Phenanthrene Amplifies Microcystin-Induced Toxicity in the Submerged Macrophyte Vallisneria natans
Abstract
1. Introduction
2. Results
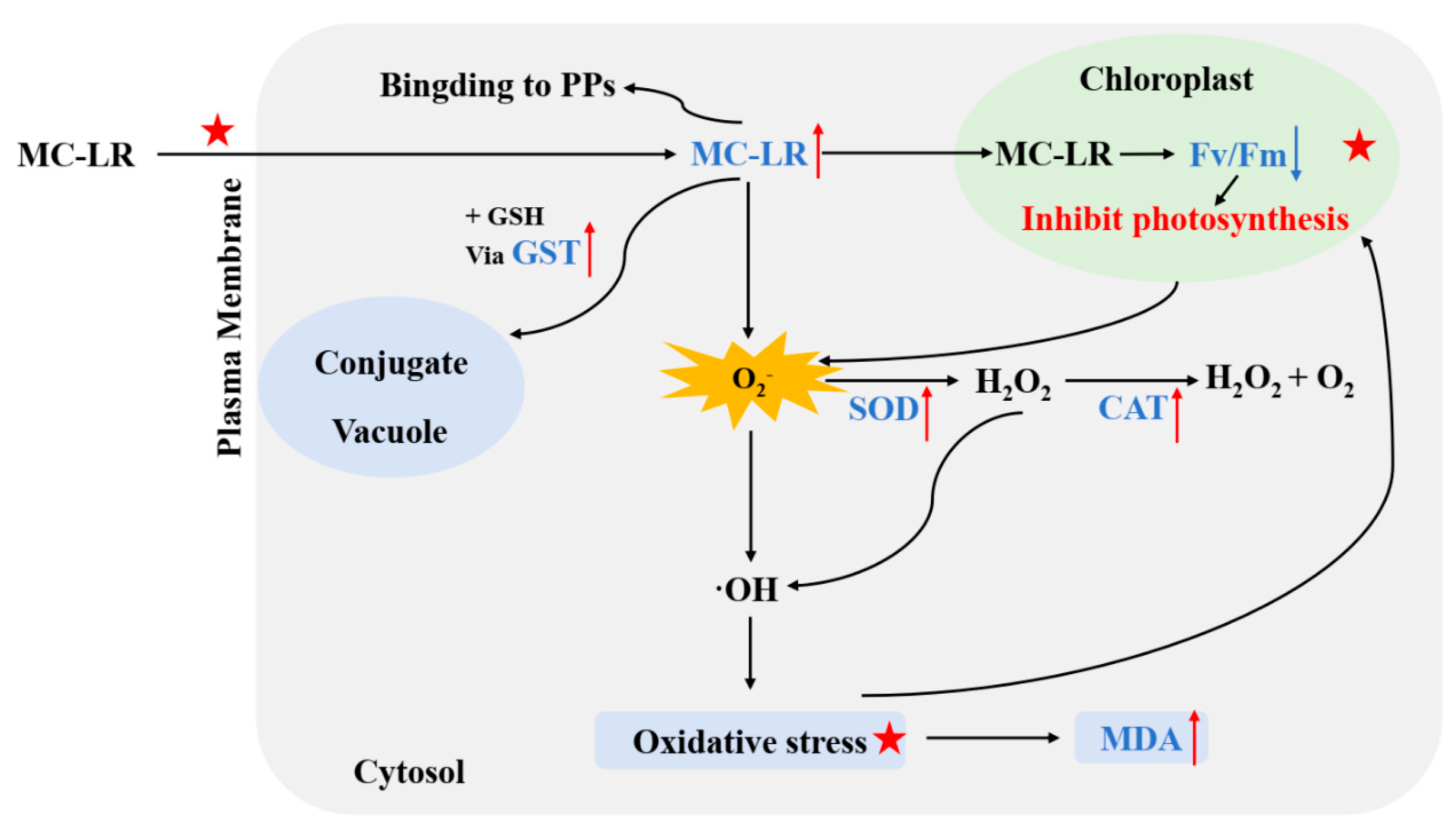
2.1. Growth and Photosynthetic Responses
2.2. Oxidative Response
2.3. Accumulation
2.4. Assessment
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Experimental Materials
5.2. Experimental Design
5.3. Analytical Methods
5.3.1. Determination of Growth Parameters and Photosynthetic Efficiency (Fv/Fm)
5.3.2. Determination of Biochemical Indicators
5.3.3. MC-LR Extraction and Accumulation Determination
5.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ho, J.C.; Michalak, A.M.; Pahlevan, N. Widespread global increase in intense lake phytoplankton blooms since the 1980s. Nature 2019, 574, 667–670. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Y.L.; Zhang, Y.B.; Shi, K.; Qian, H.M.; Yang, H.Y.; Niu, Y.K.; Qin, B.Q.; Zhu, G.W.; Woolway, R.I.; et al. The unprecedented 2022 extreme summer heatwaves increased harmful cyanobacteria blooms. Sci. Total Environ. 2023, 896, 165312. [Google Scholar] [CrossRef]
- Merder, J.; Harris, T.; Zhao, G.; Stasinopoulos, D.M.; Rigby, R.A.; Michalak, A.M. Geographic redistribution of microcystin hotspots in response to climate warming. Nat. Water 2023, 1, 844–854. [Google Scholar] [CrossRef]
- Huang, I.S.; Zimba, P.V. Cyanobacterial bioactive metabolites—A review of their chemistry and biology. Harmful Algae 2019, 83, 42–94. [Google Scholar] [CrossRef]
- Wan, X.; Cheng, C.; Gu, Y.R.; Shu, X.B.; Xie, L.Q.; Zhao, Y.Y. Acute and chronic toxicity of microcystin-LR and phenanthrene alone or in combination to the cladoceran (Daphnia magna). Ecotox. Environ. Safe. 2021, 220, 112405. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Michalak, A.M. Study role of climate change in extreme threats to water quality. Nature 2016, 535, 349–350. [Google Scholar] [CrossRef]
- Song, H.; Lavoie, M.; Fan, X.; Tan, H.; Liu, G.; Xu, P.; Fu, Z.; Paerl, H.W.; Qian, H. Allelopathic interactions of linoleic acid and nitric oxide increase the competitive ability of Microcystis aeruginosa. ISME J. 2017, 11, 1865–1876. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Otten, T.G. Blooms bite the hand that feeds them. Science 2013, 342, 433–434. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.Y.; Zhou, B.Y.; Calabrese, E.J.; Agathokleous, E. Stimulation of Microcystis aeruginosa by subtoxic concentrations of contaminants: A meta-analysis. Environ. Res. 2025, 271, 121105. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Xue, Q.J.; Gu, Y.R.; Xie, L.Q. Advance on combined toxicity of microcystins and other environmental pollutants. Asian J. Ecotox. 2021, 16, 50–62. (In Chinese) [Google Scholar]
- Mojiri, A.; Zhou, J.L.; Ohashi, A.; Ozaki, N.; Kindaichi, T. Comprehensive review of polycyclic aromatic hydrocarbons in water sources, their effects and treatments. Sci. Total Environ. 2019, 696, 133971. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Liu, X.; Lu, S.; Zhang, T.; Jin, B.; Wang, Q.; Tang, Z.; Liu, Y.; Guo, X.; Zhou, J.; et al. A review on occurrence and risk of polycyclic aromatic hydrocarbons (PAHs) in lakes of China. Sci. Total Environ. 2019, 651, 2497–2506. [Google Scholar] [CrossRef]
- Maskaoui, K.; Zhou, J.L.; Hong, H.S.; Zhang, Z.L. Contamination by polycyclic aromatic hydrocarbons in the Jiulong River estuary and western Xiamen Sea, China. Environ. Pollut. 2002, 118, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Steinman, A.D.; Shu, X.; Cao, Q.; Yao, L.; Xie, L. Combined toxic effects of microcystin–LR and phenanthrene on growth and antioxidant system of duckweed (Lemna gibba L.). Ecotoxicol. Environ. Saf. 2019, 185, 109668. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.L.; Huang, M.J.; Tan, A.J.; Lv, S.M. Joint effects of naphthalene and microcystin-LR on physiological responses and toxin bioaccumulation of Landoltia punctata. Aquat. Toxicol. 2021, 231, 105710. [Google Scholar] [CrossRef]
- Zhang, Y.; Jeppesen, E.; Liu, X.; Qin, B.; Shi, K.; Zhou, Y.; Thomaz, S.M.; Deng, J. Global loss of aquatic vegetation in lakes. Earth-Sci. Rev. 2017, 173, 259–265. [Google Scholar] [CrossRef]
- Cheng, C.; Steinman, A.D.; Xue, Q.J.; Wan, X.; Xie, L.Q. The disruption of calcium and hydrogen ion homeostasis of submerged macrophyte Vallisneria natans (Lour.) Hara caused by microcystin-LR. Aquat. Toxicol. 2023, 254, 106377. [Google Scholar] [CrossRef]
- Cook, C. Aquatic Plant; SPB-Academic Publishing: Hague, The Netherlands, 1990. [Google Scholar]
- Jiang, J.; Gu, X.; Song, R.; Wang, X.; Yang, L. Microcystin-LR induced oxidative stress and ultrastructural alterations in mesophyll cells of submerged macrophyte Vallisneria natans (Lour.) Hara. J. Hazard. Mater. 2011, 190, 188–196. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.Q.; Li, E.H.; Zhang, L.; Wang, X.L.; Song, L.R. Combined toxic effects and mechanisms of microsystin-LR and copper on Vallisneria Natans (Lour.) Hara seedlings. J. Hazard Mater. 2017, 328, 108–116. [Google Scholar] [CrossRef]
- Ren, L.; Huang, X.D.; Mcconkey, B.J.; Dixon, D.G.; Greenberg, B.M. Photoinduced toxicity of three polycyclic aromatic hydrocarbons (fluoranthene, pyrene, and naphthalene) to the duckweed Lemna gibba L. G-3. Ecotoxicol. Environ. Saf. 1994, 28, 160–171. [Google Scholar] [CrossRef]
- Zhu, X.; Kong, H.; Gao, Y.; Wu, M.; Kong, F. Low concentrations of polycyclic aromatic hydrocarbons promote the growth of Microcystis aeruginosa. J. Hazard Mater. 2012, 237–238, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.H.; Qin, Z.R.; Xia, L.L.; Zhang, D.; Mela, S.M.; Li, Y. The responding and ecological contribution of biofilm-leaves of submerged macrophytes on phenanthrene dissipation in sediments. Environ. Pollut. 2019, 246, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Wild, S.R.; Jones, K.C. The significance of polynuclear aromatic hydrocarbons applied to agricultural soils in sewage sludges in the U.K. Waste Manage. Res. 1994, 12, 49–59. [Google Scholar]
- Wang, S.; Dai, H.; Skuza, L.; Chen, Y.; Wei, S. Difference in Cd2+ flux around the root tips of different soybean (Glycine max L.) cultivars and physiological response under mild cadmium stress. Chemosphere 2022, 297, 134120. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Weisman, D.; Ye, Y.B.; Cui, B.; Huang, Y.H.; Colón-Carmona, A.; Wang, Z.H. An oxidative stress response to polycyclic aromatic hydrocarbon exposure is rapid and complex in Arabidopsis thaliana. Plant Sci. 2009, 176, 375–382. [Google Scholar] [CrossRef]
- Sharma, D.; Andersen, S.; Ottosen, C.; Rosenqvist, E. Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol. Plant. 2015, 153, 284–298. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Y.; Li, X.; Chen, Z.; Zhang, L.; Li, Y. Temporal dynamics of physiological integration intensity in Zoysia japonica under heterogeneous stress of cadmium or/and phenanthrene. Plants 2025, 14, 1230. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Wang, M.M.; Zhou, Y.H.; Xia, X.J.; Mao, W.H.; Shi, K.; Yu, J.Q. The growth, photosynthesis and antioxidant defense responses of five vegetable crops to phenanthrene stress. Ecotoxicol. Environ. Saf. 2012, 80, 132–139. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Jiang, J.; Shi, Y.; Tian, F.; Long, T.; Li, X.; Ying, R. Bioaccumulation of Microcystin-LR and Induced Physio-Biochemical Changes in Rice (Oryza sativa L.) at Vegetative Stage under Hydroponic Culture Conditions. Toxins 2024, 16, 82. [Google Scholar]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, X.R.; Sun, Y.Y.; Guo, H.Y.; Yin, D.Q. Bioaccumulation and oxidative stress in submerged macrophyte Ceratophyllum emersum L. upon exposure to pyrene. Environ. Toxicol. 2008, 23, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Wan, X.; Shu, X.B.; Xie, L.Q. Bioaccumulation and detoxication of microcystin-LR in three submerged macrophytes: The important role of glutathione biosynthesis. Chemosphere 2019, 225, 935–942. [Google Scholar] [CrossRef]
- Pflugmacher, S.; Wiegand, C.; Oberemm, A.; Beattie, K.A.; Krause, E.; Codd, G.A.; Steinberg, C.E.W. Identification of an enzymatically formed glutathione conjugate of the cyanobacterial hepatotoxin microcystin-LR: The first step of detoxication. Biochim. Biophys. Acta 1998, 1425, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xiao, B.D.; Song, L.R.; Wang, C.B.; Zhang, J.Q. Responses and toxin bioaccumulation in duckweed (Lemna minor) under microcystin-LR, linear alkybenzene sulfonate and their joint stress. J. Hazard Mater. 2012, 229–230, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Lahti, K.; Rapala, J.; Färdig, M.; Niemelä, M.; Sivonen, K. Persistence of cyanobacterial hepatotoxin microcystin-LR in particulate material and dissolved in lake water. Water Res. 1997, 31, 1005–1012. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 1–32. [Google Scholar]
- Chesworth, J.C.; Donkin, M.E.; Brown, M.T. The interactive effects of the antifouling herbicides Irgarol 1051 and Diuron on the seagrass Zostera marina (L.). Aquat. Toxicol. 2004, 66, 293–305. [Google Scholar] [CrossRef]



| Indicators | MC-LR (μg/L) | IA (%) | Phen (μg/L) | IB (%) | MC-LR + Phen (μg/L) | OIA+B (%) | EIA+B (%) | RI | Interactive Effects |
|---|---|---|---|---|---|---|---|---|---|
| Root length | 2 | 0.92 ± 7.93 | 0.2 | −1.65 ± 11.09 | 2 + 0.2 | 1.16 + 4.99 | −0.73 + 12.06 | −1.65 | Antagonism |
| 10 | −1.37 ± 6.99 | 1 | 2.96 ± 9.44 | 10 + 1 | 3.32 + 9.24 | 1.59 + 7.50 | −2.07 | Antagonism | |
| 50 | 1.59 ± 6.86 | 5 | 11.79 ± 5.04 | 50 + 5 | 27.26 + 2.97 | 13.38 + 6.12 | 2.03 * | Synergism | |
| 250 | 5.73 ± 7.53 | 25 | 24.65 ± 3.23 | 250 + 25 | 52.36 + 5.45 | 30.37 + 9.46 | 1.72 * | Synergism | |
| 1000 | 12.03 ± 11.86 | 100 | 37.96 ± 11.56 | 1000 + 100 | 65.36 + 5.77 | 49.95 + 6.12 | 1.31 * | Synergism | |
| Total fresh weight | 2 | −0.59 ± 8.66 | 0.2 | 3.22 ± 10.79 | 2 + 0.2 | −2.13 + 9.22 | 2.63 + 17.69 | −0.82 | Antagonism |
| 10 | −2.46 ± 10.91 | 1 | −3.70 ± 12.55 | 10 + 1 | 4.84 + 10.15 | −6.16 + 22.17 | −0.78 | Antagonism | |
| 50 | 3.36 ± 7.59 | 5 | −0.89 ± 7.44 | 50 + 5 | 12.60 + 6.49 | 2.46 + 13.03 | 5.04 * | Synergism | |
| 250 | 8.13 ± 4.25 | 25 | 6.72 ± 5.75 | 250 + 25 | 25.33 + 9.74 | 14.84 + 6.49 | 1.71 * | Synergism | |
| 1000 | 10.87 ± 8.79 | 100 | 16.71 ± 10.51 | 1000 + 100 | 47.69 + 5.82 | 27.56 + 14.66 | 1.73 * | Synergism | |
| Fv/Fm | 2 | −3.55 ± 7.42 | 0.2 | 5.81 ± 3.45 | 2 + 0.2 | 0.58 + 13.07 | 2.26 + 10.71 | 0.25 | Antagonism |
| 10 | −8.07 ± 14.34 | 1 | 1.73 ± 10.00 | 10 + 1 | 6.02 + 7.25 | −6.34 + 17.06 | −0.96 | Antagonism | |
| 50 | 2.31 ± 6.74 | 5 | −0.68 ± 8.08 | 50 + 5 | 9.76 + 4.21 | 1.63 + 12.98 | 6.10 * | Synergism | |
| 250 | 4.23 ± 6.74 | 25 | 5.09 ± 8.08 | 250 + 25 | 19.37 + 4.21 | 9.32 + 12.98 | 2.08 * | Synergism | |
| 1000 | 10.93 ± 3.99 | 100 | 14.23 ± 8.98 | 1000 + 100 | 38.80 + 4.12 | 25.14 + 8.59 | 1.55 * | Synergism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, X.; Zhang, Y.; Li, Y.; Yang, F.; Xie, L. Phenanthrene Amplifies Microcystin-Induced Toxicity in the Submerged Macrophyte Vallisneria natans. Toxins 2025, 17, 472. https://doi.org/10.3390/toxins17090472
Wan X, Zhang Y, Li Y, Yang F, Xie L. Phenanthrene Amplifies Microcystin-Induced Toxicity in the Submerged Macrophyte Vallisneria natans. Toxins. 2025; 17(9):472. https://doi.org/10.3390/toxins17090472
Chicago/Turabian StyleWan, Xiang, Yi Zhang, Yucong Li, Fei Yang, and Liqiang Xie. 2025. "Phenanthrene Amplifies Microcystin-Induced Toxicity in the Submerged Macrophyte Vallisneria natans" Toxins 17, no. 9: 472. https://doi.org/10.3390/toxins17090472
APA StyleWan, X., Zhang, Y., Li, Y., Yang, F., & Xie, L. (2025). Phenanthrene Amplifies Microcystin-Induced Toxicity in the Submerged Macrophyte Vallisneria natans. Toxins, 17(9), 472. https://doi.org/10.3390/toxins17090472





