How the Cobra Got Its Flesh-Eating Venom: Cytotoxicity as a Defensive Innovation and Its Co-Evolution with Hooding, Aposematic Marking, and Spitting
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Species Studied
- Non-hooding species
- Bungarus fasciatus
- Dendroaspis polylepis
- Elapsoidea boulengerii, Elapsoidea sundevali longicauda, Elapsoidea sundevali sundevali
- Walterinnesia aegyptia
- Naja annulata
- Narrow hooding species
- Aspidelaps lubricus, Aspidelaps scutatus (nar)
- Hooding species
- Hemachatus haemachatus
- African Naja
- Non-spitters = N. annulata, N. annulifera, N. haje, N. melanoleuca, N. nivea
- Spitters = N. mossambica, N. nigricollis, N. pallida
- Asian Naja
- Non-spitters = N. atra, N. kaouthia, N. naja, N. oxiana (Note that N. atra and N. kaouthia are slightly ambiguous as they are not considered as true spitting cobras, and lack the highly specialised adaptations of the other species I this category, but have been documented to spit significantly under strong duress [27,28]
- Spitters = N. philippinensis, N. siamensis, N. sumatrana
- Ophiophagus hannah clade (Four geographically different samples from O. hannah from Cambodia, Malaysia, Thailand, East Java).
3.2. Venom Preparation
3.3. Cell Lines and Cell Culture
3.4. MTT Assays
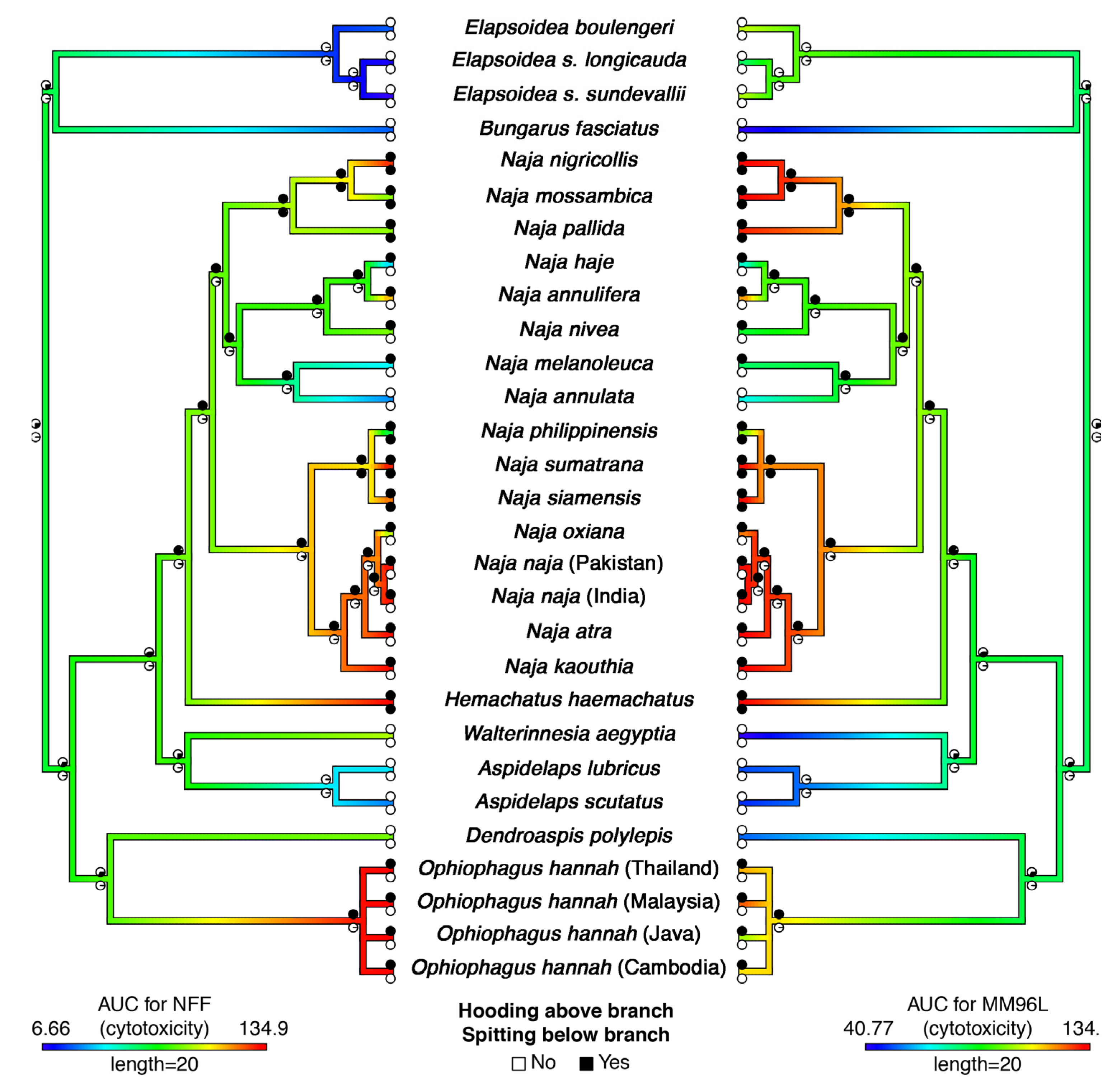
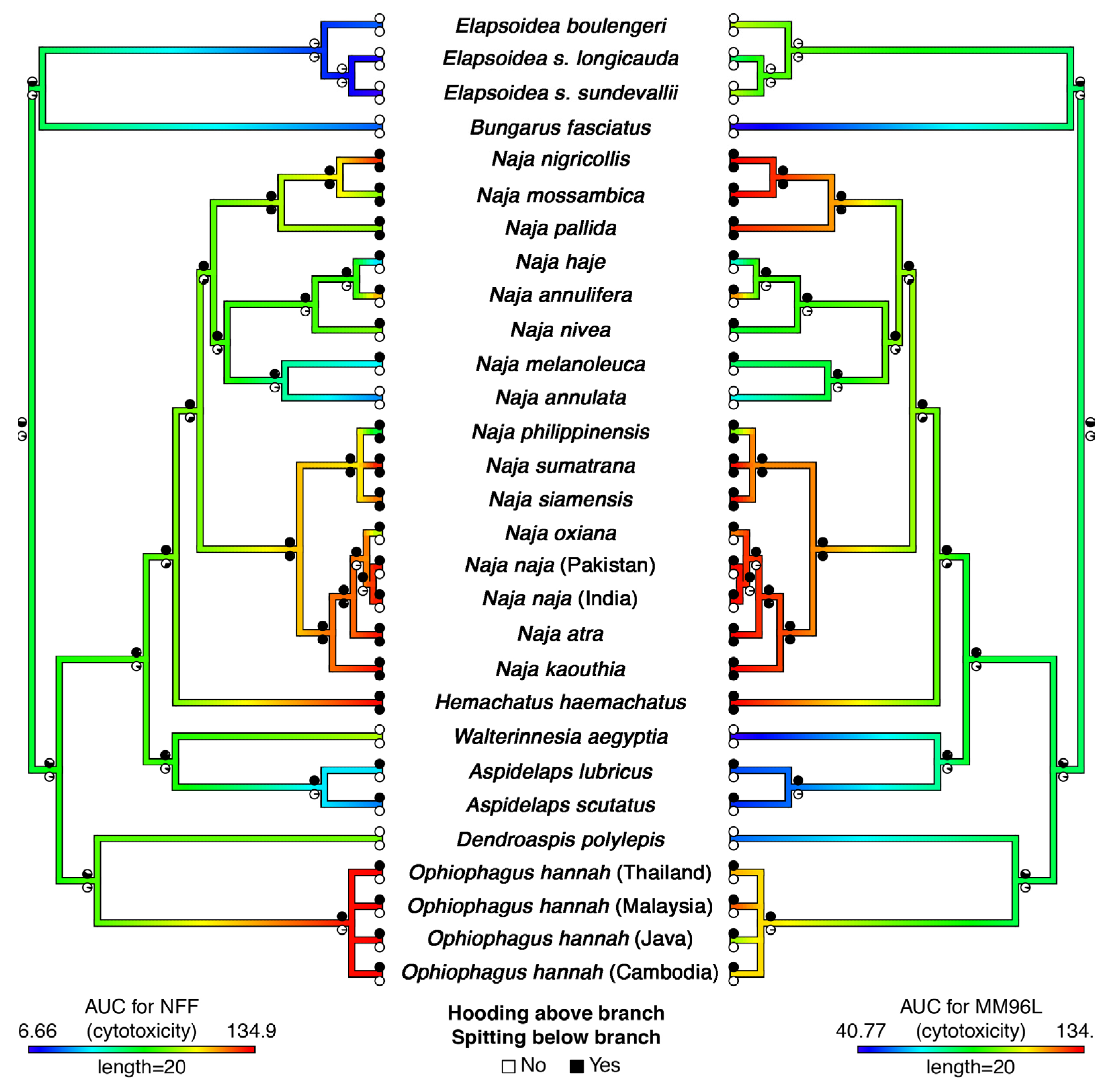
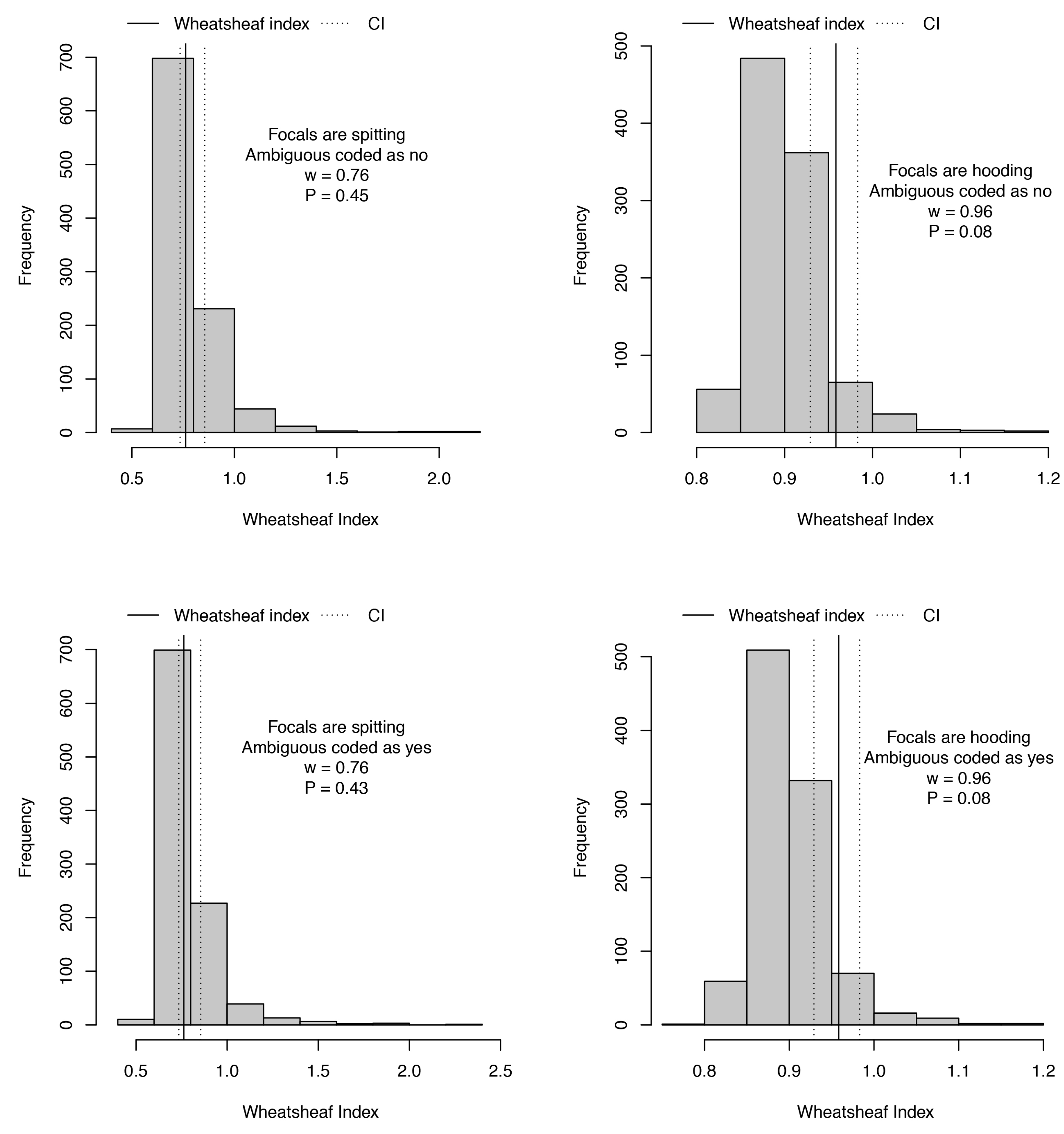
3.5. Phylogenetic Comparative Analyses
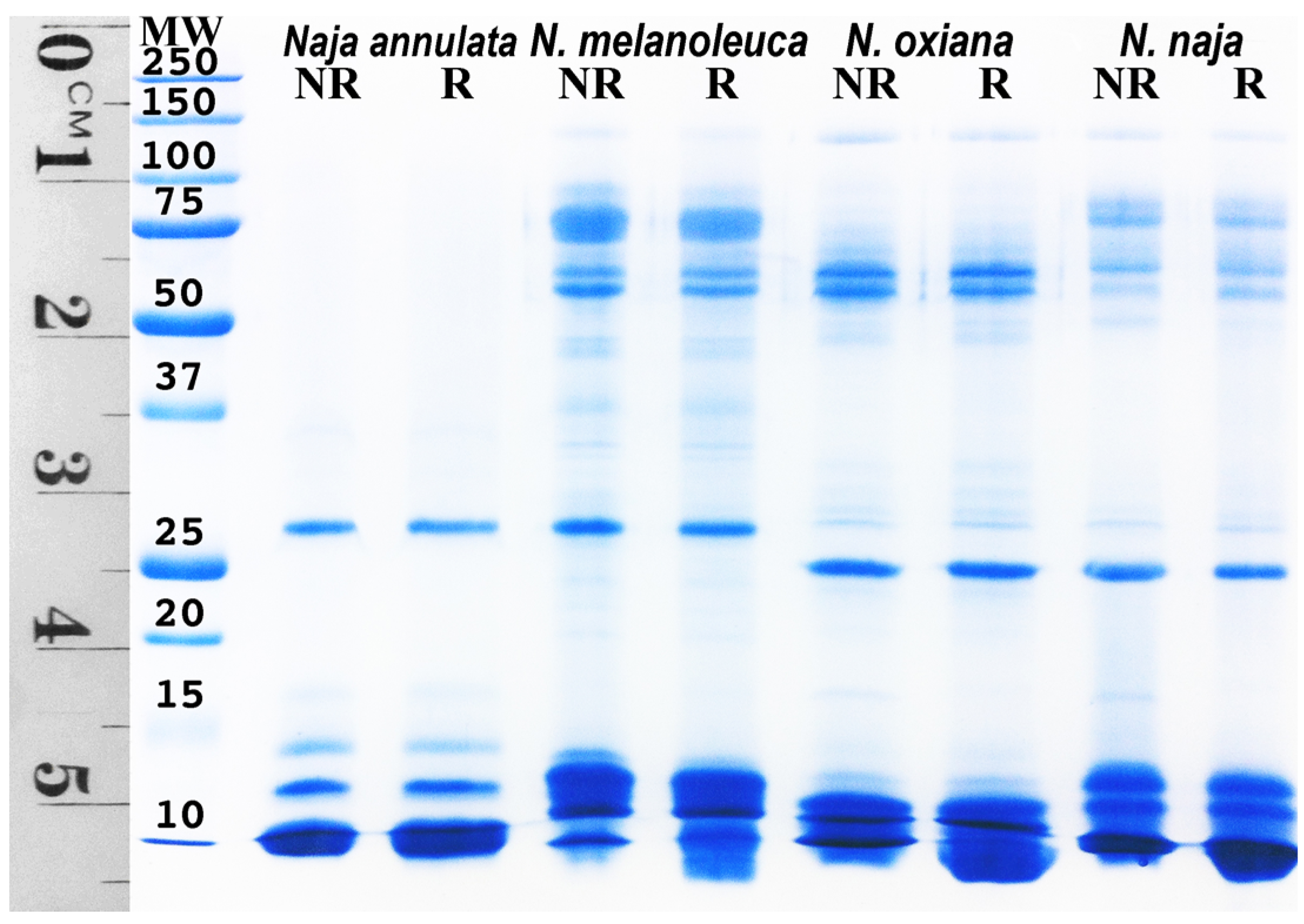
3.6. SDS-PAGE
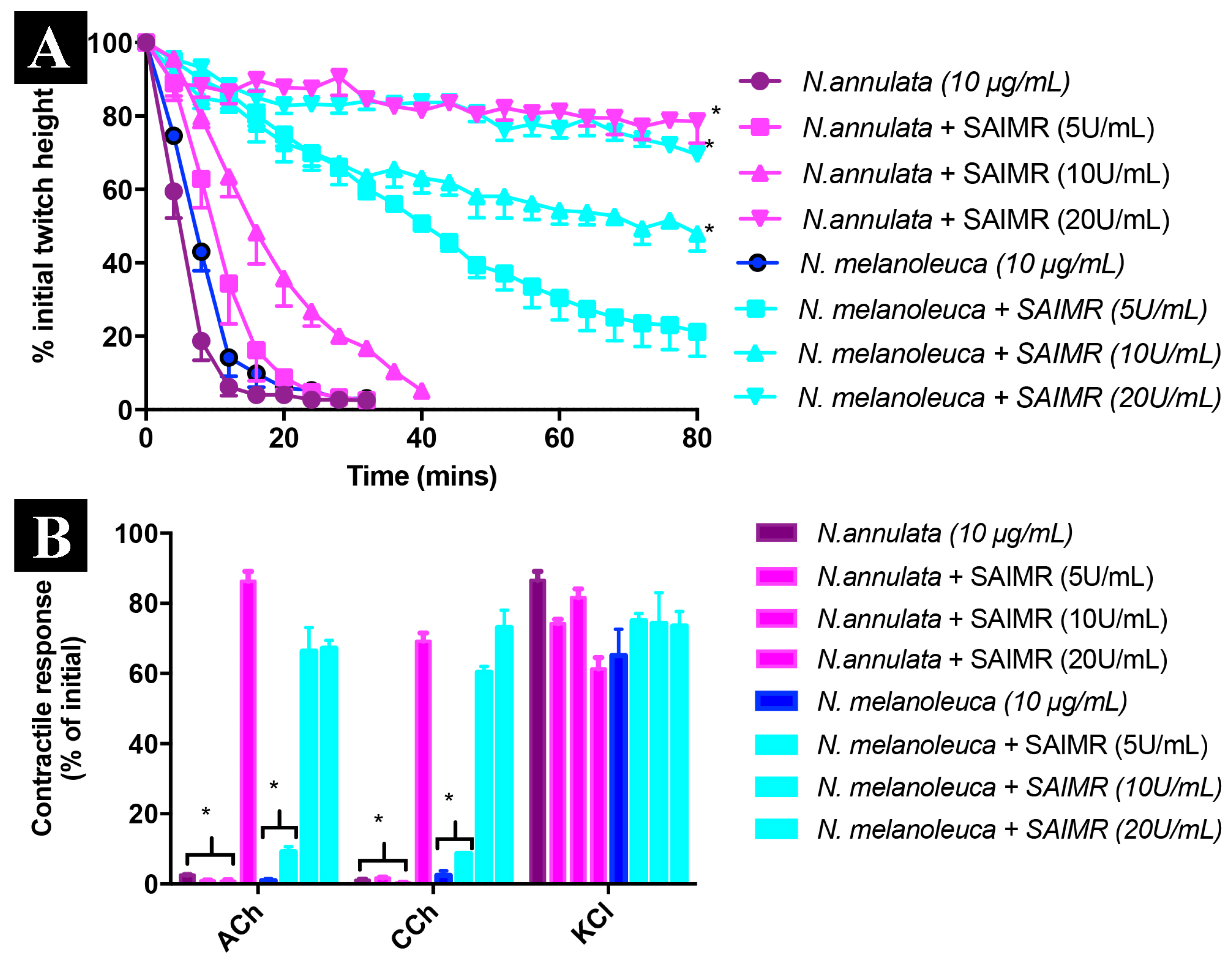
3.7. Neurotoxicity Assays
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ismail, M.; al-Bekairi, A.M.; el-Bedaiwy, A.M.; Abd-el Salam, M.A. The ocular effects of spitting cobras: II. Evidence that cardiotoxins are responsible for the corneal opacification syndrome. J. Toxicol. Clin. Toxicol. 1993, 31, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; al-Bekairi, A.M.; el-Bedaiwy, A.M.; Abd-el Salam, M.A. The ocular effects of spitting cobras: I. The ringhals cobra (Hemachatus haemachatus) venom-induced corneal opacification syndrome. J. Toxicol. Clin. Toxicol. 1993, 31, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.M.; Williams, D.; Fan, H.W.; Warrell, D.A. Snakebite envenoming from a global perspective: Towards an integrated approach. Toxicon 2010, 56, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.A.; Hargreaves, A.; Wagstaff, S.C.; Faragher, B.; Lalloo, D.G. Snake envenoming: A disease of poverty. PLoS Negl. Trop. Dis. 2009, 3, e569. [Google Scholar] [CrossRef] [PubMed]
- Chippaux, J.P. Estimate of the burden of snakebites in sub-Saharan Africa: A meta-analytic approach. Toxicon 2011, 57, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Joshi, H.S.; Mahmood, S.E.; Joshi, M.C.; Shaifali, I.; Srivastava, P.C. Clinico-epidemiological profile of snake bite cases in Western Nepal. TAF Prev. Med. Bull. 2012, 11, 57–62. [Google Scholar] [CrossRef]
- Fry, B.G.; Wuster, W.; Kini, R.M.; Brusic, V.; Khan, A.; Venkataraman, D.; Rooney, A.P. Molecular evolution and phylogeny of elapid snake venom three-finger toxins. J. Mol. Evol. 2003, 57, 110–129. [Google Scholar] [CrossRef] [PubMed]
- Sunagar, K.; Jackson, T.; Undheim, E.; Ali, S.; Antunes, A.; Fry, B. Three-fingered RAVERs: Rapid accumulation of variations in exposed residues of snake venom toxins. Toxins 2013, 5, 2172–2208. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.M.R.; Barker, N.P.; Villet, M.H.; Broadley, D.G. Phylogeny, biogeography and classification of the snake superfamily Elapoidea: A rapid radiation in the late Eocene. Cladistics 2009, 25, 38–63. [Google Scholar] [CrossRef]
- Lee, M.S.; Sanders, K.L.; King, B.; Palci, A. Diversification rates and phenotypic evolution in venomous snakes (Elapidae). R. Soc. Open Sci. 2016, 3, 150277. [Google Scholar] [CrossRef] [PubMed]
- Berthe, R.A.; de Pury, S.; Bleckmann, H.; Westhoff, G. Spitting cobras adjust their venom distribution to target distance. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2009, 195, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Berthe, R.A.; Westhoff, G.; Bleckmann, H. Potential targets aimed at by spitting cobras when deterring predators from attacking. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2013, 199, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Russell, P.; Home, E. Remarks on the voluntary expansion of the skin of the neck in the Cobra de Capello or hooded snake of the East Indies. With a description of the structures of the parts which perform that office. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1804, 94, 346–352. [Google Scholar] [CrossRef]
- Young, B.A.; Boetig, M.; Westhoff, G. Functional bases of the spatial dispersal of venom during cobra “spitting”. Physiol. Biochem. Zool. 2009, 82, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Young, B.A.; Kardong, K.V. The functional morphology of hooding in cobras. J. Exp. Biol. 2010, 213, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Young, B.A.; Dunlap, K.; Koenig, K.; Singer, M. The buccal buckle: The functional morphology of venom spitting in cobras. J. Exp. Biol. 2004, 207, 3483–3494. [Google Scholar] [CrossRef] [PubMed]
- Young, B.A.; Herzog, F.; Friedel, P.; Rammensee, S.; Bausch, A.; van Hemmen, J.L. Tears of venom: Hydrodynamics of reptilian envenomation. Phys. Rev. Lett. 2011, 106, 198103. [Google Scholar] [CrossRef] [PubMed]
- Cascardi, J.; Young, B.A.; Husic, H.D.; Sherma, J. Protein variation in the venom spat by the red spitting cobra, Naja pallida (Reptilia: Serpentes). Toxicon 1999, 37, 1271–1279. [Google Scholar] [CrossRef]
- Triep, M.; Hess, D.; Chaves, H.; Brucker, C.; Balmert, A.; Westhoff, G.; Bleckmann, H. 3D flow in the venom channel of a spitting cobra: Do the ridges in the fangs act as fluid guide vanes? PLoS ONE 2013, 8, e61548. [Google Scholar] [CrossRef] [PubMed]
- Westhoff, G.; Tzschatzsch, K.; Bleckmann, H. The spitting behavior of two species of spitting cobras. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2005, 191, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Wallach, V.; Wüster, W.; Broadley, D.G. In praise of subgenera: Taxonomic status of cobras of the genus Naja Laurenti (Serpentes: Elapidae). Zootaxa 2009, 2236, 26–36. [Google Scholar]
- Ahn, M.Y.; Lee, B.M.; Kim, Y.S. Cytotoxicity and L-amino acid oxidase activity of animal venoms. Arch. Pharm. Res. 1997, 20, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Jara, M.; Pincheira-Donoso, D. The neck flattening defensive behaviour in snakes: First record of hooding in the South American colubrid genus Philodryas. Anim. Biol. 2015, 65, 73–79. [Google Scholar] [CrossRef]
- Wuster, W.; Crookes, S.; Ineich, I.; Mane, Y.; Pook, C.E.; Trape, J.F.; Broadley, D.G. The phylogeny of cobras inferred from mitochondrial DNA sequences: Evolution of venom spitting and the phylogeography of the African spitting cobras (Serpentes: Elapidae: Naja nigricollis complex). Mol. Phylogenet. Evol. 2007, 45, 437–453. [Google Scholar] [CrossRef] [PubMed]
- Wüster, W. The cobras of the genus Naja in India. Hamadryad 1998, 23, 15–32. [Google Scholar]
- Chan, S. A Field Guide to the Venomous Land Snakes of Hong Kong; Cosmos Books Ltd.: Hong Kong, China, 2006. [Google Scholar]
- Wüster, W.; Thorpe, R.S. Dentitional phenomena in cobras revisited: Spitting and fang structure in the Asiatic species of Naja (Serpentes: Elapidae). Herpetologica 1992, 48, 424–434. [Google Scholar]
- Arbuckle, K.; Bennett, C.M.; Speed, M.P. A simple measure of the strength of convergent evolution. Methods Ecol. Evol. 2014, 5, 685–693. [Google Scholar] [CrossRef]
- Marais, J. A Complete Guide to the Snakes of Southern Africa; Struik Nature: Cape Town, South Africa, 2004. [Google Scholar]
- Kalam, Y.; Isbister, G.K.; Mirtschin, P.; Hodgson, W.C.; Konstantakopoulos, N. Validation of a cell-based assay to differentiate between the cytotoxic effects of elapid snake venoms. J. Pharmacol. Toxicol. Methods 2011, 63, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Mendez, I.; Gutierrez, J.M.; Angulo, Y.; Calvete, J.J.; Lomonte, B. Comparative study of the cytolytic activity of snake venoms from African spitting cobras (Naja spp., Elapidae) and its neutralization by a polyspecific antivenom. Toxicon 2011, 58, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Ghezellou, P.; Paiva, O.; Matainaho, T.; Ghassempour, A.; Goudarzi, H.; Kraus, F.; Sanz, L.; Williams, D.J. Snake venomics of two poorly known Hydrophiinae: Comparative proteomics of the venoms of terrestrial Toxicocalamus longissimus and marine Hydrophis cyanocinctus. J. Proteom. 2012, 75, 4091–4101. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Wuster, W.; Ramjan, S.F.R.; Jackson, T.; Martelli, P.; Kini, R.M. Analysis of Colubroidea snake venoms by liquid chromatography with mass spectrometry: Evolutionary and toxinological implications. Rapid Commun. Mass Spectrom. 2003, 17, 2047–2062. [Google Scholar] [CrossRef] [PubMed]
- Spawls, S.; Branch, B. Dangerous Snakes of Africa; Blandford Press: London, UK, 1995. [Google Scholar]
- Dutta, S.; Chanda, A.; Kalita, B.; Islam, T.; Patra, A.; Mukherjee, A.K. Proteomic analysis to unravel the complex venom proteome of eastern India Naja naja: Correlation of venom composition with its biochemical and pharmacological properties. J. Proteom. 2017, 156, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; Liu, B.S.; Chien, K.Y.; Chiang, L.C.; Huang, S.Y.; Sung, W.C.; Wu, W.G. Cobra venom proteome and glycome determined from individual snakes of Naja atra reveal medically important dynamic range and systematic geographic variation. J. Proteom. 2015, 128, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, Y.; Lee, W.; Xu, X.; Zhang, Y.; Zhao, R.; Wang, W. Venom gland transcriptomes of two elapid snakes (Bungarus multicinctus and Naja atra) and evolution of toxin genes. BMC Genom. 2011, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, L.P.; Laustsen, A.H.; Lomonte, B.; Gutierrez, J.M. Exploring the venom of the forest cobra snake: Toxicovenomics and antivenom profiling of Naja melanoleuca. J. Proteom. 2017, 150, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Laustsen, A.H.; Gutierrez, J.M.; Lohse, B.; Rasmussen, A.R.; Fernandez, J.; Milbo, C.; Lomonte, B. Snake venomics of monocled cobra (Naja kaouthia) and investigation of human IgG response against venom toxins. Toxicon 2015, 99, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Petras, D.; Sanz, L.; Segura, A.; Herrera, M.; Villalta, M.; Solano, D.; Vargas, M.; Leon, G.; Warrell, D.A.; Theakston, R.D.; et al. Snake venomics of African spitting cobras: Toxin composition and assessment of congeneric cross-reactivity of the pan-African EchiTAb-Plus-ICP antivenom by antivenomics and neutralization approaches. J. Proteome Res. 2011, 10, 1266–1280. [Google Scholar] [CrossRef] [PubMed]
- Sintiprungrat, K.; Watcharatanyatip, K.; Senevirathne, W.D.; Chaisuriya, P.; Chokchaichamnankit, D.; Srisomsap, C.; Ratanabanangkoon, K. A comparative study of venomics of Naja naja from India and Sri Lanka, clinical manifestations and antivenomics of an Indian polyspecific antivenom. J. Proteom. 2016, 132, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Suzuki-Matsubara, M.; Athauda, S.B.; Suzuki, Y.; Matsubara, K.; Moriyama, A. Comparison of the primary structures, cytotoxicities, and affinities to phospholipids of five kinds of cytotoxins from the venom of Indian cobra, Naja naja. Comp. Biochem. Physiol. Toxicol. Pharmacol. 2016, 179, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.Y.; Tan, C.H.; Fung, S.Y.; Tan, N.H. Venomics, lethality and neutralization of Naja kaouthia (monocled cobra) venoms from three different geographical regions of Southeast Asia. J. Proteom. 2015, 120, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Yap, M.K.; Fung, S.Y.; Tan, K.Y.; Tan, N.H. Proteomic characterization of venom of the medically important Southeast Asian Naja sumatrana (Equatorial spitting cobra). Acta Trop. 2014, 133, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Fung, S.Y.; Lee, M.L.; Tan, N.H. Molecular mechanism of cell death induced by king cobra (Ophiophagus hannah) venom L-amino acid oxidase. Toxicon 2015, 96, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Tan, K.Y.; Fung, S.Y.; Tan, N.H. Venom-gland transcriptome and venom proteome of the Malaysian king cobra (Ophiophagus hannah). BMC Genom. 2015, 16, 687. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.H.; Hj, M.N. Enzymatic and toxic properties of Ophiophagus hannah (king cobra) venom and venom fractions. Toxicon 1989, 27, 689–695. [Google Scholar] [CrossRef]
- Tan, N.H.; Saifuddin, M.N. Isolation and characterization of an unusual form of L-amino acid oxidase from King cobra (Ophiophagus hannah) venom. Biochem. Int. 1989, 19, 937–944. [Google Scholar] [PubMed]
- Tan, N.H.; Saifuddin, M.N. Isolation and characterization of a hemorrhagin from the venom of Ophiophagus hannah (king cobra). Toxicon 1990, 28, 385–392. [Google Scholar] [CrossRef]
- Vonk, F.J.; Casewell, N.R.; Henkel, C.V.; Heimberg, A.M.; Jansen, H.J.; McCleary, R.J.; Kerkkamp, H.M.; Vos, R.A.; Guerreiro, I.; Calvete, J.J.; et al. The king cobra genome reveals dynamic gene evolution and adaptation in the snake venom system. Proc. Natl. Acad. Sci. USA 2013, 110, 20651–20656. [Google Scholar] [CrossRef] [PubMed]
- Joubert, F.J. Snake venom toxins. The amino-acid sequences of three toxins (9B, 11 and 12A) from Hemachatus haemachatus (Ringhals) venom. Eur. J. Biochem. 1977, 74, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Rong, H.; Li, Y.; Lou, X.H.; Zhang, X.; Gao, Y.X.; Teng, M.K.; Niu, L.W. Purification, partial characterization, crystallization and preliminary X-ray diffraction of a novel cardiotoxin-like basic protein from Naja naja atra (South Anhui) venom. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2007, 63, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.Y.; Lee, B.M.; Kim, Y.S. Characterization and cytotoxicity of L-amino acid oxidase from the venom of king cobra (Ophiophagus hannah). Int. J. Biochem. Cell Biol. 1997, 29, 911–919. [Google Scholar] [CrossRef]
- Casewell, N.R.; Wuster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Casewell, N.R.; Wuster, W.; Vidal, N.; Young, B.; Jackson, T.N. The structural and functional diversification of the Toxicofera reptile venom system. Toxicon 2012, 60, 434–448. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Sunagar, K.; Casewell, N.R.; Kochva, E.; Roelants, K.; Scheib, H.; Wüster, W.; Vidal, N.; Young, B.; Burbrink, F.; et al. The origin and evolution of the Toxicofera reptile venom system. In Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery; Fry, B.G., Ed.; Oxford University Press: New York, NY, USA, 2015; pp. 1–31. [Google Scholar]
- Fry, B.G.; Undheim, E.A.; Ali, S.A.; Jackson, T.N.; Debono, J.; Scheib, H.; Ruder, T.; Morgenstern, D.; Cadwallader, L.; Whitehead, D.; et al. Squeezers and leaf-cutters: Differential diversification and degeneration of the venom system in toxicoferan reptiles. Mol. Cell. Proteom. 2013, 12, 1881–1899. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Fry, B.G.; Kini, R.M. Eggs-only diet: Its implications for the toxin profile changes and ecology of the marbled sea snake (Aipysurus eydouxii). J. Mol. Evol. 2005, 60, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Fry, B.G.; Kini, R.M. Putting the brakes on snake venom evolution: The unique molecular evolutionary patterns of Aipysuras eydouxii (Marbled sea snake) phospholipase A(2) toxins. Mol. Biol. Evol. 2005, 22, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.N.; Fry, B.G. A tricky trait: Applying the fruits of the “function debate” in the philosophy of biology to the “venom debate” in the science of toxinology. Toxins 2016, 8, 263. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Tsai, T.S.; Tsai, I.H. Functional proteomic approach to discover geographic variations of king cobra venoms from Southeast Asia and China. J. Proteom. 2013, 89, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.J.; Gutierrez, J.M.; Calvete, J.J.; Wuster, W.; Ratanabanangkoon, K.; Paiva, O.; Brown, N.I.; Casewell, N.R.; Harrison, R.A.; Rowley, P.D.; et al. Ending the drought: New strategies for improving the flow of affordable, effective antivenoms in Asia and Africa. J. Proteom. 2011, 74, 1735–1767. [Google Scholar] [CrossRef] [PubMed]
- R-Core-Team, R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2011; Available online: https://www.R-project.org/ (accessed on 27 January 2017).
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Fritz, S.A.; Purvis, A. Selectivity in mammalian extinction risk and threat types: A new measure of phylogenetic signal strength in binary traits. Conserv. Biol. 2010, 24, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Orme, D.; Freckleton, R.; Thomas, G.; Petzoldt, T.; Fritz, S.; Isaac, N.; Pearse, W. Caper: Comparative Analyses of Phylogenetics and Evolution in R. R Package Version 0.5.2. 2015. Available online: https://CRAN.R-project.org/package=caper (accessed on 1 February 2017).
- Blomberg, S.P.; Garland, T.; Ives, A.R. Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Evolution 2003, 57, 717–745. [Google Scholar] [CrossRef] [PubMed]
- Revell, L.J. phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 2012, 3, 217–223. [Google Scholar] [CrossRef]
- Yang, Z.; Kumar, S.; Nei, M. A new method of inference of ancestral nucleotide and amino acid sequences. Genetics 1995, 141, 1641–1650. [Google Scholar] [PubMed]
- Arbuckle, K.; Minter, A. Windex: Analyzing convergent evolution using the Wheatsheaf index in R. Evol. Bioinform. 2015, 11, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Jackson, T.N.; Casewell, N.R.; Low, D.H.; Rossi, S.; Baumann, K.; Fathinia, B.; Visser, J.; Nouwens, A.; Hendrikx, I.; et al. Extreme venom variation in Middle Eastern vipers: A proteomics comparison of Eristicophis macmahonii, Pseudocerastes fieldi and Pseudocerastes persicus. J. Proteom. 2015, 116, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Yang, D.C.; Jackson, T.N.; Undheim, E.A.; Koludarov, I.; Wood, K.; Jones, A.; Hodgson, W.C.; McCarthy, S.; Ruder, T.; et al. Venom proteomic characterization and relative antivenom neutralization of two medically important Pakistani elapid snakes (Bungarus sindanus and Naja naja). J. Proteom. 2013, 89, 15–23. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panagides, N.; Jackson, T.N.W.; Ikonomopoulou, M.P.; Arbuckle, K.; Pretzler, R.; Yang, D.C.; Ali, S.A.; Koludarov, I.; Dobson, J.; Sanker, B.; et al. How the Cobra Got Its Flesh-Eating Venom: Cytotoxicity as a Defensive Innovation and Its Co-Evolution with Hooding, Aposematic Marking, and Spitting. Toxins 2017, 9, 103. https://doi.org/10.3390/toxins9030103
Panagides N, Jackson TNW, Ikonomopoulou MP, Arbuckle K, Pretzler R, Yang DC, Ali SA, Koludarov I, Dobson J, Sanker B, et al. How the Cobra Got Its Flesh-Eating Venom: Cytotoxicity as a Defensive Innovation and Its Co-Evolution with Hooding, Aposematic Marking, and Spitting. Toxins. 2017; 9(3):103. https://doi.org/10.3390/toxins9030103
Chicago/Turabian StylePanagides, Nadya, Timothy N.W. Jackson, Maria P. Ikonomopoulou, Kevin Arbuckle, Rudolf Pretzler, Daryl C. Yang, Syed A. Ali, Ivan Koludarov, James Dobson, Brittany Sanker, and et al. 2017. "How the Cobra Got Its Flesh-Eating Venom: Cytotoxicity as a Defensive Innovation and Its Co-Evolution with Hooding, Aposematic Marking, and Spitting" Toxins 9, no. 3: 103. https://doi.org/10.3390/toxins9030103
APA StylePanagides, N., Jackson, T. N. W., Ikonomopoulou, M. P., Arbuckle, K., Pretzler, R., Yang, D. C., Ali, S. A., Koludarov, I., Dobson, J., Sanker, B., Asselin, A., Santana, R. C., Hendrikx, I., Van der Ploeg, H., Tai-A-Pin, J., Van den Bergh, R., Kerkkamp, H. M. I., Vonk, F. J., Naude, A., ... Fry, B. G. (2017). How the Cobra Got Its Flesh-Eating Venom: Cytotoxicity as a Defensive Innovation and Its Co-Evolution with Hooding, Aposematic Marking, and Spitting. Toxins, 9(3), 103. https://doi.org/10.3390/toxins9030103







