Green Phosphors Based on 9,10-bis((4-((3,7-dimethyloctyl)oxy) phenyl) ethynyl) Anthracene for LED
Abstract
1. Introduction
2. Experimental
2.1. General
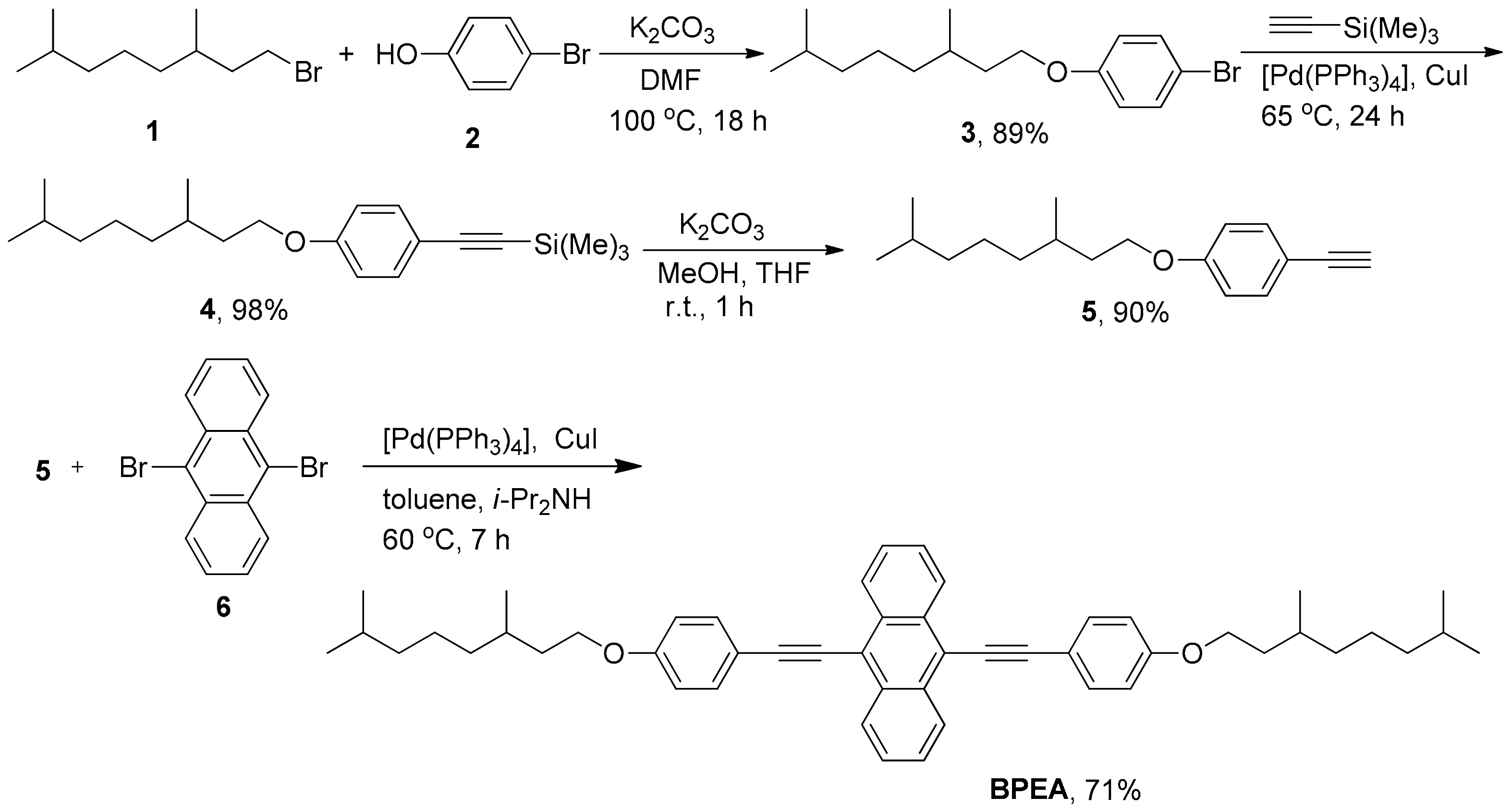
2.2. Synthesis
2.2.1. Synthesis of Compound 3
2.2.2. Synthesis of Compound 4
2.2.3. Synthesis of Compound 5
2.2.4. Synthesis of BPEA
2.3. Fabrication of LED
3. Results and Discussion
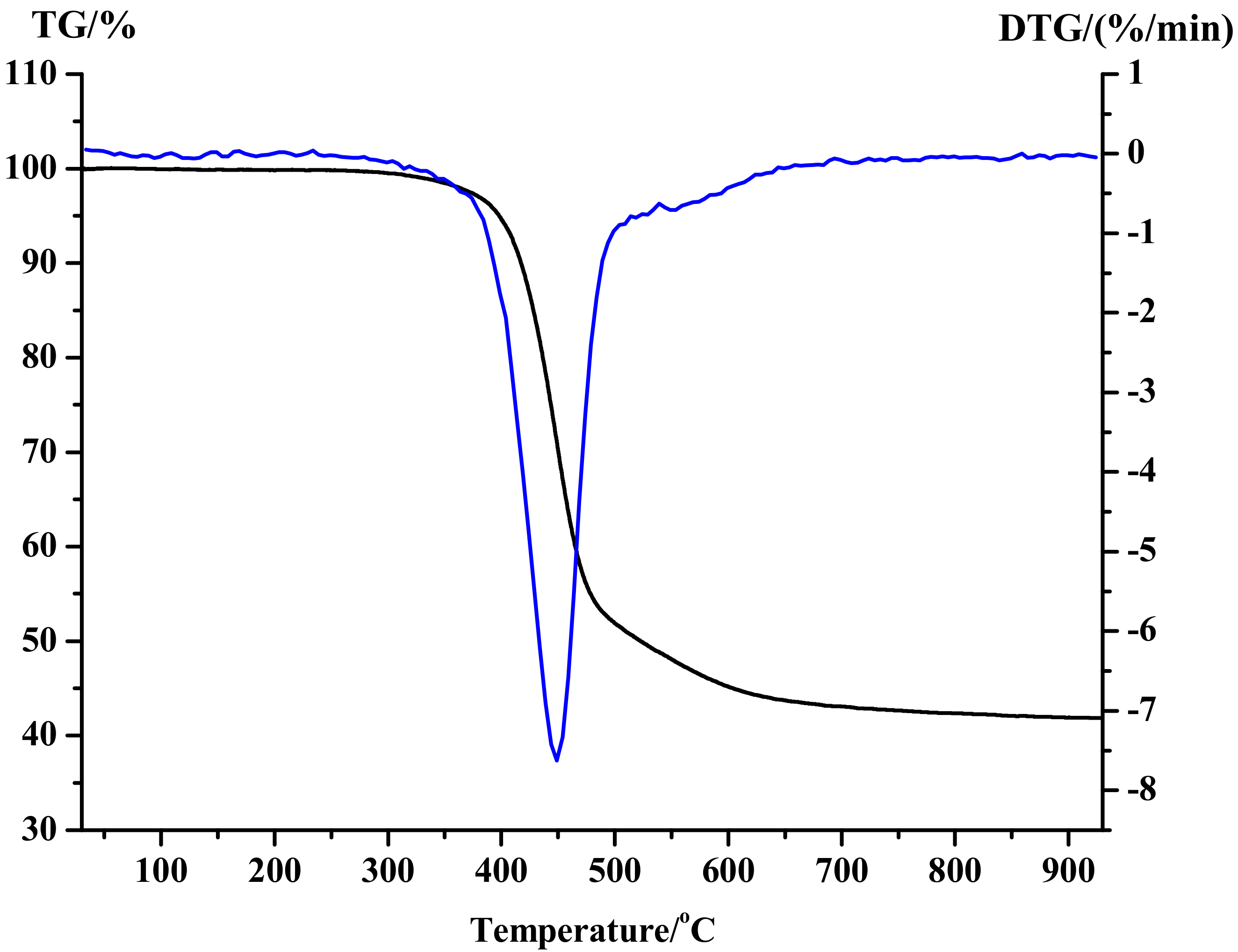
3.1. Thermal Stability
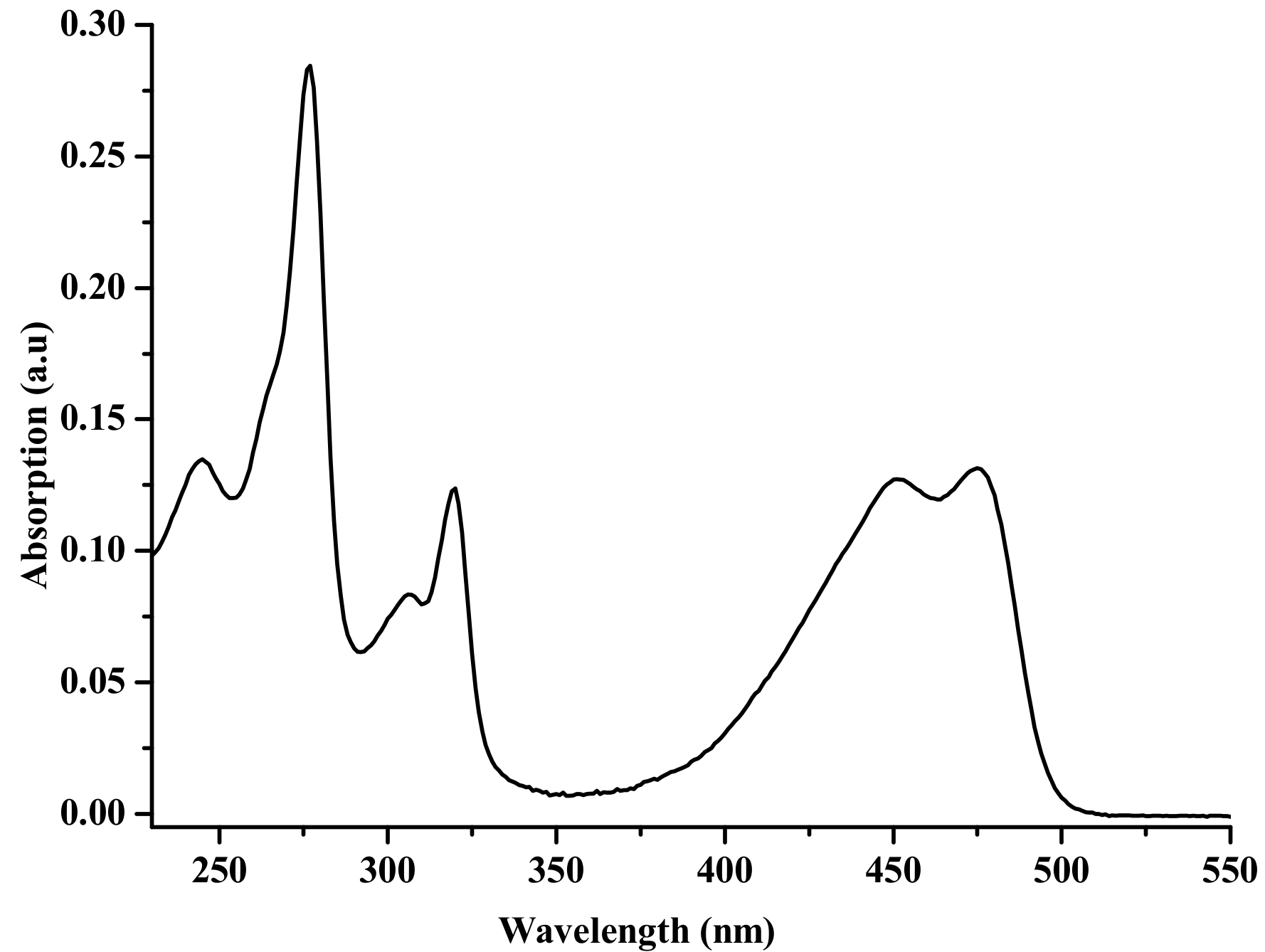
3.2. UV–vis Absorption Spectrum
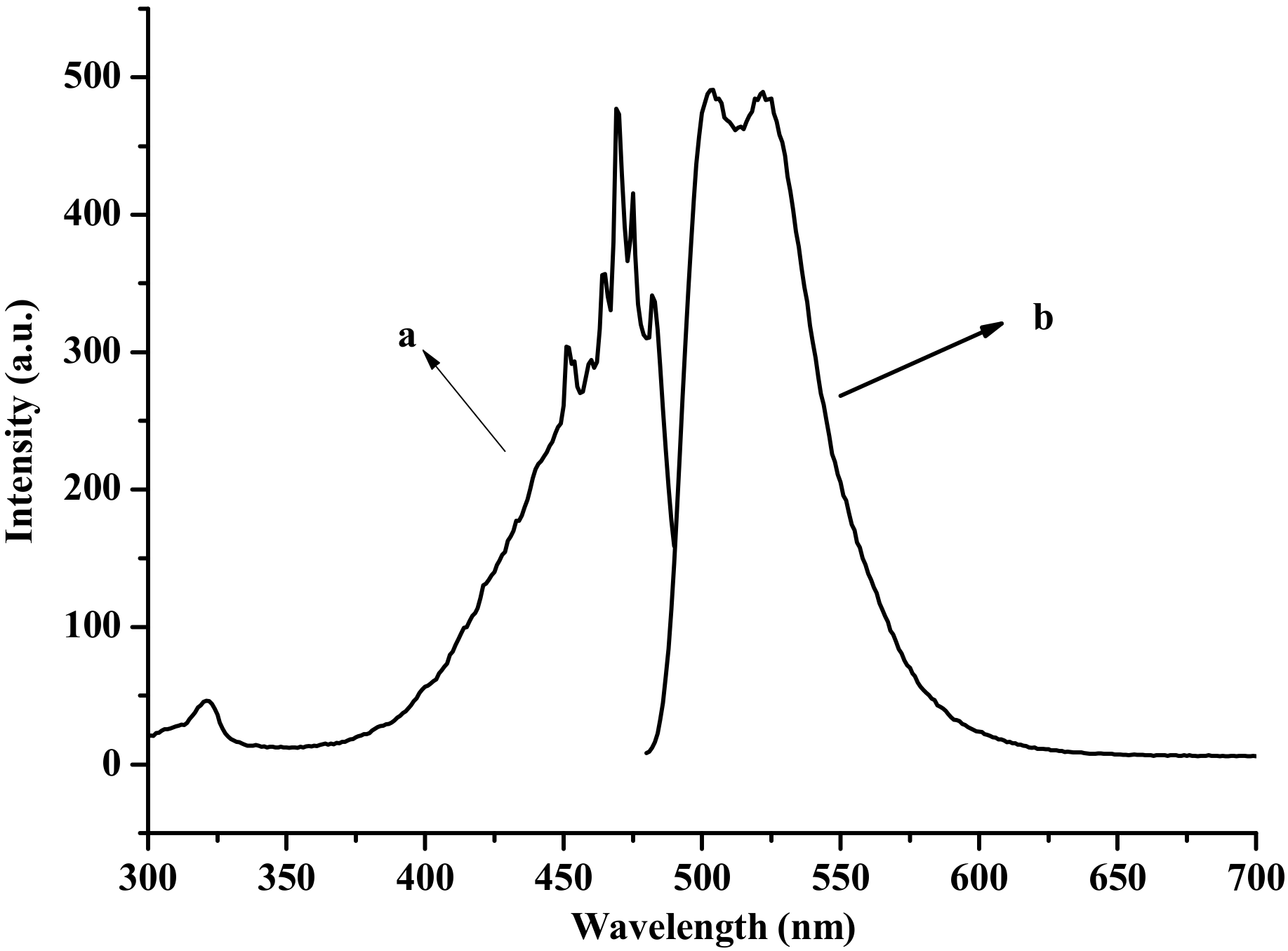
3.3. Photoluminescence Properties
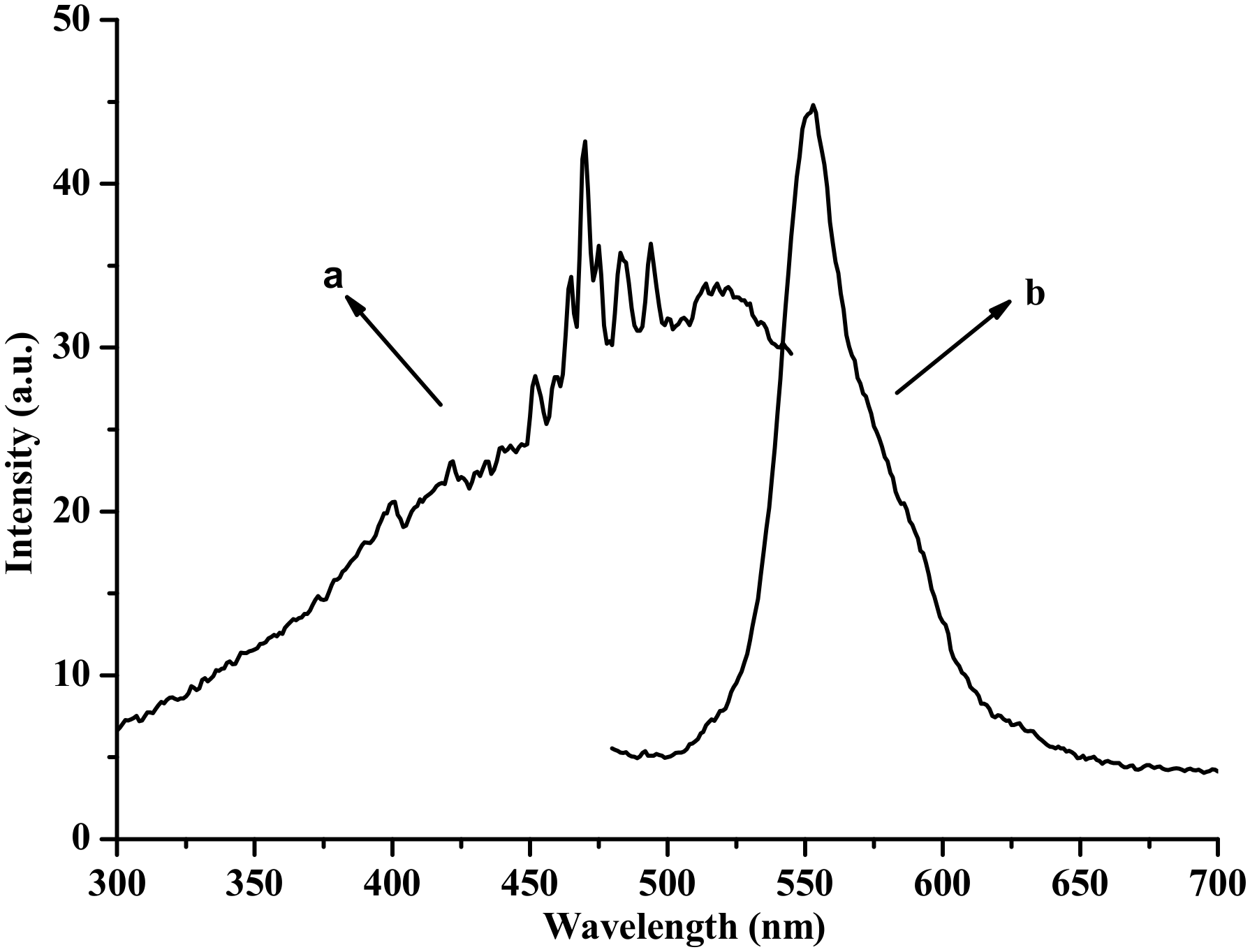
3.4. Emission Spectra of the Fabricated LED
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tan, Z.-K.; Moghaddam, R.S.; Lai, M.L.; Docampo, P.; Higler, R.; Deschler, F.; Price, M.; Sadhanala, A.; Pazos, L.M.; Credgington, D.; et al. Bright light-emitting diodes based on organometal halide perovskite. Nat. Nanotech. 2014, 9, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; Ji, E.K.; Jeong, B.W.; Jung, M.K.; Kim, E.Y.; Yoon, D.H. High power laser-driven ceramic phosphor plate for outstanding efficient white light conversion in application of automotive lighting. Sci. Rep. 2016, 6, 31206. [Google Scholar] [CrossRef] [PubMed]
- Nemitz, W.; Fulmek, P.; Nicolics, J.; Reil, F.; Wenzl, F.P. On the determination of the temperature distribution within the color conversion elements of phosphor converted LEDs. Sci. Rep. 2017, 7, 9964. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Liu, Q. Progress in discovery and structural design of color conversion phosphors for LEDs. Prog. Mater Sci. 2016, 84, 59–117. [Google Scholar] [CrossRef]
- Lorbeer, C.; Mudring, A.-V. White-Light-Emitting Single Phosphors via Triply Doped LaF3 Nanoparticles. J. Phys. Chem. C 2013, 117, 12229–12238. [Google Scholar] [CrossRef]
- Li, Y.; Gecevicius, M.; Qiu, J. Long persistent phosphors—from fundamentals to applications. Chem. Soc. Rev. 2016, 45, 2090–2136. [Google Scholar] [CrossRef]
- Xia, Z.; Meijerink, A. Ce3+-Doped garnet phosphors: Composition modification, luminescence properties and applications. Chem. Soc. Rev. 2017, 46, 275–299. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Liu, B.; Zhang, J.; Zhao, Z.; Yang, Z.; Wen, Y.; Wang, Y. Warm-white-light emission from Eu2+/Mn2+ -coactivated NaSrPO4 phosphor through energy transfer. J. Phys. Chem. Solids 2013, 74, 175–180. [Google Scholar] [CrossRef]
- Shchekin, O.B.; Schmidt, P.J.; Jin, F.; Lawrence, N.; Vampola, K.J.; Bechtel, H.; Chamberlin, D.R.; Mueller-Mach, R.; Mueller, G.O. Excitation dependent quenching of luminescence in LED phosphors. Phys. Status Solidi RRL 2016, 10, 310–314. [Google Scholar] [CrossRef]
- Wang, R.; Wei, X.; Qin, L.; Luo, Z.; Liang, C.; Tan, G. Red Light-Emitting Diode Based on Blue InGaN Chip with CdTex S(1 − x) Quantum Dots. Nanoscale Res. Lett. 2017, 12, 59. [Google Scholar] [CrossRef][Green Version]
- Schimpke, T.; Mandl, M.; Stoll, I.; Pohl-Klein, B.; Bichler, D.; Zwaschka, F.; Strube-Knyrim, J.; Huckenbeck, B.; Max, B.; Müller, M.; et al. Phosphor-converted white light from blue-emitting InGaN microrod LEDs. Phys. Status Solidi 2016, 213, 1577–1584. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, H.; Lin, Y.; Yang, M.; Li, G.; Xu, B. A new structure of p-GaN/InGaN heterojunction to enhance hole injection for blue GaN-based LEDs. J. Phys. D Appl. Phys. 2016, 49, 285106. [Google Scholar] [CrossRef]
- Shao, G.; Yu, H.; Zhang, N.; He, Y.; Feng, K.; Yang, X.; Cao, R.; Gong, M. Synthesis and photophysical properties of europium(iii)–β-diketonate complexes applied in LEDs. Phys. Chem. Chem. Phys. 2014, 16, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Ng, T.K.; ElAfandy, R.T.; Prabaswara, A.; Consiglio, G.B.; Ajia, I.A.; Roqan, I.S.; Janjua, B.; Shen, C.; Eid, J.; et al. Droop-Free, Reliable, and High-Power InGaN/GaN Nanowire Light-Emitting Diodes for Monolithic Metal-Optoelectronics. Nano Lett. 2016, 16, 4616–4623. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.H.; Goswami, S.; Evans, D.G.; Schanze, K.S.; Whitten, D.G. Photochemistry of a Model Cationic p-Phenylene Ethynylene in Water. J. Phys. Chem. Lett. 2012, 3, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Chen, H.F.; Batagoda, T.; Coburn, C.; Djurovich, P.I.; Thompson, M.E.; Forrest, S.R. Deep blue phosphorescent organic light-emitting diodes with very high brightness and efficiency. Nat. Mater. 2016, 15, 92–98. [Google Scholar] [CrossRef]
- Miki, K.; Fujita, M.; Inoue, Y.; Senda, Y.; Kowada, T.; Ohe, K. Synthesis of Strained Pyridine-Containing Cyclyne via Reductive Aromatization. J. Org. Chem. 2010, 75, 3537–3540. [Google Scholar] [CrossRef]
- Fiat Varol, S.; Sayin, S.; Eymur, S.; Merdan, Z.; Ünal, D. Optical performance of efficient blue/near UV nitropyridine-conjugated anthracene (NAMA) based light emitting diode. Org. Electron. 2016, 31, 25–30. [Google Scholar] [CrossRef]
- Nakanishi, W.; Hitosugi, S.; Piskareva, A.; Shimada, Y.; Taka, H.; Kita, H.; Isobe, H. Disilanyl Double-Pillared Bisanthracene: A Bipolar Carrier Transport Material for Organic Light-Emitting Diode Devices. Angew. Chem. 2010, 49, 7239–7242. [Google Scholar] [CrossRef]
- Du, C.; Ye, S.; Liu, Y.; Guo, Y.; Wu, T.; Liu, H.; Zheng, J.; Cheng, C.; Zhu, M.; Yu, G. Fused-seven-ring anthracene derivative with two sulfur bridges for high performance red organic light-emitting diodes. Chem. Commun. 2010, 46, 8573–8575. [Google Scholar] [CrossRef]
- Wang, B.; Mu, G.; Lv, X.; Ma, L.; Zhuang, S.; Wang, L. Tuning electron injection/transporting properties of 9,10-diphenylanthracene based electron transporters via optimizing the number of peripheral pyridine for highly efficient fluorescent OLEDs. Org. Electron. 2016, 34, 179–187. [Google Scholar] [CrossRef]
- Toyota, S.; Kawakami, T.; Iwanaga, T. Synthesis of 2,9-Diethynylanthracene Derivatives. Synthesis 2014, 46, 1667–1673. [Google Scholar] [CrossRef]
- Shi, Y.; Shi, Y.; Wei, H.; Zhai, H.; Liu, Y. A theoretical study on the electronic properties of two ring-fused derivatives of 9,10-diphenylanthracene. New J. Chem. 2017, 41, 10251–10258. [Google Scholar] [CrossRef]
- Jia, X.R.; Yu, H.J.; Chen, J.; Gao, W.J.; Fang, J.K.; Qin, Y.S.; Hu, X.; Shao, G. Stimuli-Responsive Properties of Aggregation-Induced-Emission Compounds Containing a 9,10-Distyrylanthracene Moiety. Chem. Eur. J. 2018, 24, 19053–19059. [Google Scholar] [CrossRef] [PubMed]
- Tlach, B.C.; Tomlinson, A.L.; Bhuwalka, A.; Jeffries-El, M. Tuning the Optical and Electronic Properties of 4,8-Disubstituted Benzobisoxazoles via Alkyne Substitution. J. Org. Chem. 2011, 76, 8670–8681. [Google Scholar] [CrossRef]
- He, P.; Wang, H.H.; Yan, H.G.; Hu, W.; Shi, J.X.; Gong, M.L. A strong red-emitting carbazole based europium(iii) complex excited by blue light. Dalton Trans. 2010, 39, 8919–8924. [Google Scholar] [CrossRef]
- Li, H.; Chi, Z.; Xu, B.; Zhang, X.; Yang, Z.; Li, X.; Liu, S.; Zhang, Y.; Xu, J. New aggregation-induced emission enhancement materials combined triarylamine and dicarbazolyl triphenylethylene moieties. J. Mater. Chem. 2010, 20, 6103. [Google Scholar] [CrossRef]
- Lin, H.; Wang, X.Y.; Li, C.M.; Li, X.J.; Tanabe, S.; Yu, J.Y. Spectral power distribution and quantum yields of Sm3+-doped heavy metal tellurite glass under the pumping of blue lighting emitting diode. Spectrochim. Acta Part A 2007, 67, 1417–1420. [Google Scholar] [CrossRef]
- Yuan, W.Z.; Lu, P.; Chen, S.; Lam, J.W.; Wang, Z.; Liu, Y.; Kwok, H.S.; Ma, Y.; Tang, B.Z. Changing the Behavior of Chromophores from Aggregation-Caused Quenching to Aggregation-Induced Emission: Development of Highly Efficient Light Emitters in the Solid State. Adv. Mater. 2010, 22, 2159–2163. [Google Scholar] [CrossRef]
- Shao, G.; Li, Y.; Feng, K.; Gan, F.; Gong, M. Diphenylethyne based β-diketonate europium(III) complexes as red phosphors applied in LED. Sens. Actuators B 2012, 173, 692–697. [Google Scholar] [CrossRef]
- Shao, G.; Zhang, N.; Lin, D.; Feng, K.; Cao, R.; Gong, M. A new europium(III)-β-diketonate complex based on diphenylethyne as red phosphors applied in LED. J. Lumin. 2013, 138, 195–200. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Song, H.; Xiao, J.; Yu, H.-J.; Peng, C.-F.; Shao, G. Green Phosphors Based on 9,10-bis((4-((3,7-dimethyloctyl)oxy) phenyl) ethynyl) Anthracene for LED. Micromachines 2019, 10, 703. https://doi.org/10.3390/mi10100703
Ren X, Song H, Xiao J, Yu H-J, Peng C-F, Shao G. Green Phosphors Based on 9,10-bis((4-((3,7-dimethyloctyl)oxy) phenyl) ethynyl) Anthracene for LED. Micromachines. 2019; 10(10):703. https://doi.org/10.3390/mi10100703
Chicago/Turabian StyleRen, Xuefeng, Hai Song, Jing Xiao, Hui-Juan Yu, Chi-Fang Peng, and Guang Shao. 2019. "Green Phosphors Based on 9,10-bis((4-((3,7-dimethyloctyl)oxy) phenyl) ethynyl) Anthracene for LED" Micromachines 10, no. 10: 703. https://doi.org/10.3390/mi10100703
APA StyleRen, X., Song, H., Xiao, J., Yu, H.-J., Peng, C.-F., & Shao, G. (2019). Green Phosphors Based on 9,10-bis((4-((3,7-dimethyloctyl)oxy) phenyl) ethynyl) Anthracene for LED. Micromachines, 10(10), 703. https://doi.org/10.3390/mi10100703




