Effect of Mechanical Compression on Invasion Process of Malignant Melanoma Using In Vitro Three-Dimensional Cell Culture Device
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
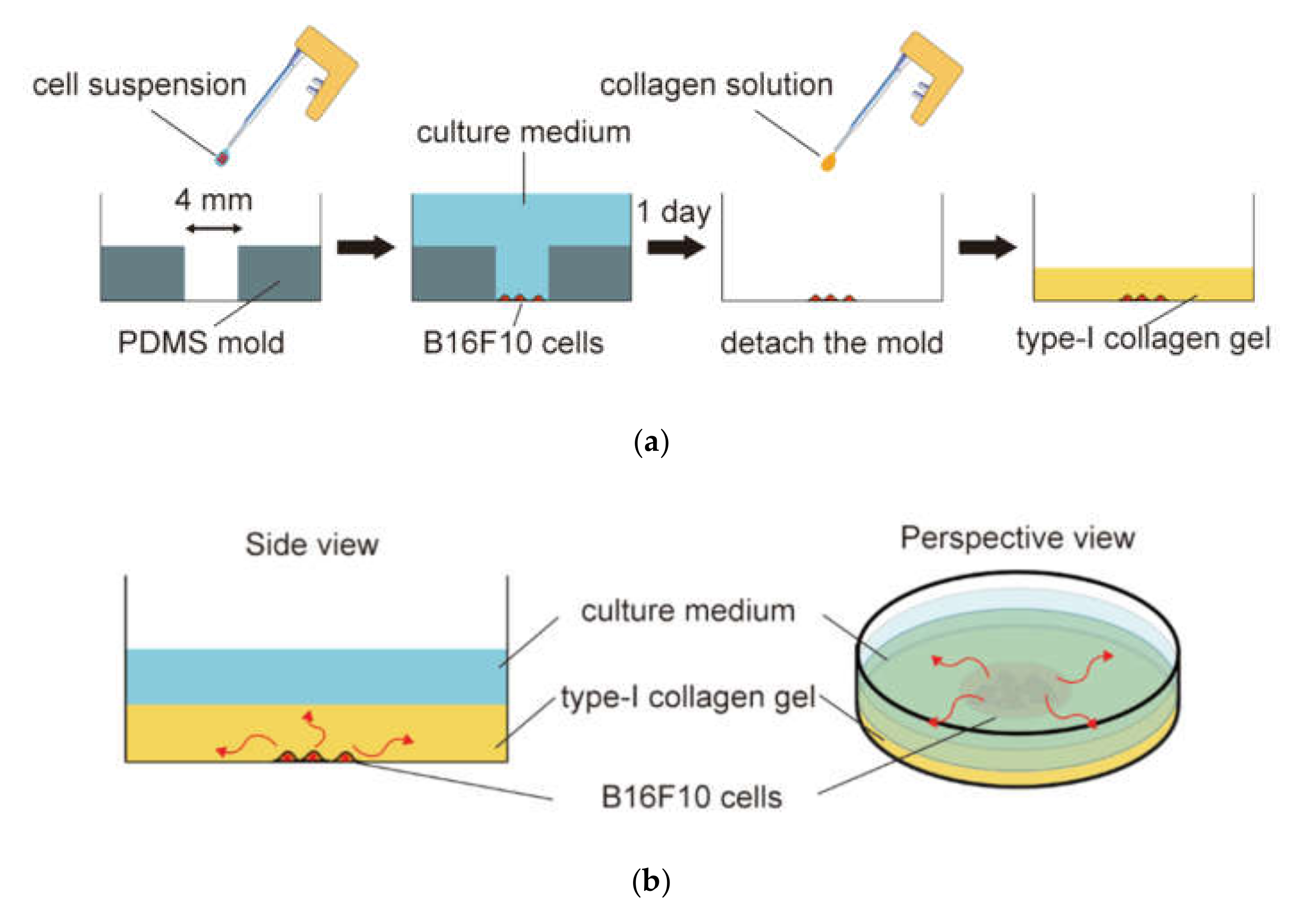
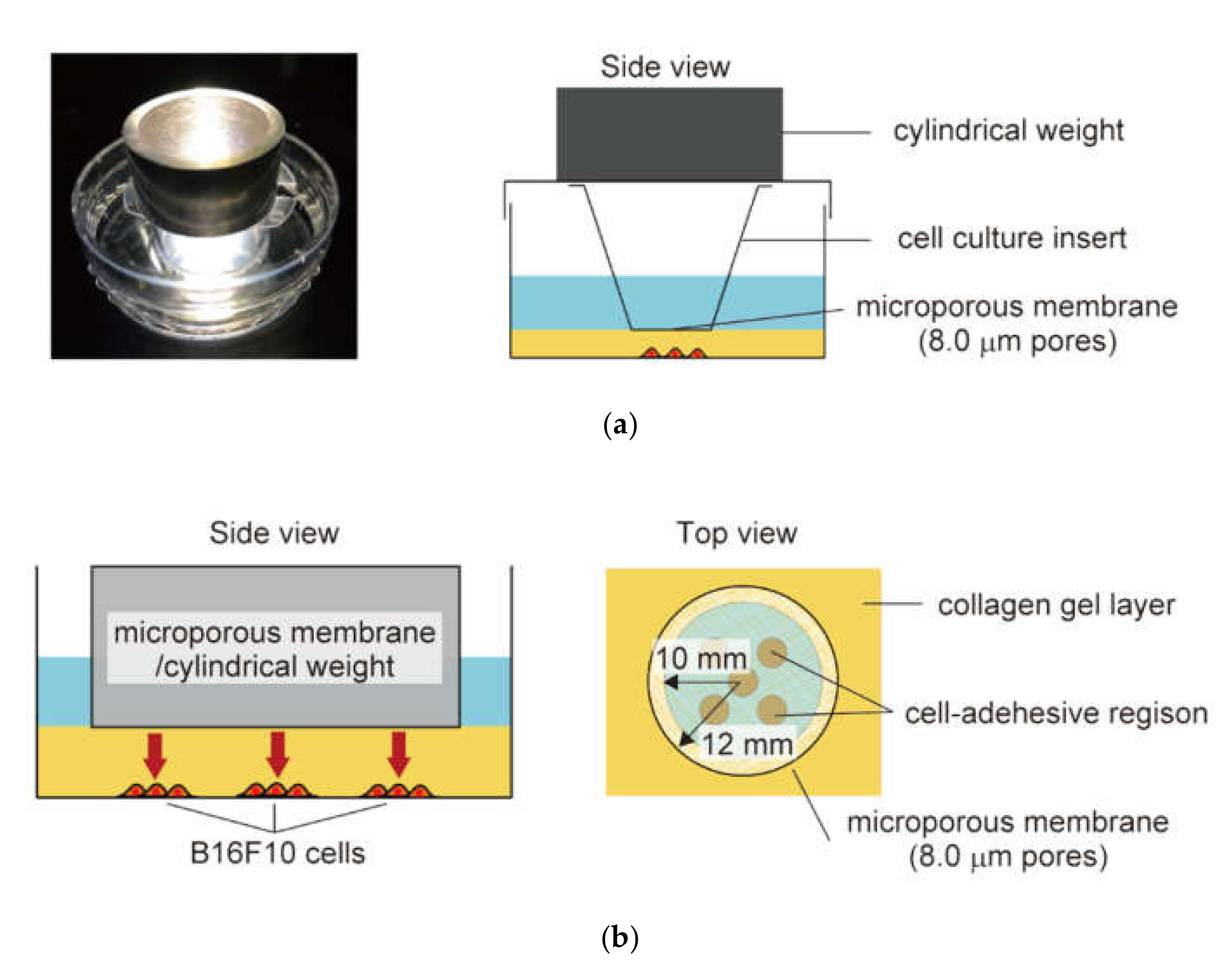
2.2. In Vitro Malignant Melanoma Model to Enable Imposing Static Compression and Monitoring of Cell Proliferation and Migration
2.3. Quantification of Cell Invasion
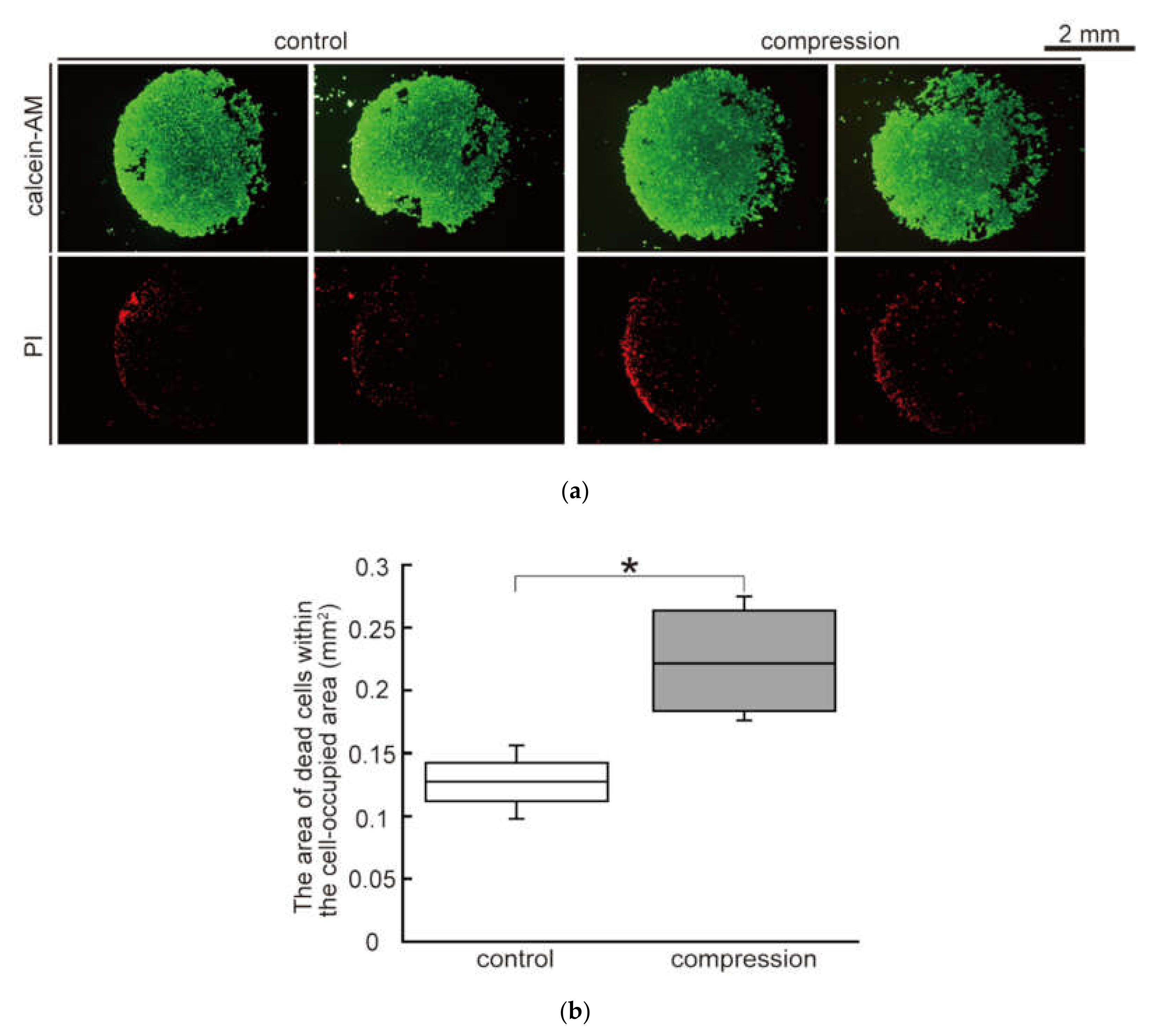
2.4. Evaluation of Cell Viability in Malignant Melanoma Model
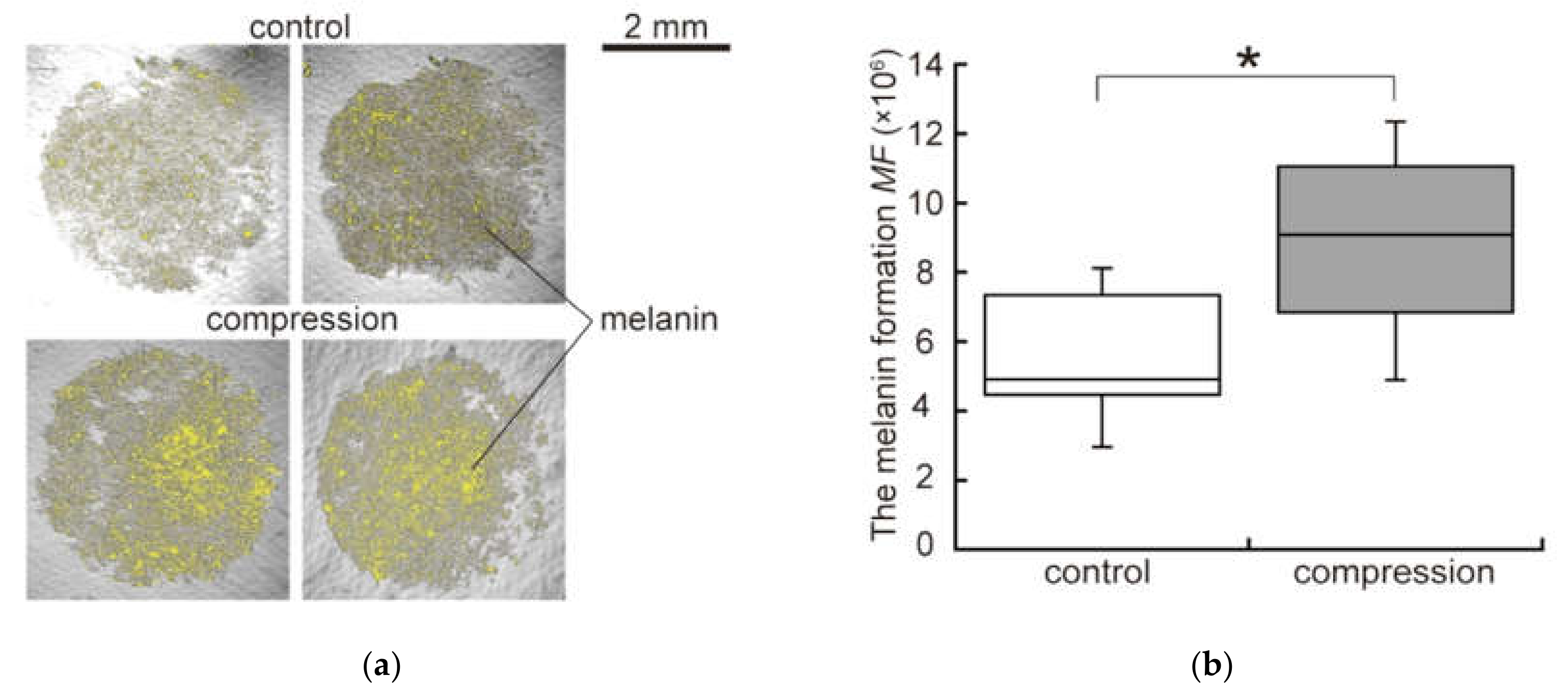
2.5. Quantification of Melanin Synthesis
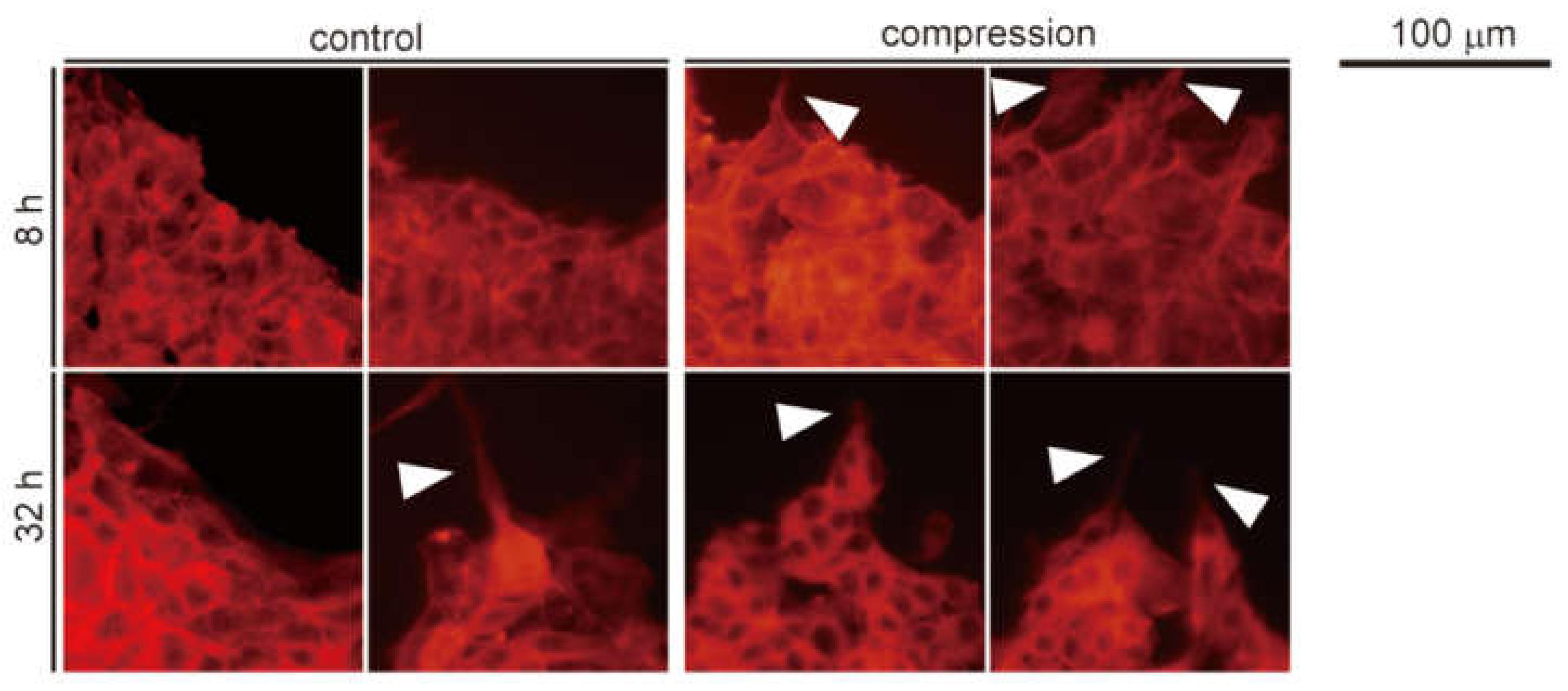
2.6. Fluorescent Staining of F-Actin Cytoskeleton
2.7. Statistical Analysis
3. Results
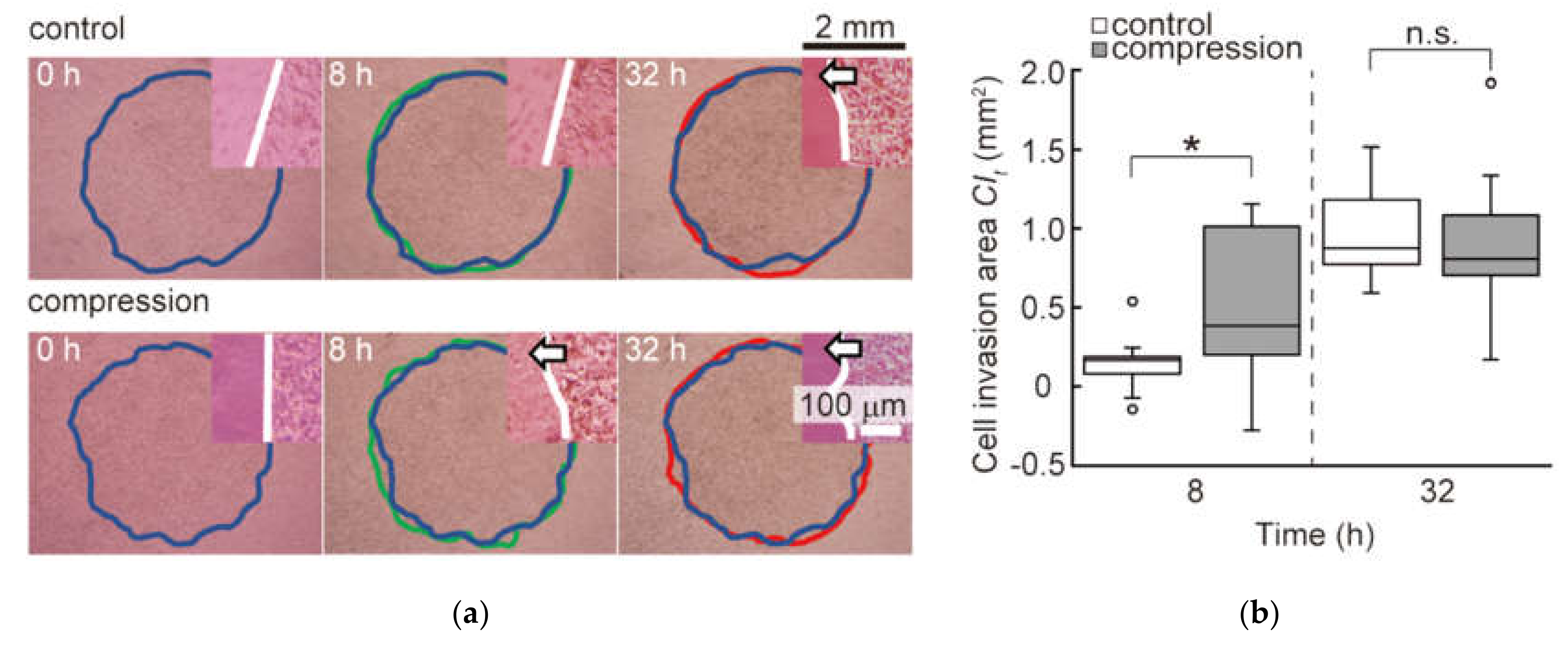
3.1. Effect of Static Compression on Invasion Process of Malignant Melanoma Cells
3.2. Cytoskeletal Reorganization of Malignant Melanoma Cells and Elongation of F-actin Filaments
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Linos, E.; Swetter, S.M.; Cockburn, M.G.; Colditz, G.A.; Clarke, C.A. Increasing burden of melanoma in the United States. J. Investig. Dermatol. 2009, 129, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Tucker, M.A. Melanoma epidemiology. Hematol. Oncol. Clin. N. Am. 2009, 23, 383–395. [Google Scholar] [CrossRef] [PubMed]
- MacKie, R.M.; Hauschild, A.; Eggermont, A.M. Epidemiology of invasive cutaneous melanoma. Ann. Oncol. 2009, 20, vi1–vi7. [Google Scholar] [CrossRef] [PubMed]
- Atallah, E.; Flaherty, L. Treatment of metastatic malignant melanoma. Curr. Treat. Options Oncol. 2005, 6, 185–193. [Google Scholar] [CrossRef]
- Baxter, L.L.; Pavan, W.J. The etiology and molecular genetics of human pigmentation disorders. Wiley. Interdiscip. Rev. Dev. Biol. 2013, 2, 379–392. [Google Scholar] [CrossRef]
- Kim, J.K.; Nelson, K.C. Dermoscopic features of common nevi: A review. G. Ital. Dermatol. Venereol. 2012, 147, 141–148. [Google Scholar]
- Chang, J.W. Acral melanoma: A unique disease in Asia. JAMA Dermatol. 2013, 149, 1272–1273. [Google Scholar] [CrossRef] [PubMed]
- Curtin, J.A.; Fridlyand, J.; Kageshita, T.; Patel, H.N.; Busam, K.J.; Kutzner, H.; Cho, K.H.; Aiba, S.; Brocker, E.B.; LeBoit, P.E.; et al. Distinct sets of genetic alterations in melanoma. N. Engl. J. Med. 2005, 353, 2135–2147. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, A.; Omodaka, T.; Okuyama, R. Melanomas and Mechanical Stress Points on the Plantar Surface of the Foot. N. Engl. J. Med. 2016, 374, 2404–2406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stucke, S.; McFarland, D.; Goss, L.; Fonov, S.; McMillan, G.R.; Tucker, A.; Berme, N.; Cenk Guler, H.; Bigelow, C.; Davis, B.L. Spatial relationships between shearing stresses and pressure on the plantar skin surface during gait. J. Biomech. 2012, 45, 619–622. [Google Scholar] [CrossRef] [Green Version]
- Cheng, G.; Tse, J.; Jain, R.K.; Munn, L.L. Micro-environmental mechanical stress controls tumor spheroid size and morphology by suppressing proliferation and inducing apoptosis in cancer cells. PLoS ONE 2009, 4, e4632. [Google Scholar] [CrossRef] [PubMed]
- Helmlinger, G.; Netti, P.A.; Lichtenbeld, H.C.; Melder, R.J.; Jain, R.K. Solid stress inhibits the growth of multicellular tumor spheroids. Nat. Biotechnol. 1997, 15, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuel, M.S.; Lopez, J.I.; McGhee, E.J.; Croft, D.R.; Strachan, D.; Timpson, P.; Munro, J.; Schroder, E.; Zhou, J.; Brunton, V.G.; et al. Actomyosin-mediated cellular tension drives increased tissue stiffness and beta-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer Cell 2011, 19, 776–791. [Google Scholar] [CrossRef] [PubMed]
- Taloni, A.; Alemi, A.A.; Ciusani, E.; Sethna, J.P.; Zapperi, S.; La Porta, C.A. Mechanical properties of growing melanocytic nevi and the progression to melanoma. PLoS ONE 2014, 9, e94229. [Google Scholar] [CrossRef]
- Farge, E. Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr. Biol. 2003, 13, 1365–1377. [Google Scholar] [CrossRef]
- Koch, T.M.; Munster, S.; Bonakdar, N.; Butler, J.P.; Fabry, B. 3D Traction forces in cancer cell invasion. PLoS ONE 2012, 7, e33476. [Google Scholar] [CrossRef]
- Koike, C.; McKee, T.D.; Pluen, A.; Ramanujan, S.; Burton, K.; Munn, L.L.; Boucher, Y.; Jain, R.K. Solid stress facilitates spheroid formation: Potential involvement of hyaluronan. Br. J. Cancer 2002, 86, 947–953. [Google Scholar] [CrossRef]
- Montel, F.; Delarue, M.; Elgeti, J.; Malaquin, L.; Basan, M.; Risler, T.; Cabane, B.; Vignjevic, D.; Prost, J.; Cappello, G.; et al. Stress clamp experiments on multicellular tumor spheroids. Phys. Rev. Lett. 2011, 107, 188102. [Google Scholar] [CrossRef]
- Montel, F.; Delarue, M.; Elgeti, J.; Vignjevic, D.; Cappello, G.; Prost, J. Isotropic stress reduces cell proliferation in tumor spheroids. N. J. Phys. 2012, 14, 055008. [Google Scholar] [CrossRef]
- Tse, J.M.; Cheng, G.; Tyrrell, J.A.; Wilcox-Adelman, S.A.; Boucher, Y.; Jain, R.K.; Munn, L.L. Mechanical compression drives cancer cells toward invasive phenotype. Proc. Natl. Acad. Sci. USA 2012, 109, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Mani, S.A.; Donaher, J.L.; Ramaswamy, S.; Itzykson, R.A.; Come, C.; Savagner, P.; Gitelman, I.; Richardson, A.; Weinberg, R.A. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004, 117, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.H.; Trapani, L.M.; Sieminski, A.L.; Mackellar, D.; Gong, H.; Kamm, R.D.; Wells, A.; Lauffenburger, D.A.; Matsudaira, P. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc. Natl. Acad. Sci. USA 2006, 103, 10889–10894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, A.D.; Wang, F.W.; Matsumoto, K.; Yamada, K.M. One-dimensional topography underlies three-dimensional fibrillar cell migration. J. Cell Biol. 2009, 184, 481–490. [Google Scholar] [CrossRef]
- Fraley, S.I.; Feng, Y.; Krishnamurthy, R.; Kim, D.H.; Celedon, A.; Longmore, G.D.; Wirtz, D. A distinctive role for focal adhesion proteins in three-dimensional cell motility. Nat. Cell Biol. 2010, 12, 598–604. [Google Scholar] [CrossRef] [Green Version]
- Wirtz, D.; Konstantopoulos, K.; Searson, P.C. The physics of cancer: The role of physical interactions and mechanical forces in metastasis. Nat. Rev. Cancer 2011, 11, 512–522. [Google Scholar] [CrossRef]
- Geraldo, S.; Simon, A.; Vignjevic, D.M. Revealing the cytoskeletal organization of invasive cancer cells in 3D. J. Vis. Exp. 2013, 80, e50763. [Google Scholar] [CrossRef]
- Kaufman, L.J.; Brangwynne, C.P.; Kasza, K.E.; Filippidi, E.; Gordon, V.D.; Deisboeck, T.S.; Weitz, D.A. Glioma expansion in collagen I matrices: Analyzing collagen concentration-dependent growth and motility patterns. Biophys. J. 2005, 89, 635–650. [Google Scholar] [CrossRef]
- Kopanska, K.S.; Alcheikh, Y.; Staneva, R.; Vignjevic, D.; Betz, T. Tensile Forces Originating from Cancer Spheroids Facilitate Tumor Invasion. PLoS ONE 2016, 11, e0156442. [Google Scholar] [CrossRef]
- Nyga, A.; Cheema, U.; Loizidou, M. 3D tumour models: Novel In Vitro approaches to cancer studies. Journal of cell communication and signaling, J. Cell Commun. Signal. 2011, 5, 239–248. [Google Scholar] [CrossRef]
- Ciarletta, P.; Foret, L.; Ben Amar, M. The radial growth phase of malignant melanoma: Multi-phase modelling, numerical simulations and linear stability analysis. J. R. Soc. Interface 2011, 8, 345–368. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Herlyn, M. Dynamics of intercellular communication during melanoma development. Mol. Med. Today. 2000, 6, 163–169. [Google Scholar] [CrossRef]
- La Porta, C.A. Mechanism of drug sensitivity and resistance in melanoma. Curr. Cancer Drug Targets 2009, 9, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.H., Jr.; Elder, D.E.; Guerry, D., 4th; Epstein, M.N.; Greene, M.H.; Van Horn, M. A study of tumor progression: The precursor lesions of superficial spreading and nodular melanoma. Hum. Pathol. 1984, 15, 1147–1165. [Google Scholar] [CrossRef]
- Danciu, C.; Falamas, A.; Dehelean, C.; Soica, C.; Radeke, H.; Barbu-Tudoran, L.; Bojin, F.; Pinzaru, S.C.; Munteanu, M.F. A characterization of four B16 murine melanoma cell sublines molecular fingerprint and proliferation behavior. Cancer Cell Int. 2013, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Marrot, L.; Belaidi, J.P.; Meunier, J.R.; Perez, P.; Agapakis-Causse, C. The human melanocyte as a particular target for UVA radiation and an endpoint for photoprotection assessment. Photochem. Photobiol. 1999, 69, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Smit, N.P.; van Nieuwpoort, F.A.; Marrot, L.; Out, C.; Poorthuis, B.; van Pelt, H.; Meunier, J.R.; Pavel, S. Increased melanogenesis is a risk factor for oxidative DNA damage—Study on cultured melanocytes and atypical nevus cells. Photochem. Photobiol. 2008, 84, 550–555. [Google Scholar] [CrossRef]
- Hirata, Y.; Katagiri, K.; Nagaoka, K.; Morishita, T.; Kudoh, Y.; Hatta, T.; Naguro, I.; Kano, K.; Udagawa, T.; Natsume, T.; et al. TRIM48 Promotes ASK1 Activation and Cell Death through Ubiquitination-Dependent Degradation of the ASK1-Negative Regulator PRMT1. Cell Rep. 2017, 21, 2447–2457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noonan, F.P.; Zaidi, M.R.; Wolnicka-Glubisz, A.; Anver, M.R.; Bahn, J.; Wielgus, A.; Cadet, J.; Douki, T.; Mouret, S.; Tucker, M.A.; et al. Melanoma induction by ultraviolet A but not ultraviolet B radiation requires melanin pigment. Nat. Commun. 2012, 3, 884. [Google Scholar] [CrossRef] [Green Version]
- Sekine, Y.; Hatanaka, R.; Watanabe, T.; Sono, N.; Iemura, S.; Natsume, T.; Kuranaga, E.; Miura, M.; Takeda, K.; Ichijo, H. The Kelch repeat protein KLHDC10 regulates oxidative stress-induced ASK1 activation by suppressing PP5. Mol. Cell 2012, 48, 692–704. [Google Scholar] [CrossRef]
- Vicente-Manzanares, M.; Choi, C.K.; Horwitz, A.R. Integrins in cell migration—The actin connection. J. Cell Sci. 2009, 122, 199–206. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morikura, T.; Miyata, S. Effect of Mechanical Compression on Invasion Process of Malignant Melanoma Using In Vitro Three-Dimensional Cell Culture Device. Micromachines 2019, 10, 666. https://doi.org/10.3390/mi10100666
Morikura T, Miyata S. Effect of Mechanical Compression on Invasion Process of Malignant Melanoma Using In Vitro Three-Dimensional Cell Culture Device. Micromachines. 2019; 10(10):666. https://doi.org/10.3390/mi10100666
Chicago/Turabian StyleMorikura, Takashi, and Shogo Miyata. 2019. "Effect of Mechanical Compression on Invasion Process of Malignant Melanoma Using In Vitro Three-Dimensional Cell Culture Device" Micromachines 10, no. 10: 666. https://doi.org/10.3390/mi10100666
APA StyleMorikura, T., & Miyata, S. (2019). Effect of Mechanical Compression on Invasion Process of Malignant Melanoma Using In Vitro Three-Dimensional Cell Culture Device. Micromachines, 10(10), 666. https://doi.org/10.3390/mi10100666




