Reliability of Protective Coatings for Flexible Piezoelectric Transducers in Aqueous Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
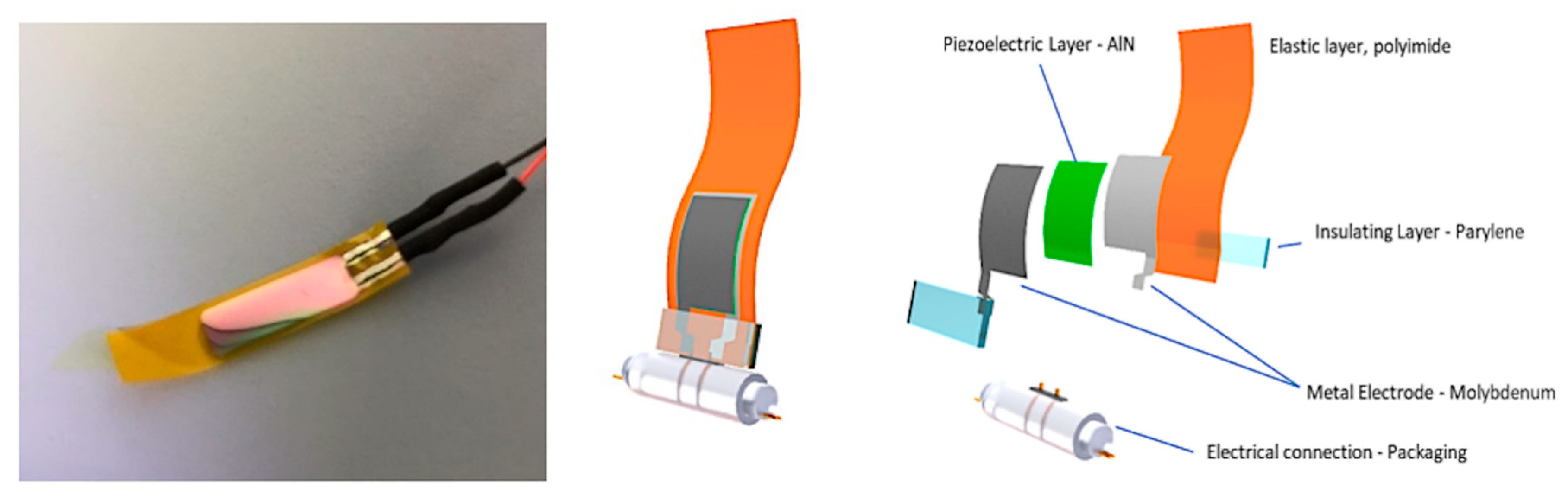
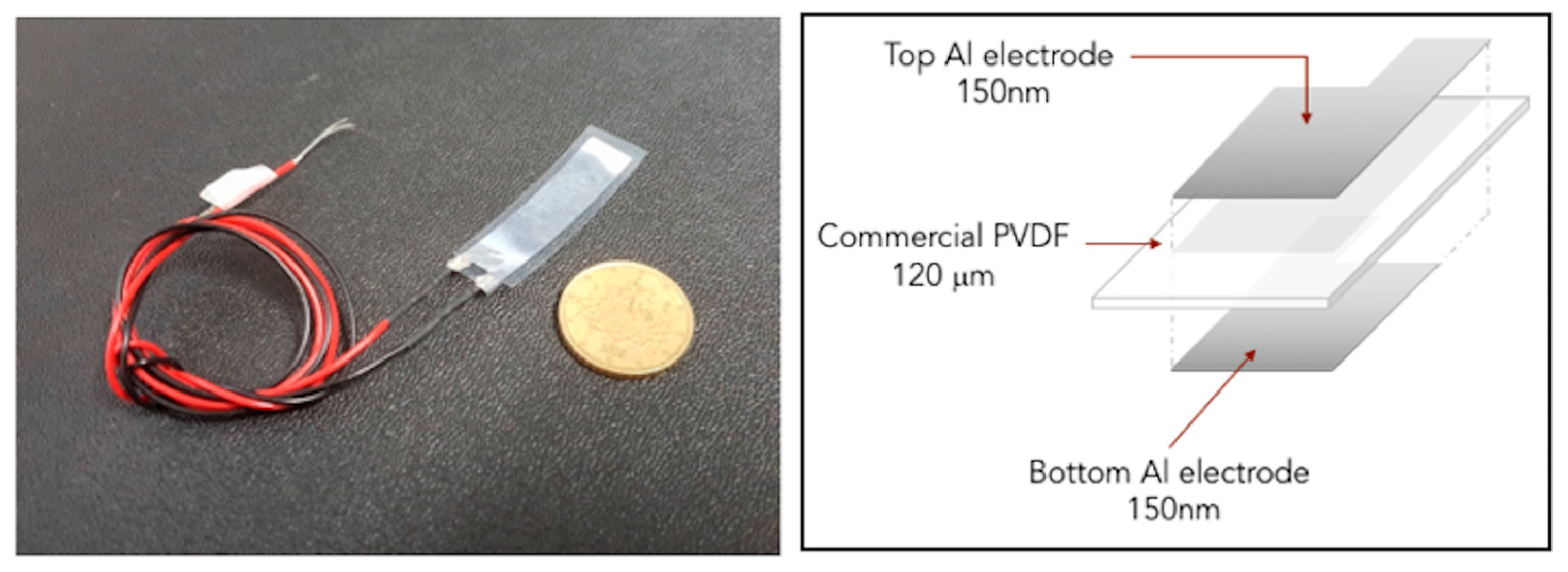
2.2. Fabrication of Flexible Transducers
2.2.1. AlN-Based Transducers
2.2.2. PVDF-Based Transducers
2.3. Preparation of Coatings
2.4. Characterization and Reliability Tests
2.4.1. Surface Characterizations
2.4.2. Exposure to Seawater
2.4.3. Water Absorption Tests
2.4.4. Corrosion Tests
2.4.5. Piezoelectric Generation in the Long-Term Period
2.4.6. Surface Treatments of Parylene Surface
3. Results
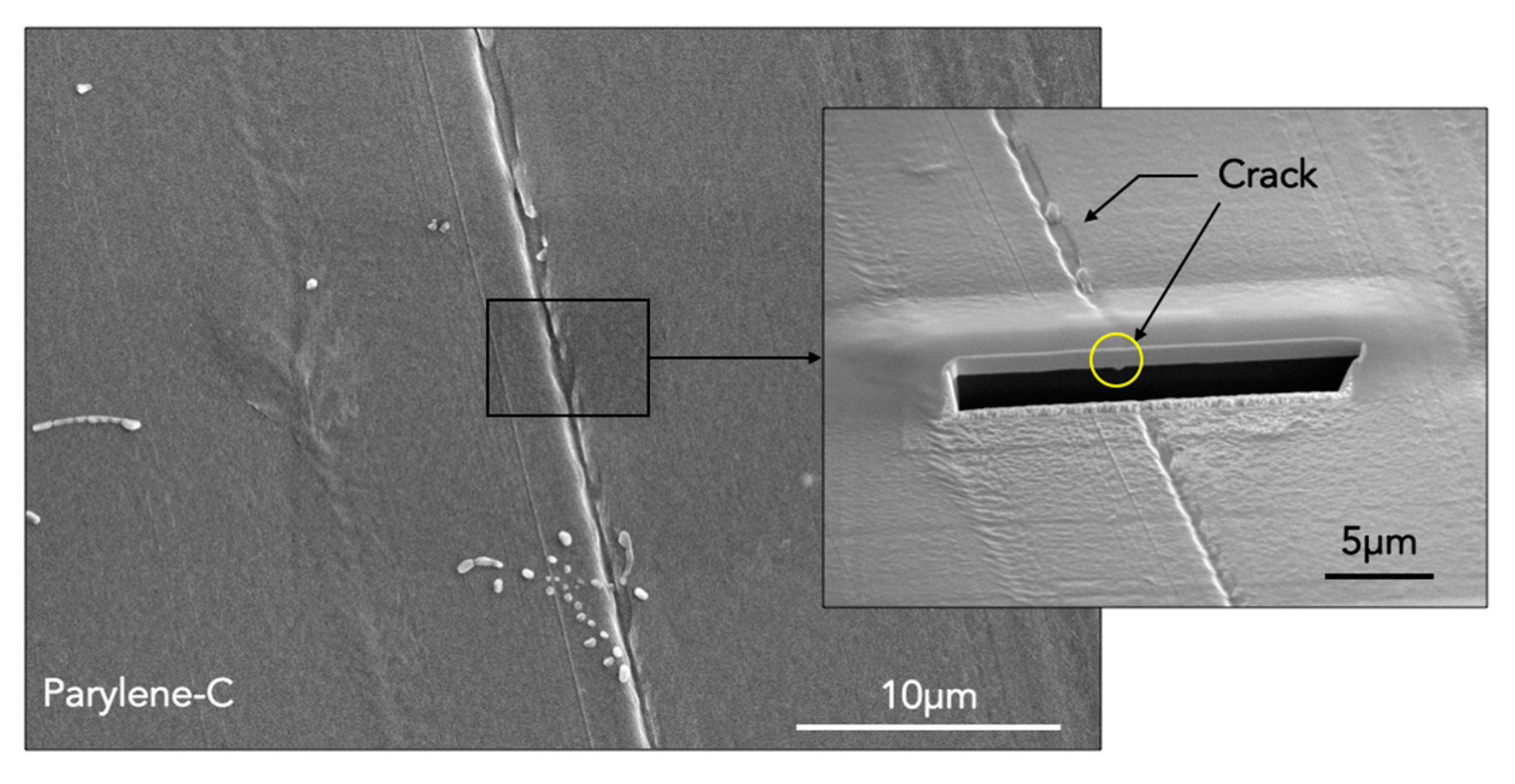
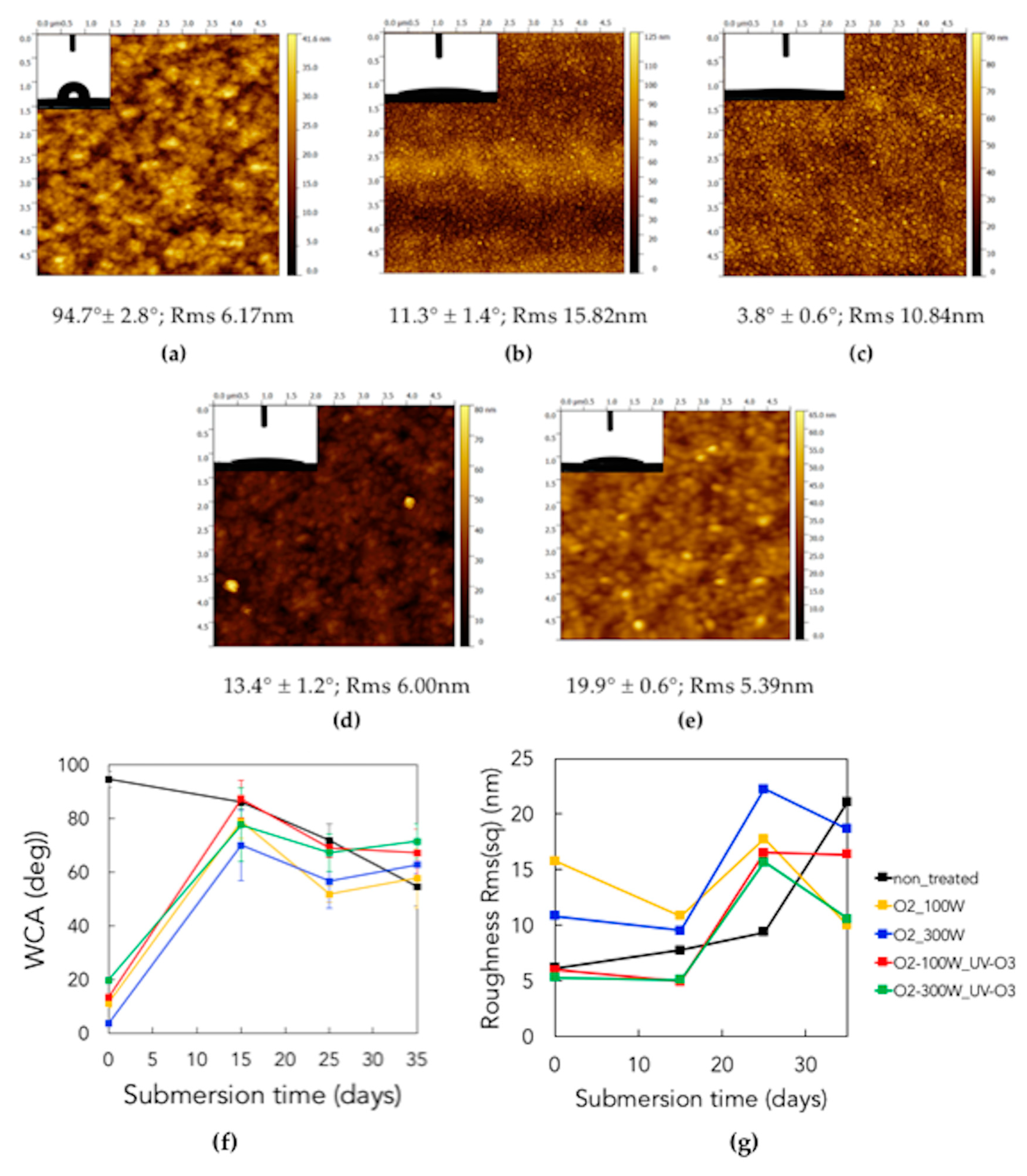
3.1. Surface Characterizations
3.2. Water Absorption Tests
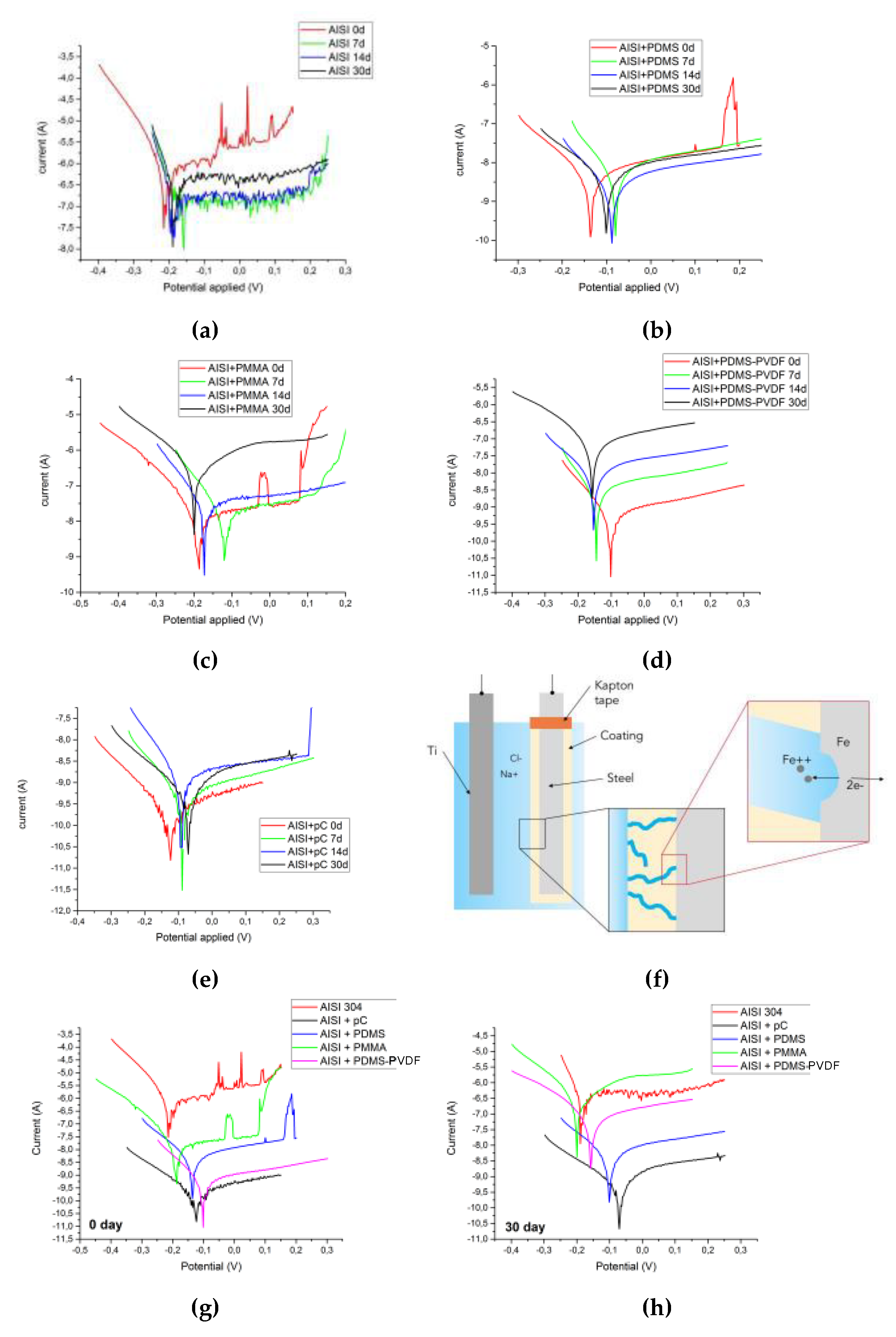
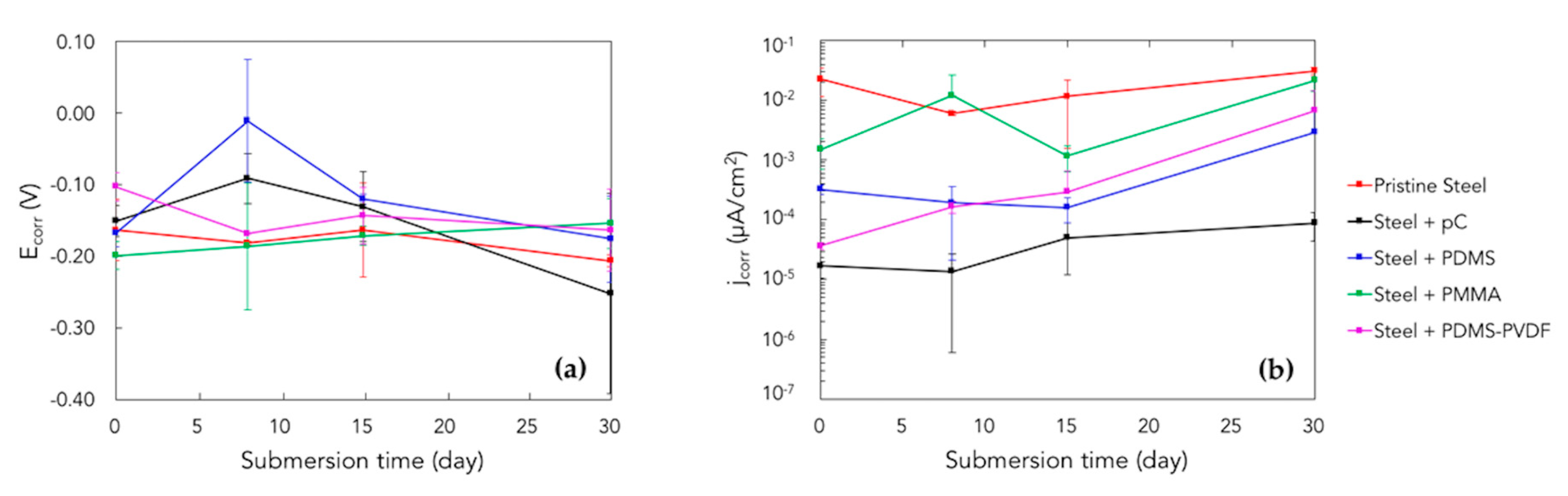
3.3. Corrosion Tests
- At 0 days the best coatings are parylene-C, PDMS and the combination PDMS-PVDF, whilst the worst is PMMA, in terms of insulation and anti-corrosive behavior;
- At 30 days the best coatings are parylene-C and PDMS, whereas PDMS-PVDF gets worse perhaps owing to inhomogeneity; PMMA confirms to be the worst coating.
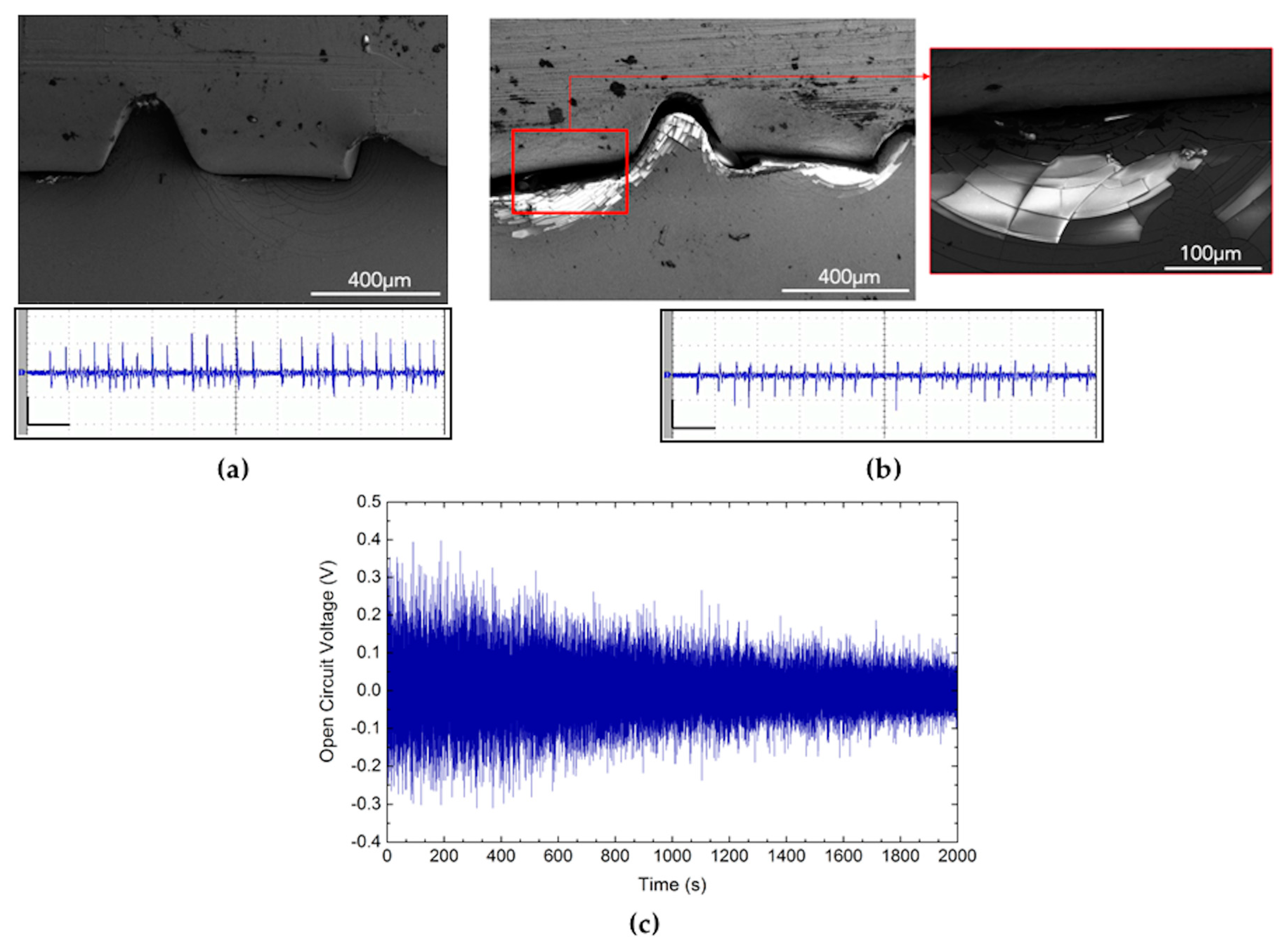
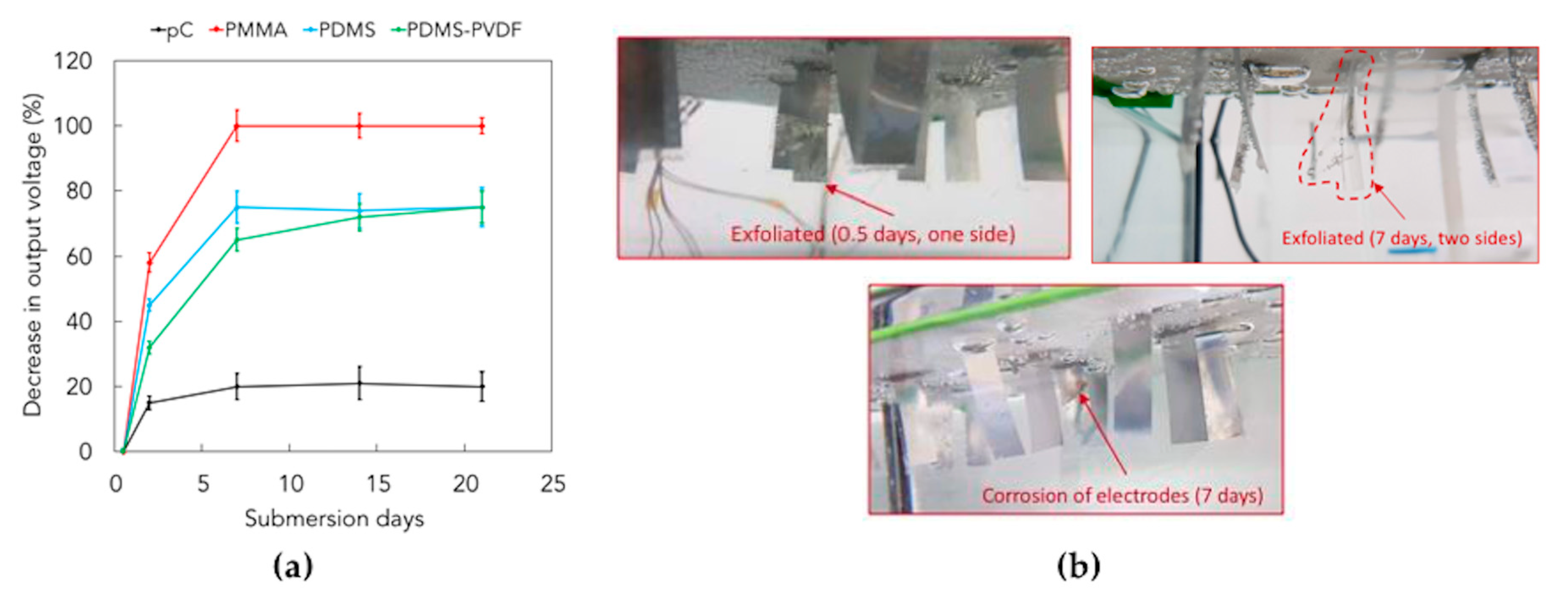
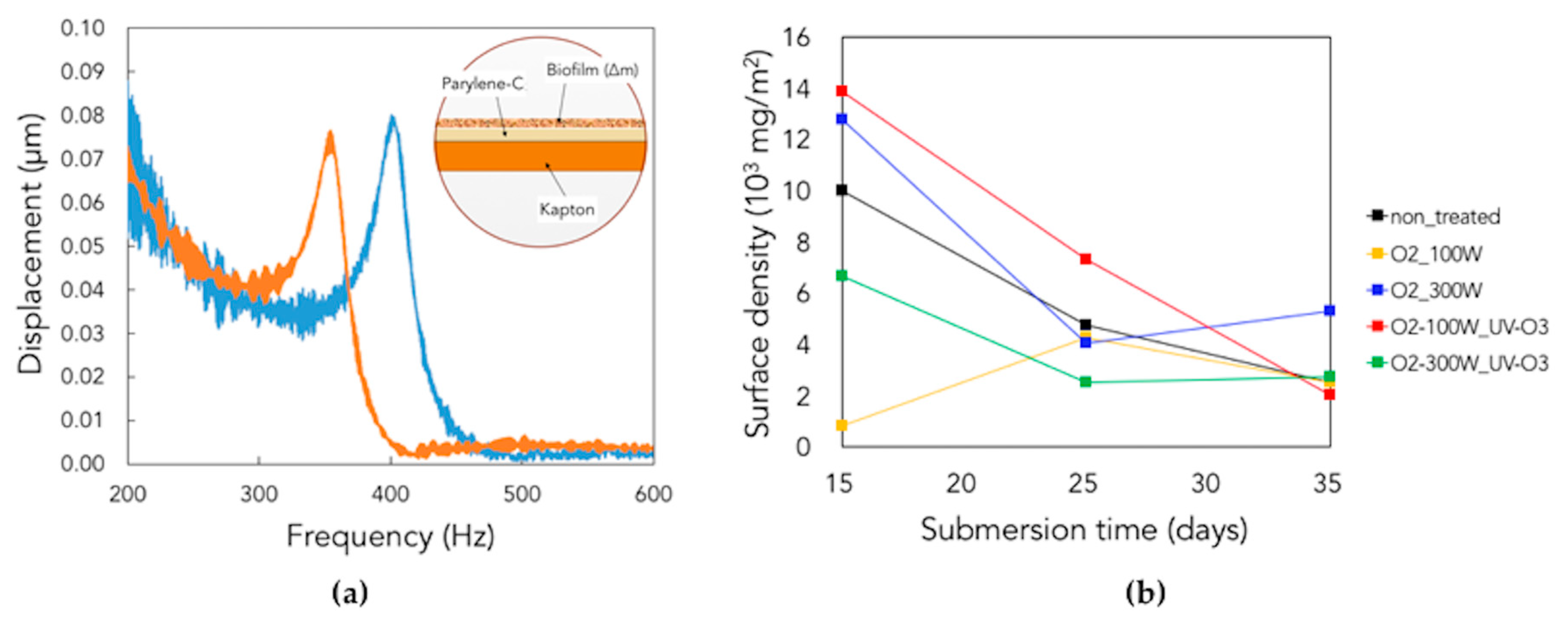
3.4. Piezoelectric Generation in the Long-Term Period
3.5. Observation of Microbial Adhesion on Pristine and Treated Parylene
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sacher, E.; Susko, J.R. Water permeation of polymer films. I. Polyimide. J. Appl. Polym. Sci. 1979, 23, 2355–2364. [Google Scholar] [CrossRef]
- Yian, Z.; Keey, S.L.; Boay, C.G. Effects of seawater exposure on mode II fatigue delamination growth of a woven E-glass/bismaleimide composite. J. Reinf. Plast. Compos. 2015, 35, 138–150. [Google Scholar] [CrossRef]
- Chiou, P.; L Bradley, W. Effects of seawater absorption on fatigue crack development in carbon/epoxy EDT specimens. Composites 1995, 26, 869–876. [Google Scholar] [CrossRef]
- Sørensen, P.A.; Dam-Johansen, K.; Weinell, C.E.; Kiil, S. Cathodic delamination of seawater-immersed anticorrosive coatings: Mapping of parameters affecting the rate. Prog. Org. Coat. 2010, 68, 283–292. [Google Scholar] [CrossRef]
- Morales, J.M.H.; Souriau, J.C.; Simon, G. Corrosion Protection of Silicon Micro Systems with Ultra-Thin Barrier Films for Miniaturized Medical Devices. ECS Trans. 2015, 69, 9–26. [Google Scholar] [CrossRef]
- Traverso, P.; Canepa, E. A review of studies on corrosion of metals and alloys in deep-sea environment. Ocean Eng. 2014, 87, 10–15. [Google Scholar] [CrossRef]
- Mohamed, A.M.A.; Abdullah, A.M.; Younan, N.A. Corrosion behavior of superhydrophobic surfaces: A review. Arab. J. Chem. 2015, 8, 749–765. [Google Scholar] [CrossRef]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef]
- Cao, S.; Wang, J.; Chen, H.; Chen, D. Progress of marine biofouling and antifouling technologies. Chin. Sci. Bull. 2011, 56, 598–612. [Google Scholar] [CrossRef]
- Railkin, A.I. Marine Biofouling: Colonization Processes and Defenses, 1st ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Matsunaga, T.; Nakayama, T.; Wake, H.; Takahashi, M.; Okochi, M.; Nakamura, N. Prevention of marine biofouling using a conductive paint electrode. Biotechnol. Bioeng. 1998, 59, 374–378. [Google Scholar] [CrossRef]
- Bixler, G.D.; Bhushan, B. Biofouling: lessons from nature. Philos. Transact. A Math. Phys. Eng. Sci. 2012, 370, 2381–2417. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C. Microbial Biofouling: Unsolved Problems, Insufficient Approaches, and Possible Solutions. In Biofilm Highlights, 1st ed.; Flemming, H.C., Wingender, J., Szewzyk, U., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 5, pp. 81–109. [Google Scholar]
- Flemming, H.C. Biofouling in water systems--cases, causes and countermeasures. Appl. Microbiol. Biotechnol. 2002, 59, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Algieri, L.; Todaro, M.T.; Guido, F.; Mastronardi, V.; Desmaële, D.; Qualtieri, A.; Giannini, C.; Sibillano, T.; De Vittorio, M. Flexible Piezoelectric Energy-Harvesting Exploiting Biocompatible AlN Thin Films Grown onto Spin-Coated Polyimide Layers. ACS Appl. Energy Mater. 2018, 1, 5203–5210. [Google Scholar] [CrossRef]
- Petroni, S.; Rizzi, F.; Guido, F.; Cannavale, A.; Donateo, T.; Ingrosso, F.; De Vittorio, M. Flexible AlN flags for efficient wind energy harvesting at ultralow cut-in wind speed. RSC Adv. 2015, 5, 14047–14052. [Google Scholar] [CrossRef]
- Mariello, M.; Guido, F.; Mastronardi, V.M.; Todaro, M.T.; Desmaële, D.; De Vittorio, M. Nanogenerators for harvesting mechanical energy conveyed by liquids. Nano Energy 2019, 57, 141–156. [Google Scholar] [CrossRef]
- Abels, C.; Mastronardi, V.M.; Guido, F.; Dattoma, T.; Qualtieri, A.; Megill, W.M.; De Vittorio, M.; Rizzi, F. Nitride-Based Materials for Flexible MEMS Tactile and Flow Sensors in Robotics. Sensors 2017, 17, 1080. [Google Scholar] [CrossRef]
- Applerot, G.; Abu-Mukh, R.; Irzh, A.; Charmet, J.; Keppner, H.; Laux, E.; Guibert, G.; Gedanken, A. Decorating Parylene-Coated Glass with ZnO Nanoparticles for Antibacterial Applications: A Comparative Study of Sonochemical, Microwave, and Microwave-Plasma Coating Routes. ACS Appl. Mater. Interfaces 2010, 2, 1052–1059. [Google Scholar] [CrossRef]
- Baek, Y.; Kang, J.; Theato, P.; Yoon, J. Measuring hydrophilicity of RO membranes by contact angles via sessile drop and captive bubble method: A comparative study. Desalination 2012, 303, 23–28. [Google Scholar] [CrossRef]
- Jbaily, A.; Yeung, R.W. Piezoelectric devices for ocean energy: a brief survey. J. Ocean Eng. Mar. Energy 2014, 1, 101–118. [Google Scholar] [CrossRef]
- Wang, Z.L.; Jiang, T.; Xu, L. Toward the blue energy dream by triboelectric nanogenerator networks. Nano Energy 2017, 39, 9–23. [Google Scholar] [CrossRef]
- Mukhopadhyay, R. When PDMS isn’t the best. Anal. Chem. 2007, 79, 3248–3253. [Google Scholar] [CrossRef] [PubMed]
- Koschwanez, J.H.; Carlson, R.H.; Meldrum, D.R. Thin PDMS films using long spin times or tert-butyl alcohol as a solvent. PloS One 2009, 4, e4572. [Google Scholar] [CrossRef] [PubMed]
- Suleman, M.S.; Lau, K.K.; Yeong, Y.F. Characterization and Performance Evaluation of PDMS/PSF Membrane for CO2/CH4 Separation under the Effect of Swelling. Procedia Eng. 2016, 148, 176–183. [Google Scholar] [CrossRef][Green Version]
- Choonee, K.; Syms, R.R.A.; Ahmad, M.M.; Zou, H. Post processing of microstructures by PDMS spray deposition. Sens. Actuators Phys. 2009, 155, 253–262. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Song, X.F.; Cui, L.Y.; Qi, Y.H. Synthesis of Polydimethylsiloxane-Modified Polyurethane and the Structure and Properties of Its Antifouling Coatings. Coatings 2018, 8, 157. [Google Scholar] [CrossRef]
- Fischer, S.C.L.; Levy, O.; Kroner, E.; Hensel, R.; Karp, J.M.; Arzt, E. Bioinspired polydimethylsiloxane-based composites with high shear resistance against wet tissue. J. Mech. Behav. Biomed. Mater. 2016, 61, 87–95. [Google Scholar] [CrossRef]
- Coan, T.; Barroso, G.S.; Motz, G.; Bolzán, A.; Machado, R. a. F. Preparation of PMMA/hBN composite coatings for metal surface protection. Mater. Res. 2013, 16, 1366–1372. [Google Scholar] [CrossRef]
- Cui, Z.; Drioli, E.; Lee, Y.M. Recent progress in fluoropolymers for membranes. Prog. Polym. Sci. 2014, 39, 164–198. [Google Scholar] [CrossRef]
- Elkasabi, Y.; Chen, H.-Y.; Lahann, J. Multipotent Polymer Coatings Based on Chemical Vapor Deposition Copolymerization. Adv. Mater. 2006, 18, 1521–1526. [Google Scholar] [CrossRef]
- Fortin, J.B.; Lu, T.M. Chemical Vapor Deposition Polymerization - The Growth and Properties of Parylene Thin Films, 1st ed.; Kluwer Academic Publishers: Boston, MA, USA, 2003. [Google Scholar]
- Fortin, J.B.; Lu, T.M. A Model for the Chemical Vapor Deposition of Poly(para-xylylene) (Parylene) Thin Films. Chem. Mater. 2002, 14, 1945–1949. [Google Scholar] [CrossRef]
- Chen, T.N.; Wuu, D.S.; Wu, C.C.; Chiang, C.C.; Chen, Y.P.; Horng, R.H. Improvements of Permeation Barrier Coatings Using Encapsulated Parylene Interlayers for Flexible Electronic Applications. Plasma Process. Polym. 2007, 4, 180–185. [Google Scholar] [CrossRef]
- Cieślik, M.; Engvall, K.; Pan, J.; Kotarba, A. Silane–parylene coating for improving corrosion resistance of stainless steel 316L implant material. Corros. Sci. 2011, 53, 296–301. [Google Scholar] [CrossRef]
- Golda-Cepa, M.; Brzychczy-Wloch, M.; Engvall, K.; Aminlashgari, N.; Hakkarainen, M.; Kotarba, A. Microbiological investigations of oxygen plasma treated parylene C surfaces for metal implant coating. Mater. Sci. Eng. C 2015, 52, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.N. Current status of environmental barrier coatings for Si-Based ceramics. Surf. Coat. Technol. 2000, 133–134, 1–7. [Google Scholar] [CrossRef]
- Saini, A.K.; Das, D.; Pathak, M.K. Thermal Barrier Coatings -Applications, Stability and Longevity Aspects. Procedia Eng. 2012, 38, 3173–3179. [Google Scholar] [CrossRef]
- Yu, J.; Ruengkajorn, K.; Crivoi, D.G.; Chen, C.; Buffet, J.C.; O’Hare, D. High gas barrier coating using non-toxic nanosheet dispersions for flexible food packaging film. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Lewis, J.S.; Weaver, M.S. Thin-film permeation-barrier technology for flexible organic light-emitting devices. IEEE J. Sel. Top. Quantum Electron. 2004, 10, 45–57. [Google Scholar] [CrossRef]
- Fredj, N.; Cohendoz, S.; Feaugas, X.; Touzain, S. Ageing of marine coating in natural and artificial seawater under mechanical stresses. Appl. Electrochem. Tech. Org. Coat. 2012, 74, 391–399. [Google Scholar] [CrossRef]
- Deyab, M.A.; Mohamed, N.H.; Moustafa, Y.M. Corrosion protection of petroleum pipelines in NaCl solution by microcrystalline waxes from waste materials: Electrochemical studies. Corros. Sci. 2017, 122, 74–79. [Google Scholar] [CrossRef]
- Deyab, M.A. Effect of carbon nano-tubes on the corrosion resistance of alkyd coating immersed in sodium chloride solution. Prog. Org. Coat. 2015, 85, 146–150. [Google Scholar] [CrossRef]
- Deyab, M.A.; Eddahaoui, K.; Essehli, R.; Benmokhtar, S.; Rhadfi, T.; De Riccardis, A.; Mele, G. Influence of newly synthesized titanium phosphates on the corrosion protection properties of alkyd coating. J. Mol. Liq. 2016, 216, 699–703. [Google Scholar] [CrossRef]
- Deyab, M.A.; Mele, G.; Al-Sabagh, A.M.; Bloise, E.; Lomonaco, D.; Mazzetto, S.E.; Clemente, C.D.S. Synthesis and characteristics of alkyd resin/M-Porphyrins nanocomposite for corrosion protection application. Prog. Org. Coat. 2017, 105, 286–290. [Google Scholar] [CrossRef]
- Li, W.; Rodger, D.; Menon, P.; Tai, Y.-C. Corrosion Behavior of Parylene-Metal-Parylene Thin Films in Saline. ECS Trans. 2008, 11, 1–6. [Google Scholar]
- Davies, P.; Evrard, G. Accelerated ageing of polyurethanes for marine applications. Polym. Degrad. Stab. 2007, 92, 1455–1464. [Google Scholar] [CrossRef]
- Pigliacelli, C.; D’Elicio, A.; Milani, R.; Terraneo, G.; Resnati, G.; Baldelli Bombelli, F.; Metrangolo, P. Hydrophobin-stabilized dispersions of PVDF nanoparticles in water. J. Fluor. Chem. 2015, 177, 62–69. [Google Scholar] [CrossRef]
- Kang, G.; Cao, Y. Application and modification of poly(vinylidene fluoride) (PVDF) membranes – A review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Calcagnile, P.; Dattoma, T.; Scarpa, E.; Qualtieri, A.; Blasi, L.; Vittorio, M.D.; Rizzi, F. A 2D approach to surface-tension-confined fluidics on parylene C. RSC Adv. 2017, 7, 15964–15970. [Google Scholar] [CrossRef]
- Calcagnile, P.; Blasi, L.; Rizzi, F.; Qualtieri, A.; Athanassiou, A.; Gogolides, E.; De Vittorio, M. Parylene C Surface Functionalization and Patterning with pH-Responsive Microgels. ACS Appl. Mater. Interfaces 2014, 6, 15708–15715. [Google Scholar] [CrossRef]
- Mariello, M.; Guido, F.; Mastronardi, V.M.; De Donato, F.; Salbini, M.; Brunetti, V.; Qualtieri, A.; Rizzi, F.; De Vittorio, M. Captive-air-bubble aerophobicity measurements of antibiofouling coatings for underwater MEMS devices. Nanomater. Nanotechnol. 2019, 9, 1847980419862075. [Google Scholar] [CrossRef]
- Gonon, P.; Hong, T.; Lesaint, O.; Bourdelais, S.; Debruyne, H. Influence of high levels of water absorption on the resistivity and dielectric permittivity of epoxy composites. Polym. Test. 2005, 24, 799–804. [Google Scholar] [CrossRef]
- Cotinaud, M.; Bonniau, P.; Bunsell, A. The effect of water absorption on the electrical properties of glass-fibre reinforced epoxy composites. J. Mater. Sci. 1982, 17, 867–877. [Google Scholar] [CrossRef]
- Maxwell, I.; Pethrick, R. Dielectric studies of water in epoxy resins. J. Appl. Polym. Sci. 1983, 28, 2363–2379. [Google Scholar] [CrossRef]
- Efros, A.; Shklovskii, B. Critical Behavior of Conductivity and Dielectric-Constant Near Metal-Non-Metal Transition Threshold. Phys. Status Solidi B 1976, 76, 475–485. [Google Scholar] [CrossRef]
- Maffezzoli, A.M.; Peterson, L.; Seferis, J.C.; Kenny, J.; Nicolais, L. Dielectric characterization of water sorption in epoxy resin matrices. Polym. Eng. Sci. 1993, 33, 75–82. [Google Scholar] [CrossRef]
- Videm, K. Phenomena disturbing electrochemical corrosion rate measurements for steel in alkaline environments. Electrochim. Acta 2001, 46, 3895–3903. [Google Scholar] [CrossRef]
- Deyab, M.A.; Keera, S.T.; El Sabagh, S.M. Chlorhexidine digluconate as corrosion inhibitor for carbon steel dissolution in emulsified diesel fuel. Corros. Sci. 2011, 53, 2592–2597. [Google Scholar] [CrossRef]
- Deyab, M.A. Application of nonionic surfactant as a corrosion inhibitor for zinc in alkaline battery solution. J. Power Sources 2015, 292, 66–71. [Google Scholar] [CrossRef]
- Deyab, M.A. Hydroxyethyl cellulose as efficient organic inhibitor of zinc–carbon battery corrosion in ammonium chloride solution: Electrochemical and surface morphology studies. J. Power Sources 2015, 280, 190–194. [Google Scholar] [CrossRef]
- Gaynor, J.F. A heterogeneous model for the chemical vapor polymerization of Poly-P-Xylylenes. Electrochem. Soc. Proc. 1997, 97-98, 176–185. [Google Scholar]
- Rogojevic, S.; Moore, J.A.; Gill, W.N. Modeling vapor deposition of low-K polymers: Parylene and polynaphthalene. J. Vac. Sci. Technol. Vac. Surf. Films 1999, 17, 266–274. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Chatterjee, S.; Biswas, N.; Datta, A.; Maiti, P.K. Periodicities in the roughness and biofilm growth on glass substrate with etching time: Hydrofluoric acid etchant. PLoS ONE 2019, 14, e0214192. [Google Scholar] [CrossRef] [PubMed]


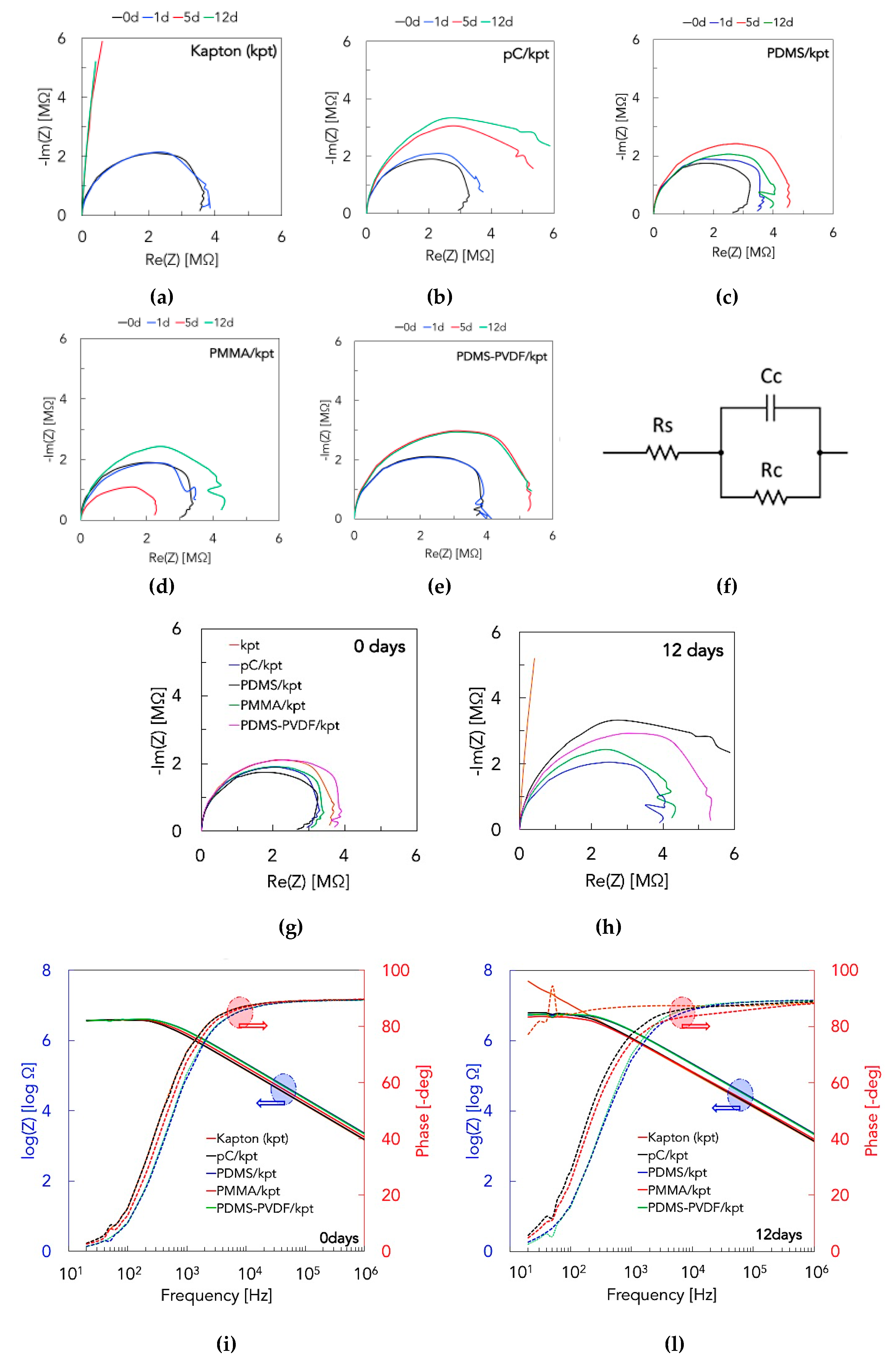

| Submersion Days | Samples | Rc [106 Ω] | Cc [10−10 F] |
|---|---|---|---|
| 0 | Kapton (kpt) | 3.5276 | 1.0910 |
| pC/kpt | 3.9573 | 1.0650 | |
| PDMS/kpt | 3.7389 | 0.7080 | |
| PMMA/kpt | 3.6062 | 0.8885 | |
| PDMS-PVDF/kpt | 3.7677 | 0.7325 | |
| 12 | Kapton (kpt) | - (low) | 1.3430 |
| pC/kpt | 6.5529 | 1.2230 | |
| PDMS/kpt | 4.0184 | 0.7404 | |
| PMMA/kpt | 4.3604 | 1.1510 | |
| PDMS-PVDF/kpt | 5.5225 | 0.7552 |
| Submersion Days | Samples | Ecorr (vs. SCE) [V] | jcorr [µAcm−2] | ηj [%] |
|---|---|---|---|---|
| 0 | Pristine AISI314 steel | −0.1635 ± 0.0426 | (2.27 ± 1.14) × 10−2 | - |
| pC | −0.1511 ± 0.0217 | (1.70 ± 0.65) × 10−5 | 99.9 | |
| PDMS | −0.1677 ± 0.0192 | (3.21 ± 0.58) × 10−4 | 98.6 | |
| PMMA | −0.1990 ± 0.0191 | (1.49 ± 0.80) × 10−3 | 93.4 | |
| PDMS-PVDF | −0.1030 ± 0.0202 | (3.67 ± 0.45) × 10−5 | 99.8 | |
| 30 | Pristine AISI314 steel | −0.2065 ± 0.0084 | (3.10 ± 0.47) × 10−2 | - |
| pC | −0.2522 ± 0.1398 | (8.84 ± 4.37) × 10−5 | 99.7 | |
| PDMS | −0.1759 ± 0.0606 | (2.90 ± 4.43) × 10−2 | 90.7 | |
| PMMA | −0.1542 ± 0.0349 | (2.15 ± 0.74) × 10−2 | 30.6 | |
| PDMS-PVDF | −0.1635 ± 0.0572 | (6.75 ± 7.96) × 10−2 | 78.2 |
| Submersion Days | Samples | Length (mm) | Width (mm) | Exp fres (Hz) | FEM fres (Hz) | Biofilm Mass (10−5 g) |
|---|---|---|---|---|---|---|
| 0 | - | 5.5 | 3.0 | 230.5 | 232 | - |
| 15 | NT | 6.0 | 3.0 | 201.25 | 201.2 | 18 |
| O2_100W | 6.0 | 3.0 | 225 | 225.5 | 1.5 | |
| O2_300W | 6.0 | 3.0 | 208.28 | 208.4 | 23 | |
| O2-100W_UV-O3 | 6.0 | 3.0 | 196.72 | 196.8 | 25 | |
| O2-300W_UV-O3 | 6.0 | 3.0 | 226.25 | 226.1 | 12 | |
| 25 | NT | 4.5 | 2.2 | 423.125 | 423.47 | 4.75 |
| O2_100W | 4.5 | 2.2 | 400.94 | 400.17 | 4.25 | |
| O2_300W | 4.5 | 2.2 | 398.75 | 398.5 | 4.00 | |
| O2-100W_UV-O3 | 4.5 | 2.2 | 354.69 | 354.9 | 7.25 | |
| O2-300W_UV-O3 | 4.5 | 2.2 | 423.44 | 424.1 | 2.50 | |
| 35 | NT | 4.5 | 2.2 | 408.75 | 408.9 | 2.50 |
| O2_100W | 4.5 | 2.2 | 430.94 | 431.1 | 2.50 | |
| O2_300W | 4.5 | 2.2 | 406.41 | 406.8 | 5.25 | |
| O2-100W_UV-O3 | 4.5 | 2.2 | 407.81 | 407.8 | 2.00 | |
| O2-300W_UV-O3 | 4.5 | 2.2 | 427.03 | 427 | 2.75 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariello, M.; Guido, F.; Mastronardi, V.M.; Giannuzzi, R.; Algieri, L.; Qualteri, A.; Maffezzoli, A.; De Vittorio, M. Reliability of Protective Coatings for Flexible Piezoelectric Transducers in Aqueous Environments. Micromachines 2019, 10, 739. https://doi.org/10.3390/mi10110739
Mariello M, Guido F, Mastronardi VM, Giannuzzi R, Algieri L, Qualteri A, Maffezzoli A, De Vittorio M. Reliability of Protective Coatings for Flexible Piezoelectric Transducers in Aqueous Environments. Micromachines. 2019; 10(11):739. https://doi.org/10.3390/mi10110739
Chicago/Turabian StyleMariello, Massimo, Francesco Guido, Vincenzo Mariano Mastronardi, Roberto Giannuzzi, Luciana Algieri, Antonio Qualteri, Alfonso Maffezzoli, and Massimo De Vittorio. 2019. "Reliability of Protective Coatings for Flexible Piezoelectric Transducers in Aqueous Environments" Micromachines 10, no. 11: 739. https://doi.org/10.3390/mi10110739
APA StyleMariello, M., Guido, F., Mastronardi, V. M., Giannuzzi, R., Algieri, L., Qualteri, A., Maffezzoli, A., & De Vittorio, M. (2019). Reliability of Protective Coatings for Flexible Piezoelectric Transducers in Aqueous Environments. Micromachines, 10(11), 739. https://doi.org/10.3390/mi10110739







