Hypoxic Physiological Environments in a Gas-Regulated Microfluidic Device
Abstract
1. Introduction
2. Materials and Methods
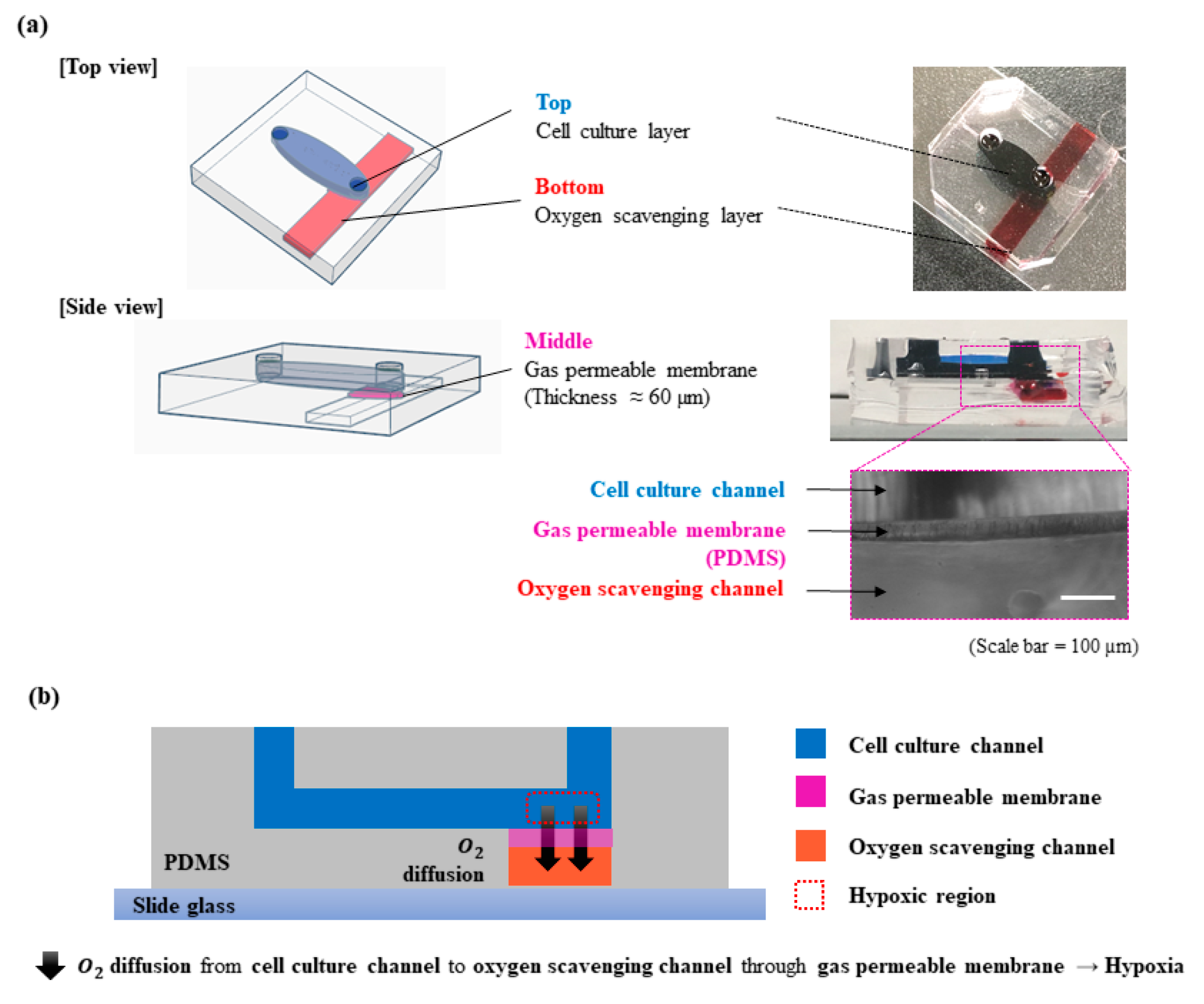
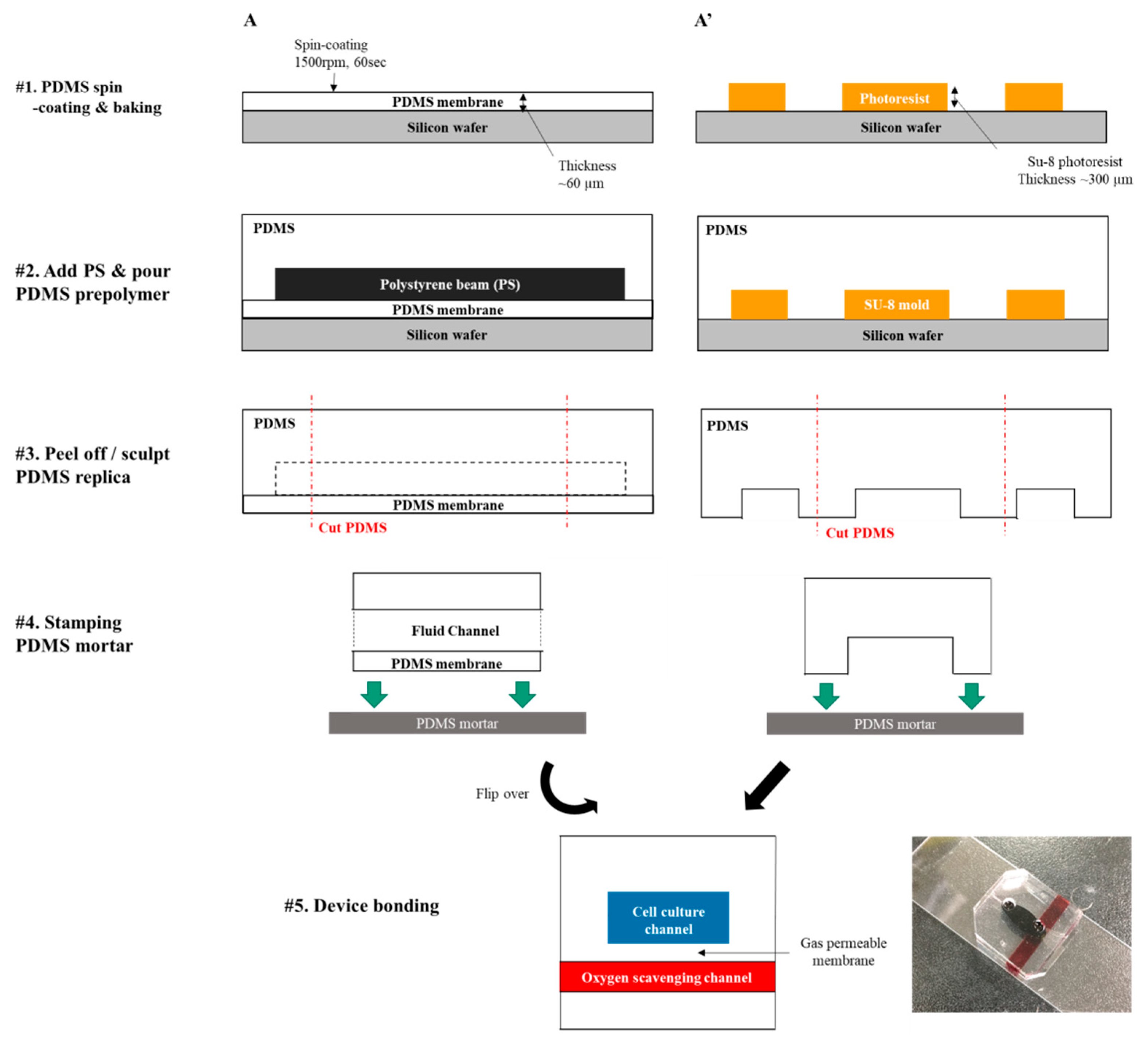
2.1. Device Fabrication
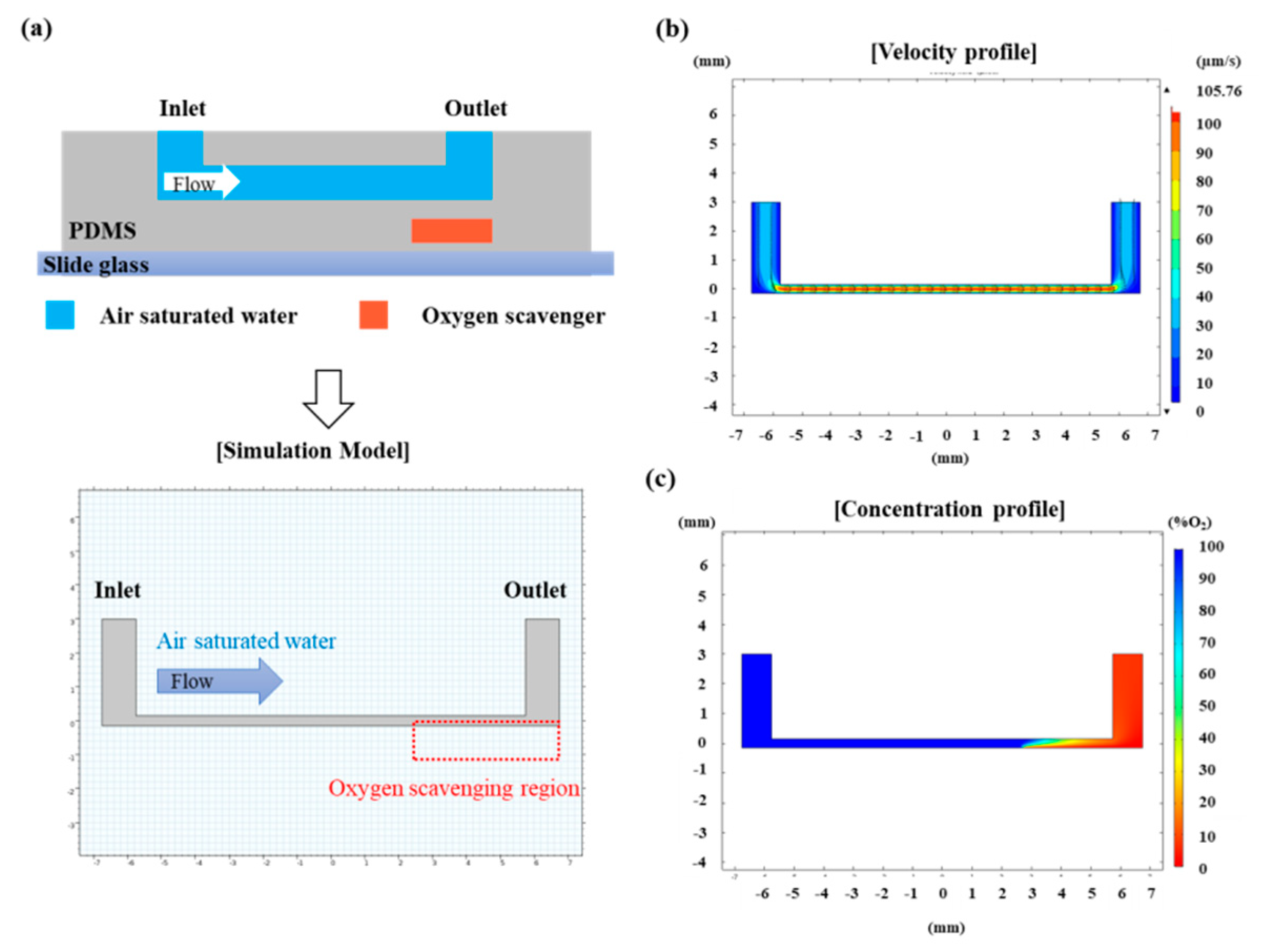
2.2. Numerical Calculation of Oxygen Concentration in the Device
2.3. Oxygen Level Control in the Microfluidic Device
2.4. Oxygen Level Measurement
2.5. Cell Culture
2.6. Cell Culture under Hypoxic Condition
3. Results and Discussion
3.1. Numerical Calculation of Oxygen Concentration Profile in the Microfluidic Channel
3.2. Recapitulation of Hypoxic Condition in the Device
3.3. Cell Viability Assay under Hypoxic Condition
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Haase, V.H. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 2013, 27, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Markus, R.; Reutens, D.C.; Kazui, S.; Read, S.; Wright, P.; Pearce, D.C.; Tochon-Danguy, H.J.; Sachinidis, J.I.; Donnan, G.A. Hypoxic tissue in ischaemic stroke: Persistence and clinical consequences of spontaneous survival. Brain 2004, 127, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Giordano, F.J. Oxygen, oxidative stress, hypoxia, and heart failure. J. Clin. Investig. 2005, 115, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, E.C. Invited Review: Physiological consequences of intermittent hypoxia: Systemic blood pressure. J. Appl. Physiol. 2001, 90, 1600–1605. [Google Scholar] [CrossRef] [PubMed]
- Brandler, M.D.; Powell, S.C.; Craig, D.M.; Quick, G.; McMahon, T.J.; Goldberg, R.N.; Stamler, J.S. A novel inhaled organic nitrate that affects pulmonary vascular tone in a piglet model of hypoxia-induced pulmonary hypertension. Pediatric Res. 2005, 58, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Fung, F.K.C.; Law, B.Y.K.; Lo, A.C.Y. Lutein attenuates both apoptosis and autophagy upon cobalt (II) chloride-induced hypoxia in rat Muller cells. PLoS ONE 2016, 11, e0167828. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, H. Methods for inducing embryoid body formation: In vitro differentiation system of embryonic stem cells. J. Biosci. Bioeng. 2007, 103, 389–398. [Google Scholar] [CrossRef]
- Yalikun, Y.; Tanaka, N.; Hosokawa, Y.; Iino, T.; Tanaka, Y. Embryonic body culturing in an all-glass microfluidic device with laser-processed 4 μm thick ultra-thin glass sheet filter. Biomed. Microdevices 2017, 19, 85. [Google Scholar] [CrossRef]
- Skolimowski, M.; Nielsen, M.W.; Emnéus, J.; Molin, S.; Taboryski, R.; Sternberg, C.; Dufva, M.; Geschke, O. Microfluidic dissolved oxygen gradient generator biochip as a useful tool in bacterial biofilm studies. Lab Chip 2010, 10, 2162–2169. [Google Scholar] [CrossRef]
- Polinkovsky, M.; Gutierrez, E.; Levchenko, A.; Groisman, A. Fine temporal control of the medium gas content and acidity and on-chip generation of series of oxygen concentrations for cell cultures. Lab Chip 2009, 9, 1073. [Google Scholar] [CrossRef]
- Brennan, M.D.; Rexius-Hall, M.L.; Elgass, L.J.; Eddington, D.T. Oxygen control with microfluidics. Lab Chip 2014, 14, 4305–4318. [Google Scholar] [CrossRef] [PubMed]
- Markov, D.A.; Lillie, E.M.; Garbett, S.P.; McCawley, L.J. Variation in diffusion of gases through PDMS due to plasma surface treatment and storage conditions. Biomed. Microdevices 2014, 16, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Mata, A.; Fleischman, A.J.; Roy, S. Characterization of Polydimethylsiloxane (PDMS) Properties for Biomedical Micro/Nanosystems. Biomed. Microdevices 2005, 7, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Chueh, B.H.; Huh, D.; Kyrtsos, C.R.; Houssin, T.; Futai, N.; Takayama, S. Leakage-Free Bonding of Porous Membranes into Layered Microfluidic Array Systems. Anal. Chem. 2007. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, A.A.; Zaidi, S.M.J. Rkcl3221 Kinetics of Catalytic Oxidation Of Aqueous Sodium Sulfite. React. Kinetics Catal. Lett. 1998, 64, 343–349. [Google Scholar] [CrossRef]
- Pavesi, A.; Piraino, F.; Fiore, G.B.; Farino, K.M.; Moretti, M.; Rasponi, M. How to embed three-dimensional flexible electrodes in microfluidic devices for cell culture applications. Lab Chip 2011, 11, 1593–1595. [Google Scholar] [CrossRef] [PubMed]
- McKeown, S.R. Defining normoxia, physoxia and hypoxia in tumours—Implications for treatment response. Br. J. Radiol. 2014. [Google Scholar] [CrossRef]
- Jiang, W.B.; Zhao, W.; Chen, H.; Wu, Y.Y.; Wang, Y.; Fu, G.S.; Yang, X.J. Baicalin protects H9c2 cardiomyocytes against hypoxia/reoxygenation-induced apoptosis and oxidative stress through activation of mitochondrial aldehyde dehydrogenase 2. Clin. Exp. Pharmacol. Physiol. 2018, 45, 303–311. [Google Scholar] [CrossRef]
- Boomsma, R.A.; Geenen, D.L. Mesenchymal Stem Cells Secrete Multiple Cytokines That Promote Angiogenesis and Have Contrasting Effects on Chemotaxis and Apoptosis. PLoS ONE 2012, 7, e35685. [Google Scholar] [CrossRef]
- Stroka, D.M.; Burkhardt, T.; Desbaillets, I.; Wenger, R.H.; Neil, D.A.H.; Bauer, C.; Gassmann, M.; Candinas, D. Hif-1 Is Expressed In Normoxic Tissue And Displays An Organ-Specific Regulation Under Systemic Hypoxia. Faseb J. 2001, 15, 2445–2453. [Google Scholar] [CrossRef]
- Bijlsma, M.F.; Groot, A.P.; Oduro, J.P.; Franken, R.J.; Schoenmakers, S.H.H.F.; Peppelenbosch, M.P.; Spek, C.A. Hypoxia induces a hedgehog response mediated by HIF-1α. J. Cell. Mol. Med. 2009, 13, 2053–2060. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, I.; Woo, J.H.; Lee, M.; Jeon, T.-J.; Kim, S.M. Hypoxic Physiological Environments in a Gas-Regulated Microfluidic Device. Micromachines 2019, 10, 16. https://doi.org/10.3390/mi10010016
Lee I, Woo JH, Lee M, Jeon T-J, Kim SM. Hypoxic Physiological Environments in a Gas-Regulated Microfluidic Device. Micromachines. 2019; 10(1):16. https://doi.org/10.3390/mi10010016
Chicago/Turabian StyleLee, Insu, Jin Hyuk Woo, Min Lee, Tae-Joon Jeon, and Sun Min Kim. 2019. "Hypoxic Physiological Environments in a Gas-Regulated Microfluidic Device" Micromachines 10, no. 1: 16. https://doi.org/10.3390/mi10010016
APA StyleLee, I., Woo, J. H., Lee, M., Jeon, T.-J., & Kim, S. M. (2019). Hypoxic Physiological Environments in a Gas-Regulated Microfluidic Device. Micromachines, 10(1), 16. https://doi.org/10.3390/mi10010016







