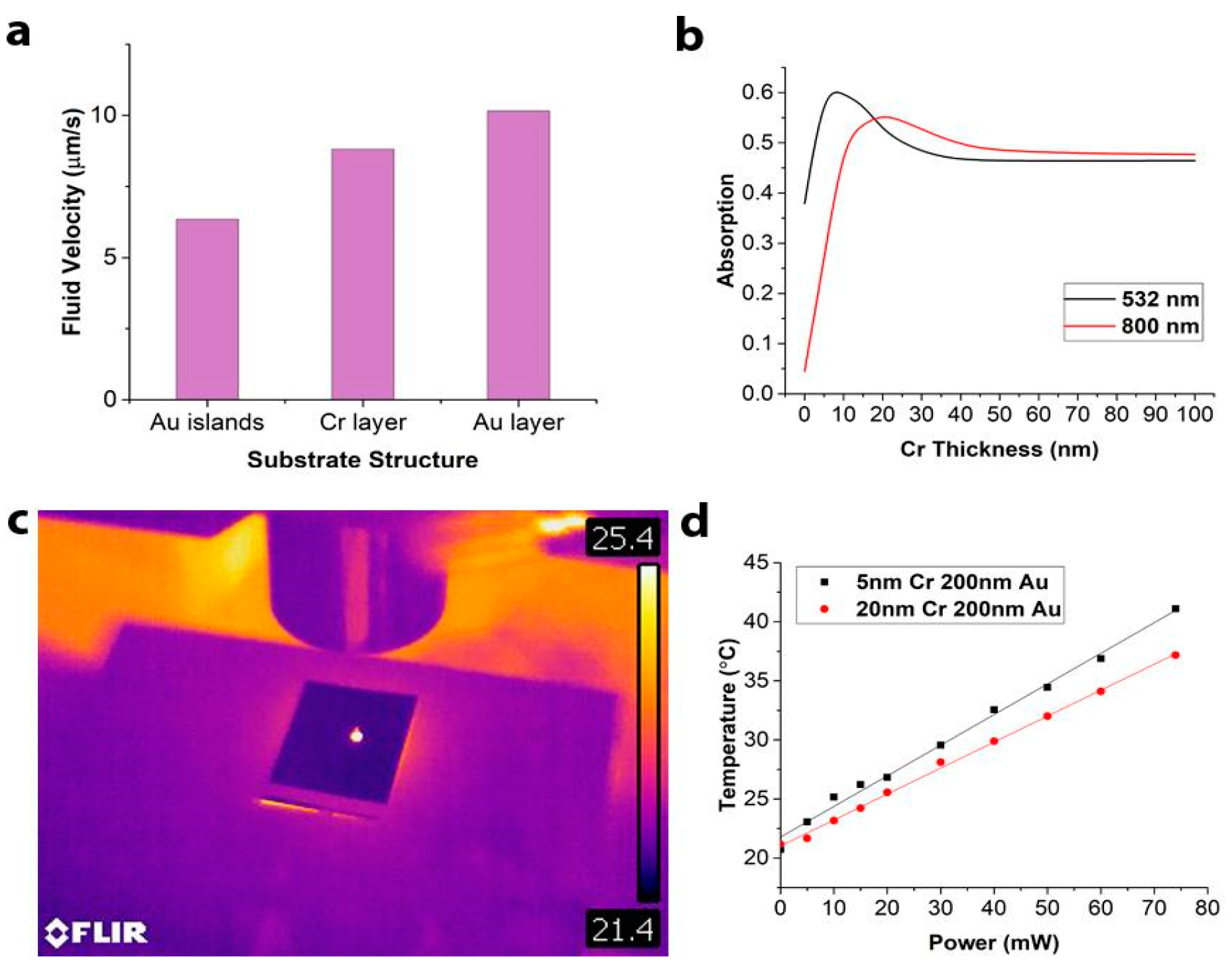
3.1. The Substrate Optimization, Fabrication, and Characterization
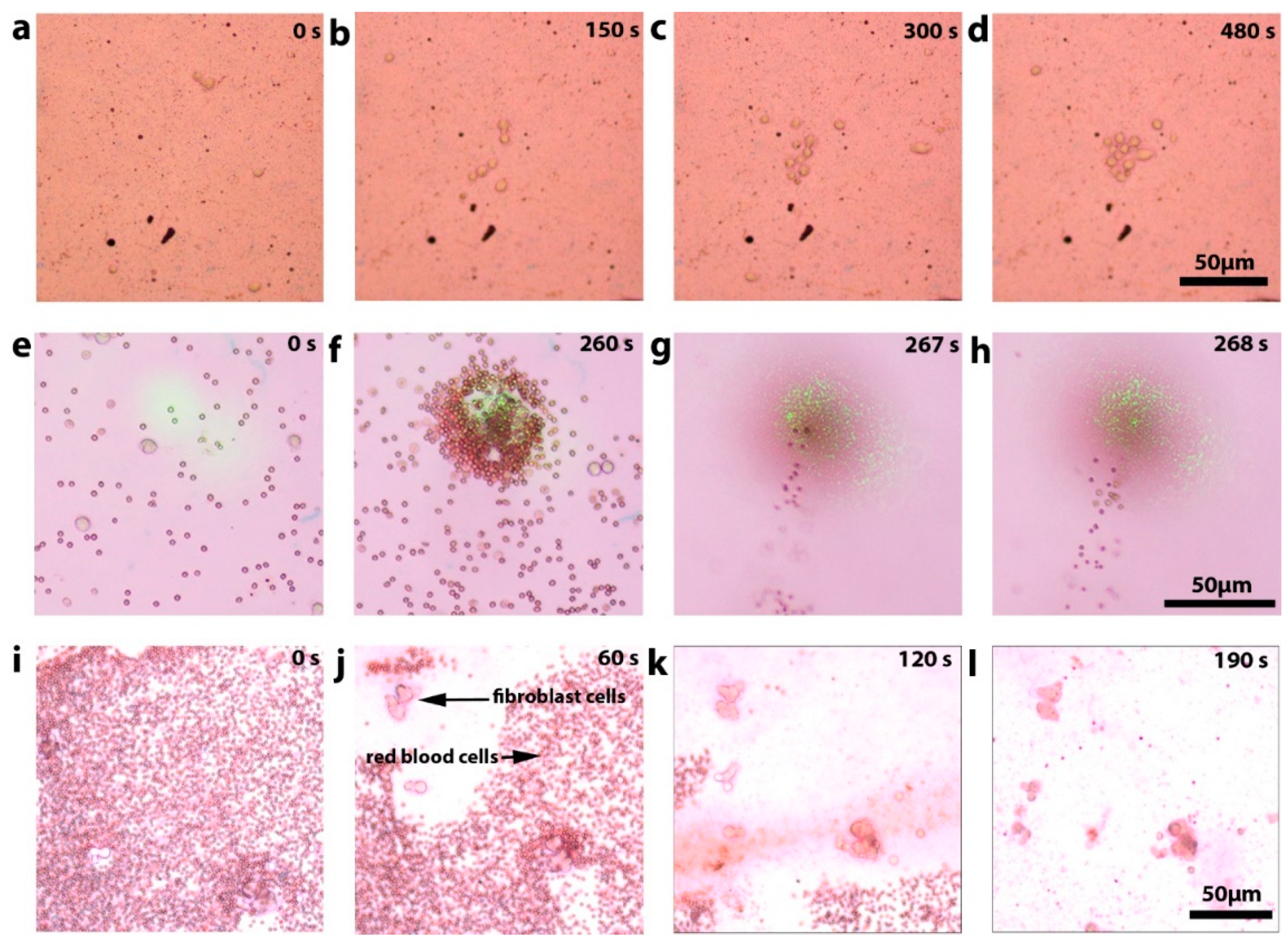
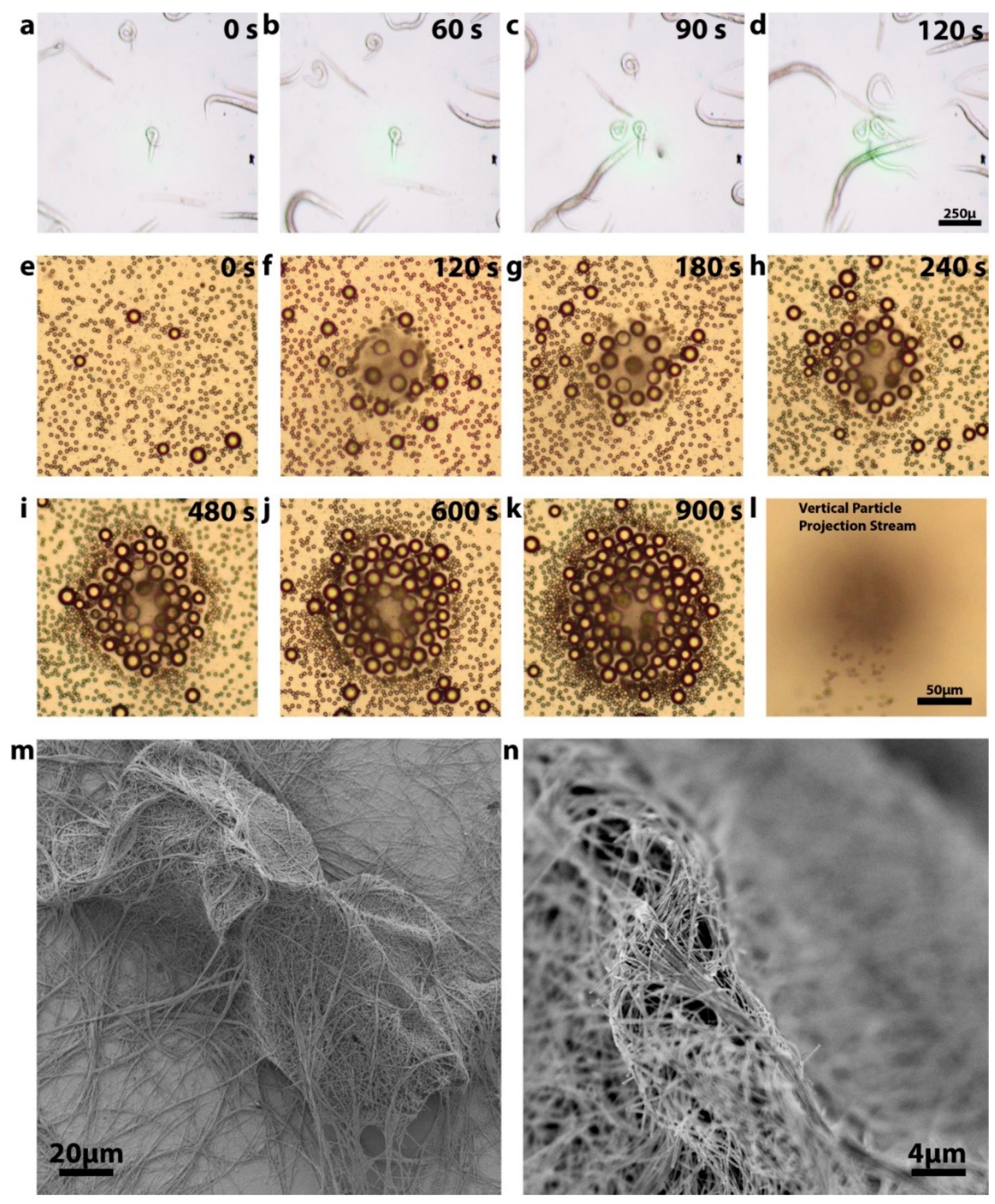
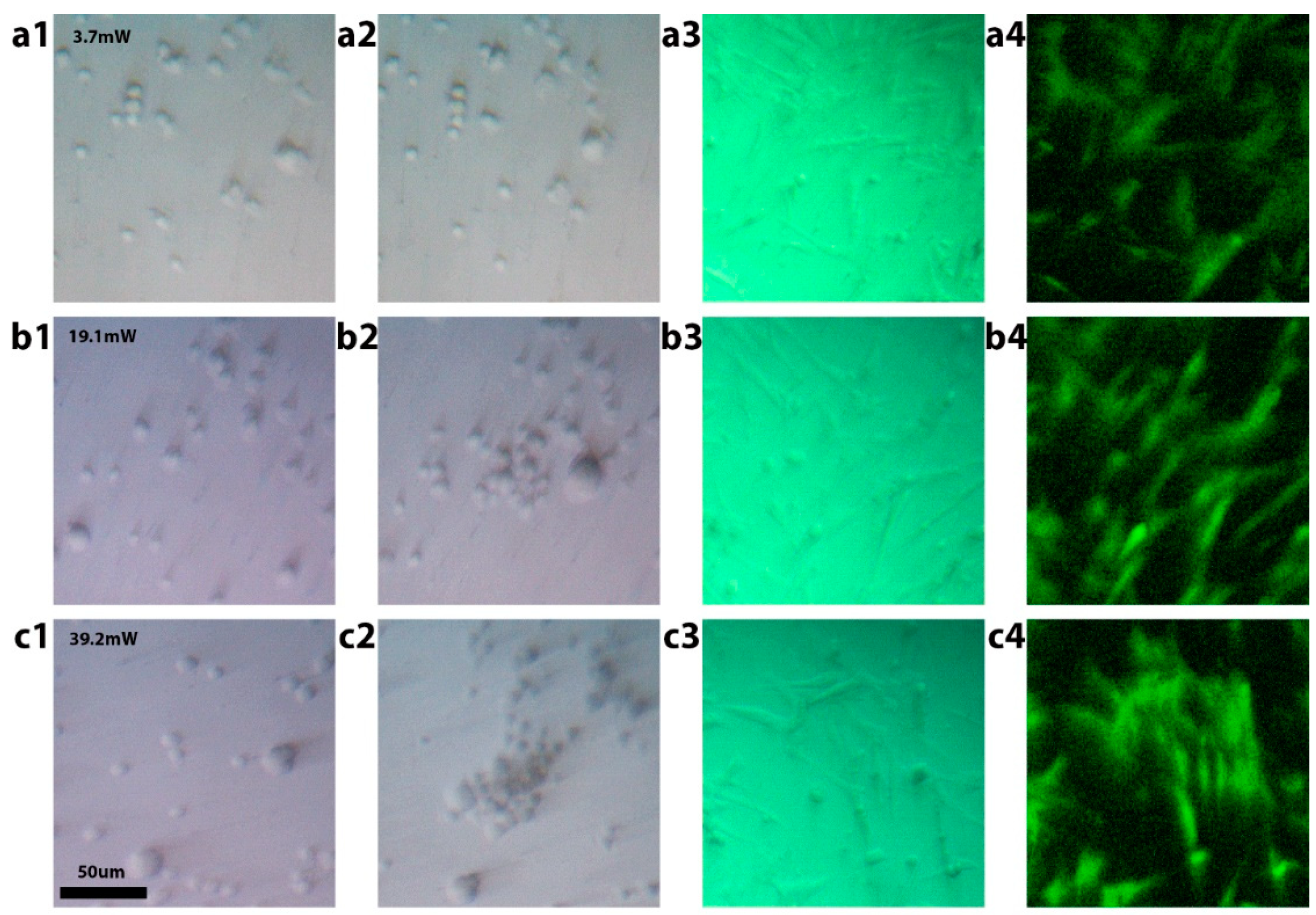
3.2. Sample Manipulation Using Optimized Substrates
3.3. Cell Viability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, W.R.; Oh, K.T.; Park, S.Y.; Yoo, N.Y.; Ahn, Y.S.; Lee, D.H.; Youn, Y.S.; Lee, D.K.; Cha, K.H.; Lee, E.S. Magnetic levitating polymeric nano/microparticular substrates for three-dimensional tumor cell culture. Colloids Surf. B Biointerf. 2011, 85, 379–384. [Google Scholar] [CrossRef]
- Durmus, N.G.; Tekin, H.C.; Guven, S.; Sridhar, K.; Yildiz, A.A.; Calibasi, G.; Ghiran, I.; Davis, R.W.; Steinmetz, L.M.; Demirci, U. Magnetic levitation of single cells. Proc. Natl. Acad. Sci. USA 2015, 112, E3661–E3668. [Google Scholar] [CrossRef]
- Thoumine, O.; Ott, A.; Cardoso, O.; Meister, J.J. Microplates: a new tool for manipulation and mechanical perturbation of individual cells. J. Biochem. Biophys. Methods 1999, 39, 47–62. [Google Scholar] [CrossRef]
- Korda, P.T.; Taylor, M.B.; Grier, D.G. Kinetically locked-in colloidal transport in an array of optical tweezers. Phys. Rev. Lett. 2002, 89, 128301. [Google Scholar] [CrossRef]
- Schuller, J.A.; Barnard, E.S.; Cai, W.; Jun, Y.C.; White, J.S.; Brongersma, M.L. Plasmonics for extreme light concentration and manipulation. Nat. Mater. 2010, 9, 193. [Google Scholar] [CrossRef]
- Kurup, G.K.; Basu, A.S. Rolling, aligning, and trapping droplets on a laser beam using marangoni optofluidic tweezers. In Proceedings of the 16th International Conference Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Beijing, China, 5–9 June 2011; pp. 266–269. [Google Scholar]
- Khan, I.; Tang, E.; Arany, P. Molecular pathway of near-infrared laser phototoxicity involves ATF-4 orchestrated ER stress. Sci. Rep. 2015, 5, 10581. [Google Scholar] [CrossRef]
- Berthelot, J.; Aćimović, S.S.; Juan, M.L.; Kreuzer, M.P.; Renger, J.; Quidant, R. Three-dimensional manipulation with scanning near-field optical nanotweezers. Nat. Nanotechnol. 2014, 9, 295–299. [Google Scholar] [CrossRef]
- Mandal, S.; Serey, X.; Erickson, D. Nanomanipulation using silicon photonic crystal resonators. Nano Lett. 2009, 10, 99–104. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, D.K.; Sonek, G.J.; Berns, M.W.; Chapman, C.F.; Tromberg, B.J. Evidence for localized cell heating induced by infrared optical tweezers. Biophys. J. 1995, 68, 2137–2144. [Google Scholar] [CrossRef]
- Chiou, P.Y.; Ohta, A.T.; Wu, M.C. Massively parallel manipulation of single cells and microparticles using optical images. Nature 2005, 436, 370. [Google Scholar] [CrossRef]
- Kurup, G.K.; Basu, A.S. Hydrodynamic particle concentration inside a microfluidic plug. In Proceedings of the 14th International Conference on Miniaturized Systems for Chemistry and Life Sciences (MicroTAS), Groningen, The Netherlands, 3–7 Octeober 2010; pp. 740–742. [Google Scholar]
- Trivedi, V.; Doshi, A.; Kurup, G.K.; Ereifej, E.; Vandevord, P.J.; Basu, A.S. A modular approach for the generation, storage, mixing, and detection of droplet libraries for high throughput screening. Lab Chip 2010, 10, 2433–2442. [Google Scholar] [CrossRef]
- Miao, X.; Wilson, B.K.; Lin, L.Y. Localized surface plasmon assisted microfluidic mixing. Appl. Phys. Lett. 2008, 92, 124108. [Google Scholar] [CrossRef]
- Donner, J.S.; Baffou, G.; McCloskey, D.; Quidant, R. Plasmon-assisted optofluidics. ACS Nano 2011, 5, 5457–5462. [Google Scholar] [CrossRef]
- Chen, J.; Kang, Z.; Kong, S.K.; Ho, H.P. Plasmonic random nanostructures on fiber tip for trapping live cells and colloidal particles. Opt. Lett. 2015, 40, 3926–3929. [Google Scholar] [CrossRef]
- Kang, Z.; Chen, J.; Wu, S.Y.; Chen, K.; Kong, S.K.; Yong, K.T.; Ho, H.P. Trapping and assembling of particles and live cells on large-scale random gold nano-island substrates. Sci. Rep. 2015, 5, 9978. [Google Scholar] [CrossRef]
- Lozan, O.; Perrin, M.; Ea-Kim, B.; Rampnoux, J.M.; Dilhaire, S.; Lalanne, P. Anomalous light absorption around subwavelength apertures in metal films. Phys. Rev. Lett. 2014, 112, 193903. [Google Scholar] [CrossRef]
- Meier, M.; Wokaun, A.; Liao, P.F. Enhanced fields on rough surfaces: dipolar interactions among particles of sizes exceeding the Rayleigh limit. JOSA B 1985, 2, 931–949. [Google Scholar] [CrossRef]
- Norman, T.J.; Grant, C.D.; Magana, D.; Zhang, J.Z.; Liu, J.; Cao, D.; Bridges, F.; Van Buuren, A. Near infrared optical absorption of gold nanoparticle aggregates. J. Phys. Chem. B 2002, 106, 7005–7012. [Google Scholar] [CrossRef]
- Jeong, Y.G.; Lee, J.S.; Shim, J.K.; Hur, W. A scaffold-free surface culture of B16F10 murine melanoma cells based on magnetic levitation. Cytotechnology 2016, 68, 2323–2334. [Google Scholar] [CrossRef]
- Namura, K.; Imafuku, S.; Kumar, S.; Nakajima, K.; Sakakura, M.; Suzuki, M. Direction control of quasi-stokeslet induced by thermoplasmonic heating of a water vapor microbubble. Sci. Rep. 2019, 9, 4770. [Google Scholar] [CrossRef]
- Flores-Flores, E.; Torres-Hurtado, S.A.; Páez, R.; Ruiz, U.; Beltrán-Pérez, G.; Neale, S.L.; Ramirez-San-Juan, J.C.; Ramos-García, R. Trapping and manipulation of microparticles using laser-induced convection currents and photophoresis. Biomed. Opt. Express 2015, 6, 4079–4087. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winskas, J.T.; Wang, H.; Zhdanov, A.; Cheemalapati, S.; Deonarine, A.; Westerheide, S.; Pyayt, A. Different Regimes of Opto-fluidics for Biological Manipulation. Micromachines 2019, 10, 802. https://doi.org/10.3390/mi10120802
Winskas JT, Wang H, Zhdanov A, Cheemalapati S, Deonarine A, Westerheide S, Pyayt A. Different Regimes of Opto-fluidics for Biological Manipulation. Micromachines. 2019; 10(12):802. https://doi.org/10.3390/mi10120802
Chicago/Turabian StyleWinskas, John T., Hao Wang, Arsenii Zhdanov, Surya Cheemalapati, Andrew Deonarine, Sandy Westerheide, and Anna Pyayt. 2019. "Different Regimes of Opto-fluidics for Biological Manipulation" Micromachines 10, no. 12: 802. https://doi.org/10.3390/mi10120802
APA StyleWinskas, J. T., Wang, H., Zhdanov, A., Cheemalapati, S., Deonarine, A., Westerheide, S., & Pyayt, A. (2019). Different Regimes of Opto-fluidics for Biological Manipulation. Micromachines, 10(12), 802. https://doi.org/10.3390/mi10120802




