In Vitro Screening of the Antifungal and Antimycotoxin Effects of a Stilbenoids-Riche Grapevine Cane Extract on Fusarium graminearum, Aspergillus flavus and Penicillium expansum
Abstract
1. Introduction
2. Results
2.1. Grapevine Cane Extract as a Growth and Toxin Inhibitor of Mycotoxinogenic Fungi
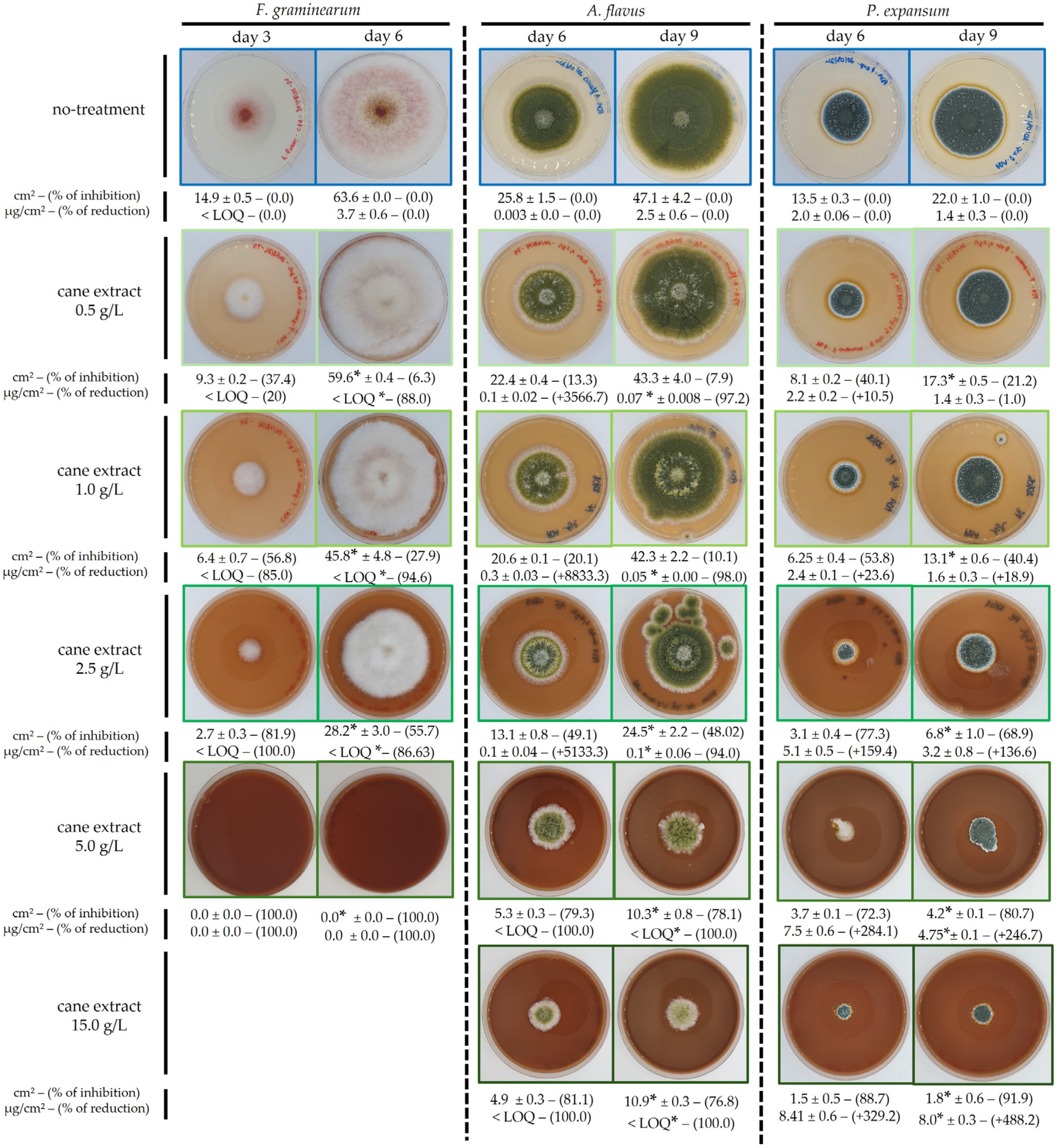
2.1.1. Growth and Mycotoxin Inhibition by GCE (Fusarium graminearum)
2.1.2. Growth and Mycotoxin Inhibition by GCE (Aspergillus flavus)
2.1.3. Growth and Mycotoxin Inhibition by GCE (Penicillium expansum)
2.2. Inhibition Through Fungal Life Cycle: From Spores to Expanding Mycelium
2.2.1. Fusarium graminearum: An Early-Stage Sensitive Fungi: Germination Inhibition and Remanence of Antifungal Effect
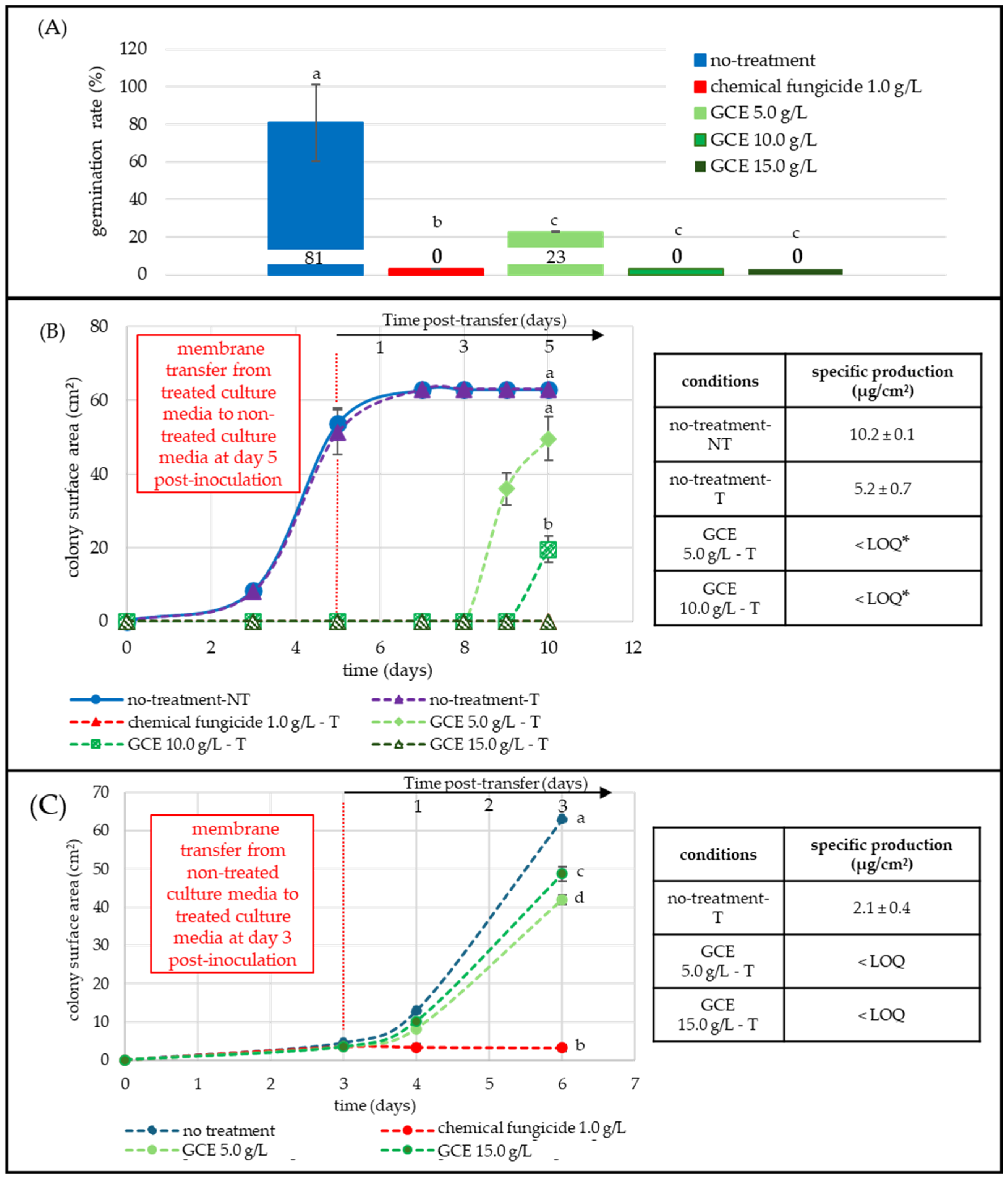
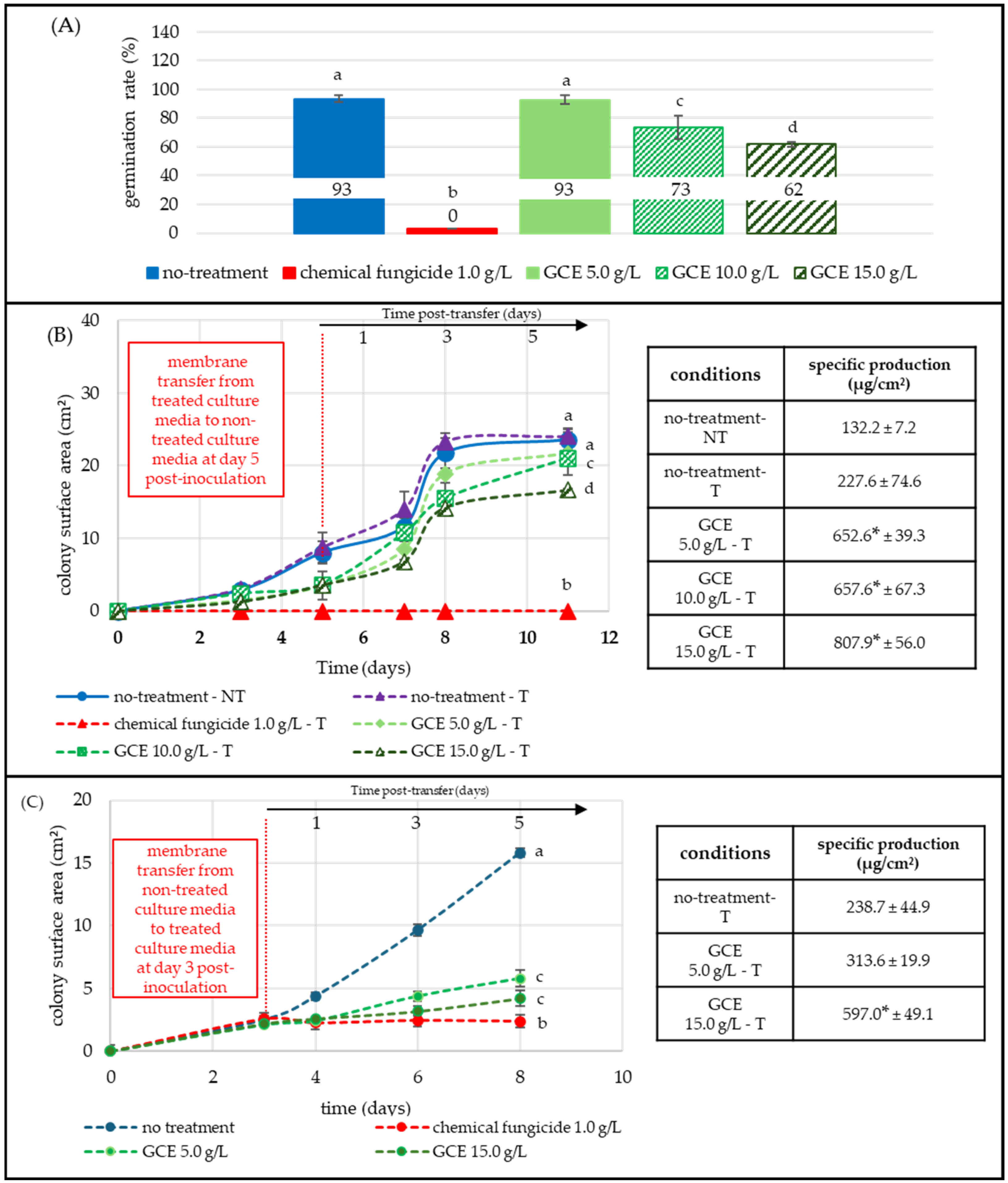
2.2.2. Aspergillus flavus: Moderate Germination Inhibition with Sustained Mycelial Suppression and Remanence of Antifungal Effect

2.2.3. Penicillium expansum: Moderate Germination Inhibition with Strong and Persistent Mycelial Suppression
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Grapevine Cane Extract (GCE)
5.2. Microorganisms, Storage Conditions, and Fungal Culture
5.2.1. Fusarium graminearum
5.2.2. Aspergillus flavus
5.2.3. Penicillium expansum
5.3. Preparation of Culture Medium
5.4. In Vitro Evaluation of Antifungal Potential of GCE
5.4.1. GCE Incorporation to Culture Medium
5.4.2. General Evaluation of Antifungal Activity
5.4.3. Sensitivity of the Fungal Targets at Various Physiological Stages
Germination Inhibition
Remanence of Antifungal and Antimycotoxin Activity
Mycelial Stage Inhibition
5.5. In Vitro Evaluation of Antimycotoxigenic Potential of GCE
5.5.1. Trichothecenes (TCTBs) Extraction and LC-MS/MS Analysis
5.5.2. Aflatoxins (AFLAs) Extraction and HPLC Analysis
5.5.3. Patulin (PAT) Extraction and HPLC Analysis
5.5.4. Quantification of Mycotoxins: Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Luo, S.; Du, H.; Kebede, H.; Liu, Y.; Xing, F. Contamination status of major mycotoxins in agricultural product and food stuff in Europe. Food Control 2021, 127, 108120. [Google Scholar] [CrossRef]
- Patriarca, A.; Pinto, V.F. Prevalence of mycotoxins in foods and decontamination. Curr. Opin. Food Sci. 2017, 14, 50–60. [Google Scholar] [CrossRef]
- Pitt, J.I.; Miller, J.D. A concise history of mycotoxin research. J. Agric. Food Chem. 2017, 65, 7021–7033. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.S.; Tardif, C.; Rouger, C.; Atanasova, V.; Richard-Forget, F.; Waffo-Téguo, P. Naturally occurring phenolic compounds as promising antimycotoxin agents: Where are we now? Compr. Rev. Food Sci. Food Saf. 2022, 21, 1161–1197. [Google Scholar] [CrossRef] [PubMed]
- IARC. IARC Monographs on the Identification of Carcinogenic Hazards to Human; International Agency for Research on Cancer: Lyon, France, 2012; Volume 1–124. [Google Scholar]
- Marques, M.M.; de Gonzalez, A.B.; Beland, F.A.; Browne, P.; Demers, P.A.; Lachenmeier, D.W.; Bahadori, T.; Barupal, D.K.; Belpoggi, F.; Comba, P.; et al. Advisory Group recommendations on priorities for the IARC Monographs. Lancet Oncol. 2019, 20, 763–764. [Google Scholar] [CrossRef]
- Gonçalves, A.; Gkrillas, A.; Dorne, J.Á.; Dall’Asta, C.; Palumbo, R.; Lima, N.; Battilani, P.; Venâncio, A.; Giorni, V.P. Pre-and postharvest strategies to minimize mycotoxin contamination in the rice food chain. Compr. Rev. Food Sci. Food Saf. 2019, 18, 441–454. [Google Scholar] [CrossRef]
- Mannaa, M.; Kim, K.D. Influence of temperature and water activity on deleterious fungi and mycotoxin production during grain storage. Mycobiology 2017, 45, 240–254. [Google Scholar] [CrossRef]
- Manu, N.; Osekre, E.A.; Opit, G.P.; Arthur, F.H.; Mbata, G.; Armstrong, P.; Danso, J.; McNeill, S.; Campbell, J. Moisture content, insect pests and mycotoxin levels of maize on farms in Tamale environs in the northern region of Ghana. J. Stored Prod. Res. 2019, 83, 153–160. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006. Official Journal of the European Union L 119 (5 May 2023), pp. 103–157. CELEX: 32023R0915. EUR-Lex (ELI). Available online: https://eur-lex.europa.eu/eli/reg/2023/915/oj (accessed on 20 July 2025).
- European Commission. Commission Regulation (EU) 2021/1399 of 24 August 2021 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Ergot Sclerotia and Ergot Alkaloids in Certain Foodstuffs (Text with EEA Relevance). Official Journal of the European Union L 301 (25 Aug 2021), pp. 1–5. CELEX: 32021R1399. EUR-Lex (ELI). Available online: https://eur-lex.europa.eu/eli/reg/2021/1399/oj (accessed on 20 July 2025).
- Mahdjoubi, C.K.; Arroyo-Manzanares, N.; Hamini-Kadar, N.; García-Campaña, A.M.; Mebrouk, K.; Gámiz-Gracia, L. Multimycotoxin occurrence and exposure assessment approach in foodstuffs from Algeria. Toxins 2020, 12, 194. [Google Scholar] [CrossRef]
- Taniwaki, M.H.; Pitt, J.I.; Copetti, M.V.; Teixeira, A.A.; Iamanaka, B.T. Understanding mycotoxin contamination across the food chain in Brazil: Challenges and opportunities. Toxins 2019, 11, 411. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Conte, G.; Fontanelli, M.; Galli, F.; Cotrozzi, L.; Pagni, L.; Pellegrini, E. Mycotoxins in feed and food and the role of ozone in their detoxification and degradation: An update. Toxins 2020, 12, 486. [Google Scholar] [CrossRef]
- Focker, M.; Van Der Fels-Klerx, H.J.; Magan, N.; Edwards, S.G.; Grahovac, M.; Bagi, F.; Budakov, D.; Suman, M.; Schatzmayr, G.; Krska, R.; et al. The impact of management practices to prevent and control mycotoxins in the European food supply chain: MyToolBox project results. World Mycotoxin J. 2021, 14, 139–154. [Google Scholar] [CrossRef]
- Nada, S.; Nikola, T.; Bozidar, U.; Ilija, D.; Andreja, R. Prevention and practical strategies to control mycotoxins in the wheat and maize chain. Food Control 2022, 136, 108855. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in occurrence, importance, and mycotoxin control strategies: Prevention and detoxification in foods. Foods 2020, 9, 137. [Google Scholar] [CrossRef]
- Brent, K.J.; Hollomon, D.W. Fungicide resistance the assessment of risk. FRAC Monogr. 1998, 2, 1–48. [Google Scholar]
- Amichot, M.; Bertrand, C.; Chauvel, B.; Corio-Costet, M.F.; Martin-Laurent, F.; Le Perchec, S.; Mamy, L. Natural products for biocontrol: Review of their fate in the environment and impacts on biodiversity. Environ. Sci. Pollut. Res. 2025, 32, 2857–2892. [Google Scholar] [CrossRef] [PubMed]
- Valette, N.; Perrot, T.; Sormani, R.; Gelhaye, E.; Morel-Rouhier, M. Antifungal activities of wood extractives. Fungal Biol. Rev. 2017, 31, 113–123. [Google Scholar] [CrossRef]
- Han, J.W.; Shim, S.H.; Jang, K.S.; Choi, Y.H.; Dang, Q.L.; Kim, H.; Choi, G.J. In vivo assessment of plant extracts for control of plant diseases: A sesquiterpene ketolactone isolated from Curcuma zedoaria suppresses wheat leaf rust. J. Environ. Sci. Health Part B 2018, 53, 135–140. [Google Scholar] [CrossRef]
- Choi, N.H.; Choi, G.J.; Min, B.S.; Jang, K.S.; Choi, Y.H.; Kang, M.S.; Park, M.; Choi, J.; Bae, B.; Kim, J.C. Effects of neolignans from the stem bark of Magnolia obovata on plant pathogenic fungi. J. Appl. Microbiol. 2009, 106, 2057–2063. [Google Scholar] [CrossRef]
- Bulgari, D.; Alias, C.; Peron, G.; Ribaudo, G.; Gianoncelli, A.; Savino, S.; Boureghda, H.; Bouznad, Z.; Monti, E.; Gobbi, E. Solid-state fermentation of Trichoderma spp.: A new way to valorize the agricultural digestate and produce value-added bioproducts. J. Agric. Food Chem. 2023, 71, 3994–4004. [Google Scholar] [CrossRef] [PubMed]
- Bouassida, M.; Mnif, I.; Hammami, I.; Triki, M.A.; Ghribi, D. Bacillus subtilis SPB1 lipopeptide biosurfactant: Antibacterial efficiency against the phytopathogenic bacteria Agrobacterium tumefaciens and compared production in submerged and solid state fermentation systems. Food Sci. Biotechnol. 2023, 32, 1595–1609. [Google Scholar] [CrossRef]
- Montibus, M.; Vitrac, X.; Coma, V.; Loron, A.; Pinson-Gadais, L.; Ferrer, N.; Verdal-Bonnin, M.-N.; Gabaston, J.; Waffo-Téguo, P.; Richard-Forget, F.; et al. Screening of wood/forest and vine by-products as sources of new drugs for sustainable strategies to control Fusarium graminearum and the production of mycotoxins. Molecules 2021, 26, 405. [Google Scholar] [CrossRef]
- Mattio, L.; Catinella, G.; Iriti, M.; Vallone, L. Inhibitory activity of stilbenes against filamentous fungi. Ital. J. Food Saf. 2021, 10, 8461. [Google Scholar] [CrossRef]
- Goufo, P.; Singh, R.K.; Cortez, I. A reference list of phenolic compounds (including stilbenes) in grapevine (Vitis vinifera L.) roots, woods, canes, stems, and leaves. Antioxidants 2020, 9, 398. [Google Scholar] [CrossRef]
- Pawlus, A.D.; Waffo-Téguo, P.; Shaver, J.; Mérillon, J.M. Stilbenoid chemistry from wine and the genus Vitis, a review. Oeno One 2012, 46, 57–111. [Google Scholar] [CrossRef]
- Jeandet, P.; Delaunois, B.; Conreux, A.; Donnez, D.; Nuzzo, V.; Cordelier, S.; Clément, C.; Courot, E. Biosynthesis, metabolism, molecular engineering, and biological functions of stilbene phytoalexins in plants. Biofactors 2010, 36, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Riviere, C.; Pawlus, A.D.; Merillon, J.M. Natural stilbenoids: Distribution in the plant kingdom and chemotaxonomic interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317–1333. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.; Bisson, J.; Waffo-Téguo, P.; Papastamoulis, Y.; Richard, T.; Corio-Costet, M.F.; Mérillon, J.-M.; Cluzet, S. Phenolics and their antifungal role in grapevine wood decay: Focus on the Botryosphaeriaceae family. J. Agric. Food Chem. 2012, 60, 11859–11868. [Google Scholar] [CrossRef]
- Xu, D.; Deng, Y.; Han, T.; Jiang, L.; Xi, P.; Wang, Q.; Jiang, Z.; Gao, L. In vitro and in vivo effectiveness of phenolic compounds for the control of postharvest gray mold of table grapes. Postharvest Biol. Technol. 2018, 139, 106–114. [Google Scholar] [CrossRef]
- Gabaston, J.; Cantos-Villar, E.; Biais, B.; Waffo-Teguo, P.; Renouf, E.; Corio-Costet, M.F.; Richard, T.; Mérillon, J.M. Stilbenes from Vitis vinifera L. waste: A sustainable tool for controlling Plasmopara viticola. J. Agric. Food Chem. 2017, 65, 2711–2718. [Google Scholar] [CrossRef]
- Pezet, R.; Gindro, K.; Viret, O.; Spring, J.L. Glycosylation and oxidative dimerization of resveratrol are respectively associated to sensitivity and resistance of grapevine cultivars to downy mildew. Physiol. Mol. Plant Pathol. 2004, 65, 297–303. [Google Scholar] [CrossRef]
- Bavaresco, L.; Petegolli, D.; Cantu, E.; Fregoni, M.; Chiusa, G.; Trevisan, M. Elicitation and accumulation of stilbene phytoalexins in grapevine berries infected by Botrytis cinerea. Vitis 1997, 36, 77–83. [Google Scholar]
- Anđelini, D.; Cvitan, D.; Prelac, M.; Pasković, I.; Černe, M.; Nemet, I.; Major, N.; Ban, S.G.; Užila, Z.; Ferri, T.Z.; et al. Biochar from Grapevine-Pruning Residues Is Affected by Grapevine Rootstock and Pyrolysis Temperature. Sustainability 2023, 15, 4851. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Biais, B.; Richard, T.; Puertas, B.; Waffo-Teguo, P.; Merillon, J.M.; Cantos-Villar, E. Grapevine cane’s waste is a source of bioactive stilbenes. Ind. Crops Prod. 2016, 94, 884–892. [Google Scholar] [CrossRef]
- Campos-Avelar, I.; Colas de la Noue, A.; Durand, N.; Cazals, G.; Martinez, V.; Strub, C.; Fontana, A.; Schorr-Galindo, S. Aspergillus flavus growth inhibition and aflatoxin B1 decontamination by Streptomyces isolates and their metabolites. Toxins 2021, 13, 340. [Google Scholar] [CrossRef]
- Baranyi, N.; Despot, D.J.; Palágyi, A.; Kiss, N.; Kocsubé, S.; Szekeres, A.; Kecskeméti, A.; Bencsik, O.; Vágvölgyi, C.; Klarić, M.Š.; et al. Identification of Aspergillus species in Central Europe able to produce G-type aflatoxins. Acta Biol. Hung. 2015, 66, 339–347. [Google Scholar] [CrossRef]
- Varga, J.; Frisvad, J.C.; Samson, R. Two new aflatoxin producing species, and an overview of Aspergillus section Flavi. Stud. Mycol. 2011, 69, 57–80. [Google Scholar] [CrossRef]
- Shukla, R.; Kumar, A.; Prasad, C.S.; Srivastava, B.; Dubey, N.K. Antimycotic and antiaflatoxigenic potency of Adenocalymma alliaceum Miers. on fungi causing biodeterioration of food commodities and raw herbal drugs. Int. Biodeterior. Biodegrad. 2008, 62, 348–351. [Google Scholar] [CrossRef]
- Zabka, M.; Pavela, R.; Slezakova, L. Antifungal effect of Pimenta dioica essential oil against dangerous pathogenic and toxinogenic fungi. Ind. Crops Prod. 2009, 30, 250–253. [Google Scholar] [CrossRef]
- Gauthier, L.; Bonnin-Verdal, M.N.; Marchegay, G.; Pinson-Gadais, L.; Ducos, C.; Richard-Forget, F.; Atanasova-Penichon, V. Fungal biotransformation of chlorogenic and caffeic acids by Fusarium graminearum: New insights in the contribution of phenolic acids to resistance to deoxynivalenol accumulation in cereals. Int. J. Food Microbiol. 2016, 221, 61–68. [Google Scholar] [CrossRef]
- Perret, C.; Pezet, R.; Tabacchi, R. Qualitative analysis of grapevine tannins by mass spectrometry and their inhibitory effect on stilbene oxidase of Botrytis cinerea. Chimia 2003, 57, 607. [Google Scholar] [CrossRef]
- Alonso-Villaverde, V.; Voinesco, F.; Viret, O.; Spring, J.L.; Gindro, K. The effectiveness of stilbenes in resistant Vitaceae: Ultrastructural and biochemical events during Plasmopara viticola infection process. Plant Physiol. Biochem. 2011, 49, 265–274. [Google Scholar] [CrossRef]
- Schnee, S.; Queiroz, E.F.; Voinesco, F.; Marcourt, L.; Dubuis, P.H.; Wolfender, J.L.; Gindro, K. Vitis vinifera canes, a new source of antifungal compounds against Plasmopara viticola, Erysiphe necator, and Botrytis cinerea. J. Agric. Food Chem. 2013, 61, 5459–5467. [Google Scholar] [CrossRef]
- Tardif, C.; Rouger, C.; Miranda, J.; Ahmed, O.S.; Richard-Forget, F.; Atanasova, V.; Waffo-Teguo, P. Targeting of Antifungal Metabolites from Grapevine Byproducts by UPLC–HRMS/MS Approaches Using Bioactivity-Based Molecular Networking. J. Agric. Food Chem. 2024, 72, 9621–9636. [Google Scholar] [CrossRef]
- Wang, H.; Lei, Y.; Yan, L.; Cheng, K.; Dai, X.; Wan, L.; Guo, W.; Cheng, L.; Liao, B. Deep sequencing analysis of transcriptomes in Aspergillus flavus in response to resveratrol. BMC Microbiol. 2015, 15, 182. [Google Scholar] [CrossRef]
- Sanzani, S.M.; De Girolamo, A.; Schena, L.; Solfrizzo, M.; Ippolito, A.; Visconti, A. Control of Penicillium expansum and patulin accumulation on apples by quercetin and umbelliferone. Eur. Food Res. Technol. 2009, 228, 381–389. [Google Scholar] [CrossRef]
- Loupit, G.; Prigent, S.; Franc, C.; De Revel, G.; Richard, T.; Cookson, S.J.; Fonayet, J.V. Polyphenol profiles of just pruned grapevine canes from wild Vitis accessions and Vitis vinifera cultivars. J. Agric. Food Chem. 2020, 68, 13397–13407. [Google Scholar] [CrossRef] [PubMed]
- Besrukow, P.; Irmler, J.; Schmid, J.; Stoll, M.; Winterhalter, P.; Schweiggert, R.; Will, F. Variability of constitutive stilbenoid levels and profiles in grape cane (Vitis vinifera L.) depending upon variety and clone, location in the vineyard, pruning time, and vintage. J. Agric. Food Chem. 2022, 70, 4342–4352. [Google Scholar] [CrossRef]
- Lambert, C.; Richard, T.; Renouf, E.; Bisson, J.; Waffo-Téguo, P.; Bordenave, L.; Ollat, N.; Mérillon, J.-M.; Cluzet, S. Comparative analyses of stilbenoids in canes of major Vitis vinifera L. cultivars. J. Agric. Food Chem. 2013, 61, 11392–11399. [Google Scholar] [CrossRef]
- Chong, J.; Poutaraud, A.; Hugueney, P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009, 177, 143–155. [Google Scholar] [CrossRef]
- Pezet, R.; Perret, C.; Jean-Denis, J.B.; Tabacchi, R.; Gindro, K.; Viret, O. δ-Viniferin, a resveratrol dehydrodimer: One of the major stilbenes synthesized by stressed grapevine leaves. J. Agric. Food Chem. 2003, 51, 5488–5492. [Google Scholar] [CrossRef]
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The fungal cell wall: Candida, Cryptococcus, and Aspergillus species. Front. Microbiol. 2020, 10, 2993. [Google Scholar] [CrossRef]
- Gow, N.A.; Latge, J.P.; Munro, C.A. The fungal cell wall: Structure, biosynthesis, and function. Microbiol. Spectr. 2017, 5, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Atanasova-Penichon, V.; Bernillon, S.; Marchegay, G.; Lornac, A.; Pinson-Gadais, L.; Ponts, N.; Zehraoui, E.; Barreau, C.; Richard-Forget, F. Bioguided isolation, characterization, and biotransformation by Fusarium verticillioides of maize kernel compounds that inhibit fumonisin production. Mol. Plant-Microbe Interact. 2014, 27, 1148–1158. [Google Scholar] [CrossRef]
- Schöneberg, T.; Kibler, K.; Sulyok, M.; Musa, T.; Bucheli, T.D.; Mascher, F.; Bertossa, M.; Voegele, R.T.; Vogelgsang, S. Can plant phenolic compounds reduce Fusarium growth and mycotoxin production in cereals? Food Addit. Contam. Part A 2018, 35, 2455–2470. [Google Scholar] [CrossRef] [PubMed]
- Ferruz, E.; Atanasova-Pénichon, V.; Bonnin-Verdal, M.N.; Marchegay, G.; Pinson-Gadais, L.; Ducos, C.; Lorán, S.; Ariño, A.; Barreau, C.; Richard-Forget, F. Effects of phenolic acids on the growth and production of T-2 and HT-2 toxins by Fusarium langsethiae and F. sporotrichioides. Molecules 2016, 21, 449. [Google Scholar] [CrossRef] [PubMed]
- Adrian, M.; Jeandet, P. Effects of resveratrol on the ultrastructure of Botrytis cinerea conidia and biological significance in plant/pathogen interactions. Fitoterapia 2012, 83, 1345–1350. [Google Scholar] [CrossRef]
- Pezet, R.; Gindro, K.; Viret, O.; Richter, H. Effects of resveratrol, viniferins and pterostilbene on Plasmopara viticola zoospore mobility and disease development. Vitis 2004, 43, 145–148. [Google Scholar]
- Phan, H.T.; Yoda, T.; Chahal, B.; Morita, M.; Takagi, M.; Mun’delanji, C.V. Structure-dependent interactions of polyphenols with a biomimetic membrane system. Biochim. Biophys. Acta (BBA)-Biomembr. 2014, 1838, 2670–2677. [Google Scholar] [CrossRef]
- Pryce, R.J.; Langcake, P. α-Viniferin: An antifungal resveratrol trimer from grapevines. Phytochemistry 1977, 16, 1452–1454. [Google Scholar] [CrossRef]
- Pont, V.; Pezet, R. Relation between the chemical structure and the biological activity of hydroxystilbenes against Botrytis cinerea. J. Phytopathol. 1990, 130, 1–8. [Google Scholar] [CrossRef]
- Crespo-Sempere, A.; Selma-Lázaro, C.; Palumbo, J.D.; González-Candelas, L.; Martínez-Culebras, P.V. Effect of oxidant stressors and phenolic antioxidants on the ochratoxigenic fungus Aspergillus carbonarius. J. Sci. Food Agric. 2016, 96, 169–177. [Google Scholar] [CrossRef]
- Palumbo, J.D.; O’Keeffe, T.L.; Mahoney, N.E. Inhibition of ochratoxin A production and growth of Aspergillus species by phenolic antioxidant compounds. Mycopathologia 2007, 164, 241–248. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Yuan, S.; Feng, X.; Wang, M. Transcriptomic insights into the antifungal effects of magnolol on the growth and mycotoxin production of Alternaria alternata. Toxins 2020, 12, 665. [Google Scholar] [CrossRef]
- Zhao, X.; Zhi, Q.Q.; Li, J.Y.; Keller, N.P.; He, Z.M. The antioxidant gallic acid inhibits aflatoxin formation in Aspergillus flavus by modulating transcription factors FarB and CreA. Toxins 2018, 10, 270. [Google Scholar] [CrossRef] [PubMed]
- Ponts, N.; Couedelo, L.; Pinson-Gadais, L.; Verdal-Bonnin, M.N.; Barreau, C.; Richard-Forget, F. Fusarium response to oxidative stress by H2O2 is trichothecene chemotype-dependent. FEMS Microbiol. Lett. 2009, 293, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Lee, S.Y.; Woo, S.Y.; Chun, H.S. Alternative oxidase: A potential target for controlling aflatoxin contamination and propagation of Aspergillus flavus. Front. Microbiol. 2020, 11, 419. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.; Cayzac, C.; Maguet, J.L.; Bidel, L.P.; Jay-Allemand, C.; Lebrun, A.; Giger, A.; Aznar, D.; Gauthier, L.; Rolet, F. Field sustainable management of apple scab disease using cane extract of Vitis vinifera. EPPO Bull. 2025, 55, 297–304. [Google Scholar] [CrossRef]
- Borges, D.F.; Lopes, E.A.; Moraes, A.R.F.; Soares, M.S.; Visôtto, L.E.; Oliveira, C.R.; Valente, V.M.M. Formulation of botanicals for the control of plant-pathogens: A review. Crop Prot. 2018, 110, 135–140. [Google Scholar] [CrossRef]
- Hadji, T.K.A.I.; Senanayake, N.S.; Dharmasena, A. Formulation of botanical extracts as nanoemulsion: Towards sustainable pest management in agriculture—A review. Trop. Agric. 2023, 171, 1–22. [Google Scholar] [CrossRef]
- Jeandet, P.; Sobarzo-Sánchez, E.; Uddin, S.; Bru, R.; Clément, C.; Jacquard, C.; Khayatkashani, M.; Batiha, G.E.-S.; Khan, H.; Morkunas, I.; et al. Resveratrol and cyclodextrins, an easy alliance: Applications in nanomedicine, green chemistry and biotechnology. Biotechnol. Adv. 2021, 53, 107844. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.F.; Monteiro, M.; Resende, D.; Braga, S.S.; Coimbra, M.A.; Silva, A.M.; Cardoso, S.M. Inclusion complex of resveratrol with γ-cyclodextrin as a functional ingredient for lemon juices. Foods 2020, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Besrukow, P.; Orbach, A.; Schweiggert, R. Modulating the Photostability of (E)-Resveratrol in Grape Cane Extract Formulations. ACS Food Sci. Technol. 2024, 4, 625–632. [Google Scholar] [CrossRef]
- Pellan, L.; Durand, N.; Martinez, V.; Fontana, A.; Schorr-Galindo, S.; Strub, C. Commercial biocontrol agents reveal contrasting comportments against two mycotoxigenic fungi in cereals: Fusarium graminearum and Fusarium verticillioides. Toxins 2020, 12, 152. [Google Scholar] [CrossRef]
- Pellan, L.; Dieye, C.A.T.; Durand, N.; Fontana, A.; Strub, C.; Schorr-Galindo, S. Biocontrol agents: Toolbox for the screening of weapons against mycotoxigenic Fusarium. J. Fungi 2021, 7, 446. [Google Scholar] [CrossRef]
- Al Riachy, R.; Strub, C.; Durand, N.; Guibert, B.; Guichard, H.; Constancias, F.; Chochois, V.; Lopez-Lauri, F.; Fontana, A.; Schorr-Galindo, S. Microbiome status of cider-apples, from orchard to processing, with a special focus on Penicillium expansum occurrence and patulin contamination. J. Fungi 2021, 7, 244. [Google Scholar] [CrossRef]
- Al Riachy, R.; Strub, C.; Durand, N.; Chochois, V.; Lopez-Lauri, F.; Fontana, A.; Schorr-Galindo, S. The influence of long-term storage on the epiphytic microbiome of postharvest apples and on Penicillium expansum occurrence and patulin accumulation. Toxins 2024, 16, 102. [Google Scholar] [CrossRef]
- Dieye, C.A.T.; Durand, N.; Schorr-Galindo, S.; Strub, C.; Fontana, A. Impacts of abiotic factors on the growth of three commercial biological control agents, on the growth and mycotoxinogenesis of Fusarium graminearum and on their interaction. J. Sci. Food Agric. 2024, 104, 932–941. [Google Scholar] [CrossRef] [PubMed]
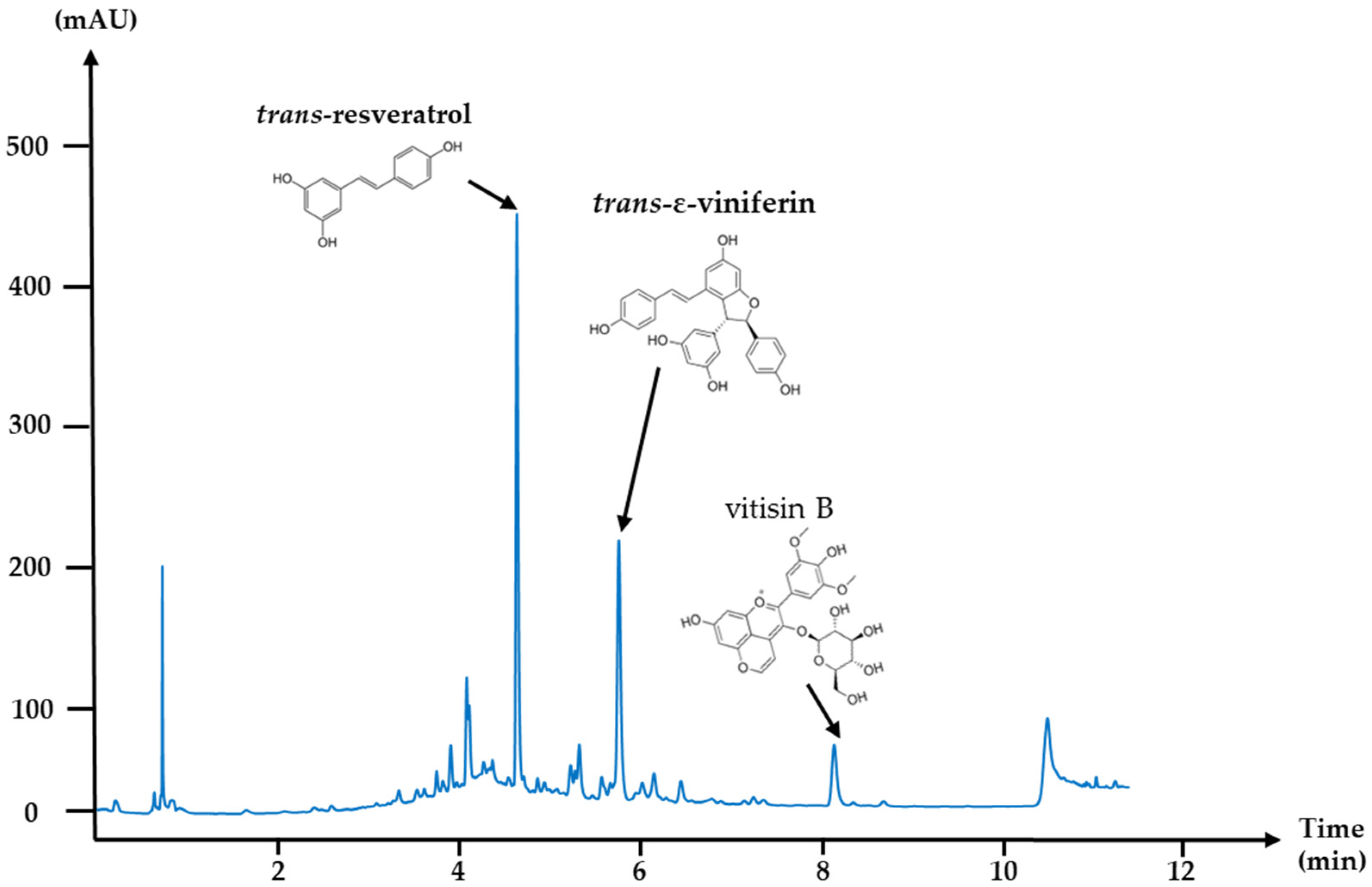
| Fungi | IC50 (g/L) | IC100 (g/L) | Spores/Germination | Mycelium/Colony | Remanence | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Growth | Toxins | Growth | Toxins | Inhibition | Sporicidal | Fungicide | Toxins | Growth | Toxins | |
| F. graminearum | 2.5 ≈55% | <0.5 ≈88% | 2.5–5.0 ≈55–100% | 1.0–2.5 ≈94–87% | 5.0 and <15.0 ≈72–100% | 15.0 | not observed 15.0 g/L ≈ 22% | <5.0 <LOQ | moderate | strong |
| A. flavus | 2.5–5.0 ≈48–77% | <0.5 ≈98% | >30.0 15.0 g/L ≈ 78% | 2.5–5.0 ≈94–100% | >15.0 ≈68% | not observed 15.0 g/L ≈ 68% | not observed 15.0 g/L ≈ 62% | <5.0 ≈99% | moderate | strong |
| P. expansum | 1.0–2.5 ≈40–69% | pro-patulin | >30.0 15.0 g/L ≈ 92% | pro-patulin | 10.0 ≈21% | not observed 15.0 g/L ≈ 33% | not observed 15.0 g/L ≈ 66% | pro-patulin | low | pro-patulin |
| Analyte | Content [% w/w] | SD [% w/w] | Detection Mode |
|---|---|---|---|
| Water | 4.59 | 1.40 | Kar-Fisher-titration method |
| monosaccharides | 1.24 | 0.81 | HPLC-RID |
| disaccharides | 0.70 | 0.68 | HPLC-RID |
| polysaccharides | 14.56 | 0.38 | HPLC-RID |
| lignin * | 70.91 | NA | HPLC-UV-vis |
| polyphenols * | 36.90 | 1.37 | folin-Ciocalteu |
| hydrolysable tannins * | 3.98 | 0.19 | absorbance |
| condensed tannins * | 0.41 | 0.07 | absorbance |
| flavonoids | 6.34 | NA | ULPC-MS, spectrometry |
| stilbenoids | 21.20 | 2.56 | UPLC-DAD |
| Extracts | Compounds | F. graminearum IC50 (µM) | Extracts | Compounds | A. flavus IC50 (µM) | Extracts | Compounds | P. expansum IC50 (µM) | Reference | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Growth | Toxins | Growth | Toxins | Growth | Toxins | |||||||
| Grapevine cane extract (GCE) IC50 growth: 2.5 g/L IC50 toxine: <0.5 g/L | piceid | 25.5 | 1.3 | Grapevine cane extract (GCE) IC50 growth: 2.5–5.0 g/L IC50 toxine: <0.5 g/L | piceid | 25.5–61.6 | 1.3 | Grapevine cane extract (GCE) IC50 growth: 1.0–2.5 g/L IC50 toxine: <30.0 g/L | piceid | 5.3–25.5 | – | |
| piceatanol | 13.5 | <LOQ | piceatanol | 13.5–39.7 | <LOQ | piceatanol | 6.4–13.5 | – | ||||
| trans-resveratrol | 138.9 | 16.4 | trans-resveratrol | 138.9–233.4 | 16.4 | trans-resveratrol | 72.4–138.9 | – | ||||
| trans-ε-viniferin | 53.2 | 10.8 | trans-ε-viniferin | 53.3–94.3 | 10.8 | trans-ε-viniferin | 23.9–53.3 | – | ||||
| vitisin B | <LOQ | <LOQ | vitisin B | <LOQ–3.8 | <LOQ | vitisin B | <LOQ | – | ||||
| Pure compound in water: 0.1 g/L | piceatanol | >410.0 (≈no inhibition) | – | Pure compound in water: 0.1 g/L | piceatanol | >410.0 (≈23% inhibition) | – | Pure compound in water: 0.1 g/L | piceatanol | >410.0 (≈no inhibition) | – | [28] |
| Sauvignon blanc canes in H2O/EtOH, 95.5/0.5, v/v IC50: >0.1 g/L | piceatanol | >8.1 | 8.1 | Pure compound in water: 0.003 g/L | trans-resveratrol | 13.2 (≈no inhibition) | 13.2 (≈47% inhibition) | Pure compound in phosphate buffer/NaoH, 9:1, v/v): 5.0 g/L | trans-resveratrol | 21,900 (≈7.2% inhibition) | 21,900 (+40%) | [50,51,52] |
| trans-resveratrol | >43.2 | 43.2 | ||||||||||
| trans-ε-viniferin | >110.9 (<50% inhibition) | 110.9 (<36% inhibition) | ||||||||||
| Tannat canes in H2O/EtOH, 95.5:0.5, v/v IC50: >0.09 g/L | piceatanol | 4.9 | 6.9 | [50] | ||||||||
| trans-resveratrol | 31.0 | 43.6 | ||||||||||
| trans-ε-viniferin | 61.2 (50% inhibition) | 86.0 (69% inhibition) | ||||||||||
| Merlot woods in H2O/EtOH, 95.5/0.5, v/v IC50: 0.07 g/L | trans-resveratrol | 73.7 | 139.5 | [50] | ||||||||
| trans-ε-viniferin | 49.8 (50% inhibition) | 94.4 (81% inhibition) | ||||||||||
| Tannat wood in H2O/EtOH, 95.5/0.5, v/v IC50: 0.07 g/L | trans-resveratrol | 16.8 | 28.8 | [50] | ||||||||
| trans-ε-viniferin | 44.7 (50% inhibition) | 76.6 (80% inhibition) | ||||||||||
| Time (min) | Solvent B (%) |
|---|---|
| 0.0 | 5 |
| 3.0 | 30 |
| 9.2 | 40 |
| 9.4 | 100 |
| 10.5 | 100 |
| 10.7 | 5 |
| 11.3 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aznar, D.; Colas de la Noue, A.; Bidel, L.P.R.; Cayzac, C.; Poss, C.; Ciordia, E.; Cozette, A.; Fontana, A.; Rolet, F.; Strub, C. In Vitro Screening of the Antifungal and Antimycotoxin Effects of a Stilbenoids-Riche Grapevine Cane Extract on Fusarium graminearum, Aspergillus flavus and Penicillium expansum. Toxins 2025, 17, 454. https://doi.org/10.3390/toxins17090454
Aznar D, Colas de la Noue A, Bidel LPR, Cayzac C, Poss C, Ciordia E, Cozette A, Fontana A, Rolet F, Strub C. In Vitro Screening of the Antifungal and Antimycotoxin Effects of a Stilbenoids-Riche Grapevine Cane Extract on Fusarium graminearum, Aspergillus flavus and Penicillium expansum. Toxins. 2025; 17(9):454. https://doi.org/10.3390/toxins17090454
Chicago/Turabian StyleAznar, Dorian, Alexandre Colas de la Noue, Luc P. R. Bidel, Caroline Cayzac, Charlie Poss, Eloïse Ciordia, Andréa Cozette, Angélique Fontana, Fanny Rolet, and Caroline Strub. 2025. "In Vitro Screening of the Antifungal and Antimycotoxin Effects of a Stilbenoids-Riche Grapevine Cane Extract on Fusarium graminearum, Aspergillus flavus and Penicillium expansum" Toxins 17, no. 9: 454. https://doi.org/10.3390/toxins17090454
APA StyleAznar, D., Colas de la Noue, A., Bidel, L. P. R., Cayzac, C., Poss, C., Ciordia, E., Cozette, A., Fontana, A., Rolet, F., & Strub, C. (2025). In Vitro Screening of the Antifungal and Antimycotoxin Effects of a Stilbenoids-Riche Grapevine Cane Extract on Fusarium graminearum, Aspergillus flavus and Penicillium expansum. Toxins, 17(9), 454. https://doi.org/10.3390/toxins17090454





