Effect of Dietary Difructose Anhydride III Supplementation on the Metabolic Profile of Japanese Black Breeding Herds with Low-Level Chronic Exposure to Zearalenone in the Dietary Feed
Abstract
1. Introduction
2. Results
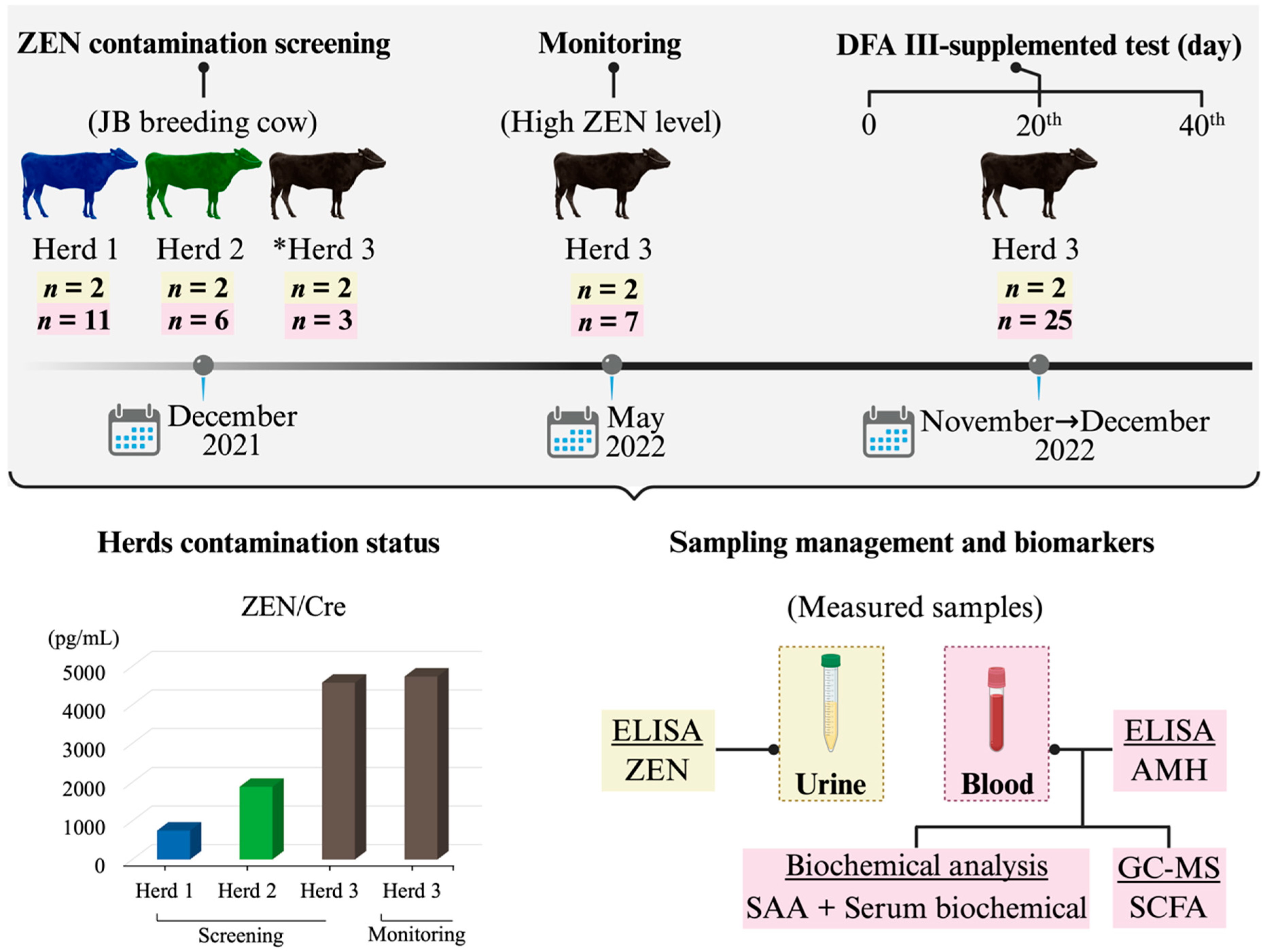
2.1. Study of Metabolic Profiles in Preliminary ZEN Contamination Screening
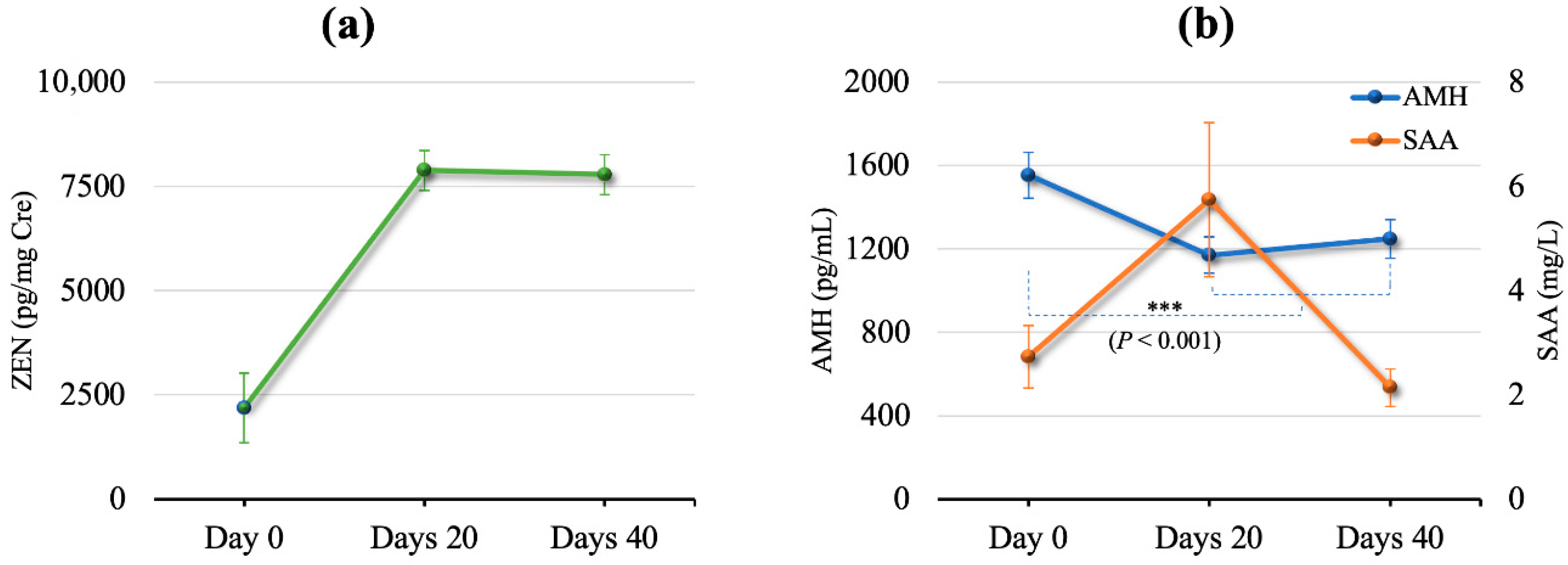
2.2. Effects of DFA III Supplementation
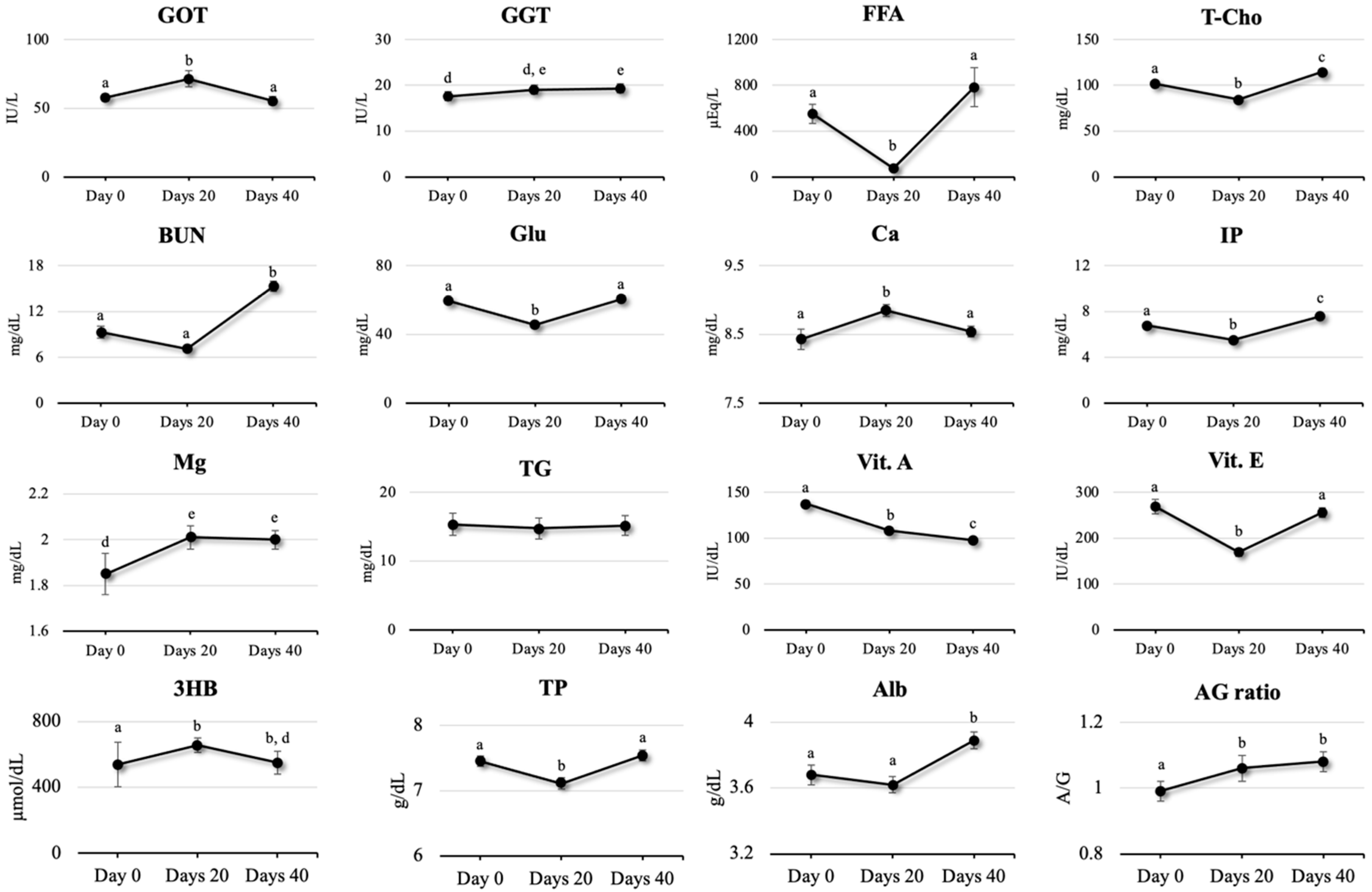
2.3. Parameters for Liver Function
2.4. Parameters for Nutritional Status
2.5. Parameters for Mineral Status
2.6. Parameters for Immune and Inflammation Status
2.7. Parameters for Vitamin Status
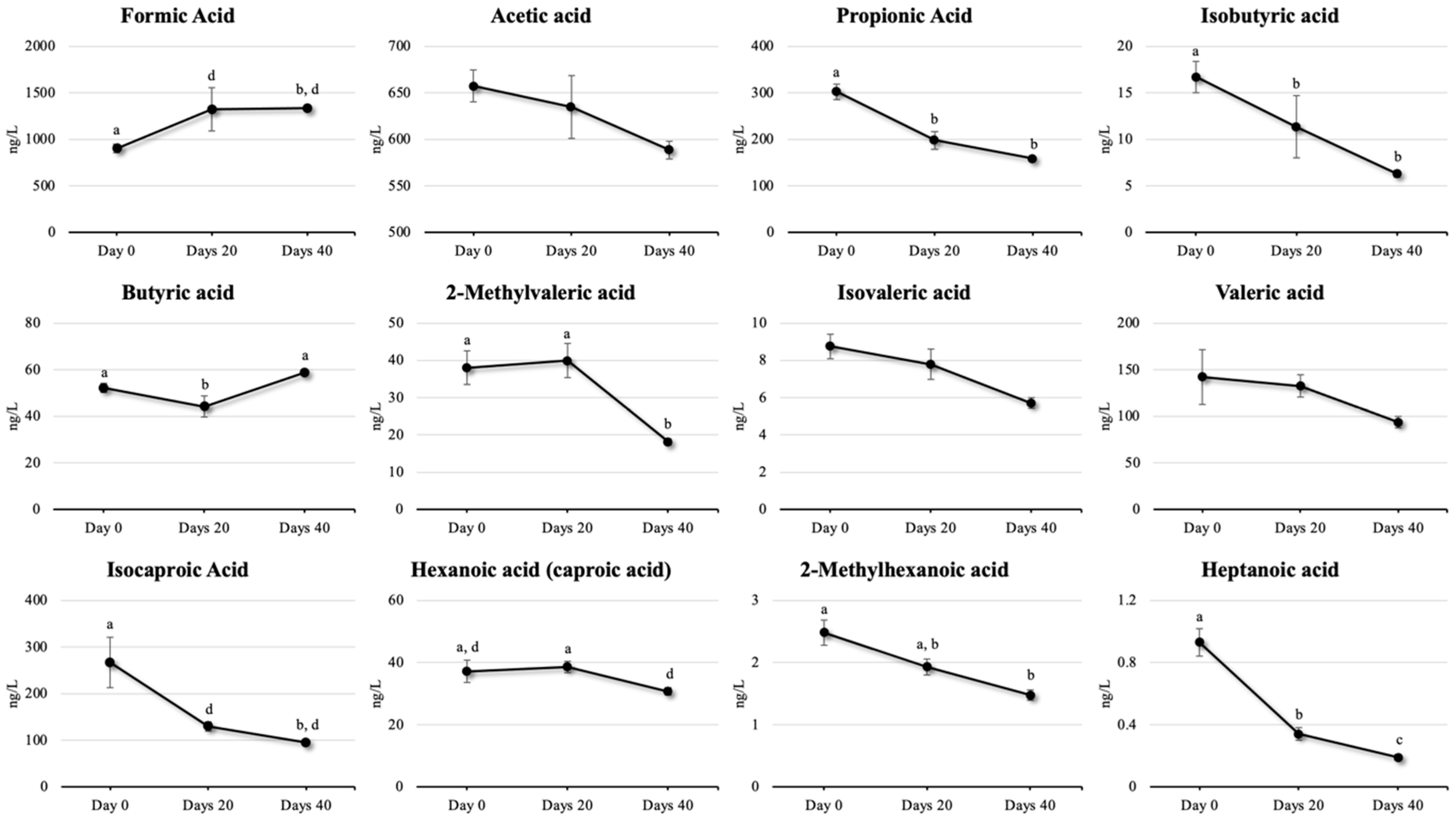
2.8. Parameters for SCFA Status
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Ethical Approval
5.2. Chemicals and Solvents
5.3. Preliminary ZEN Contamination Screening
5.4. Animals and Management
5.5. Urine and Blood Sample Collection
5.6. Measurement of ZEN, AMH, and SAA
5.7. Biochemical Analysis for Metabolic Profile Evaluation
5.8. Measurement of SCFA with GC/MS
5.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fink-Gremmels, J. The role of mycotoxins in the health and performance of dairy cows. Vet. J. 2008, 176, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Kiarie, E.G.; Yiannikouris, A.; Sun, L.; Karrow, N.A. Nutritional impact of mycotoxins in food animal production and strategies for mitigation. J. Anim. Sci. Biotechnol. 2022, 13, 69. [Google Scholar] [CrossRef] [PubMed]
- Widodo, O.S.; Pambudi, D.; Etoh, M.; Kokushi, E.; Uno, S.; Yamato, O.; Taniguchi, M.; Lamid, M.; Takagi, M. Practical application of a urinary zearalenone monitoring system for feed hygiene management of a Japanese black cattle breeding herd-relevance to anti-Müllerian hormone and serum amyloid A clarified from a two-year survey. Toxins 2023, 15, 317. [Google Scholar] [CrossRef]
- Widodo, O.S.; Uno, S.; Kokushi, E.; Yamato, O.; Mardianto, M.F.F.; Shinya, U.; Kano, Y.; Kawashima, C.; Fushimi, Y.; Ono, T.; et al. Exposure of cattle breeding herds to naturally co-contaminated zearalenone and deoxynivalenol: The relevance of a urinary mycotoxin monitoring system for herd health and food safety. Toxins 2024, 16, 402. [Google Scholar] [CrossRef]
- Gallo, A.; Minuti, A.; Bani, P.; Bertuzzi, T.; Cappelli, F.P.; Doupovec, B.; Faas, J.; Schatzmayr, D.; Trevisi, E. A mycotoxin-deactivating feed additive counteracts the adverse effects of regular levels of Fusarium mycotoxins in dairy cows. J. Dairy Sci. 2020, 103, 11314–11331. [Google Scholar] [CrossRef]
- Nadziakiewicza, M.; Kehoe, S.; Micek, P. Physico-chemical properties of clay minerals and their use as a health promoting feed additive. Animals 2019, 9, 714. [Google Scholar] [CrossRef]
- Xu, R.; Yiannikouris, A.; Shandilya, U.K.; Karrow, N.A. Comparative assessment of different yeast cell wall-based mycotoxin adsorbents using a model- and bioassay-based in vitro approach. Toxins 2023, 15, 104. [Google Scholar] [CrossRef]
- Baralić, K.; Živančević, K.; Bozic, D.; Đukić-Ćosić, D. Probiotic cultures as a potential protective strategy against the toxicity of environmentally relevant chemicals: State-of-the-art knowledge. Food Chem. Toxicol. 2023, 172, 113582. [Google Scholar] [CrossRef]
- Adegbeye, M.J.; Reddy, P.R.K.; Chilaka, C.A.; Balogun, O.B.; Elghandour, M.M.M.Y.; Rivas-Caceres, R.R.; Salem, A.Z.M. Mycotoxin toxicity and residue in animal products: Prevalence, consumer exposure and reduction strategies—A review. Toxicon 2020, 177, 96–108. [Google Scholar] [CrossRef]
- Zaura, E.; Twetman, S. Critical appraisal of Oral Pre- and probiotics for caries prevention and care. Caries Res. 2019, 53, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Akbari, P.; Fink-Gremmels, J.; Willems, R.H.A.M.; Difilippo, E.; Schols, H.A.; Schoterman, M.H.C.; Garssen, J.; Braber, S. Characterizing microbiota-independent effects of oligosaccharides on intestinal epithelial cells: Insight into the role of structure and size: Structure-activity relationships of non-digestible oligosaccharides. Eur. J. Nutr. 2017, 56, 1919–1930. [Google Scholar] [CrossRef]
- Franklin, S.T.; Newman, M.C.; Newman, K.E.; Meek, K.I. Immune parameters of dry cows fed mannan oligosaccharide and subsequent transfer of immunity to calves. J. Dairy Sci. 2005, 88, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Donovan, D.C.; Franklin, S.T.; Chase, C.C.; Hippen, A.R. Growth and health of Holstein calves fed milk replacers supplemented with antibiotics or Enteroguard. J. Dairy Sci. 2002, 85, 947–950. [Google Scholar] [CrossRef] [PubMed]
- Fleige, S.; Preißinger, W.; Meyer, H.H.; Pfaffl, M.W. Effect of lactulose on growth performance and intestinal morphology of pre-ruminant calves using a milk replacer containing Enterococcus faecium. Animal 2007, 1, 367–373. [Google Scholar] [CrossRef]
- Lee, S.I.; Kim, I.H. Difructose dianhydride improves intestinal calcium absorption, wound healing, and barrier function. Sci. Rep. 2018, 8, 7813. [Google Scholar] [CrossRef]
- Zhang, D.; Jian, Y.P.; Zhang, Y.N.; Li, Y.; Gu, L.T.; Sun, H.H.; Liu, M.D.; Zhou, H.L.; Wang, Y.S.; Xu, Z.X. Short-chain fatty acids in diseases. Cell Commun. Signal. 2023, 21, 212. [Google Scholar] [CrossRef] [PubMed]
- Crudo, F.; Aichinger, G.; Mihajlovic, J.; Varga, E.; Dellafiora, L.; Warth, B.; Dall’Asta, C.; Berry, D.; Marko, D. In vitro interactions of Alternaria mycotoxins, an emerging class of food contaminants, with the gut microbiota: A bidirectional relationship. Arch. Toxicol. 2021, 95, 2533–2549. [Google Scholar] [CrossRef]
- He, Z.; Dong, H. The roles of short-chain fatty acids derived from colonic bacteria fermentation of non-digestible carbohydrates and exogenous forms in ameliorating intestinal mucosal immunity of young ruminants. Front. Immunol. 2023, 14, 1291846. [Google Scholar] [CrossRef]
- Akhtar, M.; Naqvi, S.U.A.S.; Liu, Q.; Pan, H.; Ma, Z.; Kong, N.; Chen, Y.; Shi, D.; Kulyar, M.F.E.A.; Khan, J.A.; et al. Short chain fatty acids (SCFAs) are the potential immunomodulatory metabolites in controlling Staphylococcus aureus-mediated mastitis. Nutrients 2022, 14, 3687. [Google Scholar] [CrossRef]
- Laviano, H.D.; Gómez, G.; Escudero, R.; Nuñez, Y.; García-Casco, J.M.; Muñoz, M.; Heras-Molina, A.; López-Bote, C.; González-Bulnes, A.; Óvilo, C.; et al. Maternal supplementation of vitamin E or its combination with hydroxytyrosol increases the gut health and short chain fatty acids of piglets at weaning. Antioxidants 2023, 12, 1761. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, Z.; Bath, C.; Marett, L.; Pryce, J.; Rochfort, S. Optimised method for short-chain fatty acid profiling of bovine milk and serum. Molecules 2022, 27, 436. [Google Scholar] [CrossRef]
- Cheng, M.; Wu, H.; Zhang, W.; Mu, W. Difructose anhydride III: A 50-year perspective on its production and physiological functions. Crit. Rev. Food Sci. Nutr. 2022, 62, 6714–6725. [Google Scholar] [CrossRef]
- Teramura, M.; Nakai, T.; Itoh, M.; Sato, T.; Ohtani, M.; Kawashima, C.; Hanada, M. Short communication: Difructose anhydride III promotes calcium absorption from the duodenum in cattle. J. Dairy Sci. 2015, 98, 2533–2538. [Google Scholar] [CrossRef]
- Matsumoto, D.; Takagi, M.; Hasunuma, H.; Fushimi, Y.; Ohtani, M.; Sato, T.; Okamoto, K.; Shahada, F.; Tanaka, T.; Deguchi, E. Effects of oral administration of difructose anhydride III on selected health and blood parameters of group-housed Japanese black calves during the preweaning period. Asian Australas. J. Anim. Sci. 2009, 22, 1640–1647. [Google Scholar] [CrossRef]
- Takagi, M.; Hasunuma, H.; Matsumoto, D.; Obi, T.; Takase, K.; Ohtani, M.; Sato, T.; Watanabe, U.; Okamoto, K.; Tanaka, T.; et al. Effects of daily oral administration of difructose anhydride III on health status, blood parameters and faecal shedding of coliform bacteria of Japanese black calves during the pre-weaning period. Anim. Nutr. Feed. Technol. 2011, 11, 147–148. [Google Scholar]
- Toda, K.; Uno, S.; Kokushi, E.; Shiiba, A.; Hasunuma, H.; Matsumoto, D.; Ohtani, M.; Yamato, O.; Shinya, U.; Wijayagunawardane, M.; et al. Fructo-oligosaccharide (DFA III) feed supplementation for mitigation of mycotoxin exposure in cattle-clinical evaluation by a urinary zearalenone monitoring system. Toxins 2018, 10, 223. [Google Scholar] [CrossRef]
- Priyo, T.W., Jr.; Uno, S.; Kokushi, E.; Toda, K.; Hasunuma, H.; Matsumoto, D.; Yamato, O.; Ohtani, M.; Widodo, O.S.; Pambudi, D.; et al. Measurement of serum short-chain fatty acid concentrations in cattle after oral administration of difructose anhydride III. Vet. World 2023, 16, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Sasazaki, N.; Uno, S.; Kokushi, E.; Toda, K.; Hasunuma, H.; Matsumoto, D.; Miyashita, A.; Yamato, O.; Okawa, H.; Ohtani, M.; et al. Mitigation of sterigmatocystin exposure in cattle by difructose anhydride III feed supplementation and detection of urinary sterigmatocystin and serum amyloid A concentrations. Arch. Anim. Breed. 2021, 64, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Mineo, H.; Amano, M.; Minaminida, K.; Chiji, H.; Shigematsu, N.; Tomita, F.; Hara, H. Two-week feeding of difructose anhydride III enhances calcium absorptive activity with epithelial cell proliferation in isolated rat cecal mucosa. Nutrition 2006, 22, 312–320. [Google Scholar] [CrossRef]
- Escartín, M.; Rialp, N.; Bach, A. Effects of feeding difructose anhydride on the mineral status and milking performance of transition cows. J. Dairy Sci. 2024, 107, 4578–4586. [Google Scholar] [CrossRef]
- Nakamori, M.; Hien, V.T.T.; Khan, N.C.; Lam, N.T.; Dung, N.T.; Uotsu, N.; Shiomi, T.; Okuhara, Y.; Kise, M.; Shigematsu, N.; et al. Difructose anhydride III enhances bioavailability of water-insoluble iron in anemic Vietnamese women. J. Nutr. Sci. Vitaminol. 2010, 56, 191–197. [Google Scholar] [CrossRef]
- Teramura, M.; Wynn, S.; Reshalaitihan, M.; Kyuno, W.; Sato, T.; Ohtani, M.; Kawashima, C.; Hanada, M. Supplementation with difructose anhydride III promotes passive calcium absorption in the small intestine immediately after calving in dairy cows. J. Dairy Sci. 2015, 98, 8688–8697. [Google Scholar] [CrossRef]
- Suzuki, T.; Hara, H. Difructose anhydride III and sodium caprate activate paracellular transport via different intracellular events in Caco-2 cells. Life Sci. 2006, 79, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Inokuchi, H.; Takei, T.; Aikawa, K.; Shimizu, M. The effect of hyperosmosis on paracellular permeability in Caco-2 cell monolayers. Biosci. Biotechnol. Biochem. 2009, 73, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Htun, A.; Sato, T.; Fukuma, N.; Hanada, M. Effects of difructose anhydride III on serum immunoglobulin G concentration and health status of newborn Holstein calves during the preweaning period. J. Dairy Sci. 2018, 101, 3226–3232. [Google Scholar] [CrossRef]
- Silva, L.A.; de Mello, M.R.B.; Oliveira Pião, D.; Silenciato, L.N.; de Quadros, T.C.O.; de Souza, A.H.; Barbero, R.P. Effects of experimental exposure to zearalenone on reproductive system morphometry, plasma oestrogen levels, and oocyte quality of beef heifer. Reprod. Domest. Anim. 2021, 56, 775–782. [Google Scholar] [CrossRef]
- Hughes, R.L.; Alvarado, D.A.; Swanson, K.S.; Holscher, H.D. The prebiotic potential of inulin-type fructans: A systematic review. Adv. Nutr. 2022, 13, 492–529. [Google Scholar] [CrossRef]
- Jia, R.; Liu, W.; Zhao, L.; Cao, L.; Shen, Z. Low doses of individual and combined deoxynivalenol and zearalenone in naturally moldy diets impair intestinal functions via inducing inflammation and disrupting epithelial barrier in the intestine of piglets. Toxicol. Lett. 2020, 333, 159–169. [Google Scholar] [CrossRef]
- Ma, L.; Jiang, Y.; Lu, F.; Wang, S.; Liu, M.; Liu, F.; Huang, L.; Li, Y.; Jiao, N.; Jiang, S.; et al. Quantitative proteomic analysis of zearalenone-induced intestinal damage in weaned piglets. Toxins 2022, 14, 702. [Google Scholar] [CrossRef]
- Dolenšek, T.; Švara, T.; Knific, T.; Gombač, M.; Luzar, B.; Jakovac-Strajn, B. The influence of Fusarium mycotoxins on the liver of gilts and their suckling piglets. Animals 2021, 11, 2534. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.A.A.; Soliman, S.S.; Soliman, A.S.H.; Hanafy, A.; Jin, Y. Endoplasmic reticulum stress is involved in mycotoxin zearalenone induced inflammatory response, proliferation, and apoptosis in goat endometrial stromal cells. Reprod. Biol. 2024, 24, 100948. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.; Liu, Z.; Qian, W.; Chen, Y.; Qi, Y.; Wang, A. Induction of anti-zearalenone immune response with Mimotopes identified from a phage display peptide library. Toxicon 2021, 199, 1–6. [Google Scholar] [CrossRef]
- Nakai, T.; Murata, S.; Kikuchi, H.; Sato, T.; Sadoya, H.; Ohtani, M.; Hanada, M.; Okamoto, M. Detection of dietary difructose anhydride III in the duodenal digesta of steers. Anim. Sci. J. 2007, 78, 57–61. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-chain fatty acids: Linking diet, the microbiome and immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef]
- Garcia Diaz, T.; Ferriani Branco, A.; Jacovaci, F.A.; Cabreira Jobim, C.; Pratti Daniel, J.L.; Iank Bueno, A.V.; Gonçalves Ribeiro, M. Use of live yeast and mannan-oligosaccharides in grain-based diets for cattle: Ruminal parameters, nutrient digestibility, and inflammatory response. PLoS ONE 2018, 13, e0207127. [Google Scholar] [CrossRef] [PubMed]
- Mammi, L.M.E.; Guadagnini, M.; Mechor, G.; Cainzos, J.M.; Fusaro, I.; Palmonari, A.; Formigoni, A. The use of monensin for ketosis prevention in dairy cows during the transition period: A systematic review. Animals 2021, 11, 1988. [Google Scholar] [CrossRef]
- Tamura, A.; Nino, H.; Minobe, T.; Raneva, V.G.; Shigematsu, N.; Hara, H.; Kishida, T.; Ebihara, K. Difructose anhydride III does not contribute to body energy accumulation in rats. Biosci. Biotechnol. Biochem. 2006, 70, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, U.; Okamoto, K.; Miyamoto, A.; Otoi, T.; Yamato, O.; Tshering, C.; Takagi, M. Japanese Black breeding herd exhibiting low blood urea nitrogen: A metabolic profile study examining the effect on reproductive performance. Anim. Sci. J. 2013, 84, 389–394. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priyo, T.W., Jr.; Sasazaki, N.; Toda, K.; Hasunuma, H.; Matsumoto, D.; Kokushi, E.; Uno, S.; Yamato, O.; Obi, T.; Shinya, U.; et al. Effect of Dietary Difructose Anhydride III Supplementation on the Metabolic Profile of Japanese Black Breeding Herds with Low-Level Chronic Exposure to Zearalenone in the Dietary Feed. Toxins 2025, 17, 409. https://doi.org/10.3390/toxins17080409
Priyo TW Jr., Sasazaki N, Toda K, Hasunuma H, Matsumoto D, Kokushi E, Uno S, Yamato O, Obi T, Shinya U, et al. Effect of Dietary Difructose Anhydride III Supplementation on the Metabolic Profile of Japanese Black Breeding Herds with Low-Level Chronic Exposure to Zearalenone in the Dietary Feed. Toxins. 2025; 17(8):409. https://doi.org/10.3390/toxins17080409
Chicago/Turabian StylePriyo, Topas Wicaksono, Jr., Naoya Sasazaki, Katsuki Toda, Hiroshi Hasunuma, Daisaku Matsumoto, Emiko Kokushi, Seiichi Uno, Osamu Yamato, Takeshi Obi, Urara Shinya, and et al. 2025. "Effect of Dietary Difructose Anhydride III Supplementation on the Metabolic Profile of Japanese Black Breeding Herds with Low-Level Chronic Exposure to Zearalenone in the Dietary Feed" Toxins 17, no. 8: 409. https://doi.org/10.3390/toxins17080409
APA StylePriyo, T. W., Jr., Sasazaki, N., Toda, K., Hasunuma, H., Matsumoto, D., Kokushi, E., Uno, S., Yamato, O., Obi, T., Shinya, U., Widodo, O. S., Taura, Y., Ono, T., Taniguchi, M., & Takagi, M. (2025). Effect of Dietary Difructose Anhydride III Supplementation on the Metabolic Profile of Japanese Black Breeding Herds with Low-Level Chronic Exposure to Zearalenone in the Dietary Feed. Toxins, 17(8), 409. https://doi.org/10.3390/toxins17080409





