Prokaryotic and Eukaryotic Community Succession and Potential Parasitic Interactions During Two Alexandrium pacificum Blooms in Aotearoa New Zealand
Abstract
1. Introduction
2. Results
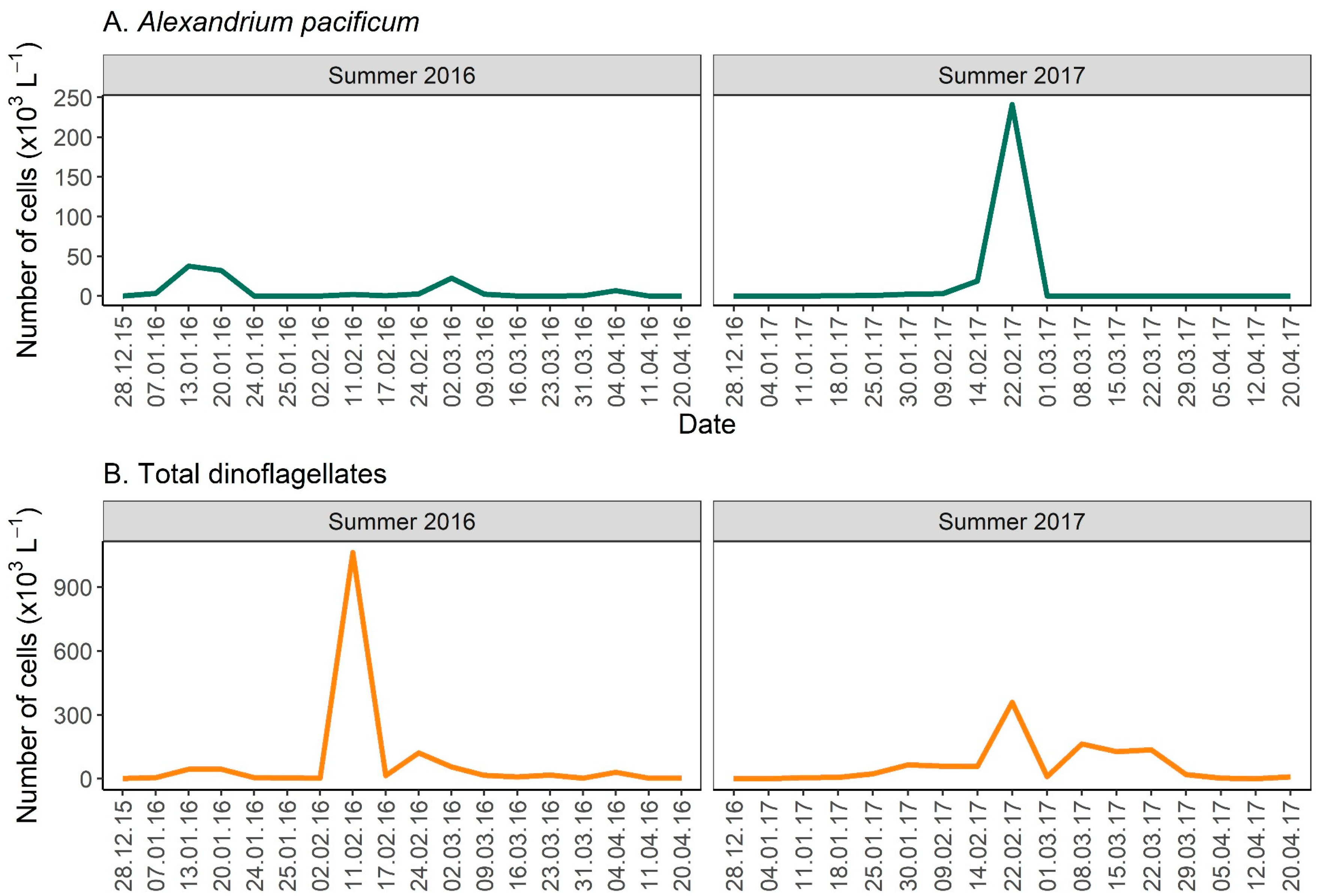
2.1. Microscopic Observations
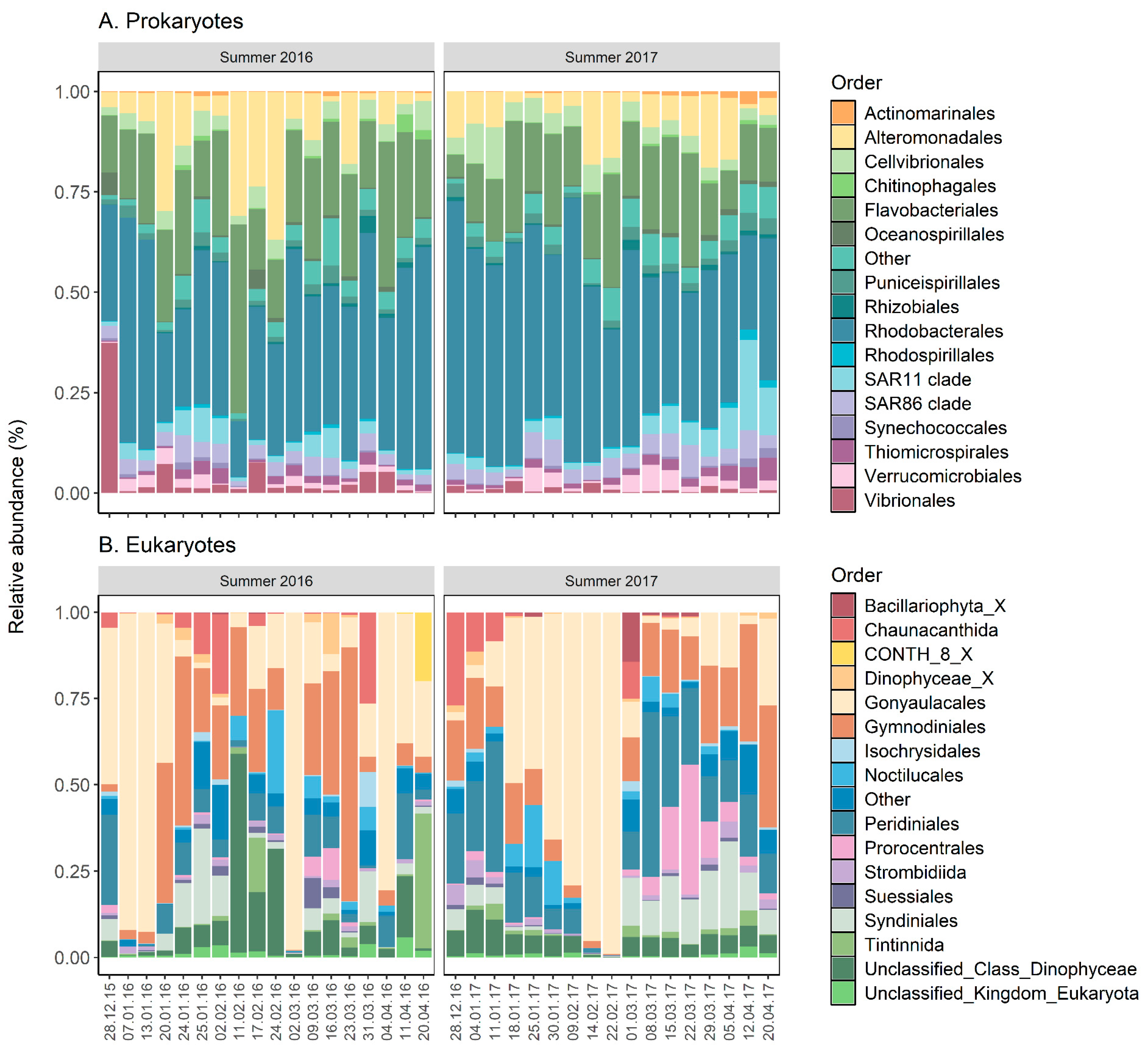
2.2. Community Composition Using Metabarcoding
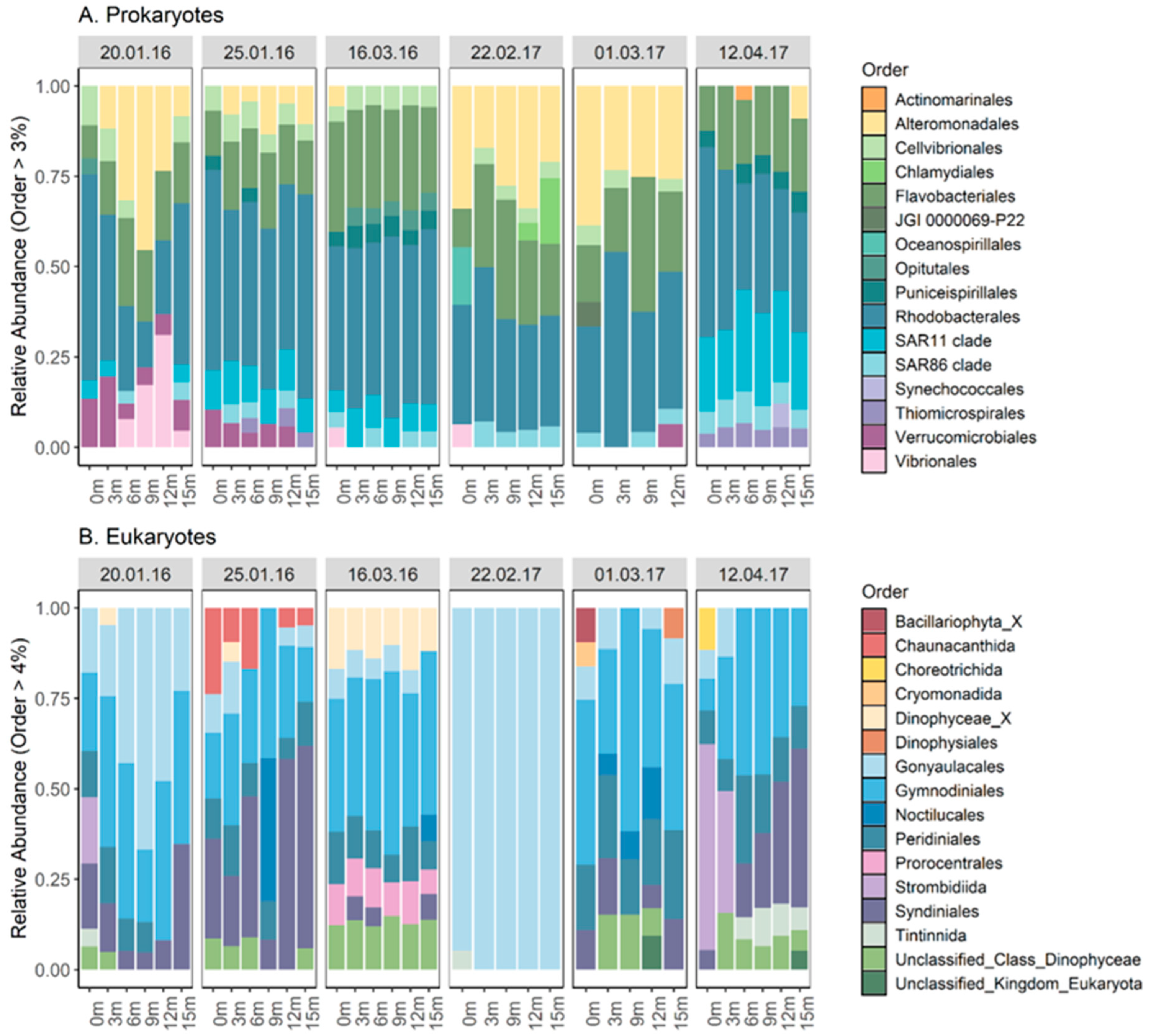
2.3. Depth Profile and Community Composition
3. Discussion
4. Conclusions
5. Materials and Methods
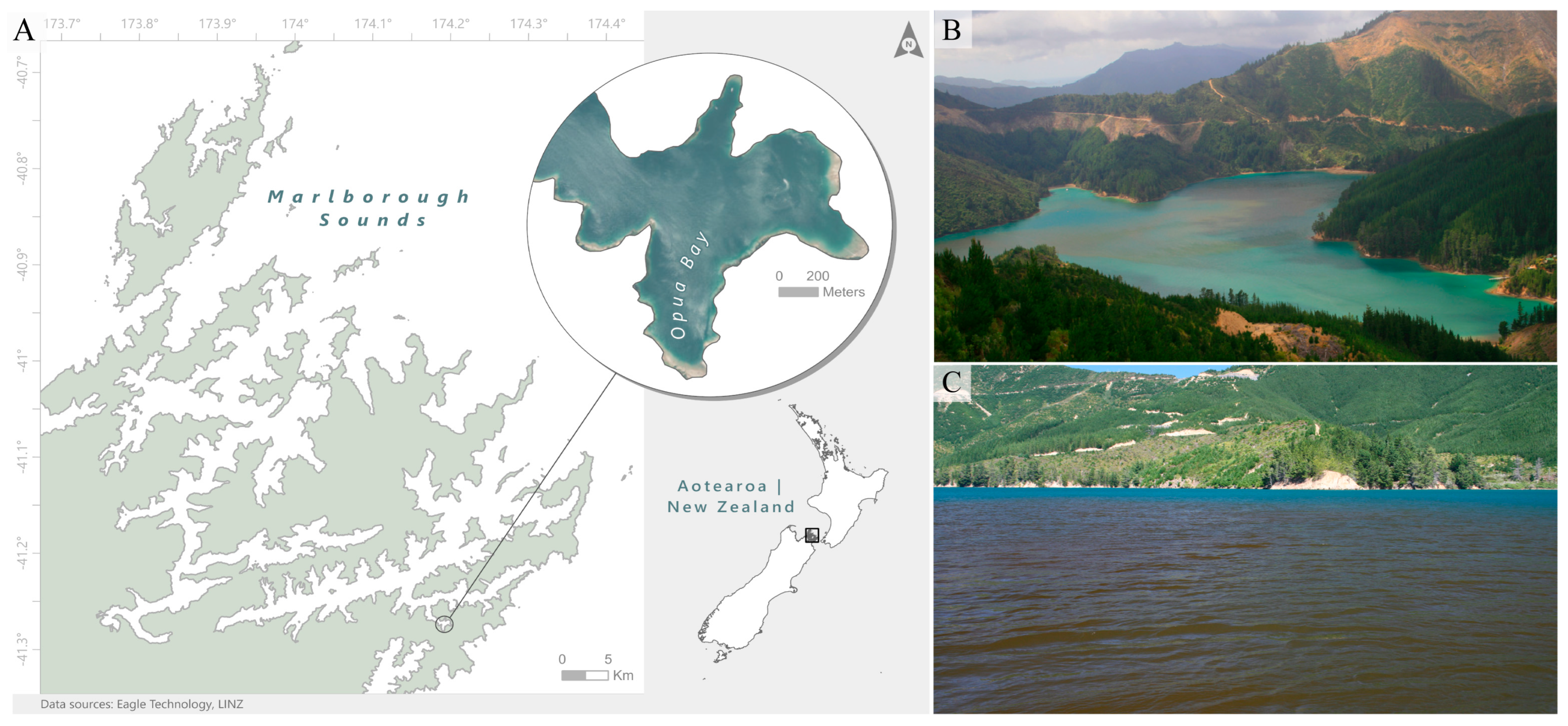
5.1. Study Site
5.2. Microscopy Analysis
5.3. DNA Extraction, Polymerase Chain Reaction, High Throughput Sequencing, and Bioinformatics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hallegraeff, G.M. Ocean climate change, phytoplankton community responses, and harmful algal blooms: A formidable predictive challenge. J. Phycol. 2010, 46, 220–235. [Google Scholar] [CrossRef]
- Lejeusne, C.; Chevaldonné, P.; Pergent-Martini, C.; Boudouresque, C.F.; Pérez, T. Climate change effects on a miniature ocean: The highly diverse, highly impacted Mediterranean Sea. Trends Ecol. Evol. 2010, 25, 250–260. [Google Scholar] [CrossRef]
- Hallegraeff, G.M. Harmful algal blooms: A global overview. Man. Harmful Mar. Microalgae 2003, 33, 1–22. [Google Scholar]
- Martino, S.; Gianella, F.; Davidson, K. An approach for evaluating the economic impacts of harmful algal blooms: The effects of blooms of toxic Dinophysis spp. on the productivity of Scottish shellfish farms. Harmful Algae 2020, 99, 101912. [Google Scholar] [CrossRef] [PubMed]
- Rolton, A.; Rhodes, L.; Hutson, K.S.; Biessy, L.; Bui, T.; MacKenzie, L.; Symonds, J.E.; Smith, K.F. Effects of harmful algal blooms on fish and shellfish species: A case study of New Zealand in a changing environment. Toxins 2022, 14, 341. [Google Scholar] [CrossRef]
- Dolah, F.M.V.; Roelke, D.; Greene, R.M. Health and ecological impacts of harmful algal blooms: Risk assessment needs. Hum. Ecol. Risk Assess. Int. J. 2001, 7, 1329–1345. [Google Scholar] [CrossRef]
- Lenzen, M.; Li, M.; Murray, S.A. Impacts of harmful algal blooms on marine aquaculture in a low-carbon future. Harmful Algae 2021, 110, 102143. [Google Scholar] [CrossRef] [PubMed]
- Ruvindy, R.; Bolch, C.J.; MacKenzie, L.; Smith, K.F.; Murray, S.A. qPCR assays for the detection and quantification of multiple paralytic shellfish toxin-producing species of Alexandrium. Front. Microbiol. 2018, 9, 3153. [Google Scholar]
- MacKenzie, A.L. The risk to New Zealand shellfish aquaculture from paralytic shellfish poisoning (PSP) toxins. N. Z. J. Mar. Freshw. Res. 2014, 48, 430–465. [Google Scholar] [CrossRef]
- Gao, Y.; Sassenhagen, I.; Richlen, M.L.; Anderson, D.M.; Martin, J.L.; Erdner, D.L. Spatiotemporal genetic structure of regional-scale Alexandrium catenella dinoflagellate blooms explained by extensive dispersal and environmental selection. Harmful Algae 2019, 86, 46–54. [Google Scholar] [CrossRef]
- Uribe, P.; Espejo, R.T. Effect of associated bacteria on the growth and toxicity of Alexandrium catenella. Appl. Environ. Microbiol. 2003, 69, 659–662. [Google Scholar] [CrossRef]
- Bravo, I.; Vila, M.; Masó, M.; Figueroa, R.I.; Ramilo, I. Alexandrium catenella and Alexandrium minutum blooms in the Mediterranean Sea: Toward the identification of ecological niches. Harmful Algae 2008, 7, 515–522. [Google Scholar] [CrossRef]
- Amin, S.A.; Parker, M.S.; Armbrust, E.V. Interactions between diatoms and bacteria. Microbiol. Mol. Biol. Rev. 2012, 76, 667–684. [Google Scholar] [CrossRef]
- Cirri, E.; Pohnert, G. Algae–bacteria interactions that balance the planktonic microbiome. New Phytol. 2019, 223, 100–106. [Google Scholar] [CrossRef]
- Fei, C.; Booker, A.; Klass, S.; Vidyarathna, N.K.; Ahn, S.H.; Mohamed, A.R.; Arshad, M.; Glibert, P.M.; Heil, C.A.; Martínez Martínez, J. Friends and foes: Symbiotic and algicidal bacterial influence on Karenia brevis blooms. ISME Commun. 2025, 5, ycae164. [Google Scholar]
- Kubanek, J.; Hicks, M.K.; Naar, J.; Villareal, T.A. Does the red tide dinoflagellate Karenia brevis use allelopathy to outcompete other phytoplankton? Limnol. Oceanogr. 2005, 50, 883–895. [Google Scholar] [CrossRef]
- Arzul, G.; Seguel, M.; Guzman, L.; Erard-Le Denn, E. Comparison of allelopathic properties in three toxic Alexandrium species. J. Exp. Mar. Biol. Ecol. 1999, 232, 285–295. [Google Scholar] [CrossRef]
- Montagnes, D.J.; Chambouvet, A.; Guillou, L.; Fenton, A. Responsibility of microzooplankton and parasite pressure for the demise of toxic dinoflagellate blooms. Aquat. Microb. Ecol. 2008, 53, 211–225. [Google Scholar] [CrossRef]
- Casas, L.; Pearman, J.K.; Irigoien, X. Metabarcoding reveals seasonal and temperature-dependent succession of zooplankton communities in the Red Sea. Front. Mar. Sci. 2017, 4, 241. [Google Scholar] [CrossRef]
- Smith, K.F.; Kohli, G.S.; Murray, S.A.; Rhodes, L.L. Assessment of the metabarcoding approach for community analysis of benthic-epiphytic dinoflagellates using mock communities. N. Z. J. Mar. Freshw. Res. 2017, 51, 555–576. [Google Scholar] [CrossRef]
- Taberlet, P.; Coissac, E.; Pompanon, F.; Brochmann, C.; Willerslev, E. Towards next-generation biodiversity assessment using DNA metabarcoding. Mol. Ecol. 2012, 21, 2045–2050. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, Q.; Simon, P.N.; Liu, J.; Liu, L.; Dai, X.; Zhang, X.; Kuang, J.; Igarashi, Y.; Pan, X. Distinct network interactions in particle-associated and free-living bacterial communities during a Microcystis aeruginosa bloom in a plateau lake. Front. Microbiol. 2017, 8, 1202. [Google Scholar] [CrossRef]
- Woodhouse, J.N.; Kinsela, A.S.; Collins, R.N.; Bowling, L.C.; Honeyman, G.L.; Holliday, J.K.; Neilan, B.A. Microbial communities reflect temporal changes in cyanobacterial composition in a shallow ephemeral freshwater lake. ISME J. 2016, 10, 1337–1351. [Google Scholar] [CrossRef]
- Zhou, J.; Richlen, M.L.; Sehein, T.R.; Kulis, D.M.; Anderson, D.M.; Cai, Z. Microbial community structure and associations during a marine dinoflagellate bloom. Front. Microbiol. 2018, 9, 1201. [Google Scholar] [CrossRef]
- Harwood, D.T.; Boundy, M.; Selwood, A.I.; van Ginkel, R.; MacKenzie, L.; McNabb, P.S. Refinement and implementation of the Lawrence method (AOAC 2005.06) in a commercial laboratory: Assay performance during an Alexandrium catenella bloom event. Harmful Algae 2013, 24, 20–31. [Google Scholar] [CrossRef]
- Ferla, M.P.; Thrash, J.C.; Giovannoni, S.J.; Patrick, W.M. New rRNA gene-based phylogenies of the Alphaproteobacteria provide perspective on major groups, mitochondrial ancestry and phylogenetic instability. PLoS ONE 2013, 8, e83383. [Google Scholar] [CrossRef]
- Morris, R.M.; Rappé, M.S.; Connon, S.A.; Vergin, K.L.; Siebold, W.A.; Carlson, C.A.; Giovannoni, S.J. SAR11 clade dominates ocean surface bacterioplankton communities. Nature 2002, 420, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Pontiller, B.; Martínez-García, S.; Joglar, V.; Amnebrink, D.; Pérez-Martínez, C.; González, J.M.; Lundin, D.; Fernández, E.; Teira, E.; Pinhassi, J. Rapid bacterioplankton transcription cascades regulate organic matter utilization during phytoplankton bloom progression in a coastal upwelling system. ISME J. 2022, 16, 2360–2372. [Google Scholar] [CrossRef]
- Herlemann, D.P.; Woelk, J.; Labrenz, M.; Jürgens, K. Diversity and abundance of “Pelagibacterales”(SAR11) in the Baltic Sea salinity gradient. Syst. Appl. Microbiol. 2014, 37, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.J.; Bestley, S.; Bissett, A.; Deagle, B.E. A globally distributed Syndiniales parasite dominates the Southern Ocean micro-eukaryote community near the sea-ice edge. ISME J. 2019, 13, 734–737. [Google Scholar] [CrossRef]
- Guillou, L.; Viprey, M.; Chambouvet, A.; Welsh, R.; Kirkham, A.; Massana, R.; Scanlan, D.J.; Worden, A.Z. Widespread occurrence and genetic diversity of marine parasitoids belonging to Syndiniales (Alveolata). Environ. Microbiol. 2008, 10, 3349–3365. [Google Scholar] [CrossRef]
- Teegarden, G.J.; Cembella, A.D. Grazing of toxic dinoflagellates, Alexandrium spp., by adult copepods of coastal Maine: Implications for the fate of paralytic shellfish toxins in marine food webs. J. Exp. Mar. Biol. Ecol. 1996, 196, 145–176. [Google Scholar] [CrossRef]
- Jansen, S.; Riser, C.W.; Wassmann, P.; Bathmann, U. Copepod feeding behaviour and egg production during a dinoflagellate bloom in the North Sea. Harmful Algae 2006, 5, 102–112. [Google Scholar] [CrossRef]
- Carlsson, P.; Granéli, E.; Finenko, G.; Maestrini, S.Y. Copepod grazing on a phytoplankton community containing the toxic dinoflagellate Dinophysis acuminata. J. Plankton Res. 1995, 17, 1925–1938. [Google Scholar] [CrossRef]
- Aylagas, E.; Borja, Á.; Rodríguez-Ezpeleta, N. Environmental status assessment using DNA metabarcoding: Towards a genetics based marine biotic index (gAMBI). PLoS ONE 2014, 9, 529. [Google Scholar] [CrossRef] [PubMed]
- Tragin, M.; Zingone, A.; Vaulot, D. Comparison of coastal phytoplankton composition estimated from the V4 and V9 regions of the 18S rRNA gene with a focus on photosynthetic groups and especially Chlorophyta. Environ. Microbiol. 2018, 20, 506–520. [Google Scholar] [CrossRef] [PubMed]
- Salonen, I.; Chronopoulou, P.; Leskinen, E.; Koho, K. Metabarcoding successfully tracks temporal changes in eukaryotic communities in coastal sediments. FEMS Microbiol. Ecol. 2019, 95, fiy226. [Google Scholar] [CrossRef]
- Amaral-Zettler, L.A.; McCliment, E.A.; Ducklow, H.W.; Huse, S.M. A method for studying protistan diversity using massively parallel sequencing of V9 hypervariable regions of small-subunit ribosomal RNA genes. PLoS ONE 2009, 4, e6372. [Google Scholar] [CrossRef]
- Van Dijk, E.L.; Auger, H.; Jaszczyszyn, Y.; Thermes, C. Ten years of next-generation sequencing technology. Trends Genet. 2014, 30, 418–426. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Z.; Deng, Y.; Shang, L.; Gobler, C.J.; Tang, Y.Z. Dependence of genome size and copy number of rRNA gene on cell volume in dinoflagellates. Harmful Algae 2021, 109, 102108. [Google Scholar] [CrossRef]
- Ruvindy, R.; Barua, A.; Bolch, C.J.; Sarowar, C.; Savela, H.; Murray, S.A. Genomic copy number variability at the genus, species and population levels impacts in situ ecological analyses of dinoflagellates and harmful algal blooms. ISME Commun. 2023, 3, 70. [Google Scholar] [CrossRef]
- Stuart, J.; Ryan, K.G.; Pearman, J.K.; Thomson-Laing, J.; Hampton, H.G.; Smith, K.F. A comparison of two gene regions for assessing community composition of eukaryotic marine microalgae from coastal ecosystems. Sci. Rep. 2024, 14, 6442. [Google Scholar] [CrossRef]
- Łach, Ł.; Khomutovska, N.; Kwiatowski, J.; Jasser, I. Testing 16S primers for proper identification of cyanobacterial communities in small water bodies. Water 2024, 16, 1357. [Google Scholar] [CrossRef]
- Keck, F.; Couton, M.; Altermatt, F. Navigating the seven challenges of taxonomic reference databases in metabarcoding analyses. Mol. Ecol. Resour. 2023, 23, 742–755. [Google Scholar]
- Nagarkar, M.; Palenik, B. Diversity and putative interactions of parasitic alveolates belonging to Syndiniales at a coastal Pacific site. Environ. Microbiol. Rep. 2023, 15, 157–169. [Google Scholar] [CrossRef]
- MacKenzie, A.; Webber, S.; Watts, A.; Tonks, A. Historical deposits of Alexandrium catenella resting cysts in the sediments of Queen Charlotte Sound, New Zealand. N. Z. J. Mar. Freshw. Res. 2016, 50, 195–208. [Google Scholar]
- Viner, A.B.; Wilkinson, V.H. Uptake of nitrate and ammonium, and distribution of related variables, in the upwelled plume of western Cook Strait/Taranaki Bight, New Zealand. N. Z. J. Mar. Freshw. Res. 1988, 22, 565–576. [Google Scholar] [CrossRef][Green Version]
- Walls, G.; Laffan, M. Native vegetation and soil patterns in the Marlborough Sounds, South Island, New Zealand. N. Z. J. Bot. 1986, 24, 293–313. [Google Scholar] [CrossRef]
- Fransen, P.; McMahon, S.; Gillespie, P.; Asher, R. Effects of logging on the marine environment at Onepua Bay, Marlborough Sounds. N. Z. For. 1998, 43, 13–18. [Google Scholar]
- Binkley, D.; Burnham, H.; Allen, H.L. Water quality impacts of forest fertilization with nitrogen and phosphorus. For. Ecol. Manag. 1999, 121, 191–213. [Google Scholar] [CrossRef]
- Amatya, D.M.; Skaggs, R.; Blanton, C.; Gilliam, J. Hydrologic and water quality effects of harvesting and regeneration of a drained pine forest. In Proceedings of the Hydrology and Management of Forested Wetlands, Proceedings of the International Conference, New Bern, NC, USA, 8–12 April 2006; p. 90. [Google Scholar]
- Beltran, B.J.; Amatya, D.M.; Youssef, M.; Jones, M.; Callahan, T.J.; Skaggs, R.W.; Nettles, J.E. Impacts of fertilization on water quality of a drained pine plantation: A worst case scenario. J. Environ. Qual. 2010, 39, 293–303. [Google Scholar] [CrossRef]
- Ensign, S.H.; Mallin, M.A. Stream water quality changes following timber harvest in a coastal plain swamp forest. Water Res. 2001, 35, 3381–3390. [Google Scholar] [CrossRef] [PubMed]
- Aristi, I.; Clapcott, J.E.; Acuña, V.; Elosegi, A.; Mills, H.; Wood, S.A.; Young, R.G. Forestry affects the abundance of Phormidium-dominated biofilms and the functioning of a New Zealand river ecosystem. Mar. Freshw. Res. 2017, 68, 1741–1751. [Google Scholar] [CrossRef][Green Version]
- Holopainen, A.-L.; Huttunen, P. Impact of forestry practices on ecology of algal communities in small brooks in the Nurmes experimental forest area, Finland. Boreal Environ. Res. 1998, 3, 63–73. [Google Scholar][Green Version]
- Herlemann, D.P.R.; Geissinger, O.; Brune, A. The termite group I phylum is highly diverse and widespread in the environment. Appl. Environ. Microbiol. 2007, 73, 6682–6685. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Zhan, A.; Hulák, M.; Sylvester, F.; Huang, X.; Adebayo, A.A.; Abbott, C.L.; Adamowicz, S.J.; Heath, D.D.; Cristescu, M.E.; MacIsaac, H. High sensitivity of 454 pyrosequencing for detection of rare species in aquatic communities. Methods Ecol. Evol. 2013, 4, 558–565. [Google Scholar] [CrossRef]
- Biessy, L.; Wood, S.A.; Chinain, M.; Roué, M.; Smith, K.F. Exploring benthic cyanobacterial diversity and co-occurring potentially harmful dinoflagellates in six islands of the South Pacific. Hydrobiologia 2021, 848, 2815–2829. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Biessy, L.; Pearman, J.K.; Hobson, M.C.; Thomson-Laing, J.; Smith, K.F.; Moy, C.M.; Riesselman, C.R.; Gilmer, G.; Vandergoes, M.J.; McLeod, R. Detection of harmful algal species in a survey of eight near pristine fjords in Fiordland Marine Area, Aotearoa-New Zealand. Total Environ. Microbiol. 2025, 1, 100017. [Google Scholar] [CrossRef]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glöckner, F.O. SILVA: A comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef] [PubMed]
- Guillou, L.; Bachar, D.; Audic, S.; Bass, D.; Berney, C.; Bittner, L.; Boutte, C.; Burgaud, G.; De Vargas, C.; Decelle, J. The Protist Ribosomal Reference database (PR2): A catalog of unicellular eukaryote small sub-unit rRNA sequences with curated taxonomy. Nucleic Acids Res. 2012, 41, 597–604. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R.J. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, 1217. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2016; p. 260. [Google Scholar]
- Oksanen, J.; Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P.; et al. Vegan: Community Ecology Package. 2019. Available online: https://vegandevs.github.io/vegan/ (accessed on 7 July 2025).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biessy, L.; Mackenzie, L.; Smith, K.F. Prokaryotic and Eukaryotic Community Succession and Potential Parasitic Interactions During Two Alexandrium pacificum Blooms in Aotearoa New Zealand. Toxins 2025, 17, 465. https://doi.org/10.3390/toxins17090465
Biessy L, Mackenzie L, Smith KF. Prokaryotic and Eukaryotic Community Succession and Potential Parasitic Interactions During Two Alexandrium pacificum Blooms in Aotearoa New Zealand. Toxins. 2025; 17(9):465. https://doi.org/10.3390/toxins17090465
Chicago/Turabian StyleBiessy, Laura, Lincoln Mackenzie, and Kirsty F. Smith. 2025. "Prokaryotic and Eukaryotic Community Succession and Potential Parasitic Interactions During Two Alexandrium pacificum Blooms in Aotearoa New Zealand" Toxins 17, no. 9: 465. https://doi.org/10.3390/toxins17090465
APA StyleBiessy, L., Mackenzie, L., & Smith, K. F. (2025). Prokaryotic and Eukaryotic Community Succession and Potential Parasitic Interactions During Two Alexandrium pacificum Blooms in Aotearoa New Zealand. Toxins, 17(9), 465. https://doi.org/10.3390/toxins17090465




