1. Introduction
Toxins produced by filamentous fungi are called mycotoxins and can contaminate food and feed products [
1]. Mycotoxin contamination can occur at various steps during food and feed production, including storage and distribution, and it decreases the quality of the product [
2]. Indeed, mycotoxin contamination reduces the efficiency of livestock and crop production in many countries [
3,
4]. Furthermore, if a previously-contaminated ingredient is added to foodstuff or animal feed, human and animal health is threatened [
5].
Fusarium species are toxigenic and pathogenic to plants or humans, and the trichothecenes and the fumonisins produced by them are the most well-known mycotoxins [
6]. Fumonisins (FUMs) were first isolated from
F. moniliforme in 1988 [
7], and they can be produced by other
Fusarium species such as
F. verticillioides,
F. proliferatum and
F. nygamai [
8]. FUMs are soluble in water, unlike other hydrophobic mycotoxins, and this property makes it difficult to study fumonisins [
9]. Fumonisin B
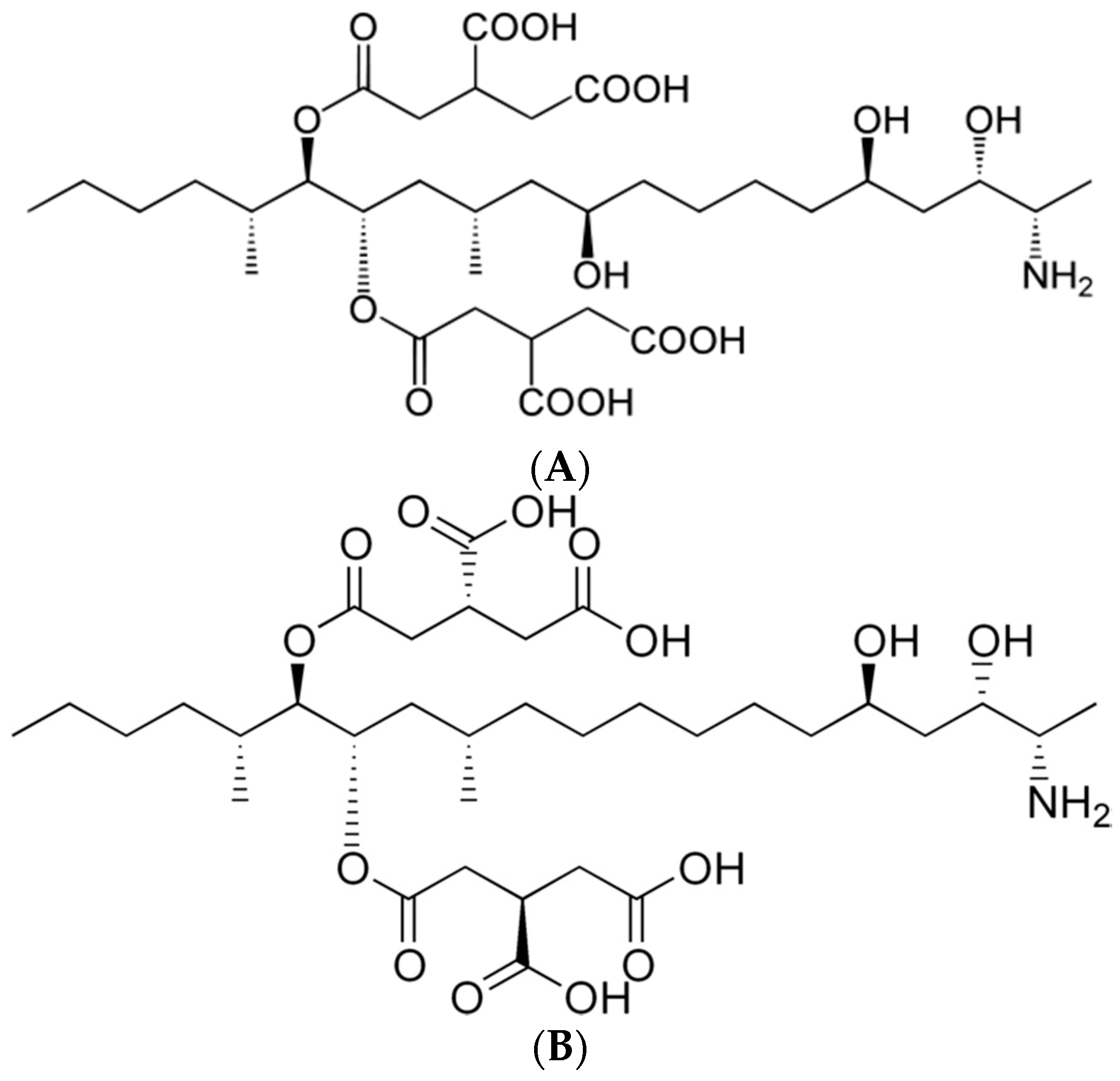
1 (FB
1) is composed of two units of propane-1,2,3-tricarboxylic acid and a 2
S-amino-12
S,16
R-dimethyl-3
S,5
R,10
R,14
S,15
R-pentahydroxy-eicosane backbone connected by a diester bridge at the C-14 and C-15 hydroxy groups [
10]. The chemical structures of FB
1 and FB
2 are shown in
Figure 1.
These toxins can disrupt sphingolipid biosynthesis leading to serious problems in membrane biosynthesis because sphingolipids regulate cell growth and differentiation through participation in communication and interactions related to the cell [
11,
12]. They also cause leukoencephalomalacia, pulmonary edema, hydrothorax, apoptosis, hepatotoxic and carcinogenic effects in animals [
7,
13,
14,
15,
16,
17,
18], as well as esophageal cancer in humans [
19]. FB
1 was assigned to group 2B, “possibly carcinogenic to humans”, by the International Agency for Research on Cancer [
20].
Because of the serious damage that mycotoxins can cause to human and animal health, mycotoxins are an important concern for the feed industry, feed supply chain and international trade [
21]. Binder et al. [
22] reported that the amount and type of fungal contamination exhibits significant regional differences. For example, in their study, European samples were mostly contaminated by deoxynivalenol (DON), T-2 toxin and zearalenone (ZEN), but Asian and Pacific samples had aflatoxins (AFs), DON, FUMs and ZEN as major contaminants. In 2015, the levels of FB
1 and glycated FB
1 in a total of 30 compound feed samples were measured, and all measured concentrations of FB
1 were below the regulation limits set by the European Union and the U.S. Food and Drug Administration [
23].
Grains, grain byproducts and vegetable proteins found in animal feed are good nutrients for the fungi that produce mycotoxins. Therefore, mycotoxins in animal feed need to be closely monitored. In 2012, 18,640 tons of compound feed were distributed in Korea, and 15,350 tons of feed ingredients were purchased by Korea from many places including China, the USA, Europe, Canada, South Africa, South East Asia, Australia and India. Feed ingredients from different countries make it difficult to control mycotoxin levels in compound feed. In one study in Slovakia, a total of 50 poultry feed samples was collected during 2003 and 2004, and the level of FUMs was measured. FB
1 and FB
2 were detected in 49 samples (98.0%) and 42 samples (84.0%), respectively [
24]. In another study, brewers’ grain, used as dairy cattle feed, was analyzed using high performance liquid chromatography (HPLC) in Brazil, and FUMs contaminated 72.5% (58) of the samples with rates between 50.3 and 908.5 μg/kg [
25]. In China, six mycotoxins were quantified in 83 feed samples including FUMs, and 82.7% were contaminated [
26].
According to the European Commission (EC), maximum levels for
Fusarium toxins were established in products intended for animal feeding in 2006 [
27], and the Food and Drug Administration (FDA) in the U.S. set guidance levels for FUMs in human food and animal feed in 2001 [
28]. In South Korea, the guidance value for
Fusarium mycotoxins in animal feed was established based on guidelines of the EC and the results of studies in Korea. The guidelines for the EC and South Korea are summarized in
Table 1.
The object of this study was to confirm the contamination level of FUMs in feed and feed ingredients and to compare the level of FUMs with the guidelines for animal feed in South Korea. For this purpose, we collected feed samples over the course of six years, analyzed the levels of FUMs contamination and assessed the risk of FUMs contamination in animal feed in Korea.
3. Discussion
Many studies related to FUMs have been performed worldwide in order to determine contamination levels in compound feed. For example, Marquardt and Madhyastha [
32] surveyed levels of various toxins in feed material and feedstuff worldwide. The highest incidence, with more than 50% positive samples, was found in North, South and Southeast Asia, South America, Southern Europe and Africa. A study in Kuwait [
33] reported that all samples of poultry feed that they tested (53 samples) were contaminated with FUMs in the range of 220–6000 μg/kg with the mean contamination level of 2733 μg/kg. Similar results were reported by Greco et al. [
34]: FUMs were detected in all of the 46 poultry feed samples with the concentration between 222 and 6000 μg/kg (median 1750 μg/kg). Kocasari et al. (2013) [
35] analyzed 180 compound feed samples in Turkey for the contamination of FUMs; approximately 10.6% of samples were contaminated by FUMs, and the mean contamination value was measured at 3190 μg/kg with range of 2690–4960 μg/kg. In the Republic of South Africa, a total of 92 commercial compound feed samples was examined for contamination with various mycotoxins, and FUMs were detected in most of the compound feed samples [
36]. Specifically, cattle feed was severely contaminated by FB
1 and FB
2. The mean contamination level of FUMs was 916 μg/kg, and the highest contamination level was 2999 μg/kg. In addition, the mean contamination value of FUMs in cattle, chicken and swine feed was 903, 975 and 313 μg/kg, respectively. The range of the contamination level of FB
2 was 103–673 μg/kg, which is lower than the contamination values for FB
1. This research showed that no compound feed samples exceeded the guidance level of the EC. Another study [
37] was conducted to determine the level of FUMs in a total of 30 pig feed samples. The mean contamination level of FUMs was 405 μg/kg, and 80% of the feed samples were contaminated by FUMs, ranging from 30–1043 μg/kg. The level of FUMs in all of samples was below 5000 μg/kg, the EC guidance value. Zachariasova et al. [
38] conducted a study in order to quantify 56 mycotoxins in 343 samples of animal feed. Those samples were non-fermented and fermented feedstuff, feedstuff supplements and complex compound feed collected from the Czech Republic (256 samples) and the United Kingdom (87 samples) between 2008 and 2012. Ninety-five compound feed samples (for pigs, chickens and laying hens, birds, rodents and dairy cows) were analyzed among the collected 343 samples, and only 13 compound feed samples for dairy cows were contaminated with FB
1; three compound feed samples for dairy cows were contaminated by FB
2. The median level of FB
1 was 10 μg/kg, and the maximum level was 58 μg/kg. In the case of FB
2, the median was 9 μg/kg, and the maximum level was 14 μg/kg. Those results were much lower than the EC legislation. In that article, FUMs were detected below 100 μg/kg in all 278 samples. In Poland, a study [
39] was performed to evaluate the level of several mycotoxins in 1384 samples including 480 complete feed samples during a four-year period. In that study, FUMs were present in 85.7% of the 14 complete feed samples analyzed. The maximum level of FUMs was measured at 1063 μg/kg. A total of 74 ready-to-consume feed samples for dairy cattle was collected in Konya, Turkey and the surrounding provinces [
40]. Seven mycotoxins were analyzed in collected feed samples, and FUMs were the most common mycotoxin; 93.2% of samples had detectable levels of FUMs. The maximum detected level was 1208 μg/kg, which was below the 50,000 μg/kg maximum residue limit in Turkey. A similar study [
41] was also performed in Turkey in which 22 cattle feed samples were collected every quarter over one year (total 88 samples) from some provinces in Turkey; 94.3% of those samples were contaminated by FUMs. The contamination level was between 0 and 3900 μg/kg. Samples collected in the summer exhibited a higher mean contamination level than in any other season.
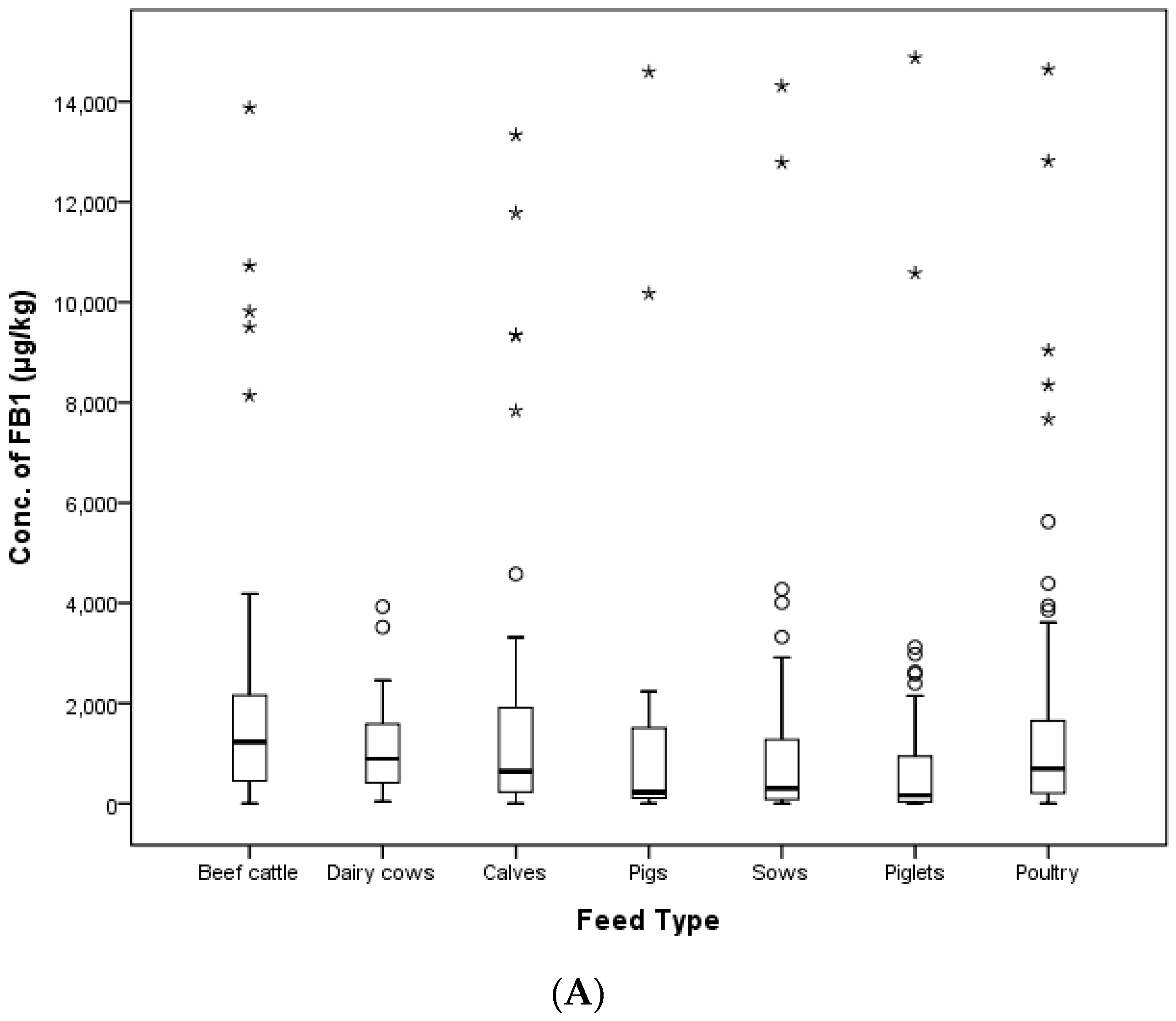
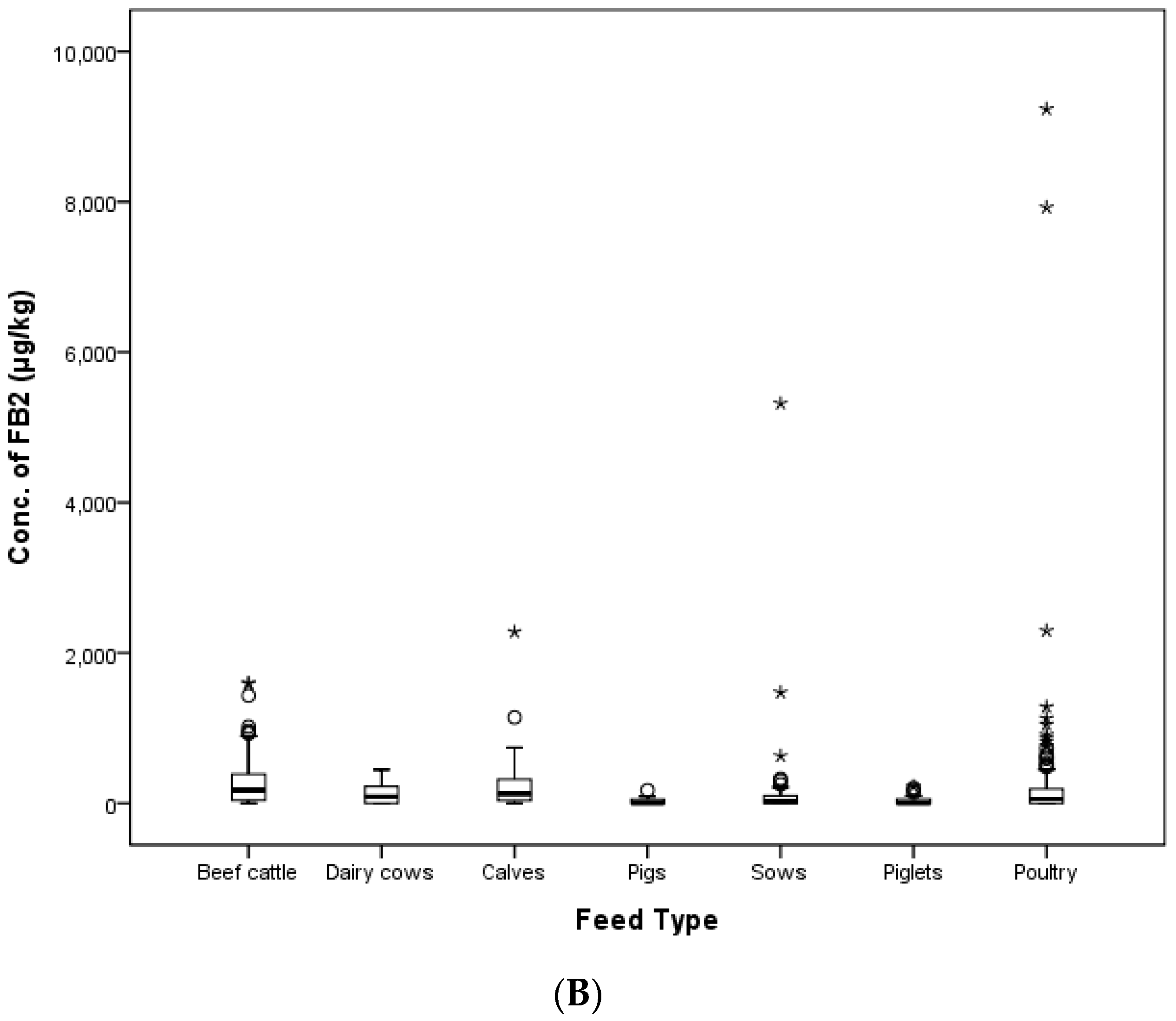
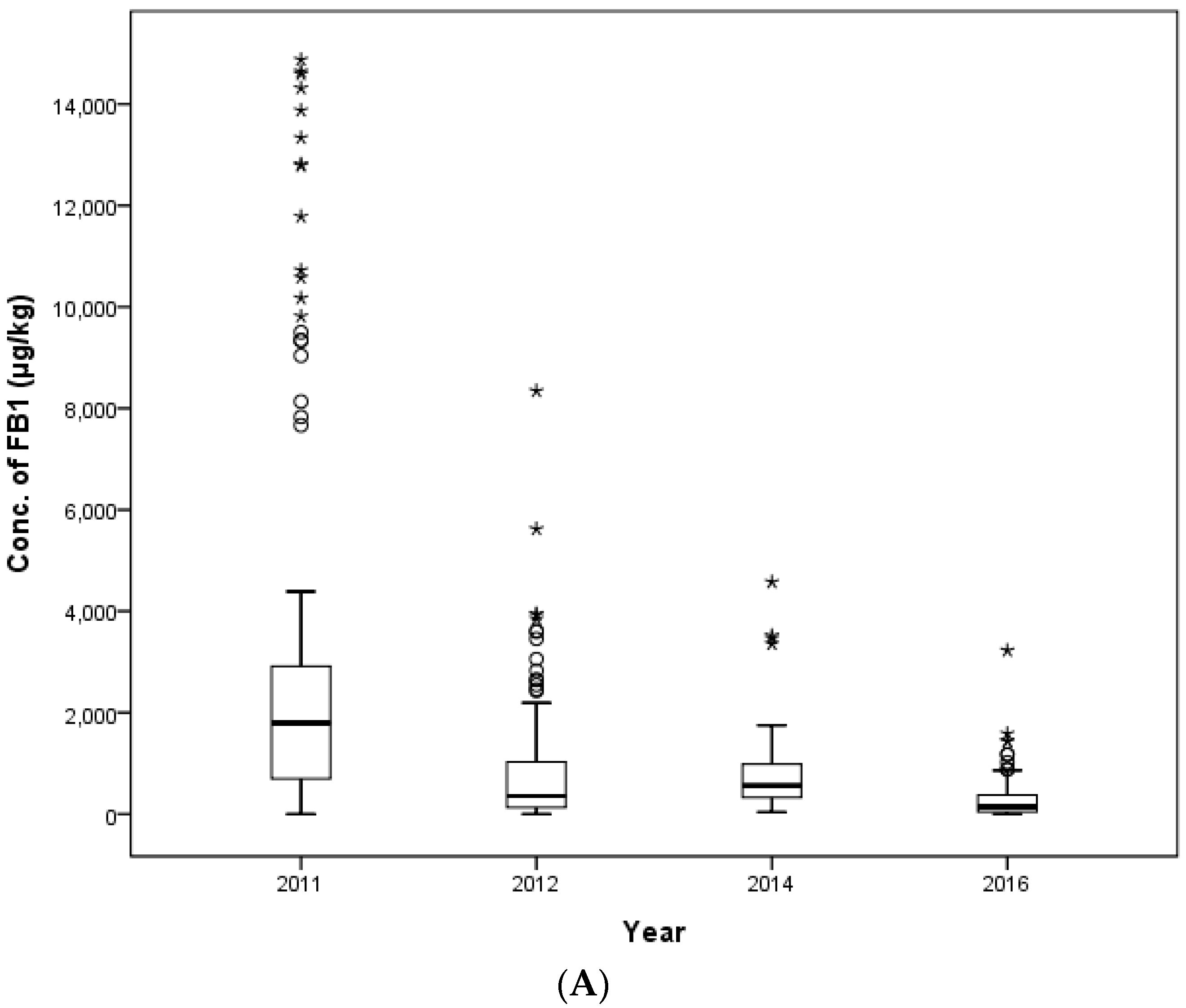
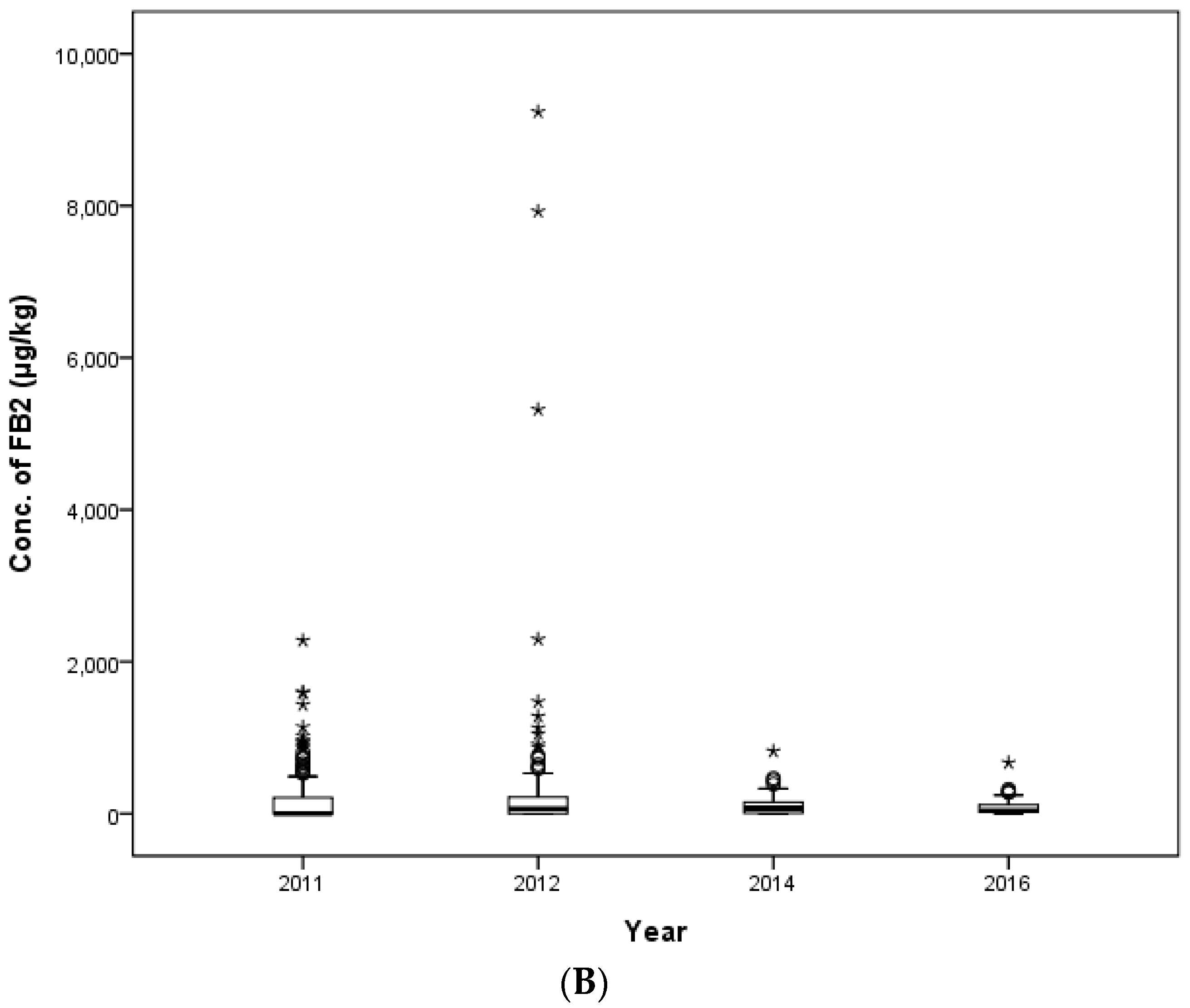
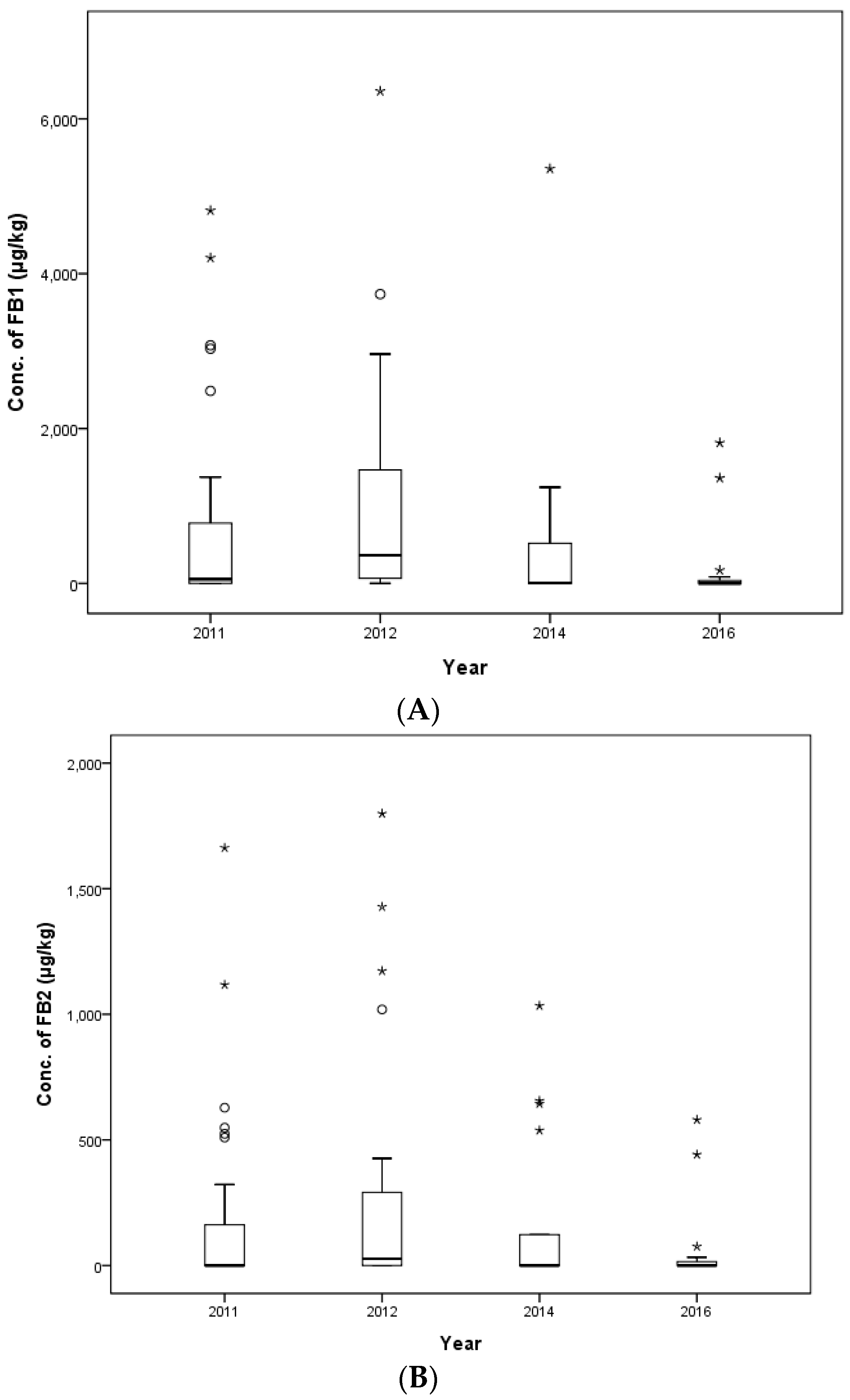
In this study, the contamination level of FUMs was estimated in 425 feed samples consisting of 145 samples of cattle feed, 140 samples of pig feed and 140 samples of poultry feed collected in 2011, 2012, 2014 and 2016 in the Republic of Korea. FB
1 and FB
2 were detected in 90.6% and 63.3% of compound feed samples. FUMs mainly contaminated cattle feed (97.9% for FB
1 and 78.6% for FB
2), followed by poultry feed (90.7% for FB
1 and 65.0% for FB
2) and pig feed (82.9% for FB
1 and 45.7% for FB
2). The incidence of FB
1 contamination in pig feed was similar to the incidence of FUMs in pig feed reported by Pleadin et al. (2012) [
37] (80%). The mean concentration of FB
1 was 1779.0 μg/kg for cattle feed, 1171.1 μg/kg for swine feed and 1382.5 μg/kg for poultry feed, and the mean concentrations of FB
2 were 251.8, 90.5 and 287.9 μg/kg for cattle, swine and poultry feed, respectively. The mean concentrations of FB
1 and FB
2 were 1382.5 and 287.9 μg/kg in poultry feed, which were lower than the estimated value (2733 μg/kg) in poultry feed in Kuwait [
33]. The maximum contamination value of FUMs was detected at 15,098 μg/kg in a weanling piglet feed sample. This contamination level was over the EC and Korean regulation limit (5000 μg/kg). The concentration of FUMs in six other pig feed samples, as well as the weanling piglet feed mentioned above also exceeded the EC and Korean guidance level. In this study, the concentration ranges of FB
1 (36.4–14,876.8 μg/kg) and FB
2 (14.9–9235.8 μg/kg) contamination were broader than the ranges from Kuwait (220–6000 μg/kg) [
33] and Turkey (2690–4960 μg/kg) [
35].
In Kuwait [
33], wheat bran, soybean meal and corn feed ingredients were contaminated by FUMs (89.8% of samples), and the mean contamination value was 2040 μg/kg with a range of 0–6000 μg/kg. Another study [
42] was conducted to determine the occurrence of various mycotoxins including fumonisins. A total of 7049 samples consisting of corn, soybean/soybean meal, wheat, dried distillers’ grains with solubles (DDGS) and finished feed samples was collected from the Americas, Europe and the Asia-Pacific region. FUMs contaminated most of these samples with mean contamination values of 1965 μg/kg. Corn from South America and Southern Europe was mainly contaminated with FUMs, and the contamination incidence was 92% and 90%, respectively. The mean contamination levels in both regions differed; the mean level of FUMs in South American samples (3226 μg/kg) was higher than that in Southern European ones (2271 μg/kg). In 2011–2014, a total of 1384 samples consisting of 295 maize samples, 143 maize silage samples, 466 small grain cereal samples and 480 complete feed samples was collected in Poland [
39]. Those samples were used to estimate the level of various mycotoxins including FUMs. Among them, 83 maize samples, 21 maize silage samples and three cereal grain samples were analyzed for levels of FUMs. FUMs were detected in 59% of the maize samples with 1885 μg/kg as the maximum level and in 53% of maize silage samples with 108 μg/kg as the maximum level. There were no positive cereal grain samples. The contamination concentration of FUMs in all of the positive samples was lower than the EC’s guidance values. In Germany, 84 maize samples were collected in 2006 (44 samples) and 2007 (40 samples). In all, 34% of maize samples were contaminated with FUMs in 2006, and no samples were contaminated with FUMs in 2007 [
43]. Researchers estimated that the samples collected in 2006 were contaminated with a higher level of FUMs than the ones in 2007 because of the high temperature and low rainfall during anthesis and early grain filling. In 2006, FB
1 was detected in 34% of samples with 1910 μg/kg as the mean concentration, and FB
2 was detected in 23% of samples with 460 μg/kg as the mean concentration. Zachariasova et al. [
38] reported that 11 maize silage samples were contaminated with a mean concentration of 35 μg/kg and 26 μg/kg for FB
1 and FB
2, respectively. In that study, FUMs were mainly found in maize, maize silage and maize-based DDGS. In our previous study, separately performed as part of another research project with different samples in 2012 [
44], the results were similar to the results of this study; most compound cattle feed was contaminated with FUMs (98%). Compound feed for early broilers was contaminated with a level of mean contamination of 3459 μg/kg and a range of 333–12,823 μg/kg. The range of contamination levels was between 0.059 and 8.239 for feed ingredient samples (83.3%).
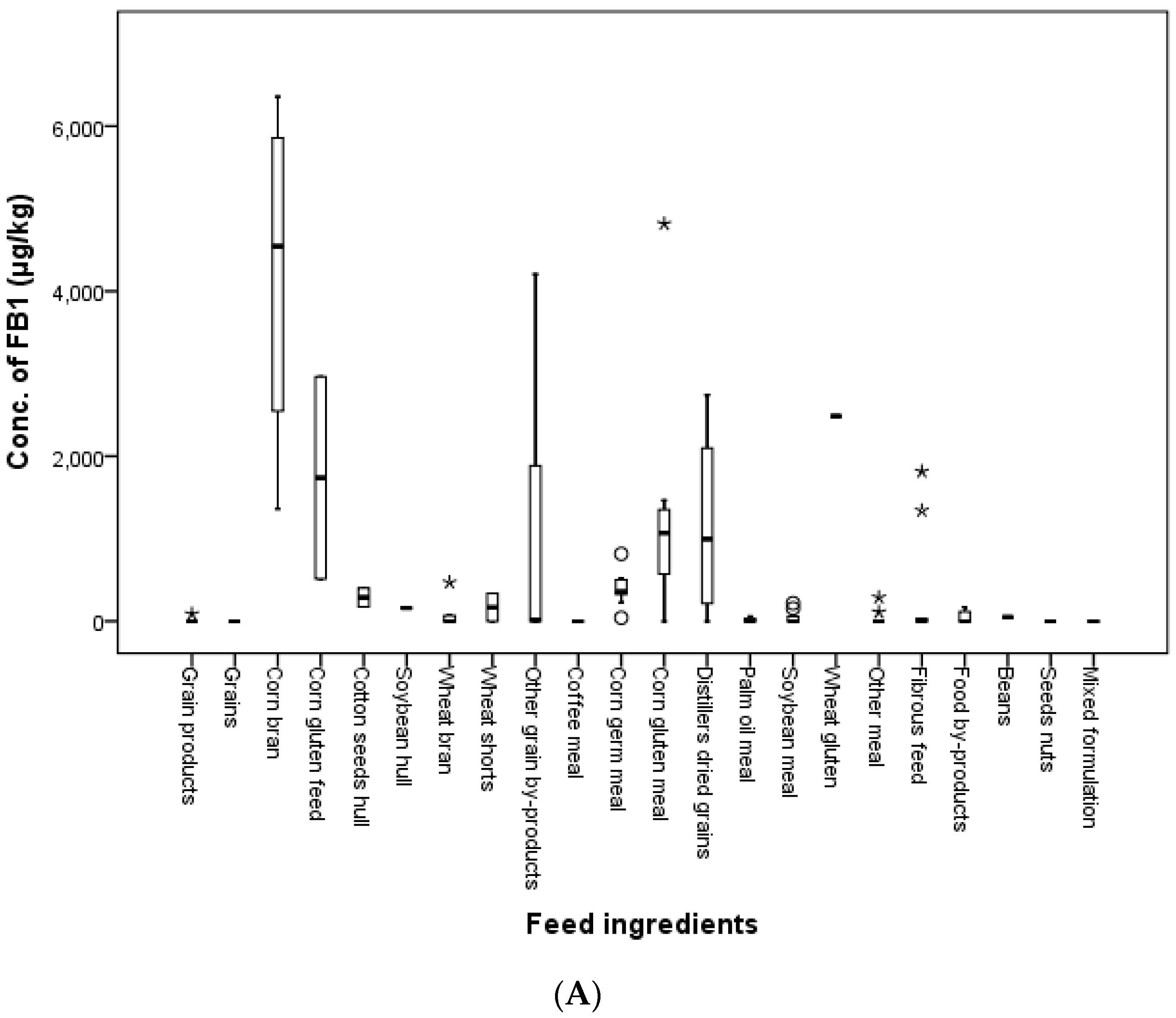
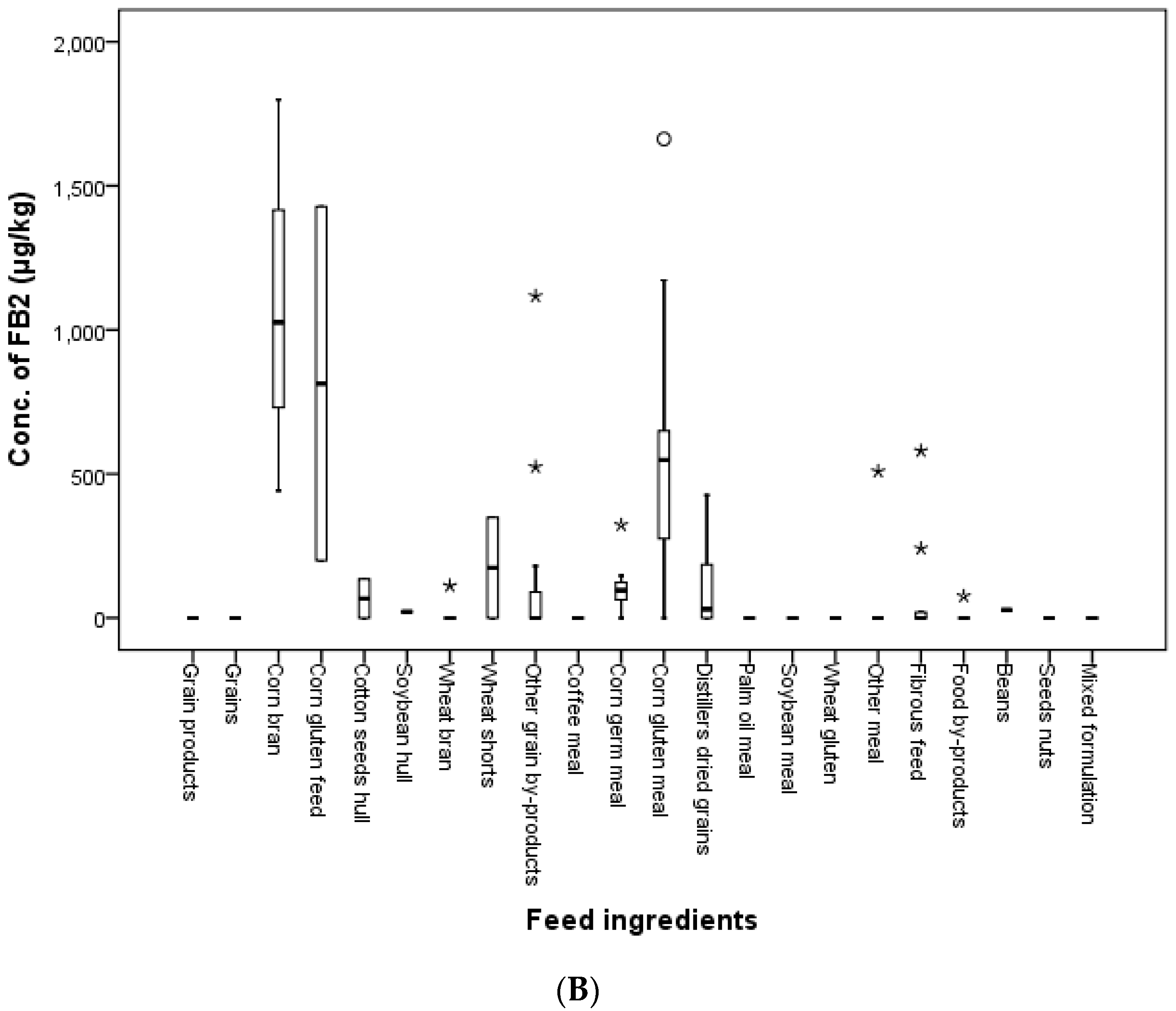
In the present study, feed ingredients were collected in 2011, 2012, 2014 and 2016 in Korea. The incidence of FB
1 was 14.3, 70.4, 63.6, 33.3 and 37.5 in grains, grain by-products, meal, fibrous feed and food by-products, respectively. FB
2 contaminated 48.1, 45.5, 44.4 and 12.5% of grain by-products, meal, fibrous feed and food by-products, respectively. FB
1 and FB
2 were found in 56.4 and 40.0% of feed ingredient samples. Kosichi et al. [
39] reported that 59% of 83 maize samples were contaminated with a mean of 1885 μg/kg FUMs. In this study, the incidence of FUMs was 92.3% in 26 samples consisting of corn gluten feed, corn bran, corn gluten meal and corn germ meal with mean levels of 1440.5 and 517.6 μg/kg for FB
1 and FB
2, respectively. The maximum detected levels of FB
1 and FB
2 were 6357.5 and 1798.9 μg/kg in corn bran and 4816.0 and 1662.7 μg/kg in corn gluten meal. As a result, all FUMs concentrations in feed ingredient samples were lower than the guidance limits established by the EC and Korea.
4. Materials and Methods
4.1. Chemicals and Reagents
Analytical standards of FB1 and FB2 were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Acetonitrile and methanol, purchased from Honeywell Burdick & Jackson (Morris Plains, NJ, USA), were used for the extraction of FB1 and FB2. Phosphate-buffered saline (PBS; Sigma-Aldrich, St. Louis, MO, USA) was used as a buffer solution for elution from the immune-affinity column. Ortho-phthaldialdehyde (OPA; Pickering Laboratories, Mountain View, CA, USA) was used for derivatization of FUMs for fluorescence analysis. Fumoniprep (R-Biopharm®, Darmstadt, Germany) and FumoniTest (Vicam Co., Milford, MA, USA) were applied for solid-phase extraction and purification. A standard solution of FB1 and FB2 was diluted with 50% acetonitrile solution (acetonitrile:water = 50:50, v:v).
4.2. Sampling of Compound Feed and Feed Ingredients
A total of 535 animal feed samples (425 compound feed and 110 feed ingredients) produced domestically between 2011 and 2016 in Korea were collected in order to evaluate the contamination level of FUMs. These samples were provided by the National Agriculture Products Quality Management Service Experiment Research Institute. These feed samples were prepared by the general guidelines on sampling [
45]. One kilogram of sample was randomly collected from every ton of compound feed and feed ingredient. Four different samples collected from the same lot were mixed and divided into another four groups according to the guidelines mentioned above. All of the divided samples of 200 g were kept at 4 °C for analysis.
Table 7 and
Table 8 describe detailed data related to collecting compound feed samples and feed ingredients. The classification of compound feed and feed ingredients is shown in the
supplementary data (Table S1–S5).
4.3. Extraction and Purification
The guidelines of the International Conference on Harmonisation (ICH) were applied to measure the level of FB
1 and FB
2 in feed samples using HPLC [
46]. The extraction solvent (acetonitrile:methanol:water = 25:25:50,
v:
v:
v; 100 mL) was added to ground feed samples (20 g), and the mixture was homogenized at 7614×
g for 10 min. The extract was filtered through filter paper (Whatman No.4, GE Healthcare Life Science, Maidstone, Kent, U.K.), and PBS solution (40 mL) was added and mixed with 10 mL of filtered extract solution. This mixed solution (10–50 mL) was loaded onto an IAC column, and the IAC column was washed with 10 mL of PBS solution before being eluted with 4 mL of solution (methanol:water = 80:20,
v:
v) under gravity. The flow rate was 2–3 mL/min at the purification step using an IAC for adequate reaction. This eluted solution was dried under nitrogen gas at 60 °C and re-dissolved with 0.2–0.5 mL of solution (acetonitrile:water = 50:50,
v:
v). The re-dissolved residues were filtered through a membrane filter (0.22-μm pore size).
The level of FUMs in animal feed was measured with HPLC analysis using an Agilent 1100 series (Santa Clara, CA, USA) including a degasser, automatic sampler, thermostated column compartment and fluorescence detector (FLD) with a ZORBAX Eclipse XDB-C18 column (4.6 mm × 250 mm, 5 μm) (Agilent, Santa Clara, CA, USA). The run time was 30 min at 30 °C. A mixture of methanol and 0.1 M monosodium phosphate (pH 3.3 adjusted with phosphoric acid) (77:23, v:v) was used as the mobile phase. A sample (20–22 μL) was injected into the HPLC system after reaction of the sample (10 μL) and OPA solution (10–12 μL) for derivatization, which was set to occur automatically by the injector. FUMs were detected at 335 nm (excitation) and 440 nm (emission). FB1 and FB2 were detected at 3.7 min and 8.7 min without a guard column and at 10.5 min and 26.7 min with a guard column, respectively.
4.4. Identification of FUMs by Mass Spectrometry
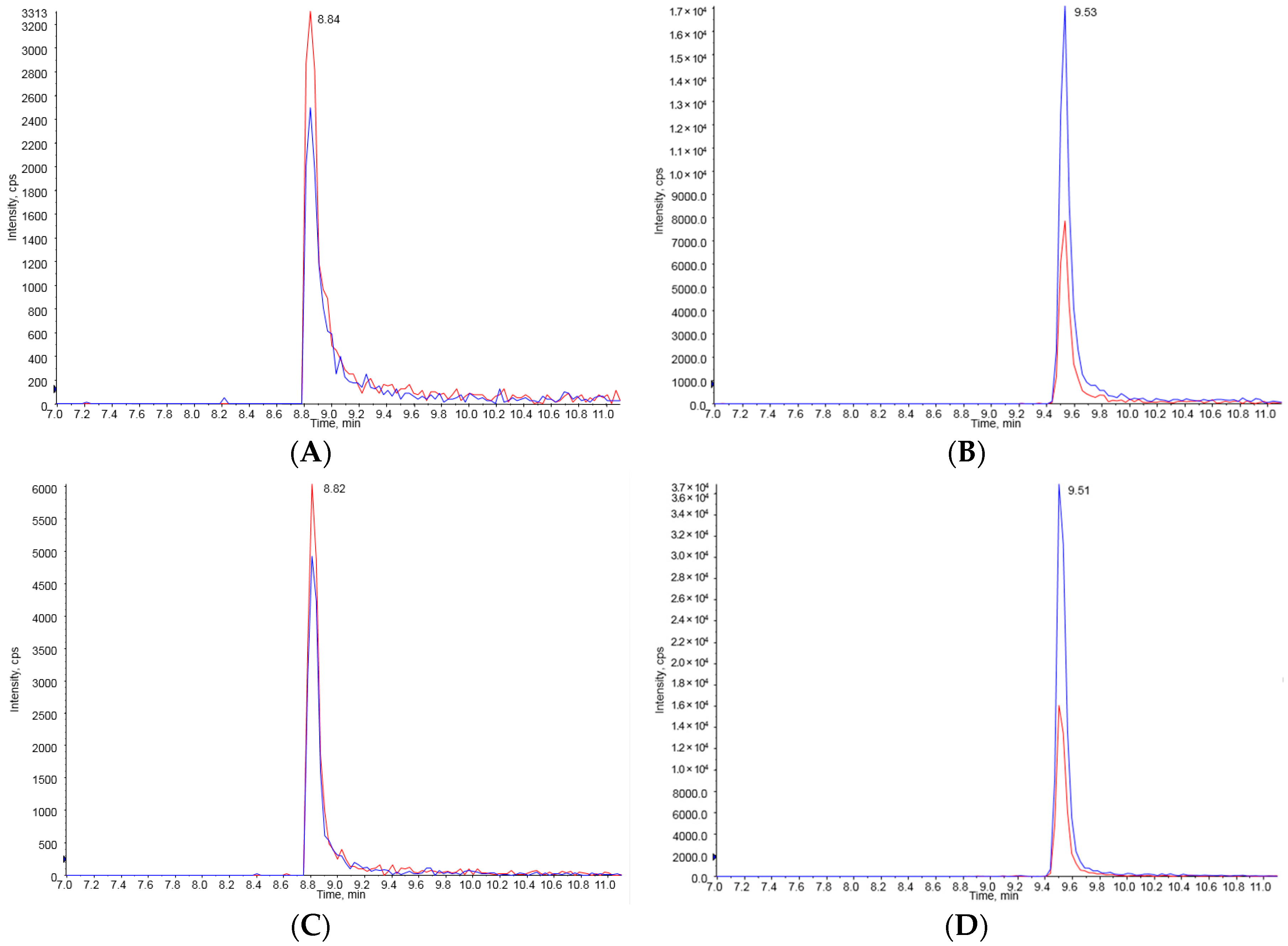
The determination of FB1 and FB2 was conducted using a positive electrospray ionization (ESI) acquisition technique with liquid chromatography-tandem mass spectrometry (API 4000, Applied Biosystems MDS SCIX, Foster, CA, USA). In order to separate FB1 and FB2 in LC-MS/MS analysis, a YMC-Pack pro C18 RS column (I.D. 3.0 mm × length 150 mm, 3-μm particle size, YMC, Kyoto, Japan) and gradient elution conditions were applied in this study. The flow rate was 0.3 mL/min, and the mobile phase was composed of water (HPLC grade) containing 2 mM ammonium acetate (0.1% formic acid) and acetonitrile containing 2 mM ammonium acetate (0.1% formic acid). Analysis was conducted with the following parameters: 20 psi curtain gas, medium collision gas, 4500 ion-spray voltage and 500 °C turbo gas. A sample (10 μL) was injected into the HPLC and LC-MS/MS system, and its retention time was 8.8 min for FB1 and 9.5 min for FB2. Detection and quantification for FB1 and FB2 were performed in the multiple reaction monitoring (MRM) mode. The precursor ions (m/z 722.373 and 706.353) for FB1 and FB2 were selected from the extracted ion chromatogram (XIC) of the FB1 and FB2 standard at 250 ppb. Each selected precursor ion was compared with that of FB1 and FB2 in the sample. Two product ions of m/z 334.300 and 318.000 were further examined in order to confirm FB1 in the standard and the sample in the MS/MS system. In addition, two product ions of m/z 336.300 and 318.300 were further checked to confirm FB2 in the standard and the sample in the MS/MS system.
4.5. Method Validation
The HPLC analytical method for FUMs was validated with parameters of linearity, LOD, LOQ, accuracy and precision according to ICH guidelines [
46]. The linearity was checked with a term expressed as a regression coefficient (r
2) after analyzing a range of FUM standard concentrations (50, 100, 250, 500 and 1000 ng/mL). The LOD was estimated at the lowest amount of FUMs that could be only detected, but not quantified, and the concentration had a signal-to-noise ratio of 3. The LOQ was calculated as the lowest amount of FUMs that could be quantitated, which was determined as the concentration of a signal-to-noise ratio of 10. Accuracy was estimated using a recovery test that analyzed blank samples spiked with a known concentration of FUMs (100, 200 and 400 μg/kg), repeatedly, and the results were expressed as a recovery ratio. A FUMs-free pig feed was used for the recovery test as a blank sample because pig feeds are composed of major components for tested feeds in an average ratio. It contains mainly corn (45%), followed by soybean meal (20%) and others (wheat, barley, wheat shorts, corn gluten meal, distiller’s dried grains, palm oil meal, calcium phosphate and syrup, etc.). The degree of repeatability was defined as precision in this study and is expressed as percent relative standard deviation (%RSD).