Enhanced Efficiencies of Perovskite Solar Cells by Incorporating Silver Nanowires into the Hole Transport Layer
Abstract
:1. Introduction
2. Experimental Setup
2.1. Materials
2.2. Device Fabrication
2.3. Measurements and Characterization
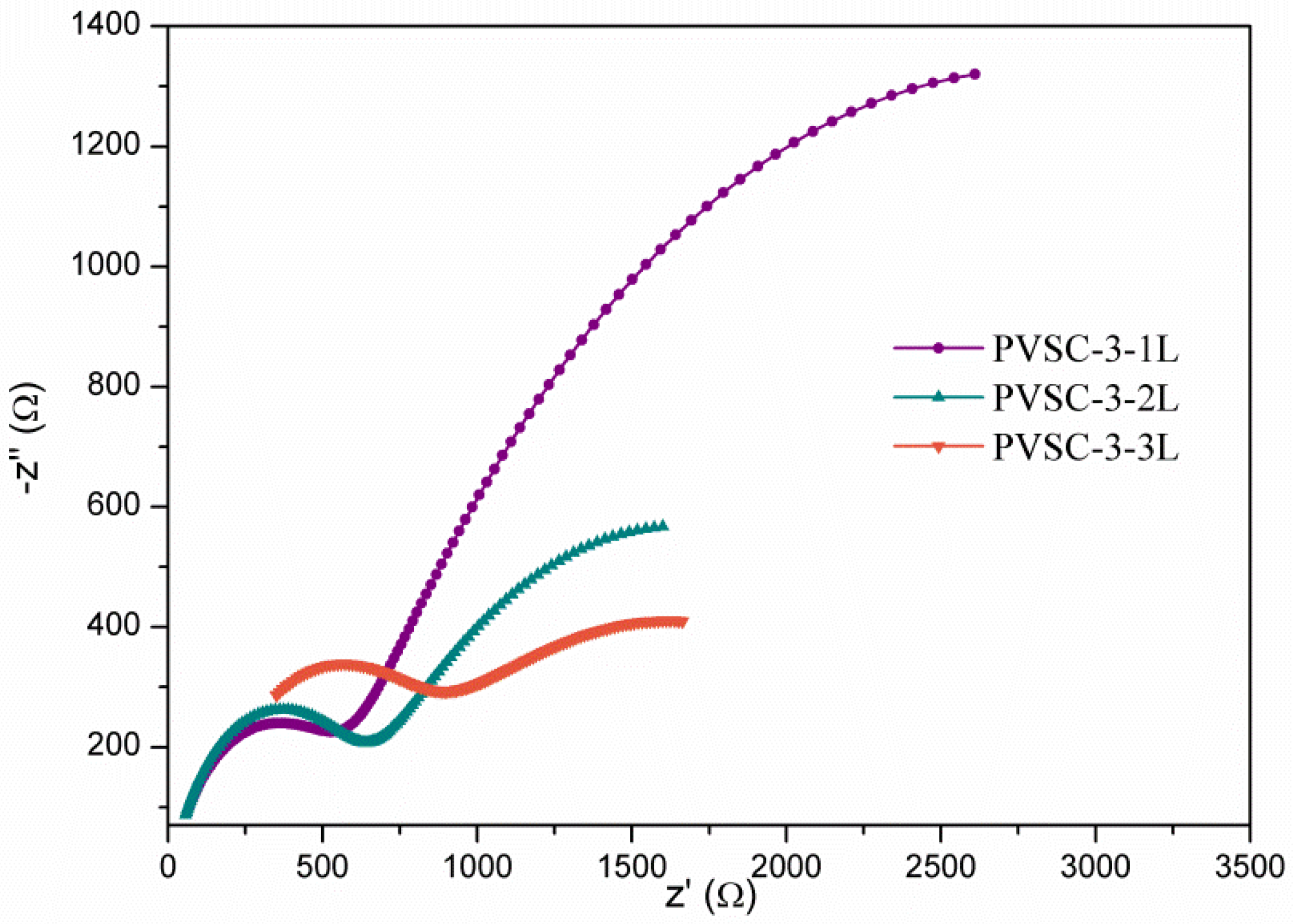
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Neophytou, M.; Griffiths, J.; Fraser, J.; Kirkus, M.; Chen, H.; Nielsen, C.B.; McCulloch, I. High mobility, hole transport materials for highly efficient PEDOT:PSS replacement in inverted perovskite solar cells. J. Mater. Chem. C 2017, 5, 4940–4945. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Liu, Z.; Lee, E.-C. High-performance metal oxide-free inverted perovskite solar cells using poly(bis(4-phenyl)(2,4,6-trimethylphenyl)amine) as the hole transport layer. J. Mater. Chem. C 2018, 6, 6975–6981. [Google Scholar] [CrossRef]
- Grätzel, M. The light and shade of perovskite solar cells. Nat. Mater. 2014, 13, 838. [Google Scholar] [CrossRef] [PubMed]
- Stranks, S.D.; Snaith, H.J. Metal-halide perovskites for photovoltaic and light-emitting devices. Nat. Nanotech. 2015, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, K.; Hu, Q.; Zhu, R.; Gong, Q. Inverted perovskite solar cells: Progresses and perspectives. Adv. Energy Mater. 2016, 6, 1600457. [Google Scholar] [CrossRef]
- Meng, L.; You, J.; Guo, T.-F.; Yang, Y. Recent advances in the inverted planar structure of perovskite solar cells. Acc. Chem. Res. 2016, 49, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Sun, K.; Li, P.; Xia, Y.; Chang, J.; Ouyang, J. Transparent conductive oxide-free perovskite solar cells with PEDOT:PSS as transparent electrode. ACS Appl. Mater. Interfaces 2015, 7, 15314–15320. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-S.; Balamurugan, R.; Liu, B.-T.; Lee, R.-H.; Chou, H.-T. MAPbI3 incorporated with carboxyl group chelated titania for planar perovskite solar cells in low-temperature process. Nanomaterials 2019, 9, 908. [Google Scholar] [CrossRef]
- Shahbazi, S.; Tajabadi, F.; Shiu, H.S.; Sedighi, R.; Jokar, E.; Gholipour, S.; Taghavinia, N.; Afshar, S.; Diau, E.W.G. An easy method to modify PEDOT:PSS/perovskite interfaces for solar cells with efficiency exceeding 15%. RSC Adv. 2016, 6, 65594–65599. [Google Scholar] [CrossRef]
- You, J.; Hong, Z.; Yang, Y.; Chen, Q.; Cai, M.; Song, T.-B.; Chen, C.-C.; Lu, S.; Liu, Y.; Zhou, H.; et al. Low-temperature solution-processed perovskite solar cells with high efficiency and flexibility. ACS Nano 2014, 8, 1674–1680. [Google Scholar] [CrossRef] [PubMed]
- Malinkiewicz, O.; Yella, A.; Lee, Y.H.; Espallargas, G.M.; Graetzel, M.; Nazeeruddin, M.K.; Bolink, H.J. Perovskite solar cells employing organic charge-transport layers. Nat. Photon. 2013, 8, 128. [Google Scholar] [CrossRef]
- Docampo, P.; Ball, J.M.; Darwich, M.; Eperon, G.E.; Snaith, H.J. Efficient organometal trihalide perovskite planar-heterojunction solar cells on flexible polymer substrates. Nat. Commun. 2013, 4, 2761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, J.P.; Zhao, L.; McGillivray, D.; Leung, K.T. High-efficiency hybrid solar cells by nanostructural modification in PEDOT:PSS with co-solvent addition. J. Mater. Chem. A 2014, 2, 2383–2389. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Ni, W.-S.; Lee, J.-Y. Effect of incorporation of ethylene glycol into PEDOT:PSS on electron phonon coupling and conductivity. J. Appl. Phys. 2015, 117, 215501. [Google Scholar]
- Lin, Y.-J.; Chin, Y.-M.; Wu, C.-Y.; Liu, D.-S. Electron-phonon coupling modification and carrier mobility enhancement in poly(3,4-ethylenedioxythiophene) doped with poly(4-styrenesulfonate) films by ultraviolet irradiation. J. Appl. Phys. 2014, 116, 093707. [Google Scholar]
- Lin, J.-H.; Zeng, J.-J.; Su, Y.-C.; Lin, Y.-J. Current transport mechanism of heterojunction diodes based on the reduced graphene oxide-based polymer composite and n-type Si. Appl. Phys. Lett. 2012, 100, 153509. [Google Scholar]
- Xiao, Z.; Bi, C.; Shao, Y.; Dong, Q.; Wang, Q.; Yuan, Y.; Wang, C.; Gao, Y.; Huang, J. Efficient, high yield perovskite photovoltaic devices grown by interdiffusion of solution-processed precursor stacking layers. Energy Environ. Sci. 2014, 7, 2619–2623. [Google Scholar] [CrossRef]
- Li, H.; Fu, K.; Boix, P.P.; Wong, L.H.; Hagfeldt, A.; Grätzel, M.; Mhaisalkar, S.G.; Grimsdale, A.C. Hole-transporting small molecules based on thiophene cores for high efficiency perovskite solar cells. ChemSusChem 2014, 7, 3420–3425. [Google Scholar] [CrossRef]
- Labban, A.E.; Chen, H.; Kirkus, M.; Barbe, J.; Del Gobbo, S.; Neophytou, M.; McCulloch, I.; Eid, J. Improved efficiency in inverted perovskite solar cells employing a novel diarylamino-substituted molecule as PEDOT:PSS replacement. Adv. Energy Mater. 2016, 6, 1502101. [Google Scholar] [CrossRef]
- You, J.; Meng, L.; Song, T.-B.; Guo, T.-F.; Yang, Y.; Chang, W.-H.; Hong, Z.; Chen, H.; Zhou, H.; Chen, Q.; et al. Improved air stability of perovskite solar cells via solution-processed metal oxide transport layers. Nat. Nanotech. 2015, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Jeng, J.-Y.; Chen, K.-C.; Chiang, T.-Y.; Lin, P.-Y.; Tsai, T.-D.; Chang, Y.-C.; Guo, T.-F.; Chen, P.; Wen, T.-C.; Hsu, Y.-J. Nickel oxide electrode interlayer in ch3nh3pbi3 perovskite/PCBM planar-heterojunction hybrid solar cells. Adv. Mater. 2014, 26, 4107–4113. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Sun, W.; Li, Y.; Yan, W.; Peng, H.; Bian, Z.; Liu, Z.; Huang, C. CuSCN-based inverted planar perovskite solar cell with an average PCE of 15.6%. Nano Lett. 2015, 15, 3723–3728. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, W.; Liu, H.; Peng, J.; Cao, H.; Shao, G.; Xia, Z.; Ma, W.; Tang, J. PbS colloidal quantum dots as an effective hole transporter for planar heterojunction perovskite solar cells. J. Mater. Chem. A 2015, 3, 515–518. [Google Scholar] [CrossRef]
- Wu, Z.; Bai, S.; Xiang, J.; Yuan, Z.; Yang, Y.; Cui, W.; Gao, X.; Liu, Z.; Jin, Y.; Sun, B. Efficient planar heterojunction perovskite solar cells employing graphene oxide as hole conductor. Nanoscale 2014, 6, 10505–10510. [Google Scholar] [CrossRef]
- Huang, X.; Wang, K.; Yi, C.; Meng, T.; Gong, X. Efficient perovskite hybrid solar cells by highly electrical conductive PEDOT:PSS hole transport layer. Adv. Energy Mater. 2016, 6, 1501773. [Google Scholar] [CrossRef]
- Kim, D.B.; Yu, J.C.; Nam, Y.S.; Kim, D.W.; Jung, E.D.; Lee, S.Y.; Lee, S.; Park, J.H.; Lee, A.-Y.; Lee, B.R.; et al. Improved performance of perovskite light-emitting diodes using a PEDOT:PSS and MoO3 composite layer. J. Mater. Chem. C 2016, 4, 8161–8165. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, C.; Liu, H.; Qin, R.; Ma, H. Poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS)–molybdenum oxide composite films as hole conductors for efficient planar perovskite solar cells. J. Mater. Chem. A 2016, 4, 9958–9966. [Google Scholar] [CrossRef]
- Wang, Z.-K.; Li, M.; Yuan, D.-X.; Shi, X.-B.; Ma, H.; Liao, L.-S. Improved hole interfacial layer for planar perovskite solar cells with efficiency exceeding 15%. ACS Appl. Mater. Interfaces 2015, 7, 9645–9651. [Google Scholar] [CrossRef]
- Luo, H.; Lin, X.; Hou, X.; Pan, L.; Huang, S.; Chen, X. Efficient and air-stable planar perovskite solar cells formed on graphene-oxide-modified PEDOT:PSS hole transport layer. Nano-Micro Lett. 2017, 9, 39. [Google Scholar] [CrossRef]
- Tsai, T.-C.; Chang, H.-C.; Chen, C.-H.; Huang, Y.-C.; Whang, W.-T. A facile dedoping approach for effectively tuning thermoelectricity and acidity of PEDOT:PSS films. Org. Electron. 2014, 15, 641–645. [Google Scholar] [CrossRef]
- Wang, Q.; Chueh, C.-C.; Eslamian, M.; Jen, A.K.Y. Modulation of PEDOT:PSS pH for efficient inverted perovskite solar cells with reduced potential loss and enhanced stability. ACS Appl. Mater. Interfaces 2016, 8, 32068–32076. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, K.-X.; Chang, J.-J.; Jiang, Y.-Y.; Xiao, Q.-S.; Li, Y. Improving the efficiency and stability of inverted perovskite solar cells with dopamine-copolymerized PEDOT:PSS as a hole extraction layer. J. Mater. Chem. A 2017, 5, 13817–13822. [Google Scholar] [CrossRef]
- Liu, B.-T.; Huang, S.-X. Transparent conductive silver nanowire electrodes with high resistance to oxidation and thermal shock. RSC Adv. 2014, 4, 59226–59232. [Google Scholar] [CrossRef]
- Kim, D.; Ko, Y.; Kim, W.; Kim, D.; You, J. Highly efficient silver nanowire/PEDPT:PSS composite microelectrodes via poly(ethylene glycol) photolithography. Opt. Mater. Express 2017, 7, 2272–2279. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.Y.; Kim, J.; Kim, J.H. Highly reliable AgNW/PEDOT:PSS hybrid films: Efficient methods for enhancing transparency and lowering resistance and haziness. J. Mater. Chem. C 2014, 2, 5636–5643. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, J.; Ou, X.-L.; Cui, H.; Xu, M.; Sun, H.-B. Ultrasmooth, highly conductive and transparent PEDOT:PSS/silver nanowire composite electrode for flexible organic light-emitting devices. Org. Electron. 2016, 31, 247–252. [Google Scholar] [CrossRef]
- Choi, D.Y.; Kang, H.W.; Sung, H.J.; Kim, S.S. Annealing-free, flexible silver nanowire–polymer composite electrodes via a continuous two-step spray-coating method. Nanoscale 2013, 5, 977–983. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.Y.; Chung, M.H.; Kim, J.; Kim, J.H. A one-step roll-to-roll process of stable AgNW/PEDOT:PSS solution using imidazole as a mild base for highly conductive and transparent films: Optimizations and mechanisms. J. Mater. Chem. C 2015, 3, 5859–5868. [Google Scholar] [CrossRef]
- Han, K.; Xie, M.; Zhang, L.; Yan, L.; Wei, J.; Ji, G.; Luo, Q.; Lin, J.; Hao, Y.; Ma, C.-Q. Fully solution processed semi-transparent perovskite solar cells with spray-coated silver nanowires/ZnO composite top electrode. Sol. Energy Mater. Sol. Cells 2018, 185, 399–405. [Google Scholar] [CrossRef]
- Xie, M.; Lu, H.; Zhang, L.; Wang, J.; Luo, Q.; Lin, J.; Ba, L.; Liu, H.; Shen, W.; Shi, L.; et al. Fully solution-processed semi-transparent perovskite solar cells with ink-jet printed silver nanowires top electrode. Sol. RRL 2018, 2, 1700184. [Google Scholar] [CrossRef]
- Fang, Y.; Wu, Z.; Li, J.; Jiang, F.; Zhang, K.; Zhang, Y.; Zhou, Y.; Zhou, J.; Hu, B. High-performance hazy silver nanowire transparent electrodes through diameter tailoring for semitransparent photovoltaics. Adv. Funct. Mater. 2018, 28, 1705409. [Google Scholar] [CrossRef]
- Jin, Y.; Sun, Y.; Wang, K.; Chen, Y.; Liang, Z.; Xu, Y.; Xiao, F. Long-term stable silver nanowire transparent composite as bottom electrode for perovskite solar cells. Nano Res. 2018, 11, 1998–2011. [Google Scholar] [CrossRef]
- Chen, H.; Li, M.; Wen, X.; Yang, Y.; He, D.; Choy, W.C.H.; Lu, H. Enhanced silver nanowire composite window electrode protected by large size graphene oxide sheets for perovskite solar cells. Nanomaterials 2019, 9, 193. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.-T.; Wang, Z.-T. Graphene oxide/poly(3,4-ethylenedioxythiophene):polystyrenesulfonate layers on silver nanowire working electrodes enhance the power conversion efficiencies of dye-sensitized solar cells in a low temperature process. RSC Adv. 2016, 6, 47185–47191. [Google Scholar] [CrossRef]
- Liu, B.-T.; Li, C.-D. Highly conductive and fine lines of silver nanowires fabricated by evaporative self-assembly. J. Taiwan Inst. Chem. Eng. 2019, 95, 569–574. [Google Scholar] [CrossRef]
- Sun, Y.; Yin, Y.; Mayers, B.T.; Herricks, T.; Xia, Y. Uniform silver nanowires synthesis by reducing AgNO3 with ethylene glycol in the presence of seeds and poly(vinyl pyrrolidone). Chem. Mater. 2002, 14, 4736–4745. [Google Scholar] [CrossRef]
- Liu, T.; Liu, W.; Zhu, Y.; Wang, S.; Wu, G.; Chen, H. All solution processed perovskite solar cells with Ag@Au nanowires as top electrode. Sol. Energy Mater. Sol. Cells 2017, 171, 43–49. [Google Scholar] [CrossRef]
- Sutanto, A.A.; Lan, S.; Cheng, C.-F.; Mane, S.B.; Wu, H.-P.; Leonardus, M.; Xie, M.-Y.; Yeh, S.-C.; Tseng, C.-W.; Chen, C.-T.; et al. Solvent-assisted crystallization via a delayed-annealing approach for highly efficient hybrid mesoscopic/planar perovskite solar cells. Sol. Energy Mater. Sol. Cells 2017, 172, 270–276. [Google Scholar] [CrossRef]
- Kang, S.; Jeong, J.; Cho, S.; Yoon, Y.J.; Park, S.; Lim, S.; Kim, J.Y.; Ko, H. Ultrathin, lightweight and flexible perovskite solar cells with an excellent power-per-weight performance. J. Mater. Chem. A 2019, 7, 1107–1114. [Google Scholar] [CrossRef]
- Kim, A.; Lee, H.; Kwon, H.-C.; Jung, H.S.; Park, N.-G.; Jeong, S.; Moon, J. Fully solution-processed transparent electrodes based on silver nanowire composites for perovskite solar cells. Nanoscale 2016, 8, 6308–6316. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Ahn, J.; Kwon, H.-C.; Ma, S.; Kim, K.; Yun, S.; Moon, J. All-solution-processed silver nanowire window electrode-based flexible perovskite solar cells enabled with amorphous metal oxide protection. Adv. Energy Mater. 2018, 8, 1702182. [Google Scholar] [CrossRef]
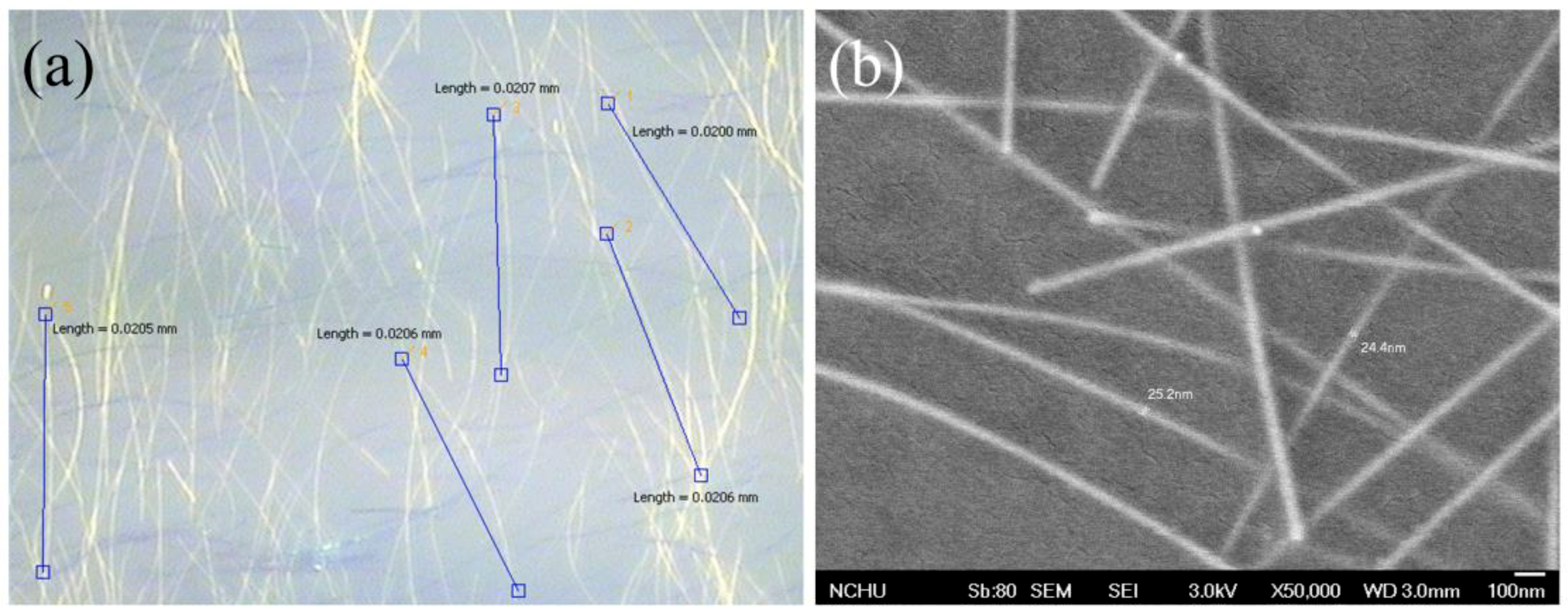
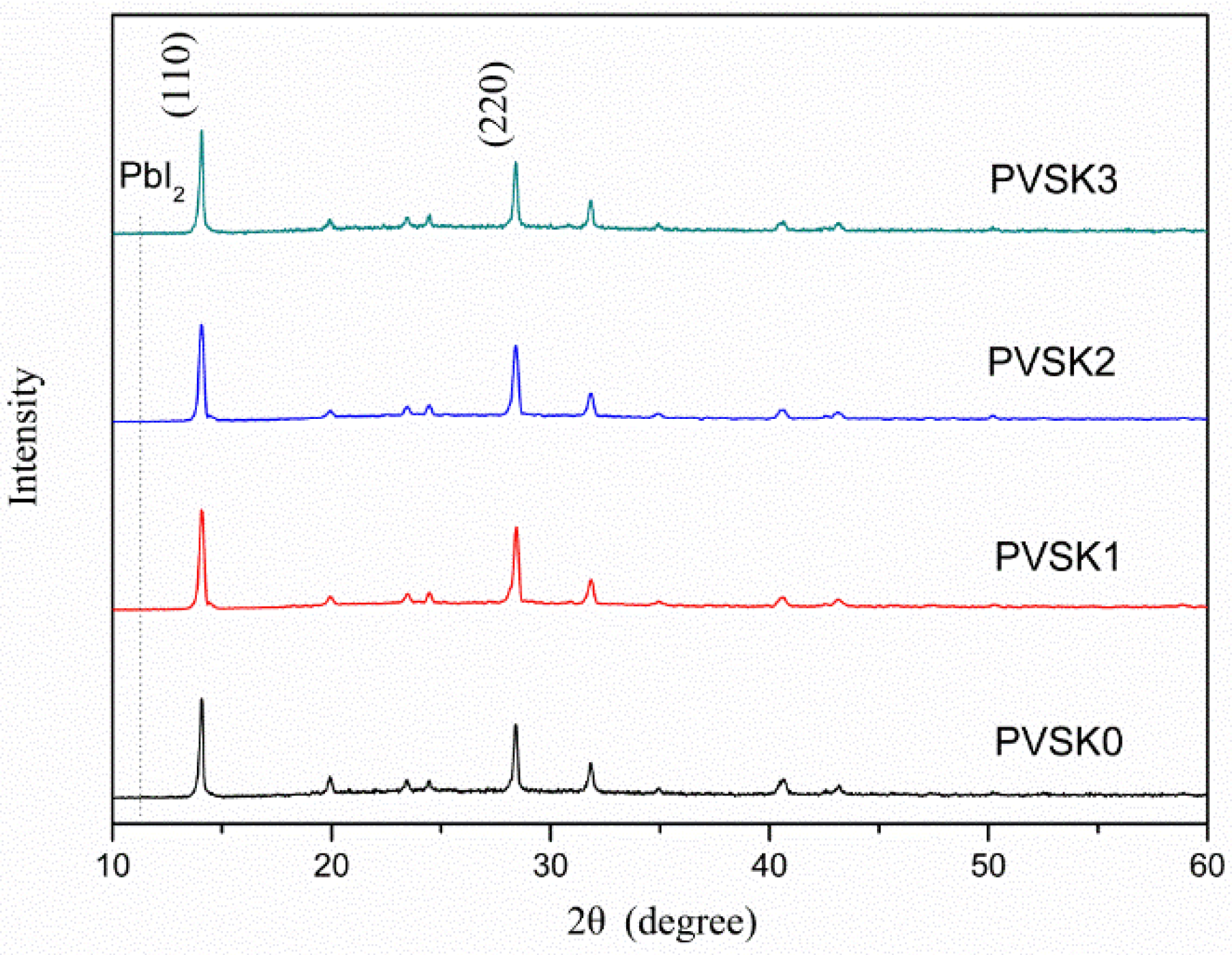
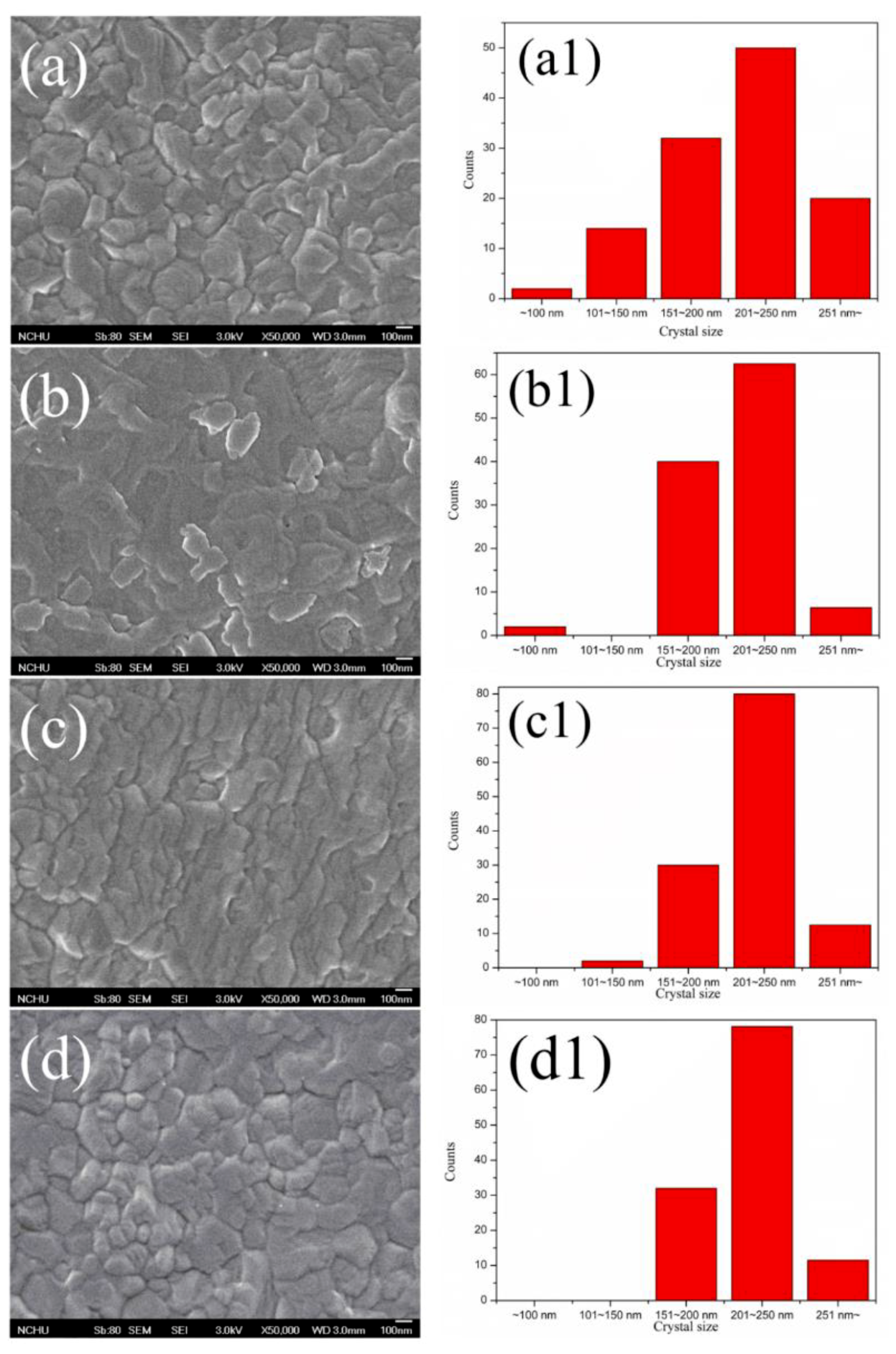
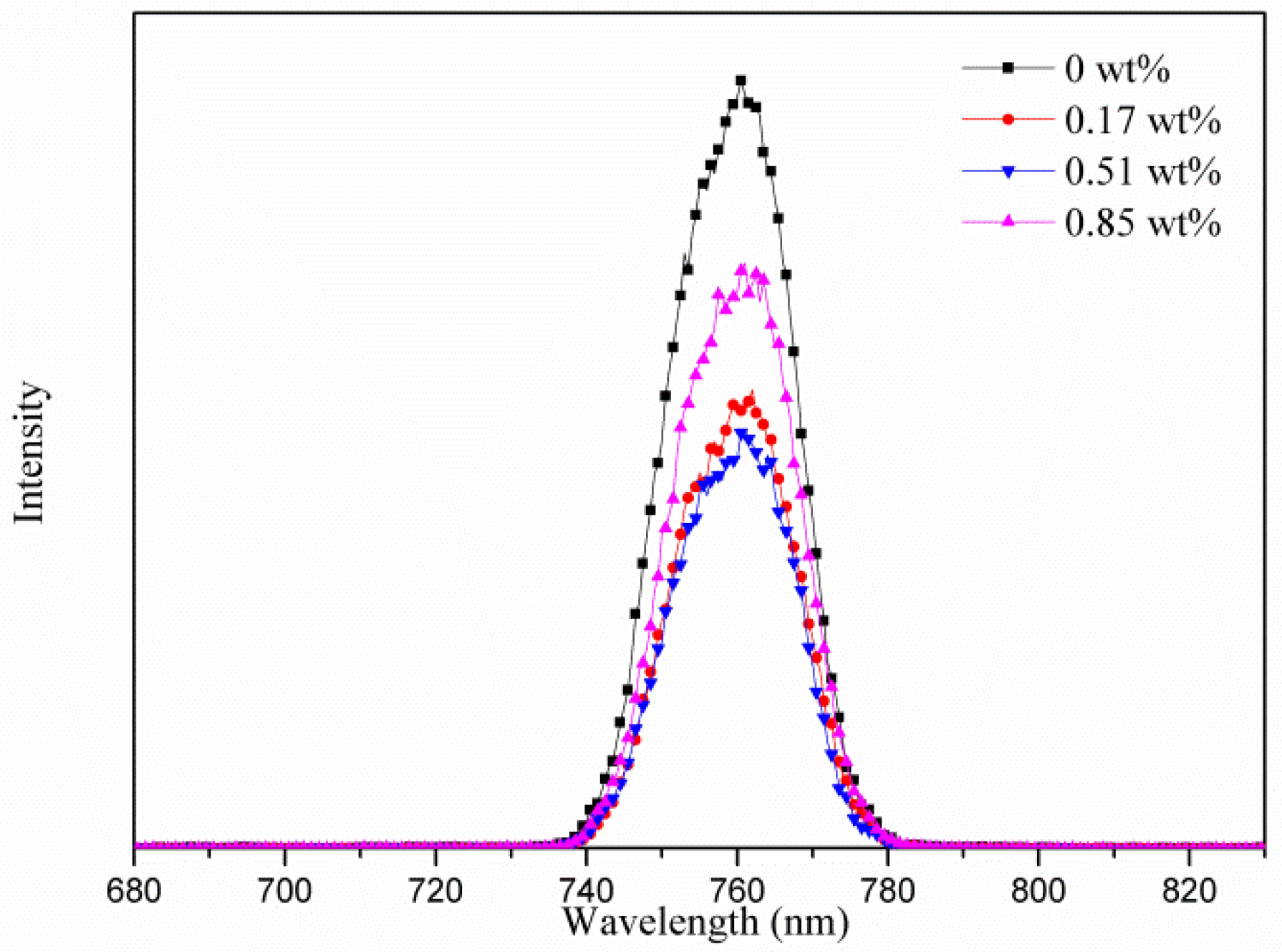
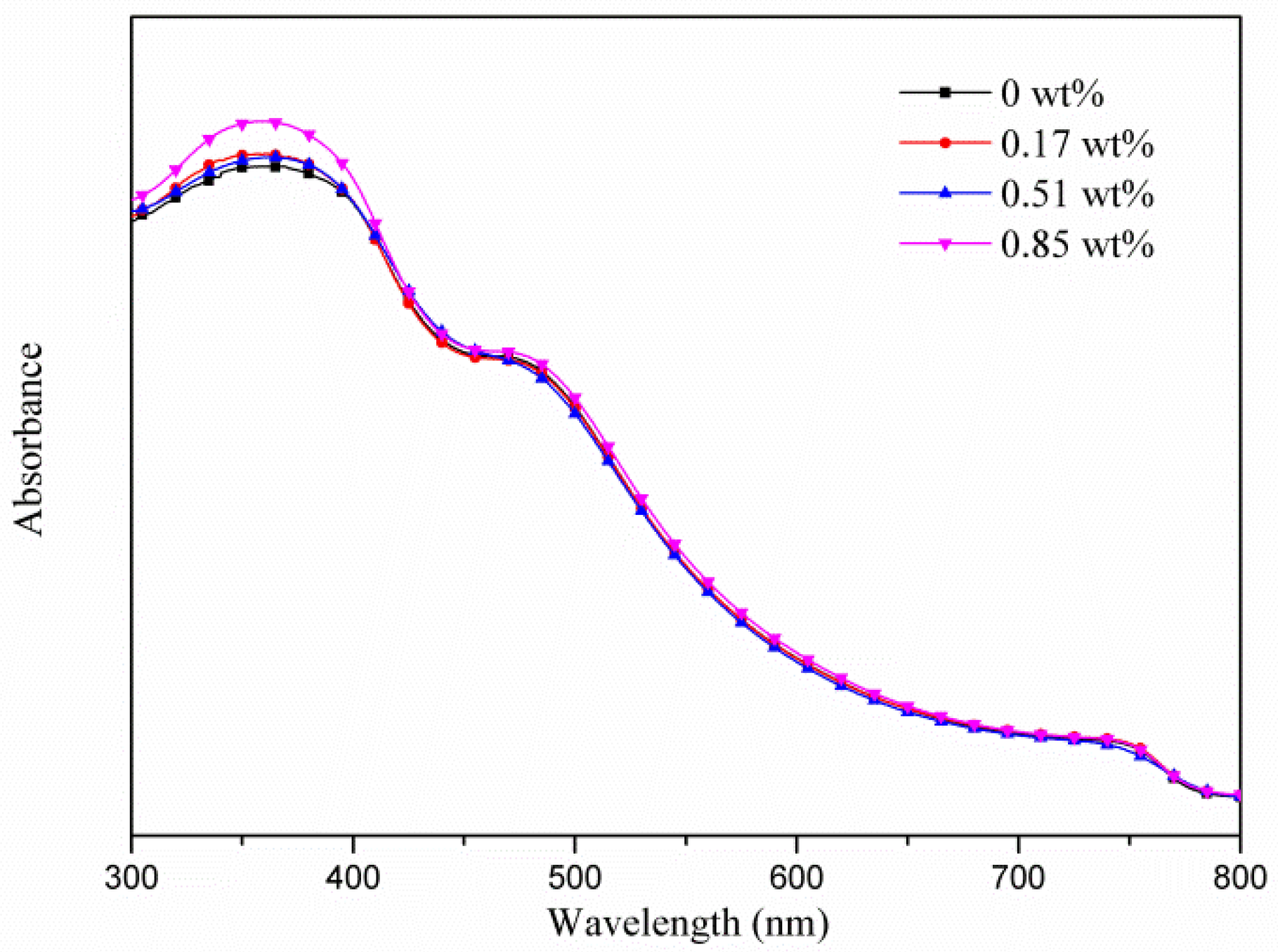
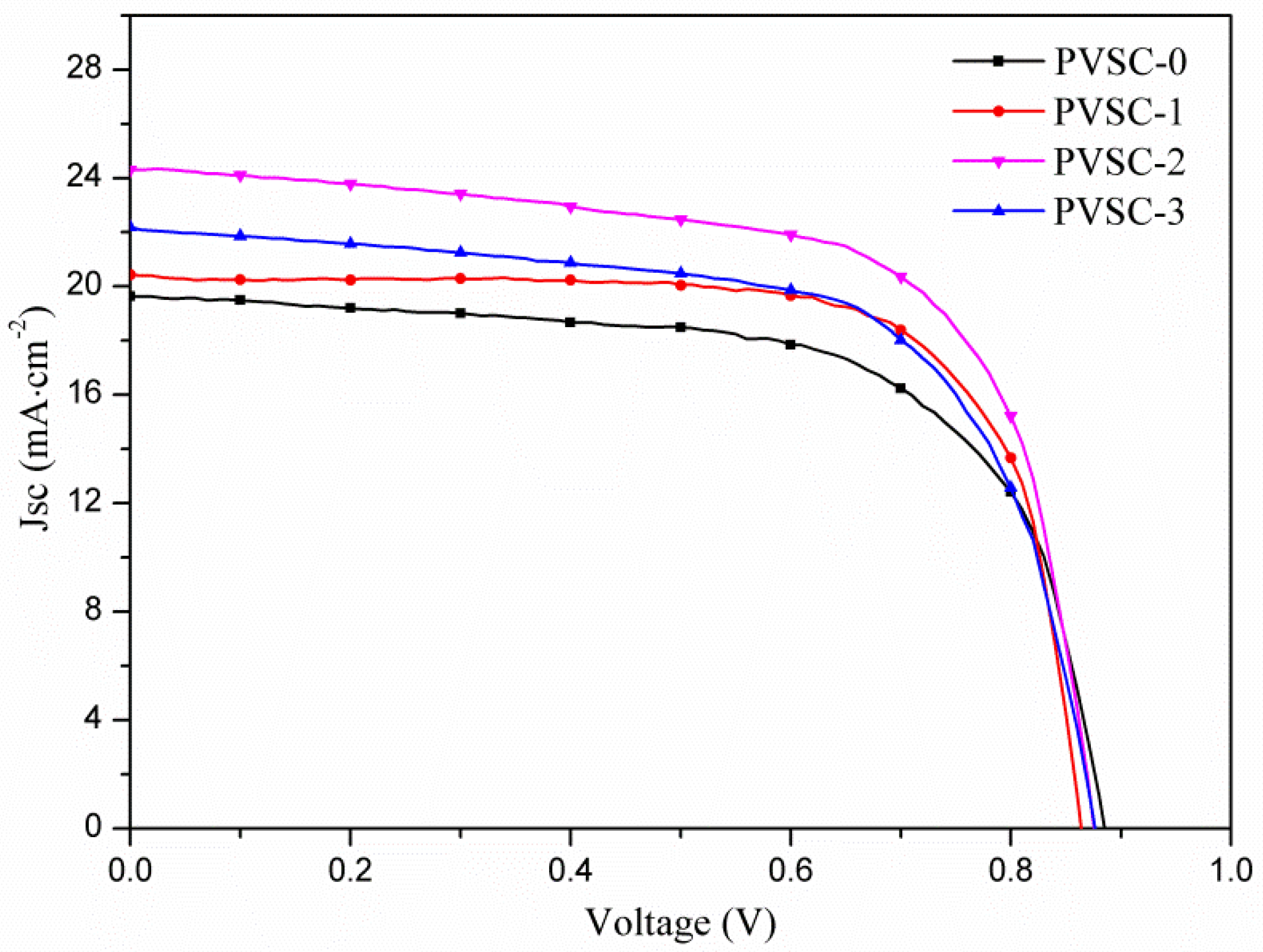
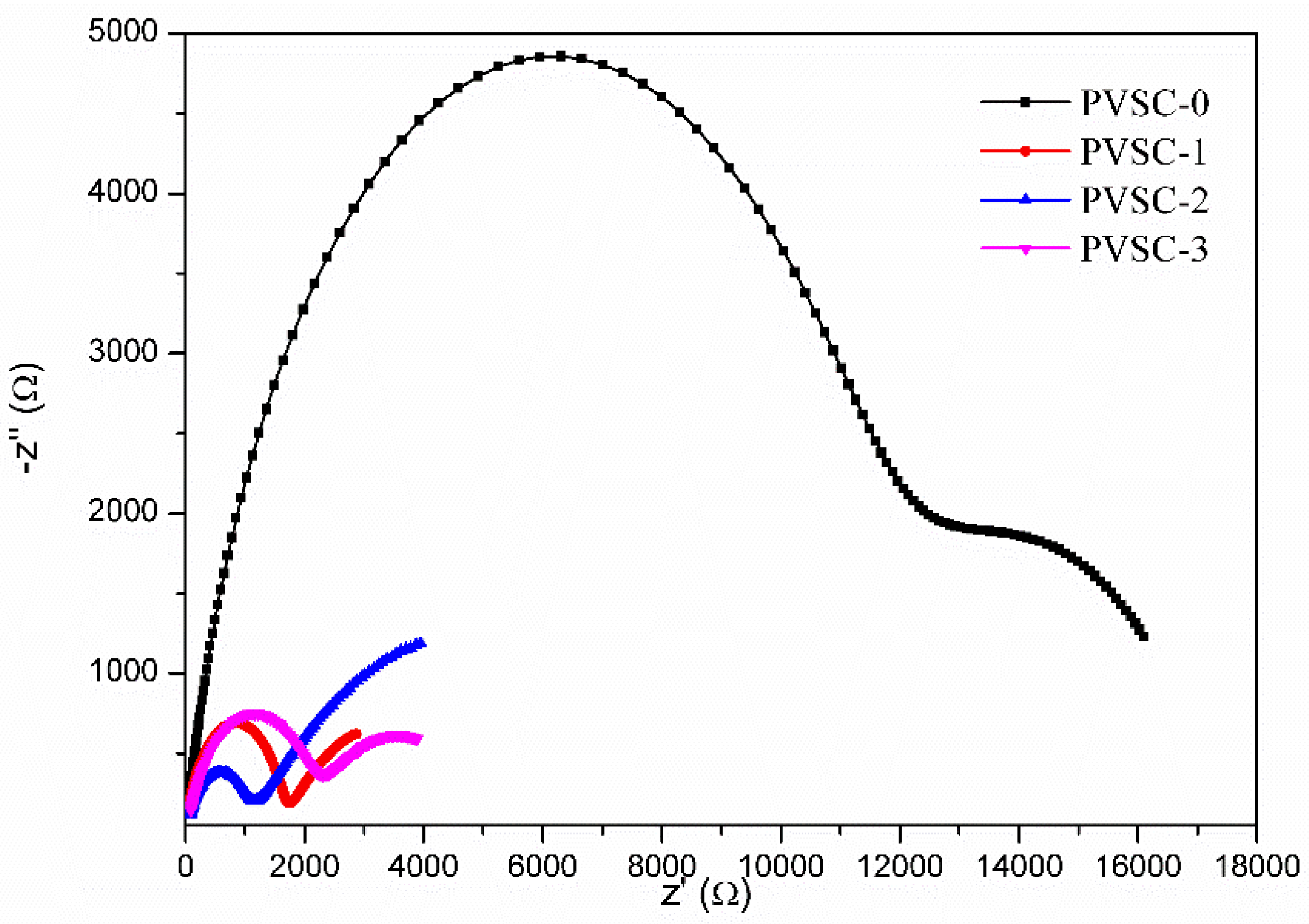
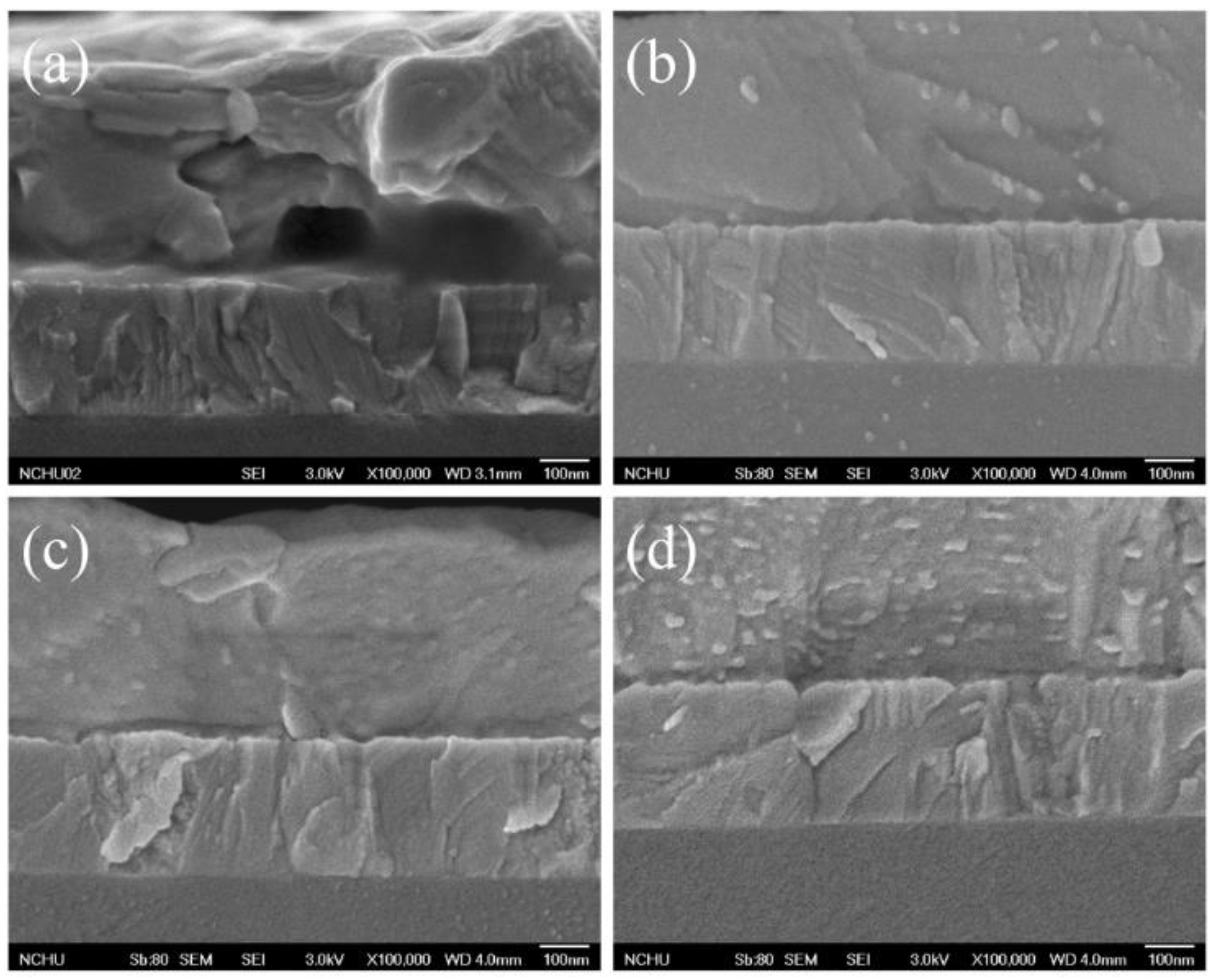
| Samples | AgNW Solution (wt%) | Voc (V) | Jsc (mA·cm−2) | FF (%) | η (%) | R1 (Ω) | R2 (kΩ) | R3 (kΩ) |
|---|---|---|---|---|---|---|---|---|
| PVSC-0 | 0 | 0.89 | 19.6 | 65.4 | 11.4 | 7.6 | 11.6 | 5.8 |
| PVSC-1 | 0.17 | 0.86 | 20.4 | 73.0 | 12.9 | 5.9 | 1.6 | 3.3 |
| PVSC-2 | 0.51 | 0.88 | 24.3 | 67.0 | 14.3 | 10.2 | 1.0 | 7.4 |
| PVSC-3 | 0.85 | 0.84 | 22.2 | 64.9 | 12.1 | 10.9 | 2.1 | 3.0 |
| Samples | AgNW Solution (wt%) | Number of Extra PEDOT:PSS Layers | Voc (V) | Jsc (mA·cm-2) | FF (%) | η (%) | R1 (Ω) | R2 (Ω) | R3 (kΩ) |
|---|---|---|---|---|---|---|---|---|---|
| PVSC-3-1L | 0.85 | 1 | 0.97 | 21.8 | 51.5 | 13.0 | 18.1 | 514.8 | 4.6 |
| PVSC-3-2L | 0.85 | 2 | 0.93 | 19.1 | 51.4 | 9.1 | 20.4 | 567.9 | 2.1 |
| PVSC-3-3L | 0.85 | 3 | 0.92 | 19.4 | 54.5 | 9.8 | 113.7 | 562.5 | 1.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.-J.; Balamurugan, R.; Liu, B.-T. Enhanced Efficiencies of Perovskite Solar Cells by Incorporating Silver Nanowires into the Hole Transport Layer. Micromachines 2019, 10, 682. https://doi.org/10.3390/mi10100682
Cheng C-J, Balamurugan R, Liu B-T. Enhanced Efficiencies of Perovskite Solar Cells by Incorporating Silver Nanowires into the Hole Transport Layer. Micromachines. 2019; 10(10):682. https://doi.org/10.3390/mi10100682
Chicago/Turabian StyleCheng, Chien-Jui, Rathinam Balamurugan, and Bo-Tau Liu. 2019. "Enhanced Efficiencies of Perovskite Solar Cells by Incorporating Silver Nanowires into the Hole Transport Layer" Micromachines 10, no. 10: 682. https://doi.org/10.3390/mi10100682
APA StyleCheng, C.-J., Balamurugan, R., & Liu, B.-T. (2019). Enhanced Efficiencies of Perovskite Solar Cells by Incorporating Silver Nanowires into the Hole Transport Layer. Micromachines, 10(10), 682. https://doi.org/10.3390/mi10100682






