The Snake with the Scorpion’s Sting: Novel Three-Finger Toxin Sodium Channel Activators from the Venom of the Long-Glanded Blue Coral Snake (Calliophis bivirgatus)
Abstract
:1. Introduction
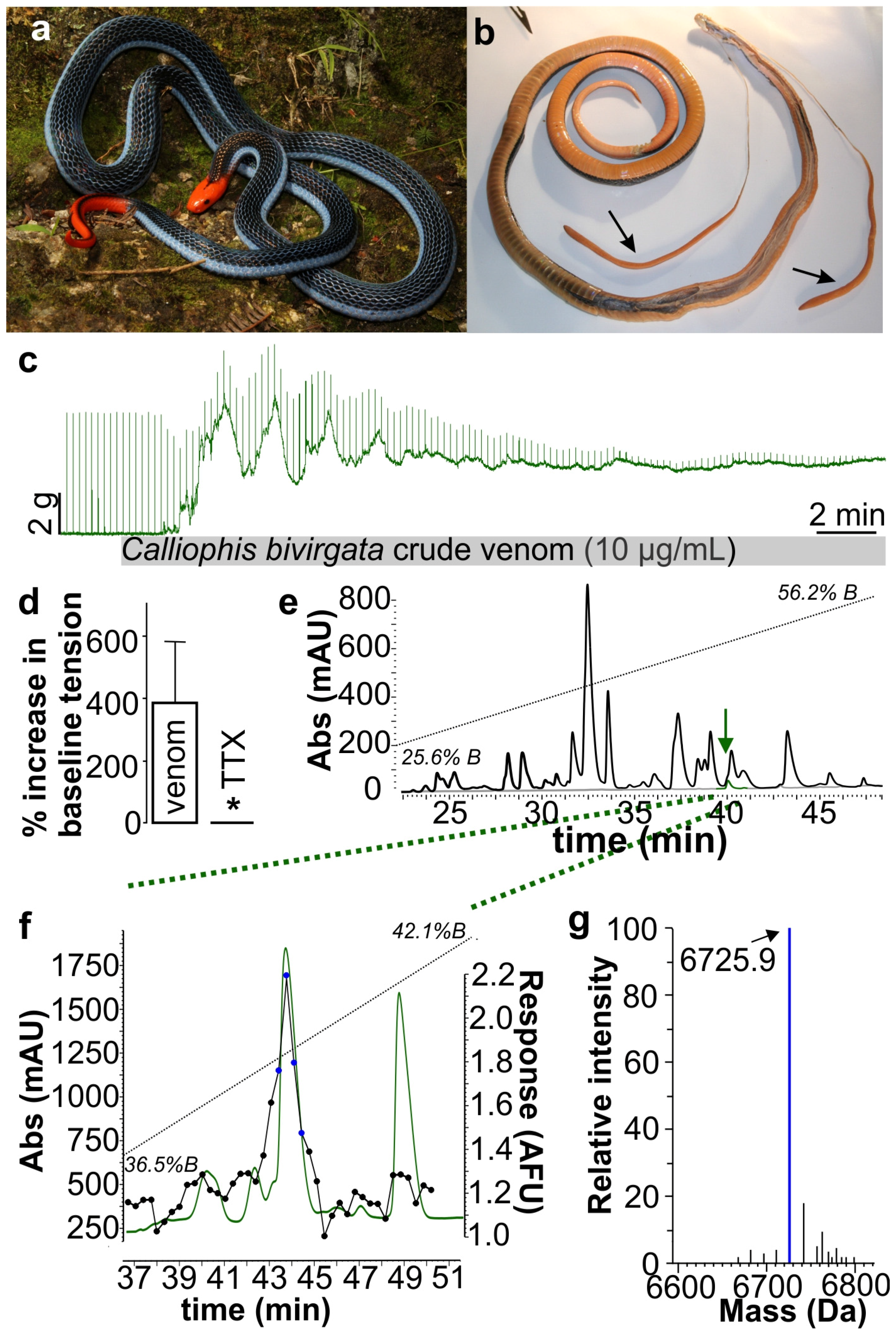
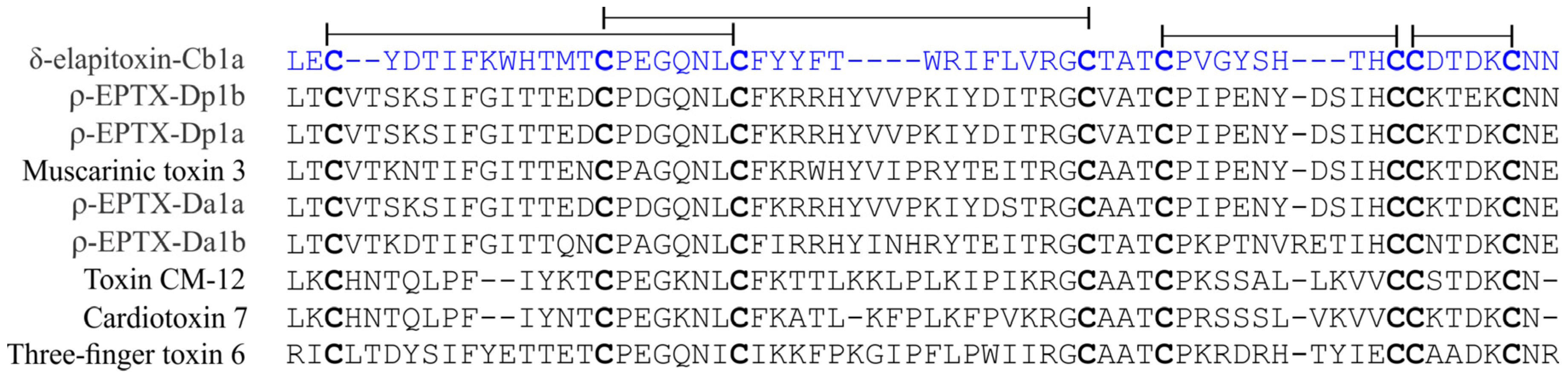
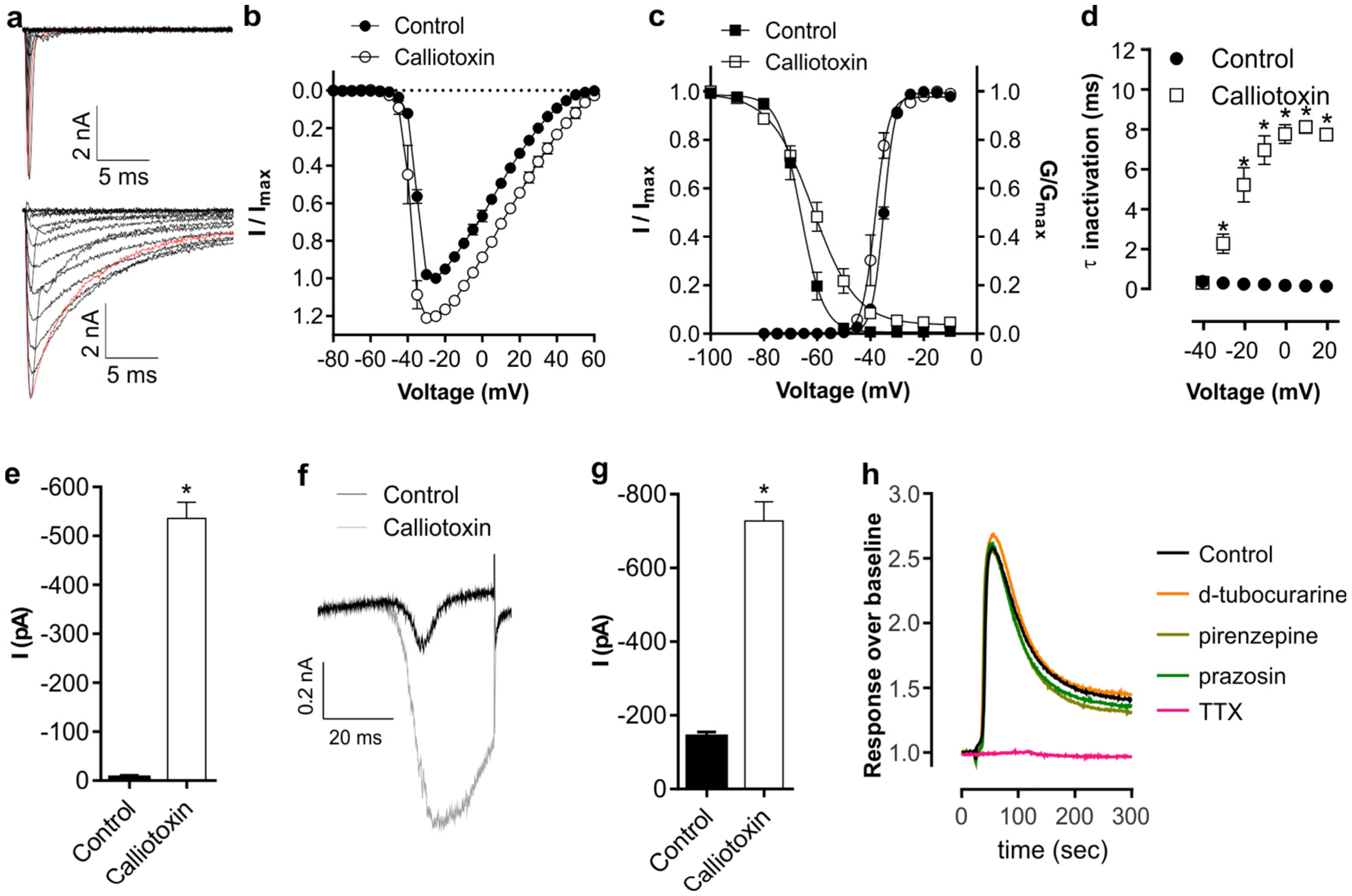
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Animal Ethics
3.3. Neurotoxicity Studies
3.3.1. Assay-Guided Fractionation
3.3.2. Mass Spectrometry
3.3.3. Edman Degradation and Venom Gland Transcriptomics
3.3.4. Electrophysiology
3.4. Data Analysis and Statistics
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jackson, T.N.; Fry, B.G. A Tricky Trait: Applying the Fruits of the “Function Debate” in the Philosophy of Biology to the “Venom Debate” in the Science of Toxinology. Toxins (Basel) 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Losos, J.B.; Hillis, D.M.; Greene, H.W. Evolution. Who speaks with a forked tongue? Science 2012, 338, 1428–1429. [Google Scholar] [CrossRef] [PubMed]
- Schwenk, K.; Wagner, G.P. Function and the evolution of phenotypic stability: Connecting pattern to process. Am. Zool. 2001, 41, 552–563. [Google Scholar] [CrossRef]
- Vitt, L.J. Walking the Natural-History Trail. Herpetologica 2013, 69, 105–117. [Google Scholar] [CrossRef]
- Gauthier, J.A.; Kearney, M.; Maisano, J.A.; Rieppel, O.; Behlke, A.D.B. Assembling the Squamate tree of life: Perspectives from the phenotype and the fossil record. Bull. Peabody Mus. Nat. Hist. 2012, 53, 3–308. [Google Scholar] [CrossRef]
- Estes, R.; de Queiroz, K.; Gauthier, J. Phylogenetic relationships of squamate reptiles. In Phylogenetic Relationships of the Lizard Families; Stanford University Press: Stanford, CA, USA, 1988; pp. 119–280. [Google Scholar]
- Pyron, R.A.; Burbrink, F.T. Extinction, ecological opportunity, and the origins of global snake diversity. Evolution 2012, 66, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Reeder, T.W.; Townsend, T.M.; Mulcahy, D.G.; Noonan, B.P.; Wood, P.L., Jr.; Sites, J.W., Jr.; Wiens, J.J. Integrated analyses resolve conflicts over squamate reptile phylogeny and reveal unexpected placements for fossil taxa. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Townsend, T.; Larson, A.; Louis, E.; Macey, J.R. Molecular phylogenetics of squamata: The position of snakes, amphisbaenians, and dibamids, and the root of the squamate tree. Syst. Biol. 2004, 53, 735–757. [Google Scholar] [CrossRef] [PubMed]
- Vidal, N.; David, P. New insights into the early history of snakes inferred from two nuclear genes. Mol. Phylogenet. Evol. 2004, 31, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Vidal, N.; Hedges, S.B. Higher-level relationships of caenophidian snakes inferred from four nuclear and mitochondrial genes. C. R. Biol. 2002, 325, 987–995. [Google Scholar] [CrossRef]
- Vidal, N.; Hedges, S.B. Molecular evidence for a terrestrial origin of snakes. Proc. Biol. Sci. R. Soc. 2004, 271 (Suppl. S4), S226–S229. [Google Scholar] [CrossRef] [PubMed]
- Vidal, N.; Hedges, S.B. The phylogeny of squamate reptiles (lizards, snakes, and amphisbaenians) inferred from nine nuclear protein-coding genes. C. R. Biol. 2005, 328, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Vidal, N.; Hedges, S.B. The molecular evolutionary tree of lizards, snakes, and amphisbaenians. C. R. Biol. 2009, 332, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Vidal, N.; Rage, J.C.; Couloux, A.; Hedges, S.B. Snakes (Serpentes). In The Timetree of Life; Oxford University Press: New York, NY, USA, 2009. [Google Scholar]
- Wiens, J.J.; Hutter, C.R.; Mulcahy, D.G.; Noonan, B.P.; Townsend, T.M.; Sites, J.W., Jr.; Reeder, T.W. Resolving the phylogeny of lizards and snakes (Squamata) with extensive sampling of genes and species. Biol. Lett. 2012, 8, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Sweet, S.S. Chasing Flamingos: Toxicofera and the Misinterpretation of Venom in Varanid Lizards; Institute for Research and Development, Suan Sunandha Rajabhat University: Bangkok, Thailand, 2016. [Google Scholar]
- Hsiang, A.Y.; Field, D.J.; Webster, T.H.; Behlke, A.D.; Davis, M.B.; Racicot, R.A.; Gauthier, J.A. The origin of snakes: Revealing the ecology, behavior, and evolutionary history of early snakes using genomics, phenomics, and the fossil record. BMC Evol. Biol. 2015, 15, 87. [Google Scholar] [CrossRef] [PubMed]
- Mackessy, S.P.; Saviola, A.J. Understanding biological roles of venoms among the Caenophidia: The importance of rear-fanged snakes. Integr. Comp. Biol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, A.D.; Swain, M.T.; Logan, D.W.; Mulley, J.F. Testing the Toxicofera: Comparative transcriptomics casts doubt on the single, early evolution of the reptile venom system. Toxicon 2014, 92, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Sweet, S.; Pianka, E.R. Monitors, Mammals, and Wallace’s Line. Mertensiella 2007, 16, 79–99. [Google Scholar]
- Ast, J.C. Mitochondrial DNA evidence and evolution in Varanoidea (Squamata). Cladistics 2001, 17, 211–226. [Google Scholar] [CrossRef]
- Thompson, G.G.; Clemente, C.J.; Withers, P.C.; Fry, B.G.; Norman, J.A. Is body shape of varanid lizards linked with retreat choice? Aust. J. Zool. 2008, 56, 351–362. [Google Scholar] [CrossRef]
- Vidal, N.; Marin, J.; Sassi, J.; Battistuzzi, F.U.; Donnellan, S.; Fitch, A.J.; Fry, B.G.; Vonk, F.J.; Rodriguez de la Vega, R.C.; Couloux, A.; et al. Molecular evidence for an Asian origin of monitor lizards followed by Tertiary dispersals to Africa and Australasia. Biol. Lett. 2012, 8, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Vidal, N.; Norman, J.A.; Vonk, F.J.; Scheib, H.; Ramjan, S.F.; Kuruppu, S.; Fung, K.; Hedges, S.B.; Richardson, M.K.; et al. Early evolution of the venom system in lizards and snakes. Nature 2006, 439, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.J.; Norton, R.S.; et al. The toxicogenomic multiverse: Convergent recruitment of proteins into animal venoms. Annu. Rev. Genom. Hum. Genet. 2009, 10, 483–511. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Sunagar, K.; Casewell, N.R.; Kochva, E.; Roelants, K.; Scheib, H.; Wüster, W.; Vidal, N.; Young, B.; Burbrink, F.; et al. The origin and evolution of the Toxicofera reptile venom system. In Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery; Fry, B.G., Ed.; Oxford University Press: New York, NY, USA, 2015; pp. 1–31. [Google Scholar]
- Fry, B.G.; Undheim, E.A.; Ali, S.A.; Jackson, T.N.; Debono, J.; Scheib, H.; Ruder, T.; Morgenstern, D.; Cadwallader, L.; Whitehead, D.; et al. Squeezers and leaf-cutters: Differential diversification and degeneration of the venom system in toxicoferan reptiles. Mol. Cell. Proteom. 2013, 12, 1881–1899. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Wroe, S.; Teeuwisse, W.; van Osch, M.J.; Moreno, K.; Ingle, J.; McHenry, C.; Ferrara, T.; Clausen, P.; Scheib, H.; et al. A central role for venom in predation by Varanus komodoensis (Komodo dragon) and the extinct giant Varanus (Megalania) priscus. Proc. Natl. Acad. Sci. USA 2009, 106, 8969–8974. [Google Scholar] [CrossRef] [PubMed]
- Vidal, N. Colubroid systematics: Evidence for an early appearance of the venom apparatus followed by extensive evolutionary tinkering. J. Toxicol. Toxin Rev. 2002, 21, 21–41. [Google Scholar] [CrossRef]
- Pyron, R.A.; Burbrink, F.T.; Colli, G.R.; de Oca, A.N.; Vitt, L.J.; Kuczynski, C.A.; Wiens, J.J. The phylogeny of advanced snakes (Colubroidea), with discovery of a new subfamily and comparison of support methods for likelihood trees. Mol. Phylogenet. Evol. 2011, 58, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Pyron, R.A.; Burbrink, F.T.; Wiens, J.J. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol. Biol. 2013, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Vidal, N.; Branch, W.R.; Pauwels, O.S.G.; Hedges, S.B.; Broadley, D.G.; Wink, M.; Cruaud, C.; Joger, U.; Nagy, Z.T. Dissecting the major African snake radiation: A molecular phylogeny of the Lamprophiidae Fitzinger (Serpentes, Caenophidia). Zootaxa 2008, 1945, 51–66. [Google Scholar]
- Vidal, N.; Delmas, A.S.; David, P.; Cruaud, C.; Couloux, A.; Hedges, S.B. The phylogeny and classification of caenophidian snakes inferred from seven nuclear protein-coding genes. C. R. Biol. 2007, 330, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Vidal, N.; Dewynter, M.; Gower, D.J. Dissecting the major American snake radiation: A molecular phylogeny of the Dipsadidae Bonaparte (Serpentes, Caenophidia). C. R. Biol. 2010, 333, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Vidal, N.; Kindl, S.G.; Wong, A.; Hedges, S.B. Phylogenetic relationships of xenodontine snakes inferred from 12S and 16S ribosomal RNA sequences. Mol. Phylogenet. Evol. 2000, 14, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G. From genome to “venome”: Molecular origin and evolution of the snake venom proteome inferred from phylogenetic analysis of toxin sequences and related body proteins. Genome Res. 2005, 15, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Vaiyapuri, S.; Sunagar, K.; Gibbins, J.M.; Jackson, T.N.W.; Reeks, T.; Fry, B.G. Kallikrein Enzymes. In Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery; Fry, B.G., Ed.; Oxford University Press: New York, NY, USA, 2015; pp. 267–280. [Google Scholar]
- Sunagar, K.; Jackson, T.N.W.; Reeks, T.; Fry, B.G. Cysteine-rich secretory proteins. In Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery; Fry, B.G., Ed.; Oxford University Press: New York, NY, USA, 2015; pp. 239–246. [Google Scholar]
- Casewell, N.R.; Huttley, G.A.; Wuster, W. Dynamic evolution of venom proteins in squamate reptiles. Nat. Commun. 2012, 3, 1066. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G. Venomous Reptiles & Their Toxins. In Venomous Reptiles & Their Toxins; Fry, B.G., Ed.; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Fry, B.G.; Richards, R.; Earl, S.; Cousin, X.; Jackson, T.N.W.; Weise, C.; Sunagar, K. Lesser-Known or Putative Reptile Toxins. In Venomous Reptiles & Their Toxins; Fry, B.G., Ed.; Oxford University Press: New York, NY, USA, 2015; pp. 364–407. [Google Scholar]
- Fry, B.G.; Roelants, K.; Winter, K.; Hodgson, W.C.; Griesman, L.; Kwok, H.F.; Scanlon, D.; Karas, J.; Shaw, C.; Wong, L.; et al. Novel venom proteins produced by differential domain-expression strategies in beaded lizards and gila monsters (genus Heloderma). Mol. Biol. Evol. 2010, 27, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Scheib, H.; van der Weerd, L.; Young, B.; McNaughtan, J.; Ramjan, S.F.; Vidal, N.; Poelmann, R.E.; Norman, J.A. Evolution of an arsenal: Structural and functional diversification of the venom system in the advanced snakes (Caenophidia). Mol. Cell. Proteom. 2008, 7, 215–246. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Vidal, N.; van der Weerd, L.; Kochva, E.; Renjifo, C. Evolution and diversification of the Toxicofera reptile venom system. J. Proteom. 2009, 72, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Winter, K.; Norman, J.A.; Roelants, K.; Nabuurs, R.J.; van Osch, M.J.; Teeuwisse, W.M.; van der Weerd, L.; McNaughtan, J.E.; Kwok, H.F.; et al. Functional and structural diversification of the Anguimorpha lizard venom system. Mol. Cell. Proteom. 2010, 9, 2369–2390. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Wuster, W. Assembling an arsenal: Origin and evolution of the snake venom proteome inferred from phylogenetic analysis of toxin sequences. Mol. Biol. Evol. 2004, 21, 870–883. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Velasco, J.; Card, D.C.; Andrew, A.L.; Shaney, K.J.; Adams, R.H.; Schield, D.R.; Casewell, N.R.; Mackessy, S.P.; Castoe, T.A. Expression of venom gene homologs in diverse python tissues suggests a new model for the evolution of snake venom. Mol. Biol. Evol. 2015, 32, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Eldredge, N.; Gould, S.J. On punctuated equilibria. Science 1997, 276, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.J. Tempo and mode in the macroevolutionary reconstruction of Darwinism. Proc. Natl. Acad. Sci. USA 1994, 91, 6764–6771. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.J.; Eldredge, N. Punctuated equilibrium comes of age. Nature 1993, 366, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.J. Punctuated equilibrium: Empirical response. Science 1986, 232, 439. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.J. Punctuated equilibrium and the fossil record. Science 1983, 219, 439–440. [Google Scholar] [CrossRef] [PubMed]
- Sunagar, K.; Moran, Y. The Rise and Fall of an Evolutionary Innovation: Contrasting Strategies of Venom Evolution in Ancient and Young Animals. PLoS Genet. 2015, 11. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.N.; Sunagar, K.; Undheim, E.A.; Koludarov, I.; Chan, A.H.; Sanders, K.; Ali, S.A.; Hendrikx, I.; Dunstan, N.; Fry, B.G. Venom down under: Dynamic evolution of Australian elapid snake toxins. Toxins (Basel) 2013, 5, 2621–2655. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.N.W.; Koludarov, I.; Ali, S.A.; Dobson, J.; Zdenek, C.N.; Dashevsky, D.; op den Brouw, B.; Masci, P.; Nouwens, A.; Josh, P.; et al. Rapid radiations and the race to redundancy: An investigation of the evolution of Australian elapid snake venoms. Toxins 2016, in press. [Google Scholar]
- Fry, B.G. Structure-function properties of venom components from Australian elapids. Toxicon 1999, 37, 11–32. [Google Scholar] [CrossRef]
- Fry, B.G.; Casewell, N.R.; Wuster, W.; Vidal, N.; Young, B.; Jackson, T.N. The structural and functional diversification of the Toxicofera reptile venom system. Toxicon 2012, 60, 434–448. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Lumsden, N.G.; Wuster, W.; Wickramaratna, J.C.; Hodgson, W.C.; Kini, R.M. Isolation of a neurotoxin (alpha-colubritoxin) from a nonvenomous colubrid: Evidence for early origin of venom in snakes. J. Mol. Evol. 2003, 57, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Roelants, K.; Norman, J.A. Tentacles of venom: Toxic protein convergence in the Kingdom Animalia. J. Mol. Evol. 2009, 68, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Wuster, W.; Kini, R.M.; Brusic, V.; Khan, A.; Venkataraman, D.; Rooney, A.P. Molecular evolution and phylogeny of elapid snake venom three-finger toxins. J. Mol. Evol. 2003, 57, 110–129. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Wuster, W.; Ramjan, S.F.R.; Jackson, T.; Martelli, P.; Kini, R.M. Analysis of Colubroidea snake venoms by liquid chromatography with mass spectrometry: Evolutionary and toxinological implications. Rapid Commun. Mass Spectrom. 2003, 17, 2047–2062. [Google Scholar] [CrossRef] [PubMed]
- Vidal, N.; Lecointre, G. Weighting and congruence: A case study based on three mitochondrial genes in pitvipers. Mol. Phylogenet. Evol. 1998, 9, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.K.; Maritz, B.; McKay, S.; Glaudas, X.; Alexander, G.J. An ambushers arsenal: Chemical crypsis in the puff adder (Bitis arietans). Proc. Biol. Sci. 2015, 282, 20152182. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.N.W.; Young, B.; McCarthy, C.J.; Kochva, E.; Vidal, N.; Underwood, G.; Fry, B.G. Endless forms most beautiful: The evolution of ophidian oral glands, including the venom system, and the use of appropriate terminology for homologous structures. Zoomorphology 2016, in press. [Google Scholar]
- Mitchell, J.S.; Heckert, A.B.; Sues, H.D. Grooves to tubes: Evolution of the venom delivery system in a Late Triassic “reptile”. Naturwissenschaften 2010, 97, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Szaniawski, H. The earliest known venomous animals recognized among conodonts. Acta Palaeontol. Pol. 2009, 54, 669–676. [Google Scholar] [CrossRef]
- Reynoso, V.H. Possible evidence of a venom apparatus in a Middle Jurassic sphenodontian from the Huizachal red beds of Tamaulipas, Mexico. J. Vertebr. Paleontol. 2005, 25, 646–654. [Google Scholar] [CrossRef]
- Cuenca-Bescos, G.; Rofes, J. First evidence of poisonous shrews with an envenomation apparatus. Naturwissenschaften 2007, 94, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Dufton, M.J. Venomous mammals. Pharmacol. Ther. 1992, 53, 199–215. [Google Scholar] [CrossRef]
- Ligabue-Braun, R.; Verli, H.; Carlini, C.R. Venomous mammals: A review. Toxicon 2012, 59, 680–695. [Google Scholar] [CrossRef] [PubMed]
- Rofes, J.; Cuenca-Bescos, G. First record of Beremendia fissidens (Mammalia, Soricidae) in the Pleistocene of the Iberian Peninsula, with a review of the biostratigraphy, biogeography and palaeoecology of the species. C. R. Palevol 2009, 8, 21–37. [Google Scholar] [CrossRef]
- Gong, E.; Martin, L.D.; Burnham, D.A.; Falk, A.R. The birdlike raptor Sinornithosaurus was venomous. Proc. Natl. Acad. Sci. USA 2010, 107, 766–768. [Google Scholar] [CrossRef] [PubMed]
- Vonk, F.J.; Admiraal, J.F.; Jackson, K.; Reshef, R.; de Bakker, M.A.G.; Vanderschoot, K.; van den Berge, I.; van Atten, M.; Burgerhout, E.; Beck, A.; et al. Evolutionary origin and development of snake fangs. Nature 2008, 454, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Stuebing, R.B.; Inger, R.F. Field Guide to the Snakes of Borneo; Natural History Publications: Borneo, Malaysia, 1993. [Google Scholar]
- Tweedie, M.W.F. The Snakes of Malaya; Singapore National Printers: Singapore, 1983. [Google Scholar]
- Gopalakrishnakone, P.; Kochva, E. Unusual aspects of the venom apparatus of the blue coral snake Maticora bivirgata. Arch. Histol. Cytol. 1990, 53, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.P.L.; Lim, F.L.K. A Guide to the Amphibians and Reptiles of Singapore; Singapore Science Centre: Singapore, 1992. [Google Scholar]
- Durkin, J.P.; Pickwell, G.V.; Trotter, J.T.; Shier, W.T. Phospholipase A2 electrophoretic variants in reptile venoms. Toxicon 1981, 19, 535–546. [Google Scholar] [CrossRef]
- Takasaki, C.; Yoshida, H.; Shimazu, T.; Teruuchi, T.; Toriba, M.; Tamiya, N. Studies on the venom components of the long-glanded coral snake, Maticora bivirgata. Toxicon 1991, 29, 191–200. [Google Scholar] [CrossRef]
- Tan, C.H.; Fung, S.Y.; Yap, M.K.; Leong, P.K.; Liew, J.L.; Tan, N.H. Unveiling the elusive and exotic: Venomics of the Malayan blue coral snake (Calliophis bivirgata flaviceps). J. Proteom. 2016, 132, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Utkin, Y.; Sunagar, K.; Jackson, T.N.W.; Reeks, T.; Fry, B.G. Three-Finger Toxins (3FTxs). In Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery; Fry, B.G., Ed.; Oxford University Press: New York, NY, USA, 2015; pp. 215–227. [Google Scholar]
- Eitan, M.; Fowler, E.; Herrmann, R.; Duval, A.; Pelhate, M.; Zlotkin, E. A scorpion venom neurotoxin paralytic to insects that affects sodium current inactivation: Purification, primary structure, and mode of action. Biochemistry 1990, 29, 5941–5947. [Google Scholar] [CrossRef] [PubMed]
- Grieco, T.M.; Raman, I.M. Production of resurgent current in NaV1.6-null Purkinje neurons by slowing sodium channel inactivation with beta-pompilidotoxin. J. Neurosci. 2004, 24, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Gur Barzilai, M.; Kahn, R.; Regev, N.; Gordon, D.; Moran, Y.; Gurevitz, M. The specificity of Av3 sea anemone toxin for arthropods is determined at linker DI/SS2-S6 in the pore module of target sodium channels. Biochem. J. 2014, 463, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Jouiaei, M.; Sunagar, K.; Federman Gross, A.; Scheib, H.; Alewood, P.F.; Moran, Y.; Fry, B.G. Evolution of an ancient venom: Recognition of a novel family of cnidarian toxins and the common evolutionary origin of sodium and potassium neurotoxins in sea anemone. Mol. Biol. Evol. 2015, 32, 1598–1610. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Jo, S.; Bean, B.P. Modulation of neuronal sodium channels by the sea anemone peptide BDS-I. J. Neurophysiol. 2012, 107, 3155–3167. [Google Scholar] [CrossRef] [PubMed]
- Moran, Y.; Gordon, D.; Gurevitz, M. Sea anemone toxins affecting voltage-gated sodium channels—Molecular and evolutionary features. Toxicon 2009, 54, 1089–1101. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, E.; Stevens, M.; Zaharenko, A.J.; Konno, K.; Tytgat, J.; Wanke, E. Voltage-gated sodium channel isoform-specific effects of pompilidotoxins. FEBS J. 2010, 277, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Shon, K.J.; Grilley, M.M.; Marsh, M.; Yoshikami, D.; Hall, A.R.; Kurz, B.; Gray, W.R.; Imperial, J.S.; Hillyard, D.R.; Olivera, B.M. Purification, characterization, synthesis, and cloning of the lockjaw peptide from Conus purpurascens venom. Biochemistry 1995, 34, 4913–4918. [Google Scholar] [CrossRef] [PubMed]
- Graudins, A.; Wilson, D.; Alewood, P.F.; Broady, K.W.; Nicholson, G.M. Cross-reactivity of Sydney funnel-web spider antivenom: Neutralization of the in vitro toxicity of other Australian funnel-web (Atrax and Hadronyche) spider venoms. Toxicon 2002, 40, 259–266. [Google Scholar] [CrossRef]
- Rash, L.D.; Birinyi-Strachan, L.C.; Nicholson, G.M.; Hodgson, W.C. Neurotoxic activity of venom from the Australian eastern mouse spider (Missulena bradleyi) involves modulation of sodium channel gating. Br. J. Pharmacol. 2000, 130, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Brazil, O.V.; Prado-Franceschi, J.; Laure, C.J. Repetitive muscle responses induced by crotamine. Toxicon 1979, 17, 61–67. [Google Scholar] [CrossRef]
- Chang, C.C.; Tseng, K.H. Effect of crotamine, a toxin of South American rattlesnake venom, on the sodium channel of murine skeletal muscle. Br. J. Pharmacol. 1978, 63, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Matavel, A.C.; Ferreira-Alves, D.L.; Beirao, P.S.; Cruz, J.S. Tension generation and increase in voltage-activated Na+ current by crotamine. Eur. J. Pharmacol. 1998, 348, 167–173. [Google Scholar] [CrossRef]
- Peigneur, S.; Orts, D.J.; Prieto da Silva, A.R.; Oguiura, N.; Boni-Mitake, M.; de Oliveira, E.B.; Zaharenko, A.J.; de Freitas, J.C.; Tytgat, J. Crotamine pharmacology revisited: Novel insights based on the inhibition of KV channels. Mol. Pharmacol. 2012, 82, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, C.T.; Carvalho-de-Souza, J.L.; Schiavon, E.; Cassola, A.C.; Wanke, E.; Troncone, L.R. Crotamine inhibits preferentially fast-twitching muscles but is inactive on sodium channels. Toxicon 2007, 50, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Yount, N.Y.; Kupferwasser, D.; Spisni, A.; Dutz, S.M.; Ramjan, Z.H.; Sharma, S.; Waring, A.J.; Yeaman, M.R. Selective reciprocity in antimicrobial activity versus cytotoxicity of hBD-2 and crotamine. Proc. Natl. Acad. Sci. USA 2009, 106, 14972–14977. [Google Scholar] [CrossRef] [PubMed]
- Prieto da Silva, A.R.B.; Fry, B.G.; Sunagar, K.; Scheib, H.; Jackson, T.N.W.; Rádis-Baptista, G.; Zaharenko, A.; de Sá, P.L., Jr.; Pereira, A.; Oguiura, N.; et al. Beta-Defensins. In Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery; Fry, B.G., Ed.; Oxford University Press: New York, NY, USA, 2015; pp. 228–238. [Google Scholar]
- Bao, Y.; Bu, P.; Jin, L.; Hongxia, W.; Yang, Q.; An, L. Purification, characterization and gene cloning of a novel phospholipase A2 from the venom of Agkistrodon blomhoffii ussurensis. Int. J. Biochem. Cell Biol. 2005, 37, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Haupt, J. The Mesothelae—A Monograph of an Exceptional Group of Spiders (Araneae: Mesothelae). Zoologica 2003, 154, 1–102. [Google Scholar]
- Foelix, R.; Erb, B. Mesothelae have venom glands. J. Arachnol. 2010, 38, 596–598. [Google Scholar] [CrossRef]
- Martini, F. The venom apparatus of the fanged blenny, Meiacanthus atrodorsalis. Am. Zool. 1988, 28, A76. [Google Scholar]
- Fishelson, L. Histology and ultrastructure of the recently found buccal toxic gland in the fish Meiacanthus nigrolineatus (Blenniidae). Copeia 1974, 1974, 386–392. [Google Scholar] [CrossRef]
- Smith, W.L.; Stern, J.H.; Girard, M.G.; Davis, M.P. Evolution of venomous cartilaginous and ray-finned fishes. Integr. Comp. Biol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Polis, G.A. The Biology of Scorpions; Stanford University Press: Palo Alto, CA, USA, 1990. [Google Scholar]
- Undheim, E.A.; Georgieva, D.N.; Thoen, H.H.; Norman, J.A.; Mork, J.; Betzel, C.; Fry, B.G. Venom on ice: First insights into Antarctic octopus venoms. Toxicon 2010, 56, 897–913. [Google Scholar] [CrossRef] [PubMed]
- Allcock, A.L.; Hochberg, F.G.; Rodhouse, P.G.K.; Thorpe, J.P. Adelieledone, a new genus of octopodid from the Southern Ocean. Antarct. Sci. 2003, 15, 415–424. [Google Scholar] [CrossRef]
- Goncalves, J.M. Estudos sobre venenos de serpents brasileiras. II. Crotalus terrificus crotaminicus, subespecie biologica. Ann. Acad. Bras. Cienc. 1956, 28, 365–367. [Google Scholar]
- Gonçalves, J.M. Purification and properties of crotamine. In Venoms; Buckley, E.E., Porges, N., Eds.; American Association for the Advancement of Science: Washington, DC, USA, 1956. [Google Scholar]
- Broad, A.J.; Sutherland, S.K.; Coulter, A.R. Lethality in mice of dangerous Australian and other snake venom. Toxicon 1979, 17, 661–664. [Google Scholar] [CrossRef]
- Trabi, M.; Sunagar, K.; Jackson, T.N.W.; Fry, B.G. Factor Xa Enzymes. In Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery; Fry, B.G., Ed.; Oxford University Press: New York, NY, USA, 2015; pp. 261–266. [Google Scholar]
- Earl, S.; Sunagar, K.; Jackson, T.N.W.; Reeks, T.; Fry, B.G. Factor Va Proteins. In Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery; Fry, B.G., Ed.; Oxford University Press: New York, NY, USA, 2015; pp. 255–260. [Google Scholar]
- Herrera, M.; Fernandez, J.; Vargas, M.; Villalta, M.; Segura, A.; Leon, G.; Angulo, Y.; Paiva, O.; Matainaho, T.; Jensen, S.D.; et al. Comparative proteomic analysis of the venom of the taipan snake, Oxyuranus scutellatus, from Papua New Guinea and Australia: Role of neurotoxic and procoagulant effects in venom toxicity. J. Proteom. 2012, 75, 2128–2140. [Google Scholar] [CrossRef] [PubMed]
- Isbister, G.K. Procoagulant Snake Toxins: Laboratory Studies, Diagnosis, and Understanding Snakebite Coagulopathy. Semin. Thromb. Hemost. 2009, 35, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Anderson, A.J. Dendrotoxins: Snake toxins that block potassium channels and facilitate neurotransmitter release. Pharmacol. Ther. 1985, 31, 33–55. [Google Scholar] [CrossRef]
- Harvey, A.L.; Karlsson, E. Dendrotoxin from the venom of the green mamba, Dendroaspis angusticeps. A neurotoxin that enhances acetylcholine release at neuromuscular junctions. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1980, 312, 1–6. [Google Scholar] [CrossRef]
- Karlsson, E.; Mbugua, P.M.; Rodriguez-Ithurralde, D. Fasciculins, anticholinesterase toxins from the venom of the green mamba Dendroaspis angusticeps. J. Physiol. (Paris) 1984, 79, 232–240. [Google Scholar]
- Osman, O.H.; Ismail, M.; El-Asmar, M.F. Pharmacological studies of snake (Dendroaspis angusticeps) venom. Toxicon 1973, 11, 185–192. [Google Scholar] [CrossRef]
- Eng, W.S.; Fry, B.G.; Sunagar, K.; Takacs, Z.; Jackson, T.N.W.; Guddat, L.W. Kunitz Peptides. In Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery; Fry, B.G., Ed.; Oxford University Press: New York, NY, USA, 2015; pp. 281–290. [Google Scholar]
- Fry, B.G.; Jackson, T.N.W.; Takacs, Z.; Reeks, T.; Sunagar, K. C-Type Natriuretic Peptides. In Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery; Oxford University Press: New York, NY, USA, 2015; pp. 318–326. [Google Scholar]
- Aman, J.W.; Imperial, J.S.; Ueberheide, B.; Zhang, M.M.; Aguilar, M.; Taylor, D.; Watkins, M.; Yoshikami, D.; Showers-Corneli, P.; Safavi-Hemami, H.; et al. Insights into the origins of fish hunting in venomous cone snails from studies of Conus tessulatus. Proc. Natl. Acad. Sci. USA 2015, 112, 5087–5092. [Google Scholar] [CrossRef] [PubMed]
- Dutertre, S.; Jin, A.H.; Vetter, I.; Hamilton, B.; Sunagar, K.; Lavergne, V.; Dutertre, V.; Fry, B.G.; Antunes, A.; Venter, D.J.; et al. Evolution of separate predation- and defence-evoked venoms in carnivorous cone snails. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Jin, A.H.; Israel, M.R.; Inserra, M.C.; Smith, J.J.; Lewis, R.J.; Alewood, P.F.; Vetter, I.; Dutertre, S. delta-Conotoxin SuVIA suggests an evolutionary link between ancestral predator defence and the origin of fish-hunting behaviour in carnivorous cone snails. Proc. Biol. Sci. R. Soc. 2015, 282. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.J. Wonderful LIFE: The Burgess Shale and the Nature of History; W.W. Norton & Company: New York, NY, USA, 1990. [Google Scholar]
- Morris, S.C.; Gould, S.J. Showdown on the Burgess Shale. Nat. Hist. Mag. 1998, 107, 48–55. [Google Scholar]
- Dennett, D.C. Darwin’s Dangerous Idea: Evolution and the Meaning of Life; Simon & Schuster: New York, NY, USA, 1996. [Google Scholar]
- Ferrada, E.; Wagner, A. Evolutionary innovations and the organization of protein functions in genotype space. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Sunagar, K.; Jackson, T.; Undheim, E.; Ali, S.; Antunes, A.; Fry, B. Three-Fingered RAVERs: Rapid Accumulation of Variations in Exposed Residues of Snake Venom Toxins. Toxins 2013, 5, 2172–2208. [Google Scholar] [CrossRef] [PubMed]
- Heyborne, W.H.; Mackessy, S.P. Identification and characterization of a taxon-specific three-finger toxin from the venom of the Green Vinesnake (Oxybelis fulgidus; family Colubridae). Biochimie 2013, 95, 1923–1932. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.E.; Mackessy, S.P. Characterization of venom (Duvernoy’s secretion) from twelve species of colubrid snakes and partial sequence of four venom proteins. Toxicon 2000, 38, 1663–1687. [Google Scholar] [CrossRef]
- Mackessy, S.P.; Sixberry, N.A.; Heyborne, W.H.; Fritts, T. Venom of the Brown Treesnake, Boiga irregularis: Ontogenetic shifts and taxa-specific toxicity. Toxicon 2006, 47, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Margres, M.J.; McGivern, J.J.; Seavy, M.; Wray, K.P.; Facente, J.; Rokyta, D.R. Contrasting modes and tempos of venom expression evolution in two snake species. Genetics 2015, 199, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, J.; Mackessy, S.P.; Fry, B.G.; Bhatia, M.; Mourier, G.; Fruchart-Gaillard, C.; Servent, D.; Menez, R.; Stura, E.; Menez, A.; et al. Denmotoxin, a three-finger toxin from the colubrid snake Boiga dendrophila (Mangrove Catsnake) with bird-specific activity. J. Biol. Chem. 2006, 281, 29030–29041. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, J.; Mackessy, S.P.; Sixberry, N.M.; Stura, E.A.; Le Du, M.H.; Menez, R.; Foo, C.S.; Menez, A.; Nirthanan, S.; Kini, R.M. Irditoxin, a novel covalently linked heterodimeric three-finger toxin with high taxon-specific neurotoxicity. FASEB J. 2009, 23, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Weldon, C.L.; Mackessy, S.P. Biological and proteomic analysis of venom from the Puerto Rican Racer (Alsophis portoricensis: Dipsadidae). Toxicon 2010, 55, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Almehdar, H.A.; Adel-Sadek, M.A.; Redwan, E.M. Immunoreactivity and two-dimensional gel-electrophoresis characterization of Egyptian cobra venom proteome. Pak. J. Pharm. Sci. 2015, 28, 59–64. [Google Scholar] [PubMed]
- Chang, L.S.; Chung, C.; Liou, J.C.; Chang, C.W.; Yang, C.C. Novel neurotoxins from Taiwan banded krait (Bungarus multicinctus) venom: Purification, characterization and gene organization. Toxicon 2003, 42, 323–330. [Google Scholar] [CrossRef]
- Eletskii, A.V.; Maslennikov, I.V.; Kukhtina, V.V.; Utkin Iu, N.; Tsetlin, V.I.; Arsen’ev, A.S. Structure and conformational heterogeneity of the weak toxin from the cobra Naja kaouthia venom. Bioorg. Khim. 2001, 27, 89–101. [Google Scholar] [PubMed]
- Kulkeaw, K.; Chaicumpa, W.; Sakolvaree, Y.; Tongtawe, P.; Tapchaisri, P. Proteome and immunome of the venom of the Thai cobra, Naja kaouthia. Toxicon 2007, 49, 1026–1041. [Google Scholar] [CrossRef] [PubMed]
- Leong, P.K.; Fung, S.Y.; Tan, C.H.; Sim, S.M.; Tan, N.H. Immunological cross-reactivity and neutralization of the principal toxins of Naja sumatrana and related cobra venoms by a Thai polyvalent antivenom (Neuro Polyvalent Snake Antivenom). Acta Trop. 2015, 149, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, H.; Liu, J.; Xu, K. Novel genes encoding six kinds of three-finger toxins in Ophiophagus hannah (king cobra) and function characterization of two recombinant long-chain neurotoxins. Biochem. J. 2006, 398, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Lyukmanova, E.N.; Shenkarev, Z.O.; Shulepko, M.A.; Paramonov, A.S.; Chugunov, A.O.; Janickova, H.; Dolejsi, E.; Dolezal, V.; Utkin, Y.N.; Tsetlin, V.I.; et al. Structural Insight into Specificity of Interactions between Nonconventional Three-finger Weak Toxin from Naja kaouthia (WTX) and Muscarinic Acetylcholine Receptors. J. Biol. Chem. 2015, 290, 23616–23630. [Google Scholar] [CrossRef] [PubMed]
- Malih, I.; Ahmad rusmili, M.R.; Tee, T.Y.; Saile, R.; Ghalim, N.; Othman, I. Proteomic analysis of Moroccan cobra Naja haje legionis venom using tandem mass spectrometry. J. Proteom. 2014, 96, 240–252. [Google Scholar]
- Nirthanan, S.; Gopalakrishnakone, P.; Gwee, M.C.; Khoo, H.E.; Kini, R.M. Non-conventional toxins from Elapid venoms. Toxicon 2003, 41, 397–407. [Google Scholar] [CrossRef]
- Ogay, A.Y.; Rzhevsky, D.I.; Murashev, A.N.; Tsetlin, V.I.; Utkin, Y.N. Weak neurotoxin from Naja kaouthia cobra venom affects haemodynamic regulation by acting on acetylcholine receptors. Toxicon 2005, 45, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Rzhevskii, D.I.; Murashev, A.N.; Kukhtina, V.V.; Tsetlin, V.I.; Utkin Iu, N. The weak neurotoxin from Naja kaouthia cobra venom decreases the arterial blood pressure in rats. Bioorg. Khim. 2001, 27, 221–223. [Google Scholar] [PubMed]
- Starkov, V.G.; Poliak Iu, L.; Vul’fius, E.A.; Kriukova, E.V.; Tsetlin, V.I.; Utkin Iu, N. New weak toxins from the cobra venom. Bioorg. Khim. 2009, 35, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.Y.; Tan, C.H.; Fung, S.Y.; Tan, N.H. Venomics, lethality and neutralization of Naja kaouthia (monocled cobra) venoms from three different geographical regions of Southeast Asia. J. Proteom. 2015, 120, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Hayes, W.K.; Lavin-Murcio, P.; Kardong, K.V. Delivery of Duvernoy’s secretion into prey by the brown tree snake, Boiga irregularis (Serpentes:Colubridae). Toxicon 1993, 31, 881–887. [Google Scholar] [CrossRef]
- Kardong, K.V. Evolutionary patterns in advanced snakes. Am. Zool. 1980, 20, 269–282. [Google Scholar] [CrossRef]
- Kardong, K.V. The evolution of the venom apparatus in snakes from colubrids to viperids and elapids. Mem. Inst. Butanan 1982, 46, 105–118. [Google Scholar]
- Kardong, K.V. Replies to Fry et al. (Toxicon 2012, 60/4, 434–448). Part B. Properties and biological roles of squamate oral products: The “venomous lifestyle” and preadaptation. Toxicon 2012, 60, 964–966. [Google Scholar] [CrossRef] [PubMed]
- Kardong, K.W. Colubrid snakes and Duvernoy’s “venom” glands. J. Toxicol. Toxin Rev. 2002, 21, 1–19. [Google Scholar] [CrossRef]
- Rochelle, M.J.; Kardong, K.V. Constriction vs envenomation in prey capture by brown tree snakes (Boiga irregularis) (Squamata, Colubridae). Herpetologica 1993, 49, 301–304. [Google Scholar]
- Lumsden, N.G.; Fry, B.G.; Kini, R.M.; Hodgson, W.C. In vitro neuromuscular activity of ‘colubrid’ venoms: Clinical and evolutionary implications. Toxicon 2004, 43, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Lumsden, N.G.; Fry, B.G.; Ventura, S.; Kini, R.M.; Hodgson, W.C. The in vitro and in vivo pharmacological activity of Boiga dendrophila (mangrove catsnake) venom. Auton. Autacoid Pharmacol. 2004, 24, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Lumsden, N.G.; Fry, B.G.; Ventura, S.; Kini, R.M.; Hodgson, W.C. Pharmacological characterisation of a neurotoxin from the venom of Boiga dendrophila (mangrove catsnake). Toxicon 2005, 45, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, S.K.; Tibbals, J. Australian Animal Toxins: The Creatures, Their Toxins and Care of the Poisoned Patient, 2nd ed.; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Modahl, C.M.; Mukherjee, A.K.; Mackessy, S.P. An analysis of venom ontogeny and prey-specific toxicity in the Monocled Cobra (Naja kaouthia). Toxicon 2016, 119, 8–20. [Google Scholar] [CrossRef] [PubMed]
- an, N.H.; Arunmozhiarasi, A.; Ponnudurai, G. A comparative study of the biological properties of Dendroaspis (mamba) snake venoms. Comp. Biochem. Physiol. Part C: Comp. Pharmacol. 1991, 99, 463–466. [Google Scholar]
- Vetter, I.; Dekan, Z.; Knapp, O.; Adams, D.J.; Alewood, P.F.; Lewis, R.J. Isolation, characterization and total regioselective synthesis of the novel muO-conotoxin MfVIA from Conus magnificus that targets voltage-gated sodium channels. Biochem. Pharmacol. 2012, 84, 540–548. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.C.; Deuis, J.R.; Dashevsky, D.; Dobson, J.; Jackson, T.N.W.; Brust, A.; Xie, B.; Koludarov, I.; Debono, J.; Hendrikx, I.; et al. The Snake with the Scorpion’s Sting: Novel Three-Finger Toxin Sodium Channel Activators from the Venom of the Long-Glanded Blue Coral Snake (Calliophis bivirgatus). Toxins 2016, 8, 303. https://doi.org/10.3390/toxins8100303
Yang DC, Deuis JR, Dashevsky D, Dobson J, Jackson TNW, Brust A, Xie B, Koludarov I, Debono J, Hendrikx I, et al. The Snake with the Scorpion’s Sting: Novel Three-Finger Toxin Sodium Channel Activators from the Venom of the Long-Glanded Blue Coral Snake (Calliophis bivirgatus). Toxins. 2016; 8(10):303. https://doi.org/10.3390/toxins8100303
Chicago/Turabian StyleYang, Daryl C., Jennifer R. Deuis, Daniel Dashevsky, James Dobson, Timothy N. W. Jackson, Andreas Brust, Bing Xie, Ivan Koludarov, Jordan Debono, Iwan Hendrikx, and et al. 2016. "The Snake with the Scorpion’s Sting: Novel Three-Finger Toxin Sodium Channel Activators from the Venom of the Long-Glanded Blue Coral Snake (Calliophis bivirgatus)" Toxins 8, no. 10: 303. https://doi.org/10.3390/toxins8100303
APA StyleYang, D. C., Deuis, J. R., Dashevsky, D., Dobson, J., Jackson, T. N. W., Brust, A., Xie, B., Koludarov, I., Debono, J., Hendrikx, I., Hodgson, W. C., Josh, P., Nouwens, A., Baillie, G. J., Bruxner, T. J. C., Alewood, P. F., Lim, K. K. P., Frank, N., Vetter, I., & Fry, B. G. (2016). The Snake with the Scorpion’s Sting: Novel Three-Finger Toxin Sodium Channel Activators from the Venom of the Long-Glanded Blue Coral Snake (Calliophis bivirgatus). Toxins, 8(10), 303. https://doi.org/10.3390/toxins8100303







