Modification of Closed-State Inactivation in Voltage-Gated Sodium Channel Nav1.7 by Two Novel Arachnid Toxins
Abstract
1. Introduction
2. Results
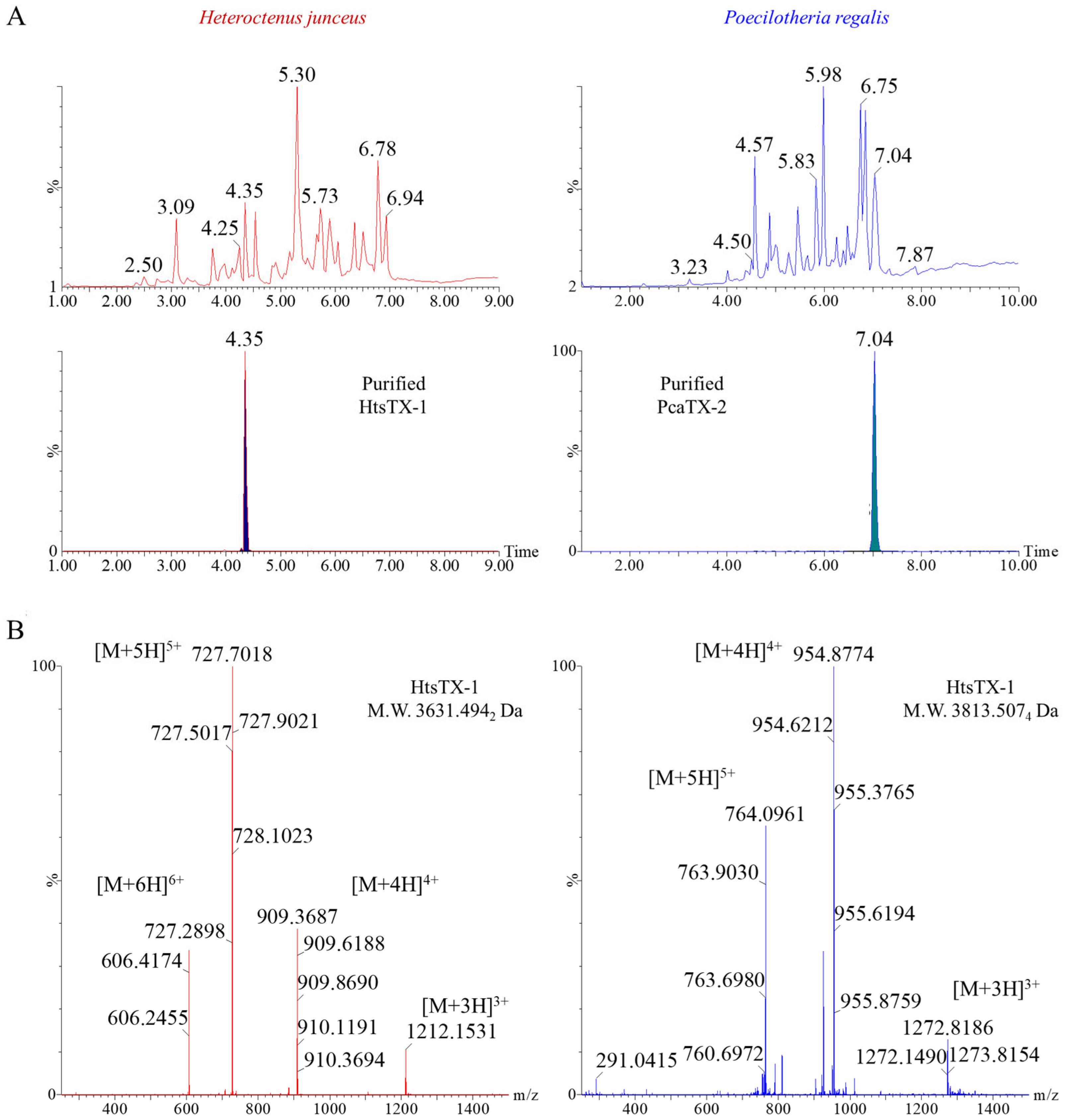
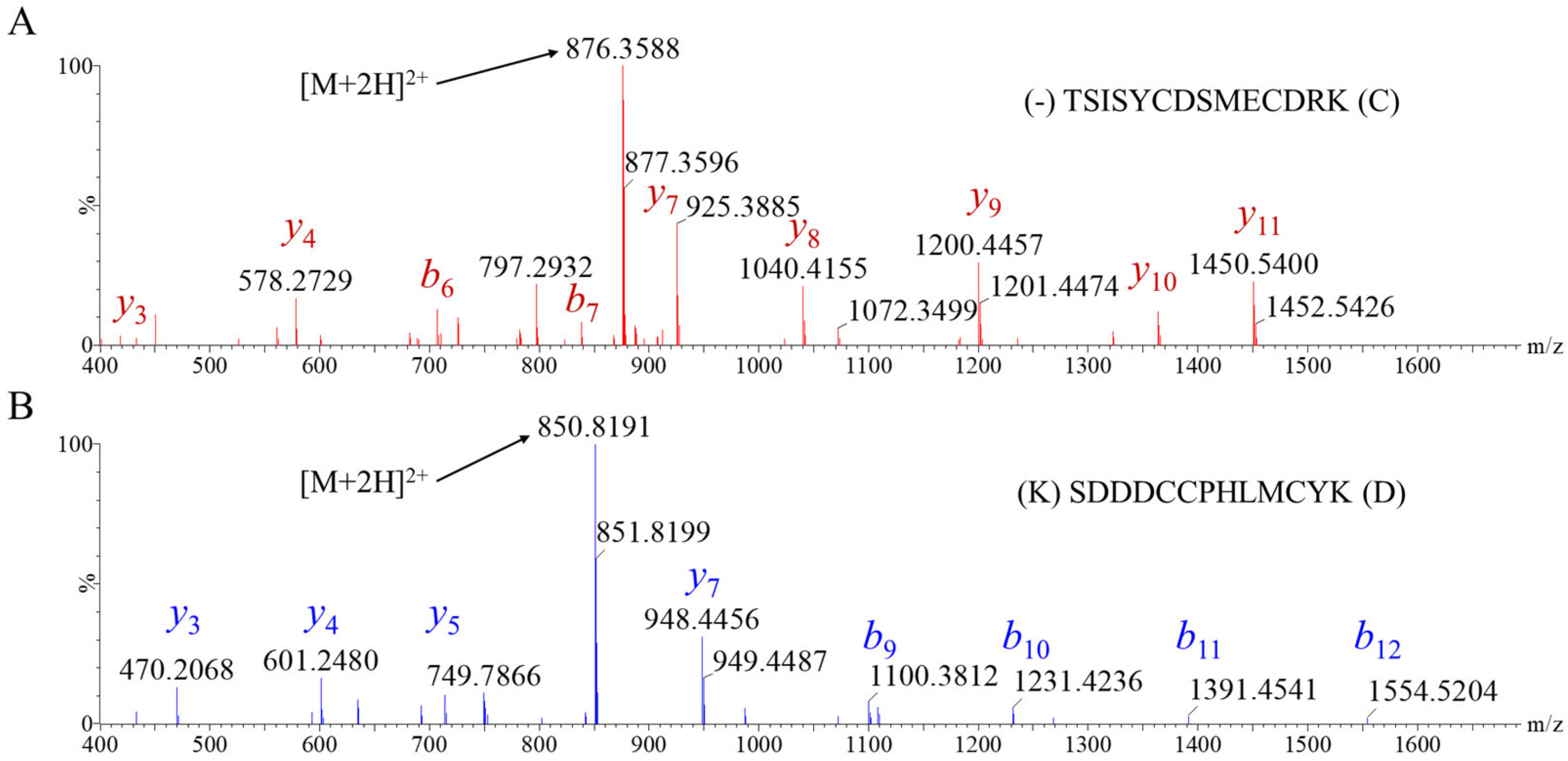
2.1. Toxin Selection and Primary Structure Determination


2.2. Effect of Toxins on Nav1.7 Currents
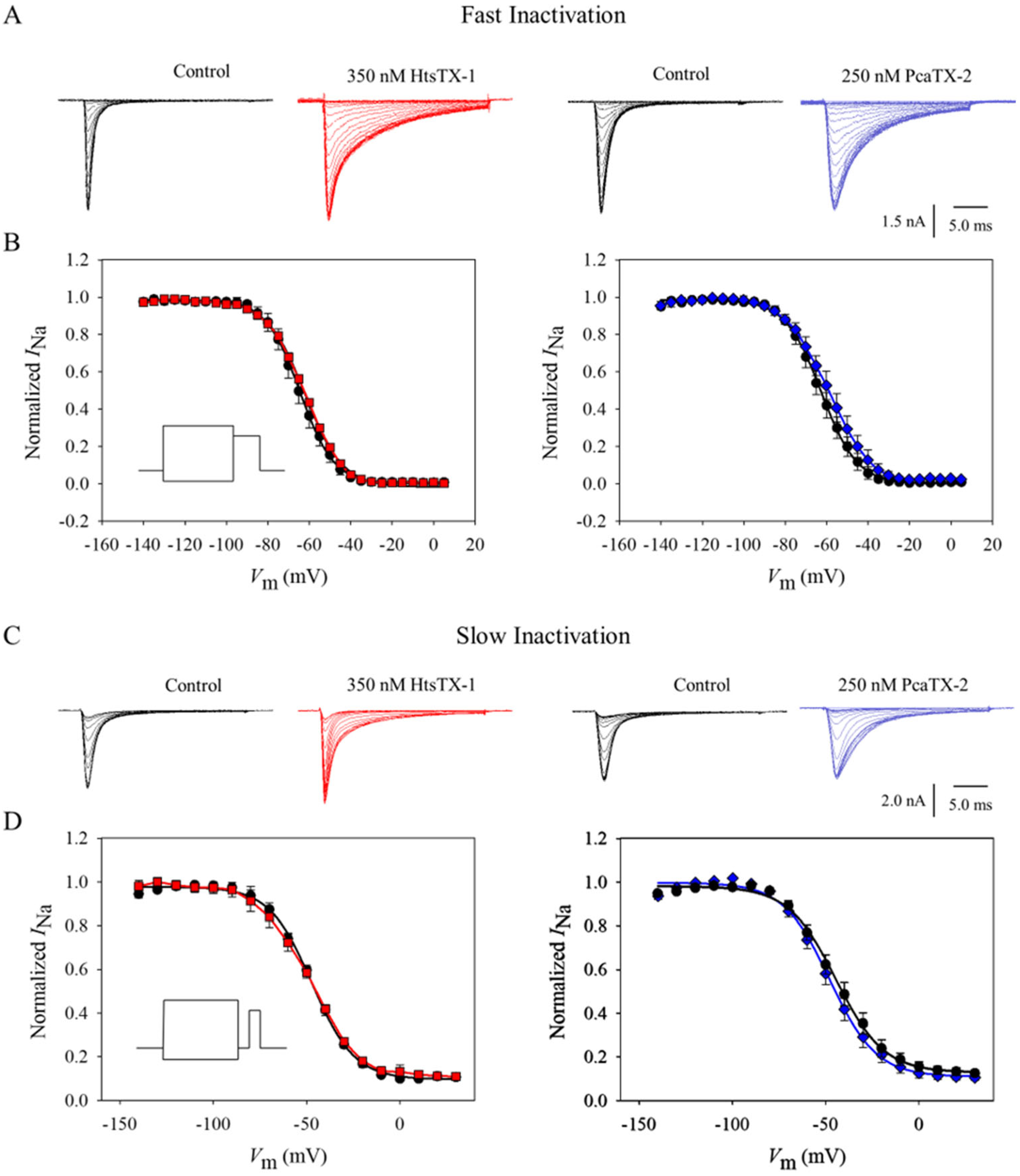
2.3. Effect of Toxins on Nav1.7 Fast and Slow Inactivation
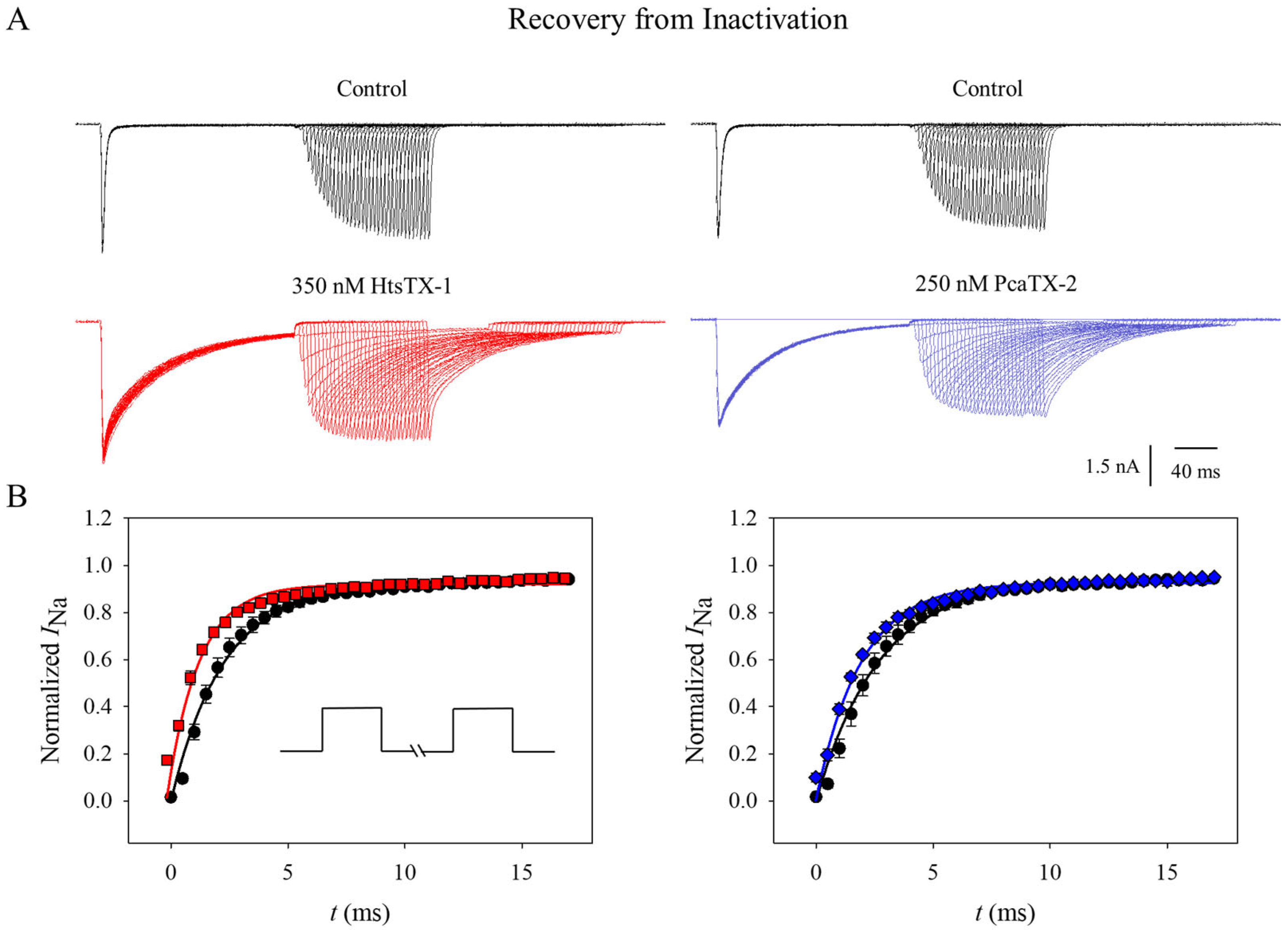
2.4. Effect of Toxins on Nav1.7 Recovery from Inactivation
2.5. Effect of Toxins on Nav1.7 Closed-State Inactivation
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Reagents and Chemicals
5.2. Venom Collection and Preparation
5.3. Reversed-Phase Chromatography (RPC) Purifications
5.4. Reversed-Phase Chromatography (RPC) Determinations
5.5. Toxin Digestions and Modifications
5.6. Accurate Mass Determinations
5.7. Cell Culture
5.8. Whole Cell Recording
5.9. Data Analysis and Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Da | Dalton |
| OSI | Open-state Inactivation |
| CSI | Closed-state Inactivation |
| HRMS | High-resolution Mass Spectrometry |
| m/z | mass to charge ratio |
| tR | Retention Time |
| ToF | Time of Flight |
| TIC | Total Ion Chromatogram |
| UPLC | Ultra-performance Liquid Chromatography |
| VSD | Voltage-gated Domain |
| VGSC | Voltage-gated Sodium Channel |
| PPI | Protein–Protein Interaction |
| HtsTX-1 | Heteroctenustoxin-1 |
| PcaTX-2 | Poecilotheriatoxin-2 |
References
- Catterall, W.A.; Raman, I.M.; Robinson, H.P.; Sejnowski, T.J.; Paulsen, O. The Hodgkin-Huxley heritage: From channels to circuits. J. Neurosci. 2012, 32, 14064–14073. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A.; Goldin, A.L.; Waxman, S.G. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol. Rev. 2005, 57, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.L.; Clark, A.J.; Huang, J.; Waxman, S.G.; Dib-Hajj, S.D. The Role of Voltage-Gated Sodium Channels in Pain Signaling. Physiol. Rev. 2019, 99, 1079–1151. [Google Scholar] [CrossRef]
- Faber, C.G.; Hoeijmakers, J.G.; Ahn, H.S.; Cheng, X.; Han, C.; Choi, J.S.; Estacion, M.; Lauria, G.; Vanhoutte, E.K.; Gerrits, M.M.; et al. Gain of function Nanu1.7 mutations in idiopathic small fiber neuropathy. Ann. Neurol. 2012, 71, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Y.; Li, S.; Xu, Z.; Li, H.; Ma, L.; Fan, J.; Bu, D.; Liu, B.; Fan, Z.; et al. Mutations in SCN9A, encoding a sodium channel alpha subunit, in patients with primary erythermalgia. J. Med. Genet. 2004, 41, 171–174. [Google Scholar] [CrossRef]
- Dib-Hajj, S.D.; Estacion, M.; Jarecki, B.W.; Tyrrell, L.; Fischer, T.Z.; Lawden, M.; Cummins, T.R.; Waxman, S.G. Paroxysmal extreme pain disorder M1627K mutation in human Nav1.7 renders DRG neurons hyperexcitable. Mol. Pain 2008, 4, 37. [Google Scholar] [CrossRef]
- Cox, J.J.; Reimann, F.; Nicholas, A.K.; Thornton, G.; Roberts, E.; Springell, K.; Karbani, G.; Jafri, H.; Mannan, J.; Raashid, Y.; et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature 2006, 444, 894–898. [Google Scholar] [CrossRef]
- Catterall, W.A. From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron 2000, 26, 13–25. [Google Scholar] [CrossRef]
- Goldin, A.L.; Snutch, T.; Lubbert, H.; Dowsett, A.; Marshall, J.; Auld, V.; Downey, W.; Fritz, L.C.; Lester, H.A.; Dunn, R.; et al. Messenger RNA coding for only the alpha subunit of the rat brain Na channel is sufficient for expression of functional channels in Xenopus oocytes. Proc. Natl. Acad. Sci. USA 1986, 83, 7503–7507. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.H.; Catterall, W.A. The VGL-chanome: A protein superfamily specialized for electrical signaling and ionic homeostasis. Sci. STKE 2004, 2004, re15. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Molecular properties of voltage-sensitive sodium channels. Annu. Rev. Biochem. 1986, 55, 953–985. [Google Scholar] [CrossRef] [PubMed]
- Yarov-Yarovoy, V.; DeCaen, P.G.; Westenbroek, R.E.; Pan, C.Y.; Scheuer, T.; Baker, D.; Catterall, W.A. Structural basis for gating charge movement in the voltage sensor of a sodium channel. Proc. Natl. Acad. Sci. USA 2012, 109, E93–E102. [Google Scholar] [CrossRef] [PubMed]
- Angsutararux, P.; Kang, P.W.; Zhu, W.; Silva, J.R. Conformations of voltage-sensing domain III differentially define NaV channel closed- and open-state inactivation. J. Gen. Physiol. 2021, 153, e202112891. [Google Scholar] [CrossRef]
- Armstrong, C.M. Na channel inactivation from open and closed states. Proc. Natl. Acad. Sci. USA 2006, 103, 17991–17996. [Google Scholar] [CrossRef]
- Vassilev, P.M.; Scheuer, T.; Catterall, W.A. Identification of an intracellular peptide segment involved in sodium channel inactivation. Science 1988, 241, 1658–1661. [Google Scholar] [CrossRef] [PubMed]
- Eaholtz, G.; Scheuer, T.; Catterall, W.A. Restoration of inactivation and block of open sodium channels by an inactivation gate peptide. Neuron 1994, 12, 1041–1048. [Google Scholar] [CrossRef]
- Kellenberger, S.; West, J.W.; Scheuer, T.; Catterall, W.A. Molecular analysis of the putative inactivation particle in the inactivation gate of brain type IIA Na+ channels. J. Gen. Physiol. 1997, 109, 589–605. [Google Scholar] [CrossRef]
- Rohl, C.A.; Boeckman, F.A.; Baker, C.; Scheuer, T.; Catterall, W.A.; Klevit, R.E. Solution structure of the sodium channel inactivation gate. Biochemistry 1999, 38, 855–861. [Google Scholar] [CrossRef]
- West, J.W.; Patton, D.E.; Scheuer, T.; Wang, Y.; Goldin, A.L.; Catterall, W.A. A cluster of hydrophobic amino acid residues required for fast Na(+)-channel inactivation. Proc. Natl. Acad. Sci. USA 1992, 89, 10910–10914. [Google Scholar] [CrossRef]
- Ulbricht, W. Sodium channel inactivation: Molecular determinants and modulation. Physiol. Rev. 2005, 85, 1271–1301. [Google Scholar] [CrossRef]
- Featherstone, D.E.; Richmond, J.E.; Ruben, P.C. Interaction between fast and slow inactivation in Skm1 sodium channels. Biophys. J. 1996, 71, 3098–3109. [Google Scholar] [CrossRef]
- Chen, H.; Xia, Z.; Dong, J.; Huang, B.; Zhang, J.; Zhou, F.; Yan, R.; Shi, Y.; Gong, J.; Jiang, J.; et al. Structural mechanism of voltage-gated sodium channel slow inactivation. Nat. Commun. 2024, 15, 3691. [Google Scholar] [CrossRef]
- Bean, B.P. Sodium channel inactivation in the crayfish giant axon. Must channels open before inactivating? Biophys. J. 1981, 35, 595–614. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, R.W.; Stevens, C.F. Inactivation of open and closed sodium channels determined separately. Cold Spring Harb. Symp. Quant. Biol. 1983, 48, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, R.W.; Stevens, C.F. Voltage-dependent gating of single sodium channels from mammalian neuroblastoma cells. J. Neurosci. 1987, 7, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Goldman, L. Sodium channel inactivation from closed states: Evidence for an intrinsic voltage dependency. Biophys. J. 1995, 69, 2369–2377. [Google Scholar] [CrossRef]
- Herzog, R.I.; Cummins, T.R.; Ghassemi, F.; Dib-Hajj, S.D.; Waxman, S.G. Distinct repriming and closed-state inactivation kinetics of Nav1.6 and Nav1.7 sodium channels in mouse spinal sensory neurons. J. Physiol. 2003, 551, 741–750. [Google Scholar] [CrossRef]
- Jarecki, B.W.; Sheets, P.L.; Jackson, J.O., 2nd; Cummins, T.R. Paroxysmal extreme pain disorder mutations within the D3/S4-S5 linker of Nav1.7 cause moderate destabilization of fast inactivation. J. Physiol. 2008, 586, 4137–4153. [Google Scholar] [CrossRef]
- Luo, S.; Sampedro Castaneda, M.; Matthews, E.; Sud, R.; Hanna, M.G.; Sun, J.; Song, J.; Lu, J.; Qiao, K.; Zhao, C.; et al. Hypokalaemic periodic paralysis and myotonia in a patient with homozygous mutation p.R1451L in Na(V)1.4. Sci. Rep. 2018, 8, 9714. [Google Scholar] [CrossRef]
- Dharmawan, T.; Nakajima, T.; Iizuka, T.; Tamura, S.; Matsui, H.; Kaneko, Y.; Kurabayashi, M. Enhanced closed-state inactivation of mutant cardiac sodium channels (SCN5A N1541D and R1632C) through different mechanisms. J. Mol. Cell Cardiol. 2019, 130, 88–95. [Google Scholar] [CrossRef]
- Kalia, J.; Milescu, M.; Salvatierra, J.; Wagner, J.; Klint, J.K.; King, G.F.; Olivera, B.M.; Bosmans, F. From foe to friend: Using animal toxins to investigate ion channel function. J. Mol. Biol. 2015, 427, 158–175. [Google Scholar] [CrossRef]
- Israel, M.R.; Tay, B.; Deuis, J.R.; Vetter, I. Sodium Channels and Venom Peptide Pharmacology. Adv. Pharmacol. 2017, 79, 67–116. [Google Scholar]
- Stevens, M.; Peigneur, S.; Tytgat, J. Neurotoxins and their binding areas on voltage-gated sodium channels. Front. Pharmacol. 2011, 2, 71. [Google Scholar] [CrossRef]
- Campos, F.V.; Chanda, B.; Beirao, P.S.; Bezanilla, F. Alpha-scorpion toxin impairs a conformational change that leads to fast inactivation of muscle sodium channels. J. Gen. Physiol. 2008, 132, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Bosmans, F.; Tytgat, J. Voltage-gated sodium channel modulation by scorpion alpha-toxins. Toxicon 2007, 49, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Maertens, C.; Cuypers, E.; Amininasab, M.; Jalali, A.; Vatanpour, H.; Tytgat, J. Potent modulation of the voltage-gated sodium channel Nav1.7 by OD1, a toxin from the scorpion Odonthobuthus doriae. Mol. Pharmacol. 2006, 70, 405–414. [Google Scholar] [CrossRef]
- Campos, F.V.; Coronas, F.I.; Beirao, P.S. Voltage-dependent displacement of the scorpion toxin Ts3 from sodium channels and its implication on the control of inactivation. Br. J. Pharmacol. 2004, 142, 1115–1122. [Google Scholar] [CrossRef]
- Rogers, J.C.; Qu, Y.; Tanada, T.N.; Scheuer, T.; Catterall, W.A. Molecular determinants of high affinity binding of alpha-scorpion toxin and sea anemone toxin in the S3-S4 extracellular loop in domain IV of the Na+ channel alpha subunit. J. Biol. Chem. 1996, 271, 15950–15962. [Google Scholar] [CrossRef]
- Thomsen, W.J.; Catterall, W.A. Localization of the receptor site for alpha-scorpion toxins by antibody mapping: Implications for sodium channel topology. Proc. Natl. Acad. Sci. USA 1989, 86, 10161–10165. [Google Scholar] [CrossRef] [PubMed]
- Cestele, S.; Qu, Y.; Rogers, J.C.; Rochat, H.; Scheuer, T.; Catterall, W.A. Voltage sensor-trapping: Enhanced activation of sodium channels by beta-scorpion toxin bound to the S3-S4 loop in domain II. Neuron 1998, 21, 919–931. [Google Scholar]
- Durek, T.; Vetter, I.; Wang, C.I.; Motin, L.; Knapp, O.; Adams, D.J.; Lewis, R.J.; Alewood, P.F. Chemical engineering and structural and pharmacological characterization of the alpha-scorpion toxin OD1. ACS Chem. Biol. 2013, 8, 1215–1222. [Google Scholar] [CrossRef]
- Corzo, G.; Escoubas, P.; Villegas, E.; Karbat, I.; Gordon, D.; Gurevitz, M.; Nakajima, T.; Gilles, N. A spider toxin that induces a typical effect of scorpion alpha-toxins but competes with beta-toxins on binding to insect sodium channels. Biochemistry 2005, 44, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Ferrat, G.; Bosmans, F.; Tytgat, J.; Pimentel, C.; Chagot, B.; Gilles, N.; Nakajima, T.; Darbon, H.; Corzo, G. Solution structure of two insect-specific spider toxins and their pharmacological interaction with the insect voltage-gated Na+ channel. Proteins 2005, 59, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Billen, B.; Vassilevski, A.; Nikolsky, A.; Debaveye, S.; Tytgat, J.; Grishin, E. Unique bell-shaped voltage-dependent modulation of Na+ channel gating by novel insect-selective toxins from the spider Agelena orientalis. J. Biol. Chem. 2010, 285, 18545–18554. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.R.; Rikli, H.G. Aspartic Acid Isomerization Characterized by High Definition Mass Spectrometry Significantly Alters the Bioactivity of a Novel Toxin from Poecilotheria. Toxins 2020, 12, 207. [Google Scholar] [CrossRef]
- Hanck, D.A.; Sheets, M.F. Site-3 toxins and cardiac sodium channels. Toxicon 2007, 49, 181–193. [Google Scholar] [CrossRef]
- Groome, J.; Lehmann-Horn, F.; Holzherr, B. Open- and closed-state fast inactivation in sodium channels: Differential effects of a site-3 anemone toxin. Channels 2011, 5, 65–78. [Google Scholar] [CrossRef]
- Tang, C.; Zhou, X.; Huang, Y.; Zhang, Y.; Hu, Z.; Wang, M.; Chen, P.; Liu, Z.; Liang, S. The tarantula toxin jingzhaotoxin-XI (kappa-theraphotoxin-Cj1a) regulates the activation and inactivation of the voltage-gated sodium channel Nav1.5. Toxicon 2014, 92, 6–13. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, X.; Tang, C.; Zhang, Y.; Tao, H.; Chen, P.; Liu, Z. Molecular basis of the inhibition of the fast inactivation of voltage-gated sodium channel Nav1.5 by tarantula toxin Jingzhaotoxin-II. Peptides 2015, 68, 175–182. [Google Scholar] [CrossRef]
- Wang, Y.; Park, K.D.; Salome, C.; Wilson, S.M.; Stables, J.P.; Liu, R.; Khanna, R.; Kohn, H. Development and characterization of novel derivatives of the antiepileptic drug lacosamide that exhibit far greater enhancement in slow inactivation of voltage-gated sodium channels. ACS Chem. Neurosci. 2011, 2, 90–106. [Google Scholar] [CrossRef]
- Bosmans, F.; Rash, L.; Zhu, S.; Diochot, S.; Lazdunski, M.; Escoubas, P.; Tytgat, J. Four novel tarantula toxins as selective modulators of voltage-gated sodium channel subtypes. Mol. Pharmacol. 2006, 69, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Karoly, R.; Lenkey, N.; Juhasz, A.O.; Vizi, E.S.; Mike, A. Fast- or slow-inactivated state preference of Na+ channel inhibitors: A simulation and experimental study. PLoS Comput. Biol. 2010, 6, e1000818. [Google Scholar] [CrossRef]
- Uchimura, N.; Higashi, H.; Nishi, S. Membrane properties and synaptic responses of the guinea pig nucleus accumbens neurons in vitro. J. Neurophysiol. 1989, 61, 769–779. [Google Scholar] [CrossRef]
- Ishii, H.; Kohno, T.; Yamakura, T.; Ikoma, M.; Baba, H. Action of dexmedetomidine on the substantia gelatinosa neurons of the rat spinal cord. Eur. J. Neurosci. 2008, 27, 3182–3190. [Google Scholar] [CrossRef] [PubMed]
- Mattia, D.; Hwa, G.G.; Avoli, M. Membrane properties of rat subicular neurons in vitro. J. Neurophysiol. 1993, 70, 1244–1248. [Google Scholar] [CrossRef] [PubMed]
- Wladyka, C.L.; Kunze, D.L. KCNQ/M-currents contribute to the resting membrane potential in rat visceral sensory neurons. J. Physiol. 2006, 575, 175–189. [Google Scholar] [CrossRef]
- Hay, M.; Lindsley, K.A. Membrane properties of area postrema neurons. Brain Res. 1995, 705, 199–208. [Google Scholar] [CrossRef]
- Wang, Z.; Van den Berg, R.J.; Ypey, D.L. Resting membrane potentials and excitability at different regions of rat dorsal root ganglion neurons in culture. Neuroscience 1994, 60, 245–254. [Google Scholar] [CrossRef]
- Amir, R.; Michaelis, M.; Devor, M. Membrane potential oscillations in dorsal root ganglion neurons: Role in normal electrogenesis and neuropathic pain. J. Neurosci. 1999, 19, 8589–8596. [Google Scholar] [CrossRef]
- Bordon, K.C.F.; Cologna, C.T.; Fornari-Baldo, E.C.; Pinheiro-Junior, E.L.; Cerni, F.A.; Amorim, F.G.; Anjolette, F.A.P.; Cordeiro, F.A.; Wiezel, G.A.; Cardoso, I.A.; et al. From Animal Poisons and Venoms to Medicines: Achievements, Challenges and Perspectives in Drug Discovery. Front. Pharmacol. 2020, 11, 1132. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.; Day, M.; Heavner, J.E. Ziconotide: An update and review. Expert. Opin. Pharmacother. 2008, 9, 1575–1583. [Google Scholar] [CrossRef]
- McGivern, J.G. Ziconotide: A review of its pharmacology and use in the treatment of pain. Neuropsychiatr. Dis. Treat. 2007, 3, 69–85. [Google Scholar] [CrossRef]
- Benzinger, G.R.; Drum, C.L.; Chen, L.Q.; Kallen, R.G.; Hanck, D.A.; Hanck, D. Differences in the binding sites of two site-3 sodium channel toxins. Pflug. Arch. 1997, 434, 742–749. [Google Scholar] [CrossRef]
- Zlotkin, E.; Kadouri, D.; Gordon, D.; Pelhate, M.; Martin, M.F.; Rochat, H. An excitatory and a depressant insect toxin from scorpion venom both affect sodium conductance and possess a common binding site. Arch. Biochem. Biophys. 1985, 240, 877–887. [Google Scholar] [CrossRef]
- Cestele, S.; Scheuer, T.; Mantegazza, M.; Rochat, H.; Catterall, W.A. Neutralization of gating charges in domain II of the sodium channel alpha subunit enhances voltage-sensor trapping by a beta-scorpion toxin. J. Gen. Physiol. 2001, 118, 291–302. [Google Scholar] [CrossRef]
- Selden, P.A.; Shear, W.A.; Sutton, M.D. Fossil evidence for the origin of spider spinnerets, and a proposed arachnid order. Proc. Natl. Acad. Sci. USA 2008, 105, 20781–20785. [Google Scholar] [CrossRef]
- Olamendi-Portugal, T.; Bartok, A.; Zamudio-Zuniga, F.; Balajthy, A.; Becerril, B.; Panyi, G.; Possani, L.D. Isolation, chemical and functional characterization of several new K(+)-channel blocking peptides from the venom of the scorpion Centruroides tecomanus. Toxicon 2016, 115, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Incorporated, Thermo-Fisher Scientific. Mass Spectrometry-Grade Endoproteinases. 2013. Available online: https://assets.fishersci.com/TFS-Assets/LSG/manuals/MAN0011638_Mass_SpectroGrade_Endoprotein_UG.pdf (accessed on 20 January 2024).
- Johnson, S.R.; Rikli, H.G.; Schmidt, J.O.; Evans, M.S. A reexamination of poneratoxin from the venom of the bullet ant Paraponera clavata. Peptides 2017, 98, 51–62. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Control | HtsTX-1 | Control | PcaTX-2 |
|---|---|---|---|---|
| I V½ (mV) | −32.42 ± 1.11 | −35.13 ± 1.21 | −33.04 ± 0.95 | −30.43 ± 0.86 |
| I Vrev (mV) | 61.1 ± 0.76 | 59.1 ± 0.62 | 57.6 ± 1.08 | 60.7 ± 1.19 |
| G V½ (mV) | −26.75 ± 0.29 | −29.35 ± 0.36 *** | −27.35 ± 0.29 | −21.62 ± 0.30 *** |
| G k | 3.41 ± 0.25 | 3.61 ± 0.31 | 3.45 ± 0.25 | 5.05 ± 0.25 ** |
| h∞ V½ (mV) | −64.49 ± 0.85 | −62.08 ± 0.67 | −62.65 ± 1.27 | −58.67 ± 1.32 |
| h∞ k | −8.19 ± 0.31 | −9.06 ± 0.26 | −8.61 ± 0.39 | −10.16 ± 0.57 |
| SI h∞ V½ (mV) | −47.12 ± 0.69 | −48.42 ± 0.42 | −44.43 ± 1.15 | −47.86 ± 1.11 |
| SI h∞ k | −11.38 ± 0.61 | −12.93 ± −0.94 | −12.81 ± 1.03 | −12.49 ± 1.00 |
| τR (ms) | 2.17 ± 0.07 | 1.69 ± 0.05 *** | 2.54 ± 0.05 | 2.11 ± 0.04 *** |
| τCSI (ms) | 57.14 ± 2.29 | 34.13 ± 3.30 *** | 58.48 ± 1.05 | 37.17 ± 2.78 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, J.W.; Rikli, H.G.; Johnson, S.R. Modification of Closed-State Inactivation in Voltage-Gated Sodium Channel Nav1.7 by Two Novel Arachnid Toxins. Toxins 2025, 17, 432. https://doi.org/10.3390/toxins17090432
Johnson JW, Rikli HG, Johnson SR. Modification of Closed-State Inactivation in Voltage-Gated Sodium Channel Nav1.7 by Two Novel Arachnid Toxins. Toxins. 2025; 17(9):432. https://doi.org/10.3390/toxins17090432
Chicago/Turabian StyleJohnson, John W., Hillary G. Rikli, and Stephen R. Johnson. 2025. "Modification of Closed-State Inactivation in Voltage-Gated Sodium Channel Nav1.7 by Two Novel Arachnid Toxins" Toxins 17, no. 9: 432. https://doi.org/10.3390/toxins17090432
APA StyleJohnson, J. W., Rikli, H. G., & Johnson, S. R. (2025). Modification of Closed-State Inactivation in Voltage-Gated Sodium Channel Nav1.7 by Two Novel Arachnid Toxins. Toxins, 17(9), 432. https://doi.org/10.3390/toxins17090432





