Bacterial Concentration Detection using a PCB-based Contactless Conductivity Sensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Bacterial Culture and Sample Preparation
2.3. E.coli Preparation for SEM
2.4. Bacteria Loading
3. Results and Discussion
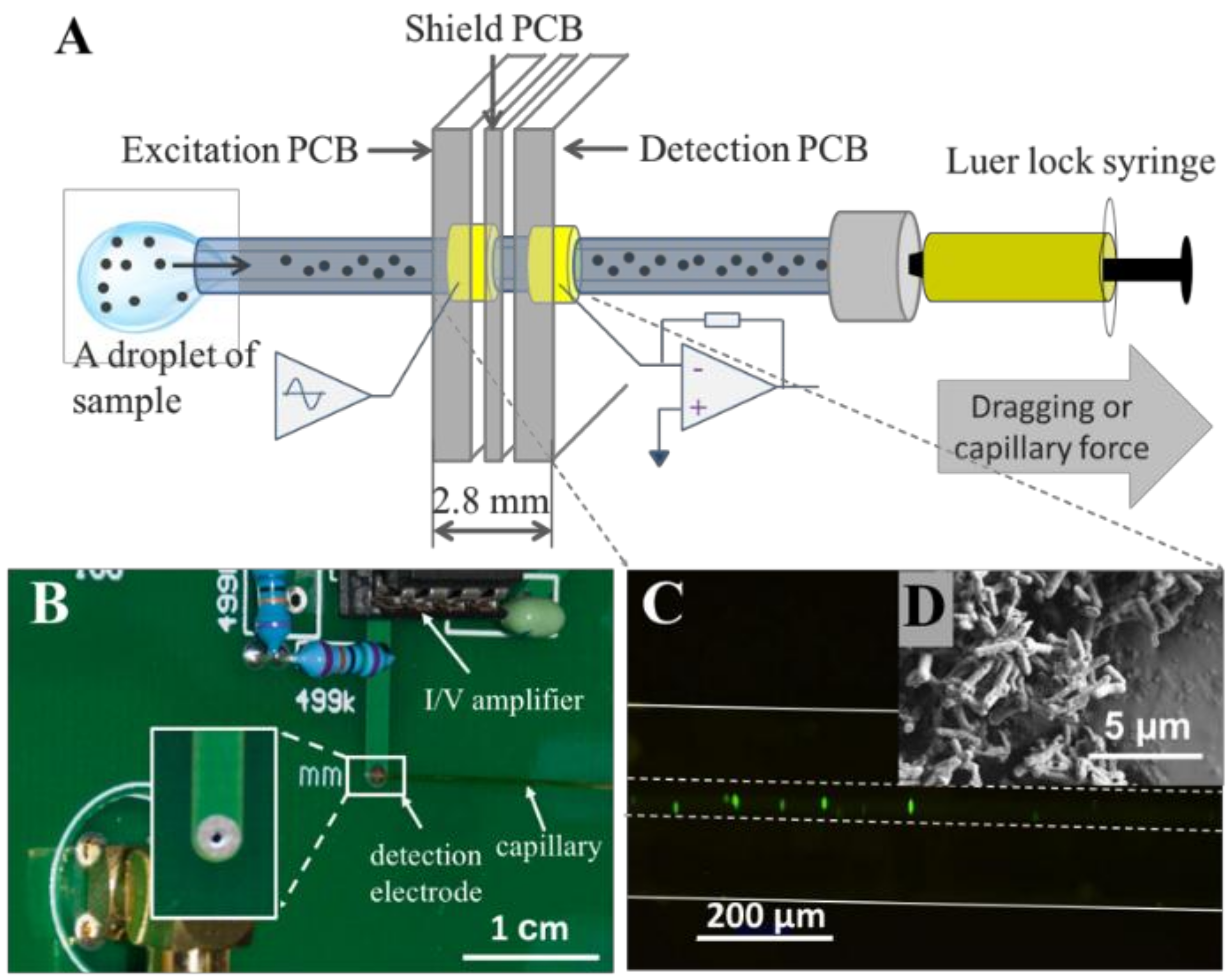
3.1. Design Strategy and Instrumentation
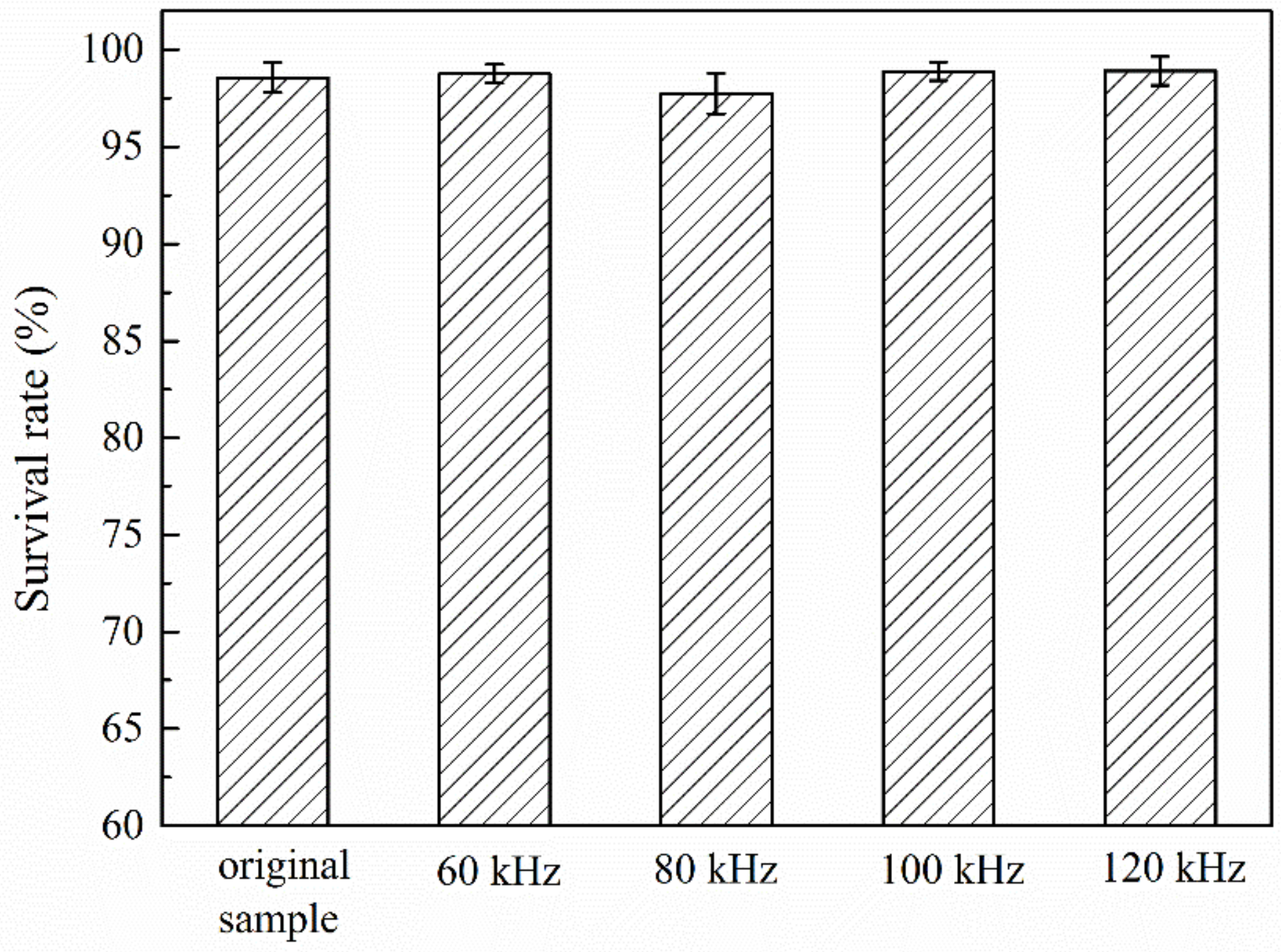
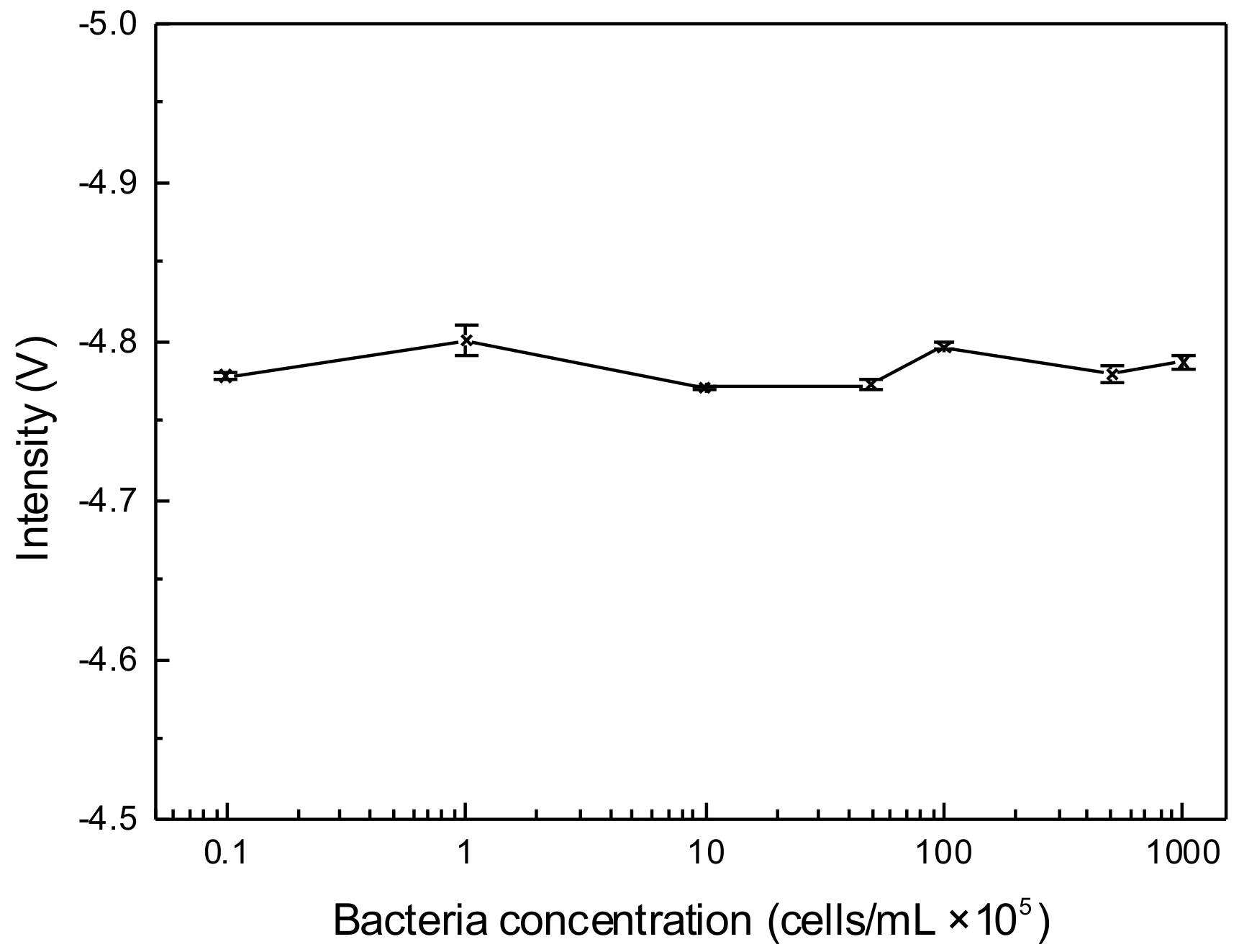
3.2. Electrical Effect of C4D on Bacteria Viability
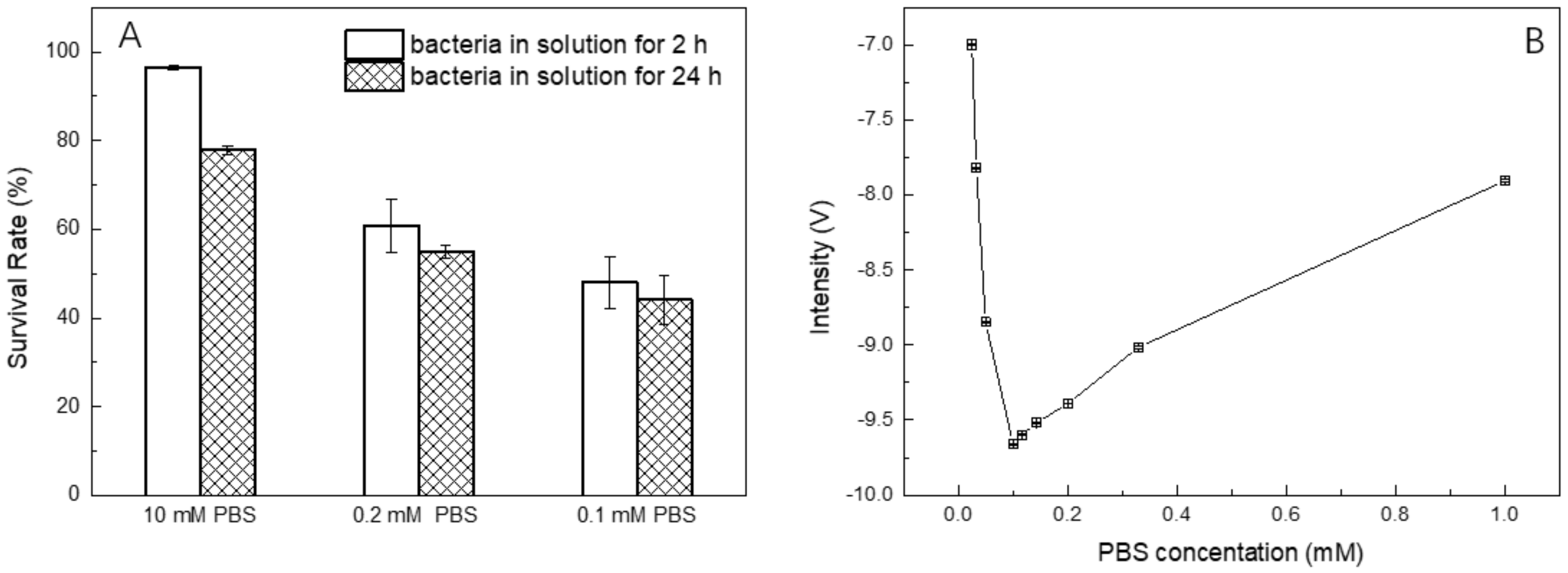
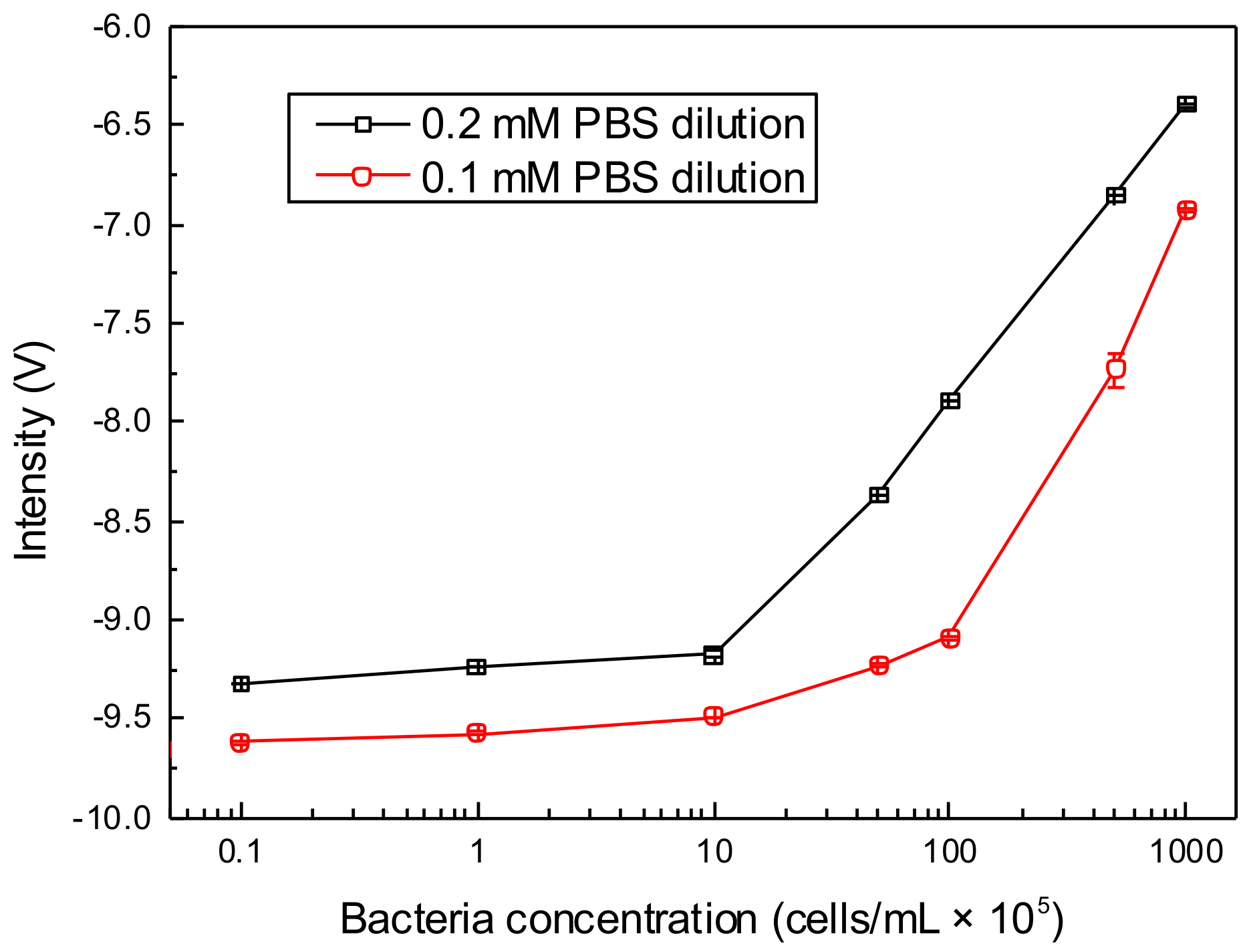
3.3. Optimization of PBS Concentration for Bacteria Counting
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Method | Operation | Recording | Label | Accuracy | Reference |
|---|---|---|---|---|---|
| Optical density (OD) | simple | automatic monitoring | no | providing the growth trend | [6] |
| Plate count | simple | manual reading | no | depending on labor | [6] |
| Flow cytometry | skilled operator needed | automatic monitoring | fluorescence | good | [7,8,9] |
| This work | simple | automatic monitoring | no | Better than OD in range of 106–108 cells/ml |
References
- Bergeron, S.; Boopathy, R.; Nathaniel, R.; Corbin, A.; LaFleur, G. Presence of antibiotic resistant bacteria and antibiotic resistance genes in raw source water and treated drinking water. Int. Biodeterior. Biodegrad. 2015, 102, 370–374. [Google Scholar] [CrossRef]
- Chen, C.W.; Hsu, C.Y.; Lai, S.M.; Syu, W.J.; Wang, T.Y.; Lai, P.S. Metal nanobullets for multidrug resistant bacteria and biofilms. Adv. Drug Deliv. Rev. 2014, 78, 88–104. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zou, X.; Zhao, J.; Shi, J.; Zhang, X.; Li, Z.; Shen, L. Sensing the quality parameters of Chinese traditional Yao-meat by using a colorimetric sensor combined with genetic algorithm partial least squares regression. Meat Sci. 2014, 98, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Hammes, F.; Berney, M.; Wang, Y.Y.; Vital, M.; Koster, O.; Egil, T. Flow-cytometric total bacterial cell counts as a descriptive microbiological parameter for drinking water treatment processes. Water Res. 2008, 42, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Hu, X.T.; Shi, J.Y.; Zou, X.B.; Huang, X.W.; Zhou, X.C.; Haroon, E.T.; Mel, H.; Malcolm, P. Bacteria counting method based on polyaniline/bacteria thin film. Biosens. Bioelectron. 2016, 81, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.X.; Zhang, H.P.; Chon, C.H.; Chen, S.; Pan, X.X.; LI, D.Q. Counting bacteria on a microfluidic chip. Anal. Chim. Acta 2010, 681, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Van der Mark, E.J.; Verberk, J.Q.; Van Dijk, J.C. Flow cytometry total cell counts: A field study assessing microbiological water quality and growth in unchlorinated drinking water distribution systems. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Czechowska, K.; Johnson, D.R.; van der Meer, J.R. Use of flow cytometric methods for single-cell analysis in environmental microbiology. Curr. Opin. Microbiol. 2008, 11, 205–212. [Google Scholar] [CrossRef]
- Lepowsky, E.; Amin, R.; Tasoglu, S. Assessing the reusability of 3D-printed photopolymer microfluidic chips for urine processing. Mciromachines 2018, 9, 520. [Google Scholar] [CrossRef]
- Chen, Y.C.; Nawaz, A.A.; Zhao, Y.H.; Huang, P.H.; McCoy, J.P.; Levine, S.J.; Wang, L.; Huang, T.J. Standing surface acoustic wave (SSAW)-based microfluidic cytometer. Lab Chip 2014, 10, 916–923. [Google Scholar] [CrossRef]
- Steil, D.; Pohlentz, G.; Legros, N.; Mormann, M.; Mellmann, A.; Karch, H.; Müthing, J. Combining Mass Spectrometry, Surface Acoustic Wave Interaction Analysis, and Cell Viability Assays for Characterization of Shiga Toxin Subtypes of Pathogenic Escherichia coli Bacteria. Anal. Chem. 2018, 90, 8989–8997. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.; Costa, T.; Carneiro, C.; Soares, R.; Jitariu, A.; Cardoso, S.; Piedade, M.; Bexiga, R.; Freitas, P. Semi-quantitative method for streptococci magnetic detection in raw milk. Biosensors 2016, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Ghali, H.; Chibli, H.; Nadeau, J.; Bianucci, P.; Peter, Y.A. Real-time detection of staphylococcus aureus using whispering gallery mode optical microdisks. Biosensors 2016, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Ge, A.; Wang, X.; Wang, S.; Yue, X.; Wang, J.; Feng, X.; Du, W.; Liu, B.F. Real-time monitoring of immune responses under pathogen invasion and drug interference by integrated microfluidic device coupled with worm-based biosensor. Biosens. Bioelectron. 2018, 110, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.M.; Misra, K.S.; Schwartz-Duval, S.A.; Daza, E.; Ostadhossein, F.; Bowman, M.; Jain, A.; Taylor, G.; McDonagh, D.; Labriola, T.L.; et al. Real-time monitoring of post-surgical and post-traumatic eye injuries using multilayered electrical biosensor chip. Appl. Mater. Interfaces 2017, 9, 8609–8622. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, Y.; Cui, F.; Xia, Y.; Chen, L.; Mou, X.; Lv, J. Monitoring of bacteria biofilms forming process by in-situ impedimetric biosensor chip. Biosens. Bioelectron. 2018, 112, 86–92. [Google Scholar] [CrossRef]
- Xu, D.; Huang, X.; Guo, J.; Ma, X. Automatic smartphone-based microfluidic biosensor system at the point of care. Biosens. Bioelectron. 2018, 110, 78–88. [Google Scholar] [CrossRef]
- Saucedo, M.N.; Gao, Y.; Pham, T.; Mulchandani, A. Lectin- and saccharide-functionalized nano-chemiresistor arrays for detection and identification of pathogenic bacteria infection. Biosensors 2018, 8, 63. [Google Scholar] [CrossRef]
- Zhang, N.; Ruan, Y.F.; Zhang, L.B.; Zhao, W.W.; Xu, J.J.; Chen, H.Y. Nanochannels photoelectrochemical biosensor. Anal. Chem. 2017, 90, 2341–2347. [Google Scholar] [CrossRef]
- Khan, S.M.; Misra, S.K.; Dighe, K.; Wang, Z.; Schwartz-Duval, A.S.; Sar, D.; Pan, D. Electrically-receptive and thermally-responsive paper-based sensor chip for rapid detection of bacterial cells. Biosens. Bioelectron. 2018, 110, 132–140. [Google Scholar] [CrossRef]
- Liebana, S.; Spricigo, A.D.; Cortes, M.P.; Barbe, J.; Llagostera, M.; Alegret, S.; Pividori, M.I. Phagomagnetci separation and electrochemical magneto-genosensing of pathogenic bacteria. Anal. Chem. 2013, 85, 3079–3086. [Google Scholar] [CrossRef] [PubMed]
- Chistyako, V.A.; Prazdnova, E.V.; Mazanko, M.S.; Bren, A.B. The use of biosensors to explore the potential of probiotic strains to reduce the SOS response and mutagenesis in bacteria. Biosensors 2018, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Le, D.Q.; Takai, M.; Suekuni, S.; Tokonami, S.; Nishino, T.; Shiigi, H.; Nagaoka, T. Development of an observation platform for bacterial activity using polypyrrole films doped with bacteria. Anal. Chem. 2015, 87, 4047–4052. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lum, J.; Callaway, Z.; Lin, J.; Bottje, W.; Li, Y. A label-free impedance immunosensor using screen-printed interdigitated electrodes and magnetic nanobeads for the detection of E. coli O157:H7. Biosensors 2015, 5, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.C.; Hannah, A.J.; Kendrick, S.L.; Tucker, N.P.; MacGregor, G.; Connolly, P. Identification and characterisation of Staphylococcus aureus on low cost screen printed carbon electrodes using impedance spectroscopy. Biosens. Bioelectron. 2018, 110, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Rehman, A.; Sims, M.; Zeng, X. Antimicrobial susceptibility assays based on the quantification of bacterial lipopolysaccharides via a label free lectin biosensor. Anal. Chem. 2015, 87, 4385–4393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Bi, H.; Liu, B.; Qiao, L. Detection of pathogenic microorganisms by microfluidics based analytical methods. Anal. Chem. 2018, 90, 5512–5520. [Google Scholar] [CrossRef]
- Thakur, B.; Zhou, G.; Chang, J.; Pu, H.; Jin, B.; Sui, X.; Yuan, X.; Yang, C.H.; Magruder, M.; Chen, J. Rapid detection of single E. coli bacteria using a graphene-based field-effect transistor device. Biosens. Bioelectron. 2018, 110, 16–22. [Google Scholar] [CrossRef]
- Braiek, M.; Rokbani, K.B.; Chrouda, A.; Mrabet, B.; Bakhrouf, A.; Maaref, A.; Jaffrezic-Renault, N. An electrochemical immunosensor for detection of staphylococcus aureus bacteria based on immobilization of antibodies on self-assembled monolayers-functionalized gold electrode. Biosensors 2012, 2, 417–426. [Google Scholar] [CrossRef]
- Miyajima, K.; Koshida, T.; Arakawa, T.; Kudo, H.; Saito, H.; Yano, K.; Mitsubayashi, K. Fiber-optic fluoroimmunoassay system with a flow-through cell for rapid on-site determination of Escherichia coli O157:H7 by monitoring fluorescence dynamics. Biosensors 2013, 3, 120–131. [Google Scholar] [CrossRef]
- Xiao, N.; Wang, C.; Yu, C. A self-referencing detection of microorganisms using surface enhanced raman scattering nanoprobes in a test-in-a-tube platform. Biosensors 2013, 3, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Mosier-Boss, A.P. Review on SERS of bacteria. Biosensors 2017, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Knoll, W.; Dostalek, J. Bacterial pathogen surface plasmon resonance biosensor advanced by long range surface plasmons and magnetic nanoparticle assays. Anal. Chem. 2012, 84, 8345–8350. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.C.; Ji, H.F.; Yang, S.J.; Huang, Z.Y.; Wang, B.L.; Li, H.Q. New C4D sensor with a simulated inductor. Sensors 2018, 16, 165. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.D.; Li, X.H.; Fan, L.; Zheng, L.; Wu, M.M.; Zhang, S.X.; Huang, Q.L. Determination of inorganic cations and anions in chitooligosaccharides by ion chromatography with conductivity detection. Mar. Drugs 2017, 15, 51–59. [Google Scholar]
- Gubartallah, E.A.; Makahleh, A.; Quirino, J.P.; Saad, B. Determination of biogenic amines in seawater using capillary electrophoresis with capacitively coupled contactless conductivity cetection. Molecules 2018, 23, 1112. [Google Scholar] [CrossRef] [PubMed]
- Kaml, I.; Vcelakova, K.; Kenndler, E. Characterisation and identification of proteinaceous binding media (animal glues) from their amino acid profile by capillary zone electrophoresis. J. Sep. Sci. 2004, 27, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Kuban, P.; Hauser, P.C. Application of an external contactless conductivity detector for the analysis of beverages by microchip capillary electrophoresis. Electrophoresis 2005, 26, 3169–3178. [Google Scholar] [CrossRef]
- Guijit, M.R.; Armstrong, P.J.; Candish, E.; Lefleur, V.; Percey, J.W.; Shabala, S.; Hauser, C.P.; Breadmore, C.M. Microfluidic chips for capillary electrophoresis with integrated electrodes for capacitively coupled conductivity detection based on printed circuit board technology. Sens. Actuator B Chem. 2011, 159, 307–313. [Google Scholar] [CrossRef]
- Coltro, W.K.T.; Da Silva, J.A.F.; Carrilho, E. Rapid prototyping of polymeric electrophoresis microchips with integrated copper electrodes for contactless conductivity detection. Anal. Methods 2011, 3, 168–172. [Google Scholar] [CrossRef]
- Vazquez, M.; Frankenfeld, C.; Coltro, W.K.T.; Carrilho, E.; Diamond, D.; Lunte, S.M. Dual contactless conductivity and amperometric detection on hybrid PDMS/glass electrophoresis microchips. Analyst 2010, 1, 96–103. [Google Scholar] [CrossRef]
- Da Silva, J.A.F.; Guzman, N.; do Lago, L.C. Contactless conductivity detection for capillary electrophoresis Hardware improvements and optimization of the input-signal amplitude and frequency. J. Chromatogr. A 2002, 942, 249–258. [Google Scholar] [CrossRef]
- Francisco, K.J.M.; do Lago, C.L. A compact and high-resolution version of a capacitively coupled contactless conductivity detector. Electrophoresis 2009, 30, 3458–3464. [Google Scholar] [CrossRef] [PubMed]
- Jaanus, M.; Udal, A.; Kukk, V.; Umbleja, K.; Gorbatsova, J.; Molder, L. Improved C5D electronic realization of conductivity detector for capillary electrophoresis. Electron. Elektrotech. 2016, 22, 29–32. [Google Scholar] [CrossRef]
- Emaminejad, S.; Javanmard, M.; Dutton, R.W.; Davis, R.W. Microfluidic diagnostic tool for the developing world: Contactless impedance flow cytometry. Lab Chip 2012, 12, 4499–4507. [Google Scholar] [CrossRef]
- Sun, D.P.; Lu, J.; Chen, Z.G. Microfluidic contactless conductivity cytometer for electrical cell sensing and counting. RSC Adv. 2015, 5, 59306–59313. [Google Scholar] [CrossRef]
- Yang, L. Electrical impedance spectroscopy for detection of bacterial cells in suspensions using interdigitated microelectrodes. Talanta 2004, 74, 1621–1629. [Google Scholar] [CrossRef]
- Coates, J.D.; Ellis, D.J.; Blunt-Harris, E.L.; Gaw, G.V.; Roden, E.E.; Lovley, D.R. Recovery of humic-reducing bacteria from a diversity of environments. Appl. Environ. Microbiol. 1998, 61, 1504–1509. [Google Scholar]
- Wang, H.Y.; Bhunia, A.K.; Lu, C. A microfluidic flow-through device for high throughput electrical lysis of bacterial cells based on continuous dc voltage. Biosens. Bioelectron. 2006, 22, 582–588. [Google Scholar] [CrossRef]
- Islam, M.S.; Shahid, A.; Kuryllo, K.; Li, Y.F.; Deen, M.J.; Selvaganapathy, P.R. Electrophoretic concentration and electrical lysis of bacteria in a microfluidic device using a nanoporous membrane. Micromachines 2017, 8, 45. [Google Scholar] [CrossRef]
- Hulsheger, H.; Potel, J.; Niemann, E.G. Electric field effects on bacteria and yeast cells. Radiat. Environ. Biophys. 1983, 22, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Dosoky, N.S.; Mustafa, G.; Patel, D.; Berdiev, B.; Williams, J.D. Electrophysiology of epithelial sodium channel (ENaC) embedded in supported lipid bilayer using a single nanopore chip. Langmuir 2017, 33, 13680–13688. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.W.; Wang, Y.; Zhang, X.; Sun, C.F.; Li, M.G.; Yan, J.W.; Mao, B.W. Electrochemical impedance spectroscopy and atomic force microscopic studies of electrical and mechanical properties of nano-black lipid membranes and size dependence. Langmuir 2012, 28, 14739–14746. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Dosoky, N.S.; Patel, D.; Weimer, J.; Williams, J.D. Lipid bilayer membrane in a silicon based micron sized cavity accessed by atomic force microscopy and electrochemical impedance spectroscopy. Biosensors 2017, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Shawki, M.M.; Gaballah, A. The effect of low AC electric field on bacterial cell death. Rom. J. Biophys. 2015, 25, 163–172. [Google Scholar]
- Syed, M.S.; Rafeie, M.; Vandamme, D.; Asadnia, M.; Henderson, R.; Taylor, R.A.; Warkiani, M.E. Selective separation of microalgae cells using inertial microfluidics. Bioresour. Technol. 2018, 252, 91–99. [Google Scholar] [CrossRef]
- Liu, W.; Wang, C.; Ding, H.; Shao, J.; Ding, Y. AC electric field induced dielectrophoretic assembly behavior of gold nanoparticles in a wide frequency range. Appl. Surf. Sci. 2016, 370, 184–192. [Google Scholar] [CrossRef]
- Lapizco-Encinas, B.H.; Simmons, B.A.; Cummings, E.B.; Fintschenko, Y. Dielectrophoretic concentration and separation of live and dead bacteria in an array of insulators. Anal. Chem. 2004, 76, 1571–1579. [Google Scholar] [CrossRef]
- Rojas, E.; Theriot, A.J.; Huang, K.C. Response of Escherichia coli growth rate to osmotic shock. Proc. Natl. Acad. Sci. USA 2014, 111, 7807–7812. [Google Scholar] [CrossRef]
- Brito-Neto, J.G.A.; da Silva, J.A.F.; do Lago, L.; Blanes, C.L. Understanding Capacitively Coupled Contactless Conductivity Detection in Capillary and Microchip Electrophoresis. Part 1. Fundamentals. Electroanalysis 2005, 17, 1198–1206. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.-Y.; Li, Z.-Y.; Zhang, Y.; Zang, X.-Q.; Ueno, K.; Misawa, H.; Sun, K. Bacterial Concentration Detection using a PCB-based Contactless Conductivity Sensor. Micromachines 2019, 10, 55. https://doi.org/10.3390/mi10010055
Zhang X-Y, Li Z-Y, Zhang Y, Zang X-Q, Ueno K, Misawa H, Sun K. Bacterial Concentration Detection using a PCB-based Contactless Conductivity Sensor. Micromachines. 2019; 10(1):55. https://doi.org/10.3390/mi10010055
Chicago/Turabian StyleZhang, Xiao-Yan, Zhe-Yu Li, Yu Zhang, Xiao-Qian Zang, Kosei Ueno, Hiroaki Misawa, and Kai Sun. 2019. "Bacterial Concentration Detection using a PCB-based Contactless Conductivity Sensor" Micromachines 10, no. 1: 55. https://doi.org/10.3390/mi10010055
APA StyleZhang, X.-Y., Li, Z.-Y., Zhang, Y., Zang, X.-Q., Ueno, K., Misawa, H., & Sun, K. (2019). Bacterial Concentration Detection using a PCB-based Contactless Conductivity Sensor. Micromachines, 10(1), 55. https://doi.org/10.3390/mi10010055





