Progress in the Field of Micro-Electrocorticography
Abstract
1. Introduction
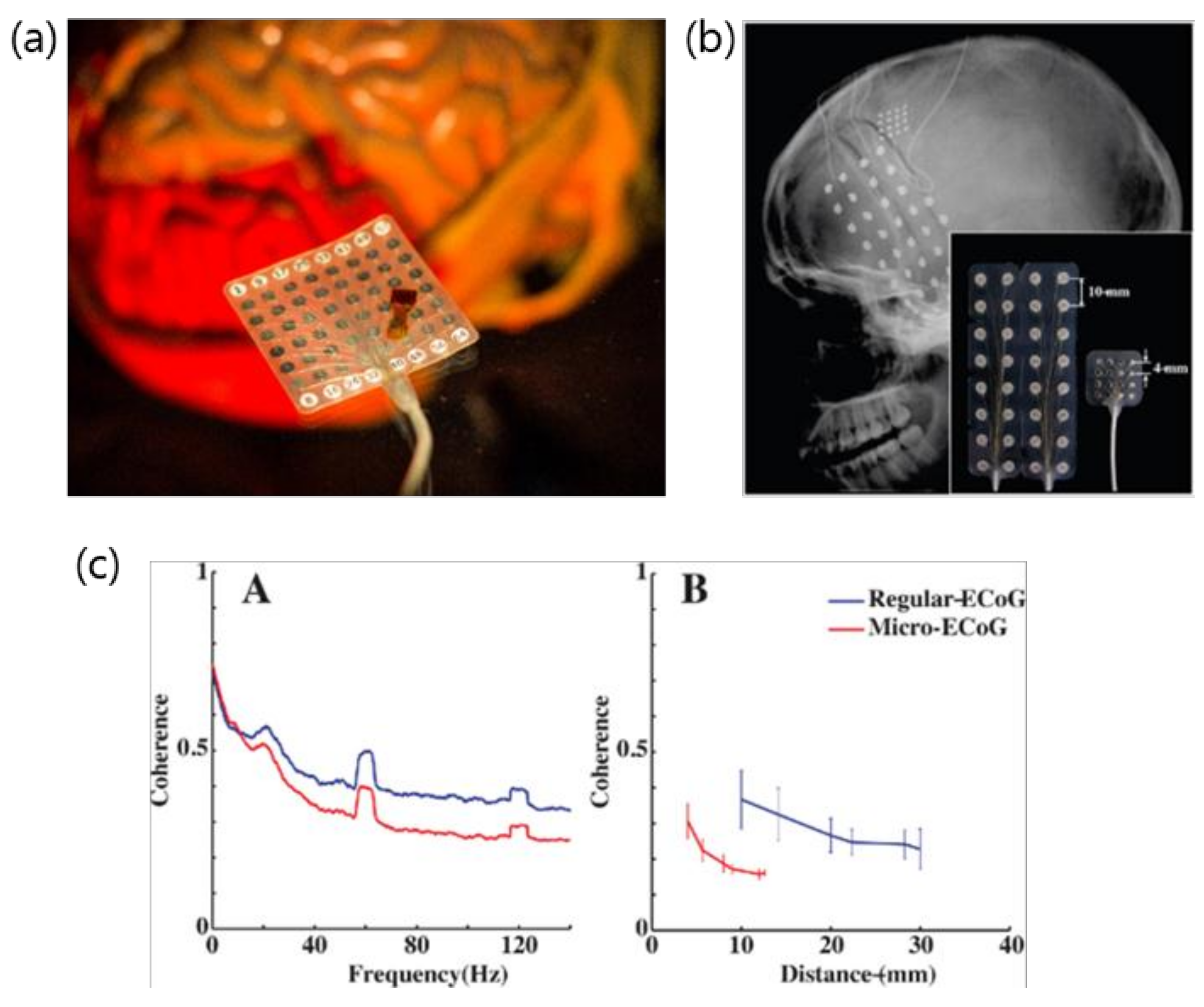
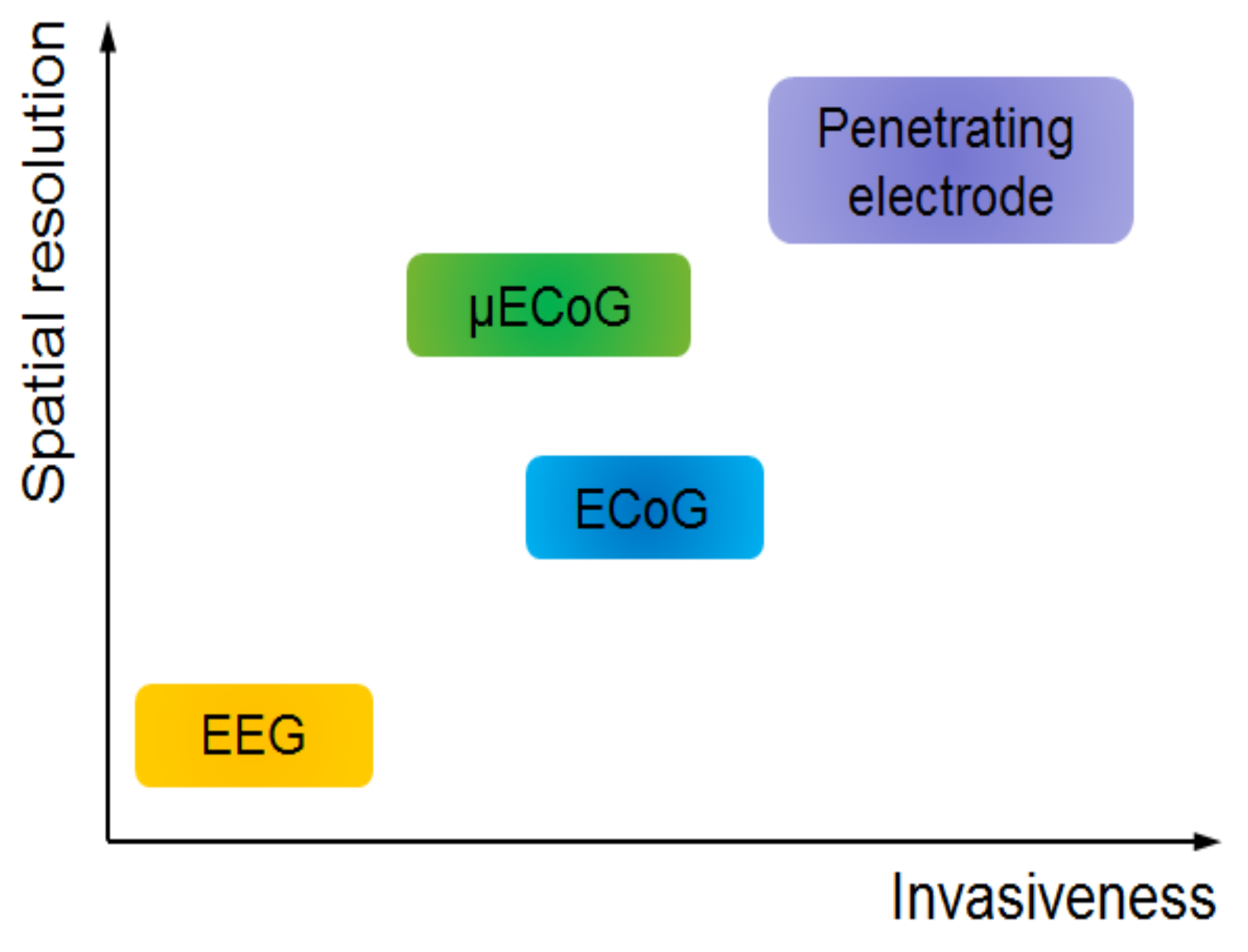
2. Evolution of ECoG into μECoG
3. Micro-ECoG: Electrodes and Substrates
3.1. Platinum
3.2. Sputtered Iridium Oxide
3.3. ITO
3.4. Graphene
3.5. Bioresorbable Silicon
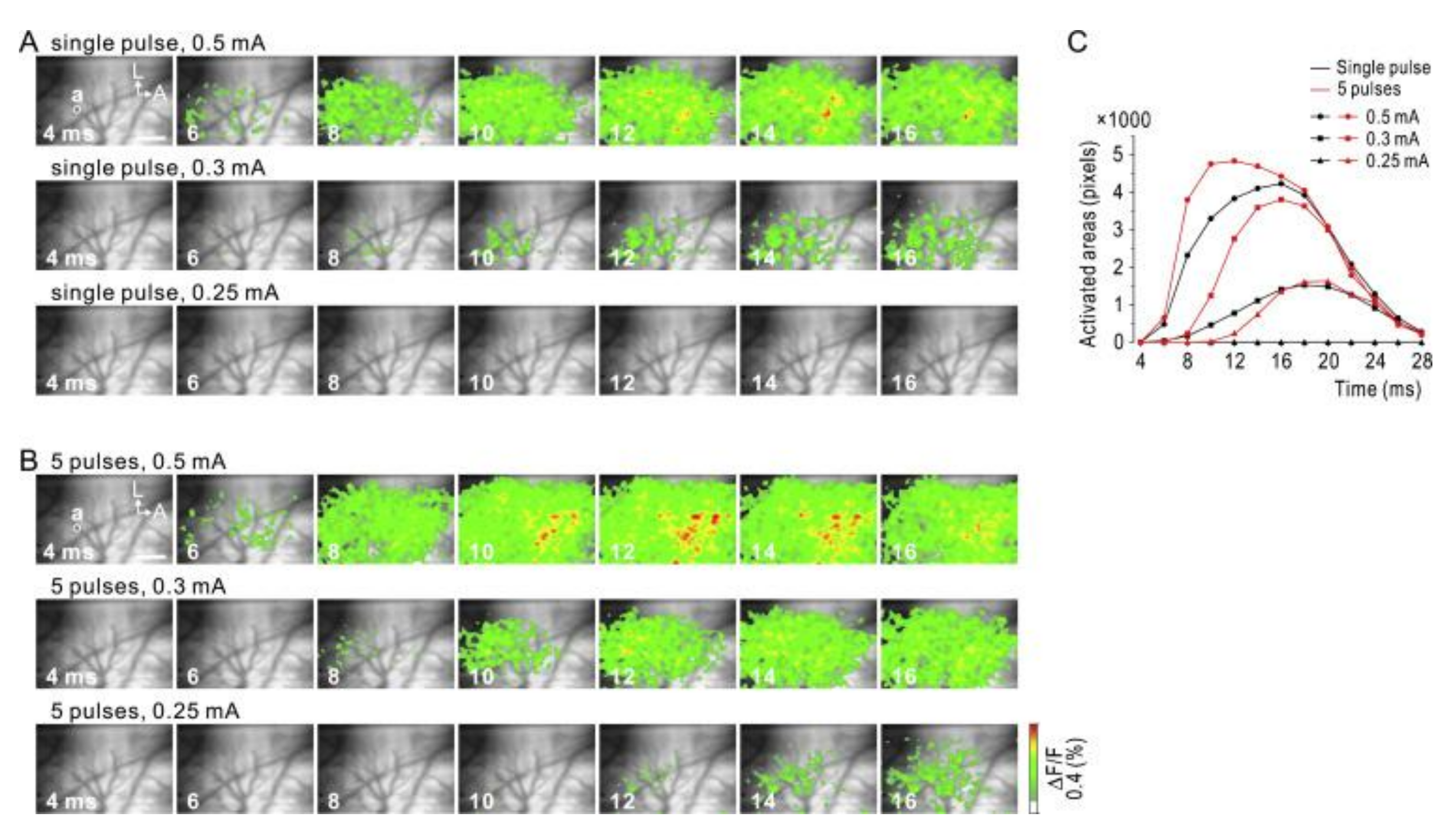
4. Host Response to µECoG Devices
5. Role of ECoG and µECoG in Human Disease and BCI
6. Discussion and Future Direction
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Serruya, M.D.; Hatsopoulos, N.G.; Paninski, L.; Fellows, M.R.; Donoghue, J.P. Brain-machine interface: Instant neural control of a movement signal. Nature 2002, 416, 141–142. [Google Scholar] [CrossRef] [PubMed]
- Carmena, J.M.; Lebedev, M.A.; Crist, R.E.; O’Doherty, J.E.; Santucci, D.M.; Dimitrov, D.F.; Patil, P.G.; Henriquez, C.S.; Nicolelis, M.A. Learning to control a brain–machine interface for reaching and grasping by primates. PLoS Biol. 2003, 1, e42. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, L.R.; Serruya, M.D.; Friehs, G.M.; Mukand, J.A.; Saleh, M.; Caplan, A.H.; Branner, A.; Chen, D.; Penn, R.D.; Donoghue, J.P. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 2006, 442, 164. [Google Scholar] [CrossRef] [PubMed]
- Collinger, J.L.; Wodlinger, B.; Downey, J.E.; Wang, W.; Tyler-Kabara, E.C.; Weber, D.J.; McMorland, A.J.; Velliste, M.; Boninger, M.L.; Schwartz, A.B. High-performance neuroprosthetic control by an individual with tetraplegia. Lancet 2013, 381, 557–564. [Google Scholar] [CrossRef]
- Petroff, O.A.; Spencer, D.D.; Goncharova, I.I.; Zaveri, H.P. A comparison of the power spectral density of scalp EEG and subjacent electrocorticograms. Clin. Neurophysiol. 2016, 127, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Degenhart, A.D.; Collinger, J.L.; Vinjamuri, R.; Sudre, G.P.; Adelson, P.D.; Holder, D.L.; Leuthardt, E.C.; Moran, D.W.; Boninger, M.L. Human motor cortical activity recorded with Micro-ECoG electrodes, during individual finger movements. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 586–589. [Google Scholar]
- Viventi, J.; Kim, D.-H.; Vigeland, L.; Frechette, E.S.; Blanco, J.A.; Kim, Y.-S.; Avrin, A.E.; Tiruvadi, V.R.; Hwang, S.-W.; Vanleer, A.C. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat. Neurosci. 2011, 14, 1599–1605. [Google Scholar] [CrossRef]
- Leuthardt, E.C.; Schalk, G.; Wolpaw, J.R.; Ojemann, J.G.; Moran, D.W. A brain–computer interface using electrocorticographic signals in humansThe authors declare that they have no competing financial interests. J. Neural Eng. 2004, 1, 63. [Google Scholar] [CrossRef]
- Schalk, G.; Miller, K.; Anderson, N.; Wilson, J.; Smyth, M.; Ojemann, J.; Moran, D.; Wolpaw, J.; Leuthardt, E. Two-dimensional movement control using electrocorticographic signals in humans. J. Neural Eng. 2008, 5, 75. [Google Scholar] [CrossRef]
- Towle, V.L.; Yoon, H.-A.; Castelle, M.; Edgar, J.C.; Biassou, N.M.; Frim, D.M.; Spire, J.-P.; Kohrman, M.H. ECoG gamma activity during a language task: Differentiating expressive and receptive speech areas. Brain 2008, 131, 2013–2027. [Google Scholar] [CrossRef]
- Hill, N.J.; Gupta, D.; Brunner, P.; Gunduz, A.; Adamo, M.A.; Ritaccio, A.; Schalk, G. Recording human electrocorticographic (ECoG) signals for neuroscientific research and real-time functional cortical mapping. J. Vis. Exp. 2012, 64, e3993. [Google Scholar] [CrossRef]
- Miran, S.; Akram, S.; Sheikhattar, A.; Simon, J.Z.; Zhang, T.; Babadi, B. Real-Time Tracking of Selective Auditory Attention From M/EEG: A Bayesian Filtering Approach. Front. Neurosci. 2018, 12, 262. [Google Scholar] [CrossRef] [PubMed]
- Buzsaki, G. Rhythms of the Brain; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Ray, S.; Maunsell, J.H. Do gamma oscillations play a role in cerebral cortex? Trends Cogn. Sci. 2015, 19, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Penfield, W.; Steelman, H. The Treatment of Focal Epilepsy by Cortical Excision. Ann. Surg. 1947, 126, 740–761. [Google Scholar] [CrossRef]
- Lycke, R.J.; Schendel, A.; Williams, J.C.; Otto, K.J. In vivo evaluation of a μECoG array for chronic stimulation. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 1294–1297. [Google Scholar]
- Kellis, S.; Sorensen, L.; Darvas, F.; Sayres, C.; O’Neill, K.; Brown, R.B.; House, P.; Ojemann, J.; Greger, B. Multi-scale analysis of neural activity in humans: Implications for micro-scale electrocorticography. Clin. Neurophysiol. 2016, 127, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Schalk, G.; Kubanek, J.; Miller, K.; Anderson, N.; Leuthardt, E.; Ojemann, J.; Limbrick, D.; Moran, D.; Gerhardt, L.; Wolpaw, J. Decoding two-dimensional movement trajectories using electrocorticographic signals in humans. J. Neural Eng. 2007, 4, 264. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, J.; Hu, X.; Zhang, L.; Mao, L.; Jiang, X.; Liou, A.K.; Leak, R.K.; Gao, Y.; Chen, J. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J. Cereb. Blood Flow Metab. 2013, 33, 1864–1874. [Google Scholar] [CrossRef] [PubMed]
- Morishita, S.; Sato, K.; Watanabe, H.; Nishimura, Y.; Isa, T.; Kato, R.; Nakamura, T.; Yokoi, H. Brain-machine interface to control a prosthetic arm with monkey ECoGs during periodic movements. Front. Neurosci. 2014, 8, 417. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Hirata, M.; Saitoh, Y.; Goto, T.; Kishima, H.; Fukuma, R.; Yokoi, H.; Kamitani, Y.; Yoshimine, T. Real-time control of a prosthetic hand using human electrocorticography signals: Technical note. J. Neurosurg. 2011, 114, 1715–1722. [Google Scholar] [CrossRef]
- Rubehn, B.; Bosman, C.; Oostenveld, R.; Fries, P.; Stieglitz, T. A MEMS-based flexible multichannel ECoG-electrode array. J. Neural Eng. 2009, 6, 036003. [Google Scholar] [CrossRef]
- Chao, Z.C.; Nagasaka, Y.; Fujii, N. Long-term asynchronous decoding of arm motion using electrocorticographic signals in monkey. Front. Neuroeng. 2010, 3, 3. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Hirata, M.; Saitoh, Y.; Kishima, H.; Goto, T.; Fukuma, R.; Yokoi, H.; Kamitani, Y.; Yoshimine, T. Prosthetic arm control by paralyzed patients using electrocorticograms. Neurosci. Res. 2010, 68, e83. [Google Scholar] [CrossRef]
- Shimoda, K.; Nagasaka, Y.; Chao, Z.C.; Fujii, N. Decoding continuous three-dimensional hand trajectories from epidural electrocorticographic signals in Japanese macaques. J. Neural Eng. 2012, 9, 036015. [Google Scholar] [CrossRef] [PubMed]
- Breshears, J.D.; Gaona, C.M.; Roland, J.L.; Sharma, M.; Anderson, N.R.; Bundy, D.T.; Freudenburg, Z.V.; Smyth, M.D.; Zempel, J.; Limbrick, D.D. Decoding motor signals from the pediatric cortex: Implications for brain-computer interfaces in children. Pediatrics 2011, 128, e160–e168. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.J.; O’Brien, T.J.; Berkovic, S.F.; Murphy, M.; Morokoff, A.; Fabinyi, G.; D’Souza, W.; Yerra, R.; Archer, J.; Litewka, L. Prediction of seizure likelihood with a long-term, implanted seizure advisory system in patients with drug-resistant epilepsy: A first-in-man study. Lancet Neurol. 2013, 12, 563–571. [Google Scholar] [CrossRef]
- Derix, J.; Iljina, O.; Schulze-Bonhage, A.; Aertsen, A.; Ball, T. “Doctor” or “darling”? Decoding the communication partner from ECoG of the anterior temporal lobe during non-experimental, real-life social interaction. Front. Hum. Neurosci. 2012, 6, 251. [Google Scholar] [CrossRef]
- Bleichner, M.; Freudenburg, Z.; Jansma, J.; Aarnoutse, E.; Vansteensel, M.; Ramsey, N. Give me a sign: Decoding four complex hand gestures based on high-density ECoG. Brain Struct. Funct. 2016, 221, 203–216. [Google Scholar] [CrossRef]
- Leuthardt, E.C.; Gaona, C.; Sharma, M.; Szrama, N.; Roland, J.; Freudenberg, Z.; Solis, J.; Breshears, J.; Schalk, G. Using the electrocorticographic speech network to control a brain–computer interface in humans. J. Neural Eng. 2011, 8, 036004. [Google Scholar] [CrossRef]
- Felton, E.A.; Wilson, J.A.; Williams, J.C.; Garell, P.C. Electrocorticographically controlled brain-computer interfaces using motor and sensory imagery in patients with temporary subdural electrode implants: Report of four cases. J. Neurosurg. 2007, 106, 495–500. [Google Scholar] [CrossRef]
- Vinjamuri, R.; Weber, D.; Degenhart, A.; Collinger, J.; Sudre, G.; Adelson, P.; Holder, D.; Boninger, M.L.; Schwartz, A.; Crammond, D. A fuzzy logic model for hand posture control using human cortical activity recorded by micro-ECoG electrodes. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 4339–4342. [Google Scholar]
- Kellis, S.; Miller, K.; Thomson, K.; Brown, R.; House, P.; Greger, B. Decoding spoken words using local field potentials recorded from the cortical surface. J. Neural Eng. 2010, 7, 056007. [Google Scholar] [CrossRef]
- Kellis, S.; Miller, K.; Thomson, K.; Brown, R.; House, P.; Greger, B. Classification of spoken words using surface local field potentials. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 3827–3830. [Google Scholar]
- Bundy, D.T.; Zellmer, E.; Gaona, C.M.; Sharma, M.; Szrama, N.; Hacker, C.; Freudenburg, Z.V.; Daitch, A.; Moran, D.W.; Leuthardt, E.C. Characterization of the effects of the human dura on macro-and micro-electrocorticographic recordings. J. Neural Eng. 2014, 11, 016006. [Google Scholar] [CrossRef]
- Rouse, A.G.; Williams, J.J.; Wheeler, J.J.; Moran, D.W. Cortical adaptation to a chronic micro-electrocorticographic brain computer interface. J. Neurosci. 2013, 33, 1326–1330. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Sato, M.-A.; Suzuki, T.; Nambu, A.; Nishimura, Y.; Kawato, M.; Isa, T. Reconstruction of movement-related intracortical activity from micro-electrocorticogram array signals in monkey primary motor cortex. J. Neural Eng. 2012, 9, 036006. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Leuthardt, E.C.; Gaona, C.M.; Brunner, P.; Wolpaw, J.R.; Schalk, G. Spatiotemporal dynamics of electrocorticographic high gamma activity during overt and covert word repetition. Neuroimage 2011, 54, 2960–2972. [Google Scholar] [CrossRef] [PubMed]
- Price, C.J. The anatomy of language: Contributions from functional neuroimaging. J. Anat. 2000, 197, 335–359. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.J.; Rouse, A.G.; Thongpang, S.; Williams, J.C.; Moran, D.W. Differentiating closed-loop cortical intention from rest: Building an asynchronous electrocorticographic BCI. J. Neural Eng. 2013, 10, 046001. [Google Scholar] [CrossRef]
- Krusienski, D.J.; Shih, J.J. Control of a visual keyboard using an electrocorticographic brain–computer interface. Neurorehabil. Neural Repair 2011, 25, 323–331. [Google Scholar] [CrossRef]
- Weremfo, A.; Carter, P.; Hibbert, D.B.; Zhao, C. Investigating the interfacial properties of electrochemically roughened platinum electrodes for neural stimulation. Langmuir 2015, 31, 2593–2599. [Google Scholar] [CrossRef] [PubMed]
- Negi, S.; Bhandari, R.; Rieth, L.; Solzbacher, F. In vitro comparison of sputtered iridium oxide and platinum-coated neural implantable microelectrode arrays. Biomed. Mater. 2010, 5, 015007. [Google Scholar] [CrossRef]
- Park, D.-W.; Schendel, A.A.; Mikael, S.; Brodnick, S.K.; Richner, T.J.; Ness, J.P.; Hayat, M.R.; Atry, F.; Frye, S.T.; Pashaie, R. Graphene-based carbon-layered electrode array technology for neural imaging and optogenetic applications. Nat. Commun. 2014, 5, 5258. [Google Scholar] [CrossRef]
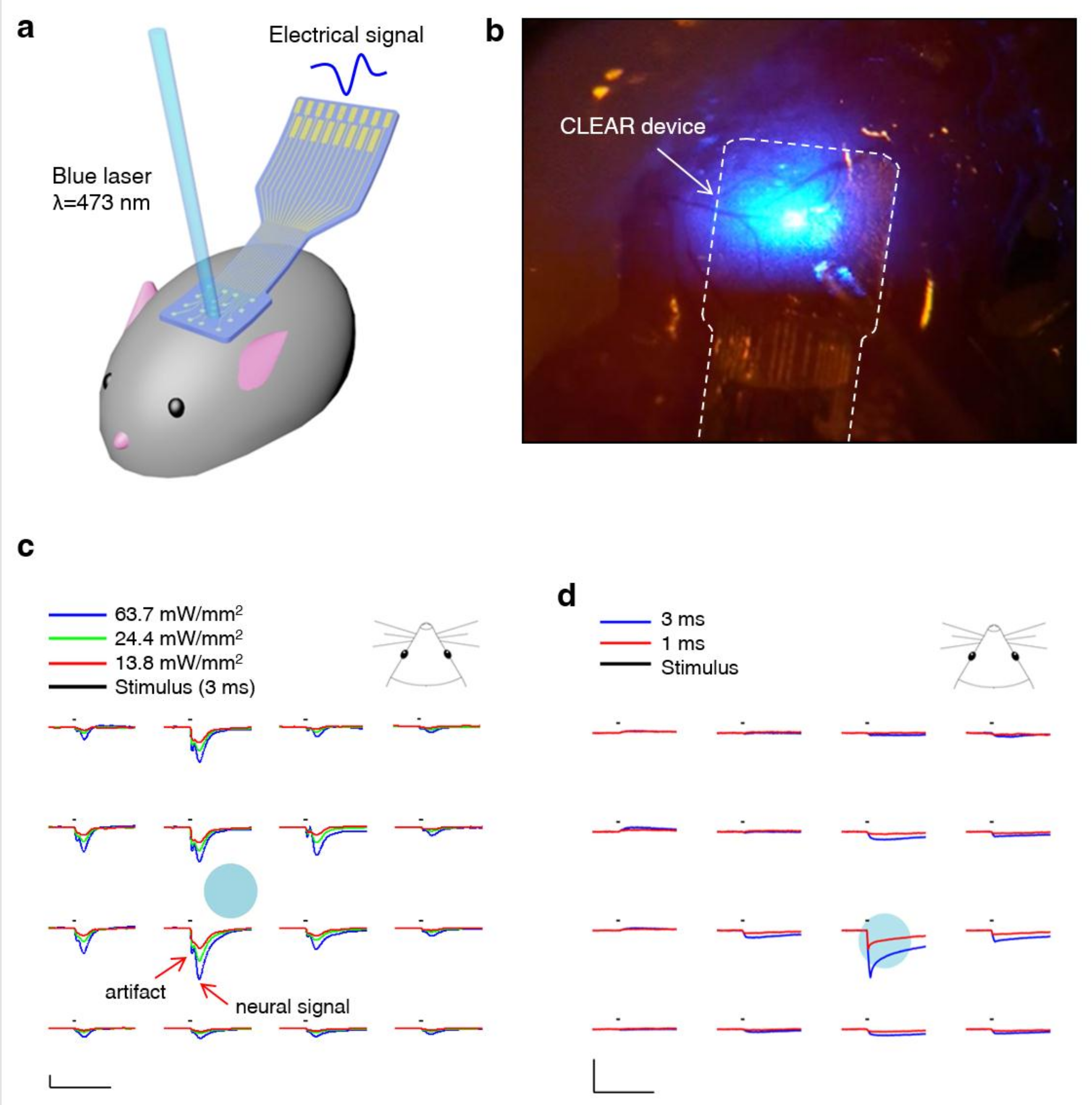
- Richner, T.J.; Thongpang, S.; Brodnick, S.K.; Schendel, A.A.; Falk, R.W.; Krugner-Higby, L.A.; Pashaie, R.; Williams, J.C. Optogenetic micro-electrocorticography for modulating and localizing cerebral cortex activity. J. Neural Eng. 2014, 11, 016010. [Google Scholar] [CrossRef]
- Cogan, S.F.; Plante, T.; Ehrlich, J. Sputtered iridium oxide films (SIROFs) for low-impedance neural stimulation and recording electrodes. In Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, IEMBS’04, San Francisco, CA, USA, 1–5 September 2004; pp. 4153–4156. [Google Scholar]
- Polikov, V.S.; Tresco, P.A.; Reichert, W.M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 2005, 148, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ledochowitsch, P.; Olivero, E.; Blanche, T.; Maharbiz, M.M. A transparent μECoG array for simultaneous recording and optogenetic stimulation. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 2937–2940. [Google Scholar]
- Kwon, K.Y.; Sirowatka, B.; Weber, A.; Li, W. Opto-μECoG array: A hybrid neural interface with transparent μECoG electrode array and integrated LEDs for optogenetics. IEEE Trans. Biomed. Circuits Syst. 2013, 7, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Kunori, N.; Takashima, I. A transparent epidural electrode array for use in conjunction with optical imaging. J. Neurosci. Methods 2015, 251, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Wander, J.; Sarma, D.; Su, D.; Fetz, E.; Ojemann, J.G. Direct electrical stimulation of the somatosensory cortex in humans using electrocorticography electrodes: A qualitative and quantitative report. J. Neural Eng. 2013, 10, 036021. [Google Scholar] [CrossRef] [PubMed]
- Kuzum, D.; Takano, H.; Shim, E.; Reed, J.C.; Juul, H.; Richardson, A.G.; de Vries, J.; Bink, H.; Dichter, M.A.; Lucas, T.H. Transparent and flexible low noise graphene electrodes for simultaneous electrophysiology and neuroimaging. Nat. Commun. 2014, 5, 5259. [Google Scholar] [CrossRef] [PubMed]
- Park, D.-W.; Brodnick, S.K.; Ness, J.P.; Atry, F.; Krugner-Higby, L.; Sandberg, A.; Mikael, S.; Richner, T.J.; Novello, J.; Kim, H.; et al. Fabrication and utility of a transparent graphene neural electrode array for electrophysiology, in vivo imaging, and optogenetics. Nat. Protoc. 2016, 11, 2201. [Google Scholar] [CrossRef] [PubMed]
- Park, D.-W.; Ness, J.P.; Brodnick, S.K.; Esquibel, C.; Novello, J.; Atry, F.; Baek, D.-H.; Kim, H.; Bong, J.; Swanson, K.I.; et al. Electrical Neural Stimulation and Simultaneous in Vivo Monitoring with Transparent Graphene Electrode Arrays Implanted in GCaMP6f Mice. ACS Nano 2018, 12, 148–157. [Google Scholar] [CrossRef]
- Britt, J.P.; McDevitt, R.A.; Bonci, A. Use of channelrhodopsin for activation of CNS neurons. Curr. Protoc. Neurosci. 2012, 58, 2.16.1–2.16.19. [Google Scholar]
- Thunemann, M.; Lu, Y.; Liu, X.; Kılıç, K.; Desjardins, M.; Vandenberghe, M.; Sadegh, S.; Saisan, P.A.; Cheng, Q.; Weldy, K.L.; et al. Deep 2-photon imaging and artifact-free optogenetics through transparent graphene microelectrode arrays. Nat. Commun. 2018, 9, 2035. [Google Scholar] [CrossRef]
- Chang, W.H.; Kim, H.; Sun, W.; Kim, J.Y.; Shin, Y.-I.; Kim, Y.-H. Effects of extradural cortical stimulation on motor recovery in a rat model of subacute stroke. Restor. Neurol. Neurosci. 2015, 33, 589–596. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Y.; Cai, W.; Borysiak, M.; Han, B.; Chen, D.; Piner, R.D.; Colombo, L.; Ruoff, R.S. Transfer of large-area graphene films for high-performance transparent conductive electrodes. Nano Lett. 2009, 9, 4359–4363. [Google Scholar] [CrossRef] [PubMed]
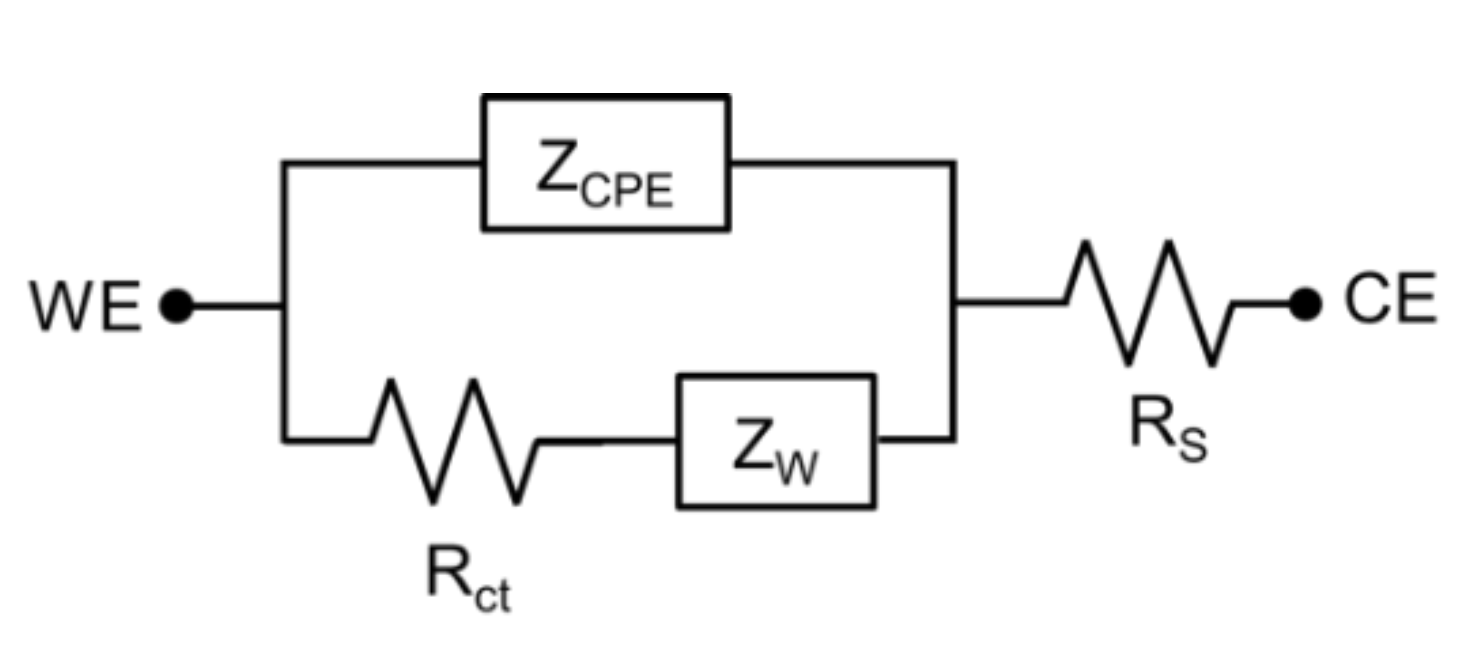
- Williams, J.C.; Hippensteel, J.A.; Dilgen, J.; Shain, W.; Kipke, D.R. Complex impedance spectroscopy for monitoring tissue responses to inserted neural implants. J. Neural Eng. 2007, 4, 410. [Google Scholar] [CrossRef] [PubMed]
- Cogan, S.F. Neural stimulation and recording electrodes. Annu. Rev. Biomed. Eng. 2008, 10, 275–309. [Google Scholar] [CrossRef]
- Khodagholy, D.; Gelinas, J.N.; Thesen, T.; Doyle, W.; Devinsky, O.; Malliaras, G.G.; Buzsáki, G. NeuroGrid: Recording action potentials from the surface of the brain. Nat. Neurosci. 2015, 18, 310–315. [Google Scholar] [CrossRef]
- Gierthmuehlen, M.; Ball, T.; Henle, C.; Wang, X.; Rickert, J.; Raab, M.; Freiman, T.; Stieglitz, T.; Kaminsky, J. Evaluation of μECoG electrode arrays in the minipig: Experimental procedure and neurosurgical approach. J. Neurosci. Methods 2011, 202, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Castagnola, E.; Maiolo, L.; Maggiolini, E.; Minotti, A.; Marrani, M.; Maita, F.; Pecora, A.; Angotzi, G.N.; Ansaldo, A.; Boffini, M. PEDOT-CNT-coated low-impedance, ultra-flexible, and brain-conformable micro-ECoG arrays. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 23, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Orsborn, A.L.; Wang, C.; Chiang, K.; Maharbiz, M.M.; Viventi, J.; Pesaran, B. Semi-chronic chamber system for simultaneous subdural electrocorticography, local field potentials, and spike recordings. In Proceedings of the 2015 7th International IEEE/EMBS Conference on Neural Engineering (NER), Montpellier, France, 22–24 April 2015; pp. 398–401. [Google Scholar]
- Schendel, A.A.; Thongpang, S.; Brodnick, S.K.; Richner, T.J.; Lindevig, B.D.; Krugner-Higby, L.; Williams, J.C. A cranial window imaging method for monitoring vascular growth around chronically implanted micro-ECoG devices. J. Neurosci. Methods 2013, 218, 121–130. [Google Scholar] [CrossRef]
- Thongpang, S.; Richner, T.J.; Brodnick, S.K.; Schendel, A.; Kim, J.; Wilson, J.A.; Hippensteel, J.; Krugner-Higby, L.; Moran, D.; Ahmed, A.S. A micro-electrocorticography platform and deployment strategies for chronic BCI applications. Clin. EEG Neurosci. 2011, 42, 259–265. [Google Scholar] [CrossRef]
- Schendel, A.A.; Nonte, M.W.; Vokoun, C.; Richner, T.J.; Brodnick, S.K.; Atry, F.; Frye, S.; Bostrom, P.; Pashaie, R.; Thongpang, S. The effect of micro-ECoG substrate footprint on the meningeal tissue response. J. Neural Eng. 2014, 11, 046011. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Viventi, J.; Amsden, J.J.; Xiao, J.; Vigeland, L.; Kim, Y.-S.; Blanco, J.A.; Panilaitis, B.; Frechette, E.S.; Contreras, D. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat. Mater. 2010, 9, 511–517. [Google Scholar] [CrossRef]
- Yu, K.J.; Kuzum, D.; Hwang, S.-W.; Kim, B.H.; Juul, H.; Kim, N.H.; Won, S.M.; Chiang, K.; Trumpis, M.; Richardson, A.G. Bioresorbable silicon electronics for transient spatiotemporal mapping of electrical activity from the cerebral cortex. Nat. Mater. 2016, 15, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-W.; Tao, H.; Kim, D.-H.; Cheng, H.; Song, J.-K.; Rill, E.; Brenckle, M.A.; Panilaitis, B.; Won, S.M.; Kim, Y.-S. A physically transient form of silicon electronics. Science 2012, 337, 1640–1644. [Google Scholar] [CrossRef] [PubMed]
- Tsytsarev, V.; Taketani, M.; Schottler, F.; Tanaka, S.; Hara, M. A new planar multielectrode array: Recording from a rat auditory cortex. J. Neural Eng. 2006, 3, 293. [Google Scholar] [CrossRef]
- Nicolelis, M.A.; Dimitrov, D.; Carmena, J.M.; Crist, R.; Lehew, G.; Kralik, J.D.; Wise, S.P. Chronic, multisite, multielectrode recordings in macaque monkeys. Proc. Natl. Acad. Sci. USA 2003, 100, 11041–11046. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.C.; Rennaker, R.L.; Kipke, D.R. Long-term neural recording characteristics of wire microelectrode arrays implanted in cerebral cortex. Brain Res. Protoc. 1999, 4, 303–313. [Google Scholar] [CrossRef]
- Bai, Q.; Wise, K.D.; Anderson, D.J. A high-yield microassembly structure for three-dimensional microelectrode arrays. IEEE Trans. Biomed. Eng. 2000, 47, 281–289. [Google Scholar] [PubMed]
- Vetter, R.J.; Williams, J.C.; Hetke, J.F.; Nunamaker, E.A.; Kipke, D.R. Chronic neural recording using silicon-substrate microelectrode arrays implanted in cerebral cortex. IEEE Trans. Biomed. Eng. 2004, 51, 896–904. [Google Scholar] [CrossRef]
- Ludwig, K.A.; Langhals, N.B.; Joseph, M.D.; Richardson-Burns, S.M.; Hendricks, J.L.; Kipke, D.R. Poly (3, 4-ethylenedioxythiophene)(PEDOT) polymer coatings facilitate smaller neural recording electrodes. J. Neural Eng. 2011, 8, 014001. [Google Scholar] [CrossRef]
- Kozai, T.D.Y.; Langhals, N.B.; Patel, P.R.; Deng, X.; Zhang, H.; Smith, K.L.; Lahann, J.; Kotov, N.A.; Kipke, D.R. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nat. Mater. 2012, 11, 1065–1073. [Google Scholar] [CrossRef]
- Jones, K.E.; Campbell, P.K.; Normann, R.A. A glass/silicon composite intracortical electrode array. Ann. Biomed. Eng. 1992, 20, 423–437. [Google Scholar] [CrossRef]
- Normann, R.A.; Maynard, E.M.; Rousche, P.J.; Warren, D.J. A neural interface for a cortical vision prosthesis. Vis. Res. 1999, 39, 2577–2587. [Google Scholar] [CrossRef]
- Suner, S.; Fellows, M.R.; Vargas-Irwin, C.; Nakata, G.K.; Donoghue, J.P. Reliability of signals from a chronically implanted, silicon-based electrode array in non-human primate primary motor cortex. IEEE Trans. Neural Syst. Rehabil. Eng. 2005, 13, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Herwik, S.; Kisban, S.; Aarts, A.; Seidl, K.; Girardeau, G.; Benchenane, K.; Zugaro, M.; Wiener, S.; Paul, O.; Neves, H. Fabrication technology for silicon-based microprobe arrays used in acute and sub-chronic neural recording. J. Micromech. Microeng. 2009, 19, 074008. [Google Scholar] [CrossRef]
- Corps, K.N.; Roth, T.L.; McGavern, D.B. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015, 72, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, F.; Mander, K.A.; Leonard, A.V.; Vink, R. Neurogenic inflammation after traumatic brain injury and its potentiation of classical inflammation. J. Neuroinflamm. 2016, 13, 264. [Google Scholar] [CrossRef]
- Corrigan, F.; Vink, R.; Turner, R.J. Inflammation in acute CNS injury: A focus on the role of substance P. Br. J. Pharmacol. 2016, 173, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Leach, J.; Achyuta, A.K.H.; Murthy, S.K. Bridging the divide between neuroprosthetic design, tissue engineering and neurobiology. Front. Neuroeng. 2010, 2, 18. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Xie, Y.; Schumacher, A.; Löffler, S.; Kirch, R.D.; Al-Hasani, J.; Rapoport, D.H.; Kruse, C.; Moser, A.; Tronnier, V.; et al. A simple implantation method for flexible, multisite microelectrodes into rat brains. Front. Neuroeng. 2013, 6, 6. [Google Scholar] [CrossRef]
- Jorfi, M.; Skousen, J.L.; Weder, C.; Capadona, J.R. Progress towards biocompatible intracortical microelectrodes for neural interfacing applications. J. Neural Eng. 2014, 12, 011001. [Google Scholar] [CrossRef]
- Moshayedi, P.; Ng, G.; Kwok, J.C.; Yeo, G.S.; Bryant, C.E.; Fawcett, J.W.; Franze, K.; Guck, J. The relationship between glial cell mechanosensitivity and foreign body reactions in the central nervous system. Biomaterials 2014, 35, 3919–3925. [Google Scholar] [CrossRef]
- Polikov, V.S.; Block, M.L.; Fellous, J.-M.; Hong, J.-S.; Reichert, W.M. In vitro model of glial scarring around neuroelectrodes chronically implanted in the CNS. Biomaterials 2006, 27, 5368–5376. [Google Scholar] [CrossRef] [PubMed]
- Arulmoli, J.; Pathak, M.M.; McDonnell, L.P.; Nourse, J.L.; Tombola, F.; Earthman, J.C.; Flanagan, L.A. Static stretch affects neural stem cell differentiation in an extracellular matrix-dependent manner. Sci. Rep. 2015, 5, 8499. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.G.; Reddy, G.G.; Kawasaki, H.; Oya, H.; Miller, L.E.; Howard III, M.A. Decoding movement-related cortical potentials from electrocorticography. Neurosurg. Focus 2009, 27, E11. [Google Scholar] [CrossRef] [PubMed]
- Klopfleisch, R. Macrophage reaction against biomaterials in the mouse model–Phenotypes, functions and markers. Acta Biomater. 2016, 43, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Bekar, L.K.; Furber, K.; Walz, W. Vimentin-expressing proximal reactive astrocytes correlate with migration rather than proliferation following focal brain injury. Brain Res. 2004, 1024, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Schouenborg, J.; Garwicz, M.; Danielsen, N. Reducing surface area while maintaining implant penetrating profile lowers the brain foreign body response to chronically implanted planar silicon microelectrode arrays. Brain Mach. Interfaces Implic. Sci. Clin. Pract. Soc. 2011, 194, 167. [Google Scholar]
- Busch, S.A.; Silver, J. The role of extracellular matrix in CNS regeneration. Curr. Opin. Neurobiol. 2007, 17, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Seymour, J.P.; Kipke, D.R. Neural probe design for reduced tissue encapsulation in CNS. Biomaterials 2007, 28, 3594–3607. [Google Scholar] [CrossRef]
- Goldring, S.; Gregorie, E.M. Surgical management of epilepsy using epidural recordings to localize the seizure focus: Review of 100 cases. J. Neurosurg. 1984, 60, 457–466. [Google Scholar] [CrossRef]
- Goldring, S. A method for surgical management of focal epilepsy, especially as it relates to children. J. Neurosurg. 1978, 49, 344–356. [Google Scholar] [CrossRef]
- Kubanek, J.; Miller, K.; Ojemann, J.; Wolpaw, J.; Schalk, G. Decoding flexion of individual fingers using electrocorticographic signals in humans. J. Neural Eng. 2009, 6, 066001. [Google Scholar] [CrossRef] [PubMed]
- Schalk, G.; McFarland, D.J.; Hinterberger, T.; Birbaumer, N.; Wolpaw, J.R. BCI2000: A general-purpose brain-computer interface (BCI) system. IEEE Trans. Biomed. Eng. 2004, 51, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.M.; Peppard, A.; Magnuson, B. Nutrition considerations in traumatic brain injury. Nutr. Clin. Pract. 2008, 23, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Heck, C.N.; King-Stephens, D.; Massey, A.D.; Nair, D.R.; Jobst, B.C.; Barkley, G.L.; Salanova, V.; Cole, A.J.; Smith, M.C.; Gwinn, R.P. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: Final results of the RNS System Pivotal trial. Epilepsia 2014, 55, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Morrell, M.J. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 2011, 77, 1295–1304. [Google Scholar] [CrossRef]
- Dohmen, C.; Sakowitz, O.W.; Fabricius, M.; Bosche, B.; Reithmeier, T.; Ernestus, R.I.; Brinker, G.; Dreier, J.P.; Woitzik, J.; Strong, A.J. Spreading depolarizations occur in human ischemic stroke with high incidence. Ann. Neurol. 2008, 63, 720–728. [Google Scholar] [CrossRef]
- Fabricius, M.; Fuhr, S.; Bhatia, R.; Boutelle, M.; Hashemi, P.; Strong, A.J.; Lauritzen, M. Cortical spreading depression and peri-infarct depolarization in acutely injured human cerebral cortex. Brain 2006, 129, 778–790. [Google Scholar] [CrossRef]
- Fabricius, M.; Fuhr, S.; Willumsen, L.; Dreier, J.P.; Bhatia, R.; Boutelle, M.G.; Hartings, J.A.; Bullock, R.; Strong, A.J.; Lauritzen, M. Association of seizures with cortical spreading depression and peri-infarct depolarisations in the acutely injured human brain. Clin. Neurophysiol. 2008, 119, 1973–1984. [Google Scholar] [CrossRef]
- Strong, A.J.; Fabricius, M.; Boutelle, M.G.; Hibbins, S.J.; Hopwood, S.E.; Jones, R.; Parkin, M.C.; Lauritzen, M. Spreading and synchronous depressions of cortical activity in acutely injured human brain. Stroke 2002, 33, 2738–2743. [Google Scholar] [CrossRef]
- Baba, T.; Kameda, M.; Yasuhara, T.; Morimoto, T.; Kondo, A.; Shingo, T.; Tajiri, N.; Wang, F.; Miyoshi, Y.; Borlongan, C.V. Electrical stimulation of the cerebral cortex exerts antiapoptotic, angiogenic, and anti-inflammatory effects in ischemic stroke rats through phosphoinositide 3-kinase/Akt signaling pathway. Stroke 2009, 40, e598–e605. [Google Scholar] [CrossRef]
- Kang, C.; Yang, C.-Y.; Kim, J.H.; Moon, S.-K.; Lee, S.; Park, S.-A.; Han, E.-H.; Zhang, L.-Q. The effect of continuous epidural electrical stimulation on neuronal proliferation in cerebral ischemic rats. Ann. Rehabil. Med. 2013, 37, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Kleim, J.A.; Bruneau, R.; VandenBerg, P.; MacDonald, E.; Mulrooney, R.; Pocock, D. Motor cortex stimulation enhances motor recovery and reduces peri-infarct dysfunction following ischemic insult. Neurol. Res. 2003, 25, 789–793. [Google Scholar] [CrossRef] [PubMed]
- O’Bryant, A.J.; Adkins, D.L.; Sitko, A.A.; Combs, H.L.; Nordquist, S.K.; Jones, T.A. Enduring Poststroke Motor Functional Improvements by a Well–Timed Combination of Motor Rehabilitative Training and Cortical Stimulation in Rats. Neurorehabil. Neural Repair 2016, 30, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Plautz, E.J.; Barbay, S.; Frost, S.B.; Friel, K.M.; Dancause, N.; Zoubina, E.V.; Stowe, A.M.; Quaney, B.M.; Nudo, R.J. Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: A feasibility study in primates. Neurol. Res. 2003, 25, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Teskey, G.C.; Flynn, C.; Goertzen, C.D.; Monfils, M.H.; Young, N.A. Cortical stimulation improves skilled forelimb use following a focal ischemic infarct in the rat. Neurol. Res. 2003, 25, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.; Lutsep, H.L.; Weinand, M.; Cramer, S.C. Motor cortex stimulation for the enhancement of recovery from stroke: A prospective, multicenter safety study. Neurosurgery 2006, 58, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.; Lutsep, H.; Cramer, S.C.; Weinand, M. Motor cortex stimulation for enhancement of recovery after stroke: Case report. Neurol. Res. 2003, 25, 815–818. [Google Scholar] [CrossRef]
- Huang, M.; Harvey, R.L.; Stoykov, M.E.; Ruland, S.; Weinand, M.; Lowry, D.; Levy, R. Cortical stimulation for upper limb recovery following ischemic stroke: A small phase II pilot study of a fully implanted stimulator. Top. Stroke Rehabil. 2008, 15, 160–172. [Google Scholar] [CrossRef]
- Levy, R.; Ruland, S.; Weinand, M.; Lowry, D.; Dafer, R.; Bakay, R. Cortical stimulation for the rehabilitation of patients with hemiparetic stroke: A multicenter feasibility study of safety and efficacy. J. Neurosurg. 2008, 108, 707–714. [Google Scholar] [CrossRef]
- Levy, R.M.; Harvey, R.L.; Kissela, B.M.; Winstein, C.J.; Lutsep, H.L.; Parrish, T.B.; Cramer, S.C.; Venkatesan, L. Epidural Electrical Stimulation for Stroke Rehabilitation Results of the Prospective, Multicenter, Randomized, Single-Blinded Everest Trial. Neurorehabil. Neural Repair 2016, 30, 107–119. [Google Scholar] [CrossRef]
- Plow, E.B.; Carey, J.R.; Nudo, R.J.; Pascual-Leone, A. Invasive cortical stimulation to promote recovery of function after stroke a critical appraisal. Stroke 2009, 40, 1926–1931. [Google Scholar] [CrossRef]
- Miranda, R.A.; Casebeer, W.D.; Hein, A.M.; Judy, J.W.; Krotkov, E.P.; Laabs, T.L.; Manzo, J.E.; Pankratz, K.G.; Pratt, G.A.; Sanchez, J.C. DARPA-funded efforts in the development of novel brain–computer interface technologies. J. Neurosci. Methods 2015, 244, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Leuthardt, E.C.; Freudenberg, Z.; Bundy, D.; Roland, J. Microscale recording from human motor cortex: Implications for minimally invasive electrocorticographic brain-computer interfaces. Neurosurg. Focus 2009, 27, E10. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Collinger, J.L.; Degenhart, A.D.; Tyler-Kabara, E.C.; Schwartz, A.B.; Moran, D.W.; Weber, D.J.; Wodlinger, B.; Vinjamuri, R.K.; Ashmore, R.C. An electrocorticographic brain interface in an individual with tetraplegia. PLoS One 2013, 8, e55344. [Google Scholar] [CrossRef] [PubMed]
- Fifer, M.S.; Acharya, S.; Benz, H.L.; Mollazadeh, M.; Crone, N.E.; Thakor, N.V. Towards electrocorticographic control of a dexterous upper limb prosthesis. IEEE Pulse 2012, 3, 38–42. [Google Scholar] [CrossRef]
- Maharbiz, M.M.; Muller, R.; Alon, E.; Rabaey, J.M.; Carmena, J.M. Reliable Next-Generation Cortical Interfaces for Chronic Brain–Machine Interfaces and Neuroscience. Proc. IEEE 2017, 105, 73–82. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Shokoueinejad, M.; Iskandar, B.J.; Medow, J.E.; Webster, J.G. A Novel Intracranial Pressure Readout Circuit for Passive Wireless LC Sensor. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 1123–1132. [Google Scholar] [CrossRef]
- Iskandar, B.J.; Medow, J.; Luzzio, C.; Webster, J.G.; Maragheh, M.S.; Wang, F.; Zhang, X. Cerebrospinal-Fluid Shunt Valve System. U.S. Patent 15/473,126, 4 October 2018. [Google Scholar]
- Ma, Z.; Williams, J.C.; Park, D.-W.; Schendel, A.A.; Mikael, S.T. Transparent and Flexible Neural Electrode Arrays. US Patent 9,861,288, 9 January 2018. [Google Scholar]
| Layout | Substrate Materials | Recording Site Materials | Size/Impedance | Notes | Reference (Year) |
|---|---|---|---|---|---|
| 2D planar array | Polyimide | Pt | 1 mm2 1.5–5 kΩ | 255 channels LFP and ECoG recording awake monkey for 4 months | [22] (2009) |
| 2D planar array | Parylene C | Au-PEDOT:PSS | 10 × 10 µm2 0.2 MΩ | LFP and ECoG recording in freely moving rat and humans 256 channels | [61] (2015) |
| 2D planar array | Parylene C | Graphene | Diameter: 150–200 µm, 100–600 kΩ | Transparency evoked potential by light (Optogenetics)(lifetime >70 days) | [44] (2014) |
| Parylene C | Pt | Diameter: 150–200 µm 50–300 kΩ | (lifetime >70 days) | ||
| 2D planar array | Silicone rubber | Pt | - | SEP recording (µECoG) and stimulation | [62] (2011) |
| 2D planar array | Parylene C | Sputtered indium tin oxide (ITO) | 49-channel (Pitch of 800 μm ) 16-channel (Pitch of 200 μm) | Design, fabrication, and characterization | [48] (2011) |
| 2D planar array | Parylene C | Sputtered indium tin oxide (ITO) | Diameter: 200 µm 100–200 kΩ | Optogenetics with integrated LEDs | [49] (2013) |
| 2D planar array | Polyimide | Au-PEDOT | 100 µm × 100 µm ~2.1 kΩ | recording from rat somatosensory cortex in vivo | [63] (2015) |
| 2D planar array | Parylene C | PEDOT:PSS | 10 × 10 µm 210–50 kΩ | Spike recording from surface (NeuroGrid),256 channel | [61] (2015) |
| 2D planar array | Polyimide | Pt | 300 × 300 µm2 ~20 kΩ | Multiplexing with integrated transistors Electrographic seizures | [7] (2011) |
| 2D planar array in a chamber system | Polyimide | Au | Diameter: 200 µm 24–45 kΩ | 124-channel µECoG and 32-channel microdrive, Multi-unit, LFP, µECoG comparison | [64] (2015) |
| 2D planar array, perforated | Parylene C | Pt | Diameter: 200 µm | 16 channel, optimizing vascular imaging. | [65] (2013) |
| 2D planar array | Polyimide | Pt and Au | Diameter: 300 µm 5–10 kΩ | 32-channel µECoG | [66] (2011) |
| 2D planar array | Parylene C | Pt | Diameter: 200 µm<1000 kΩ | 16 channel µECoG arrays, varying array footprint. | [67] (2014) |
| 2D planar array | Silk | Au | 30 electrodes | Mesh structure for conformal contact | [68] (2010) |
| 2D planar array | Polyimide | Pt | 360 channels each electrode 300 um × 300 um | Multiplexed using Si transistors | [7] (2011) |
| 2D planar array | PLGA | Si | 256 channels overall 3 cm × 3.5 cm | Bioresorbable | [69,70] (2016/2012) |
| Electrode Type | Layout | Substrate Materials | Recording Site Materials | Size/Impedance | Notes | Reference (Year) |
|---|---|---|---|---|---|---|
| Micro wire | 3D array | N/A | Stainless | 50 µm ×50 µm 64 channels | Primary auditory cortex (rat, ECoG recording) | [71] (2006) |
| 3D array | N/A | Stainless Or Tungsten | 50 µm × 50 µm Teflon coated | Single cortical neurons (monkey) | [72] (2003) | |
| 3D array | N/A | Tungsten | 35 µm2 | Cerebral cortex (rat) | [73] (1999) | |
| Michigan | Assembled 3D array | Si | Ir | 100 µm2, 2 MΩ | LFP | [74] (2000) |
| Michigan | Assembled 3D array | 15 µm thickness of Si | Ir | 177 µm2, 0.72 MΩ 312 µm2, 1.65 MΩ | Cerebral cortex (rat) Chronic recording (127 days) | [75] (2004) |
| Michigan | 2D array | Si | PEDOT & Au | Gold, 9.1 MΩ PEDOT, 0.37 MΩ | Single unit implanted in layer V (rat) | [76] (2011) |
| Michigan | 2D array | Si | PEDOT | - | PEDOT VS Carbon A new set of materials to make fundamental Chronic single unit spikes in cortex | [77] (2012) |
| Utah | 10 × 10 3D array | Doped Si | Ti/Pt (50/240 nm) | Width 80 µm, length 1500 µm | Insulated with polyimide | [78] (1992) |
| Utah | 10 × 10 3D array | Doped Si | Pt/Ir | 100–300 kΩ | Tip exposed (500 µm) Cat auditory & visual cortex | [79] (1999) |
| Utah | 10 × 10 3D array | Doped Si | Pt/Ir | 1600 µm2 100–750 kΩ | Tip exposed (40 µm) Primary motor cortex (M1, monkey) | [80] (2005) |
| Utah | 10 × 103D array | Doped Si | Pt | 125 kΩ 2 mC·cm−2 | Cortical stimulation/recording (>90 days in vitro) | [43] (2010) |
| Doped Si | Sputtered iridium oxide film (SIROF) | 6 kΩ 0.3 mC·cm−2 | Cortical stimulation/recording (>90 days in vitro) | |||
| Utah | Unrestricted freedom in the 2D probe | 300 µm thickness of Si | Ti/Au/Pt (30/200/100 nm) | 1–2 MΩ | 72 channels Recording LFP in layers 1, 2, and 3 for 15 days | [81] (2009) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shokoueinejad, M.; Park, D.-W.; Jung, Y.H.; Brodnick, S.K.; Novello, J.; Dingle, A.; Swanson, K.I.; Baek, D.-H.; Suminski, A.J.; Lake, W.B.; et al. Progress in the Field of Micro-Electrocorticography. Micromachines 2019, 10, 62. https://doi.org/10.3390/mi10010062
Shokoueinejad M, Park D-W, Jung YH, Brodnick SK, Novello J, Dingle A, Swanson KI, Baek D-H, Suminski AJ, Lake WB, et al. Progress in the Field of Micro-Electrocorticography. Micromachines. 2019; 10(1):62. https://doi.org/10.3390/mi10010062
Chicago/Turabian StyleShokoueinejad, Mehdi, Dong-Wook Park, Yei Hwan Jung, Sarah K. Brodnick, Joseph Novello, Aaron Dingle, Kyle I. Swanson, Dong-Hyun Baek, Aaron J. Suminski, Wendell B. Lake, and et al. 2019. "Progress in the Field of Micro-Electrocorticography" Micromachines 10, no. 1: 62. https://doi.org/10.3390/mi10010062
APA StyleShokoueinejad, M., Park, D.-W., Jung, Y. H., Brodnick, S. K., Novello, J., Dingle, A., Swanson, K. I., Baek, D.-H., Suminski, A. J., Lake, W. B., Ma, Z., & Williams, J. (2019). Progress in the Field of Micro-Electrocorticography. Micromachines, 10(1), 62. https://doi.org/10.3390/mi10010062





