Effectiveness of Kaolinite with and Without Polyaluminum Chloride (PAC) in Removing Toxic Alexandrium minutum
Abstract
1. Introduction
2. Results
2.1. Removal Efficiencies
2.2. Surface Charge
3. Discussion
3.1. Removal Efficiency Mechanism
| Clay Type | Clay (gL−1) | Cell Density (Cells L−1) | Removal Efficiency | Tested Species | Ref. |
|---|---|---|---|---|---|
| Kaolinite clay (unmodified) | 1 | - | 80% | Noctiluca scintillans Prorocentrum minimum | [70] |
| Yellow loess modified with biosurfactant sophorolipid ratio (1:5) | 10 | 1.3 to 2.1 × 106 | 95%—after 30 min | Cochlodinium polykrikoides | [71] |
| Kaolin with PAC ratio (1:5) | 0.5 | 8 to 9 × 109 & 2.2 to 2.5 × 109 | 80–82% | Aureococcus anophagefferens Phaeocystis globosa | [48] |
| MC II (kaolin with PAC) ratio (5:1) | 0.2 | 1.0 × 106 | 57% after 8 h 95% after 48 h | Karenia brevis | [53] |
| MC II (kaolin with PAC) ratio (5:1) | 0.5 | 1.0 × 106 | 95% after 24 h | Karenia brevis | [55] |
| Kaolin with PAC ratio (5:1) | 0.3 | 3.0 × 106 | 83.67% | Karenia mikimotoi | [72] |
| Dry bentonite with PAC ratio (1:2) | 0.5 | 1.9 × 108 | 50% | Prymnesium parvum | [40] |
| Wet bentonite with PAC ratio (1:2) | 0.1 | 1.0 × 103 | 100% | Prymnesium parvum | [40] |
3.2. Ecological and Environmental Impact
3.3. Cost Estimate
4. Conclusions and Future Directions
5. Materials and Methods
5.1. Removal Efficiency
5.1.1. Clay Preparation
5.1.2. Alexandrium minutum Stock Culture Preparation and Maintenance
5.1.3. Experimental Design
5.1.4. Data Analysis
5.2. Surface Charge Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MC | Modified clay |
| KPAC | Kaolinite–polyaluminum chloride clay |
| KNAC | Kaolinite natural clay |
| RE | Removal efficiency |
| WA | Western Australia |
| PAC | Polyaluminum chloride |
| HAB | Harmful algal bloom |
| PST | Paralytic shellfish toxin |
| NaCl | Sodium chloride |
| NaOH | Sodium hydroxide |
| HCL | Hydrochloric acid |
References
- Lewis, A.M.; Coates, L.N.; Turner, A.D.; Percy, L.; Lewis, J.; Mock, T. A review of the global distribution of Alexandrium minutum (Dinophyceae) and comments on ecology and associated paralytic shellfish toxin profiles, with a focus on Northern Europe. J. Phycol. 2018, 54, 581–598. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Alpermann, T.J.; Cembella, A.D.; Collos, Y.; Masseret, E.; Montresor, M. The globally distributed genus Alexandrium: Multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae 2012, 14, 10–35. [Google Scholar] [CrossRef]
- Hallegraeff, G.M.; Schweibold, L.; Jaffrezic, E.; Rhodes, L.; MacKenzie, L.; Hay, B.; Farrell, H. Overview of Australian and New Zealand harmful algal species occurrences and their societal impacts in the period 1985 to 2018, including a compilation of historic records. Harmful Algae 2021, 102, 101848. [Google Scholar] [CrossRef]
- Anderson, D.M.; Fensin, E.; Gobler, C.J.; Hoeglund, A.E.; Hubbard, K.A.; Kulis, D.M.; Landsberg, J.H.; Lefebvre, K.A.; Provoost, P.; Richlen, M.L. Marine harmful algal blooms (HABs) in the United States: History, current status and future trends. Harmful Algae 2021, 102, 101975. [Google Scholar] [CrossRef]
- Manfrin, C.; De Moro, G.; Torboli, V.; Venier, P.; Pallavicini, A.; Gerdol, M. Physiological and molecular responses of bivalves to toxic dinoflagellates. Invertebr. Surviv. J. 2012, 9, 184–199. [Google Scholar]
- Anderson, D.M.; Kulis, D.M.; Qi, Y.-Z.; Zheng, L.; Lu, S.; Lin, Y.-T. Paralytic shellfish poisoning in Southern China. Toxicon 1996, 34, 579–590. [Google Scholar] [CrossRef]
- Garcı, C.; del Carmen Bravo, M.A.; Lagos, M.; Lagos, N. Paralytic shellfish poisoning: Post-mortem analysis of tissue and body fluid samples from human victims in the Patagonia fjords. Toxicon 2004, 43, 149–158. [Google Scholar] [CrossRef]
- Landsberg, J.H.; Hall, S.; Johannessen, J.N.; White, K.D.; Conrad, S.M.; Abbott, J.P.; Flewelling, L.J.; Richardson, R.W.; Dickey, R.W.; Jester, E.L.E.; et al. Saxitoxin puffer fish poisoning in the United States, with the first report of Pyrodinium bahamense as the putative toxin source. Environ. Health Perspect. 2006, 114, 1502–1507. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, L.E. Saxitoxin, a toxic marine natural product that targets a multitude of receptors. Nat. Prod. Rep. 2006, 23, 200–222. [Google Scholar] [CrossRef] [PubMed]
- Deeds, J.R.; Landsberg, J.H.; Etheridge, S.M.; Pitcher, G.C.; Longan, S.W. Non-traditional vectors for paralytic shellfish poisoning. Mar. Drugs 2008, 6, 308–348. [Google Scholar] [CrossRef]
- Gainey, L.F., Jr. A compendium of the responses of bivalve molluscs to toxic dinoflagellate. J. Shellfish Res. 1998, 7, 623–628. [Google Scholar]
- Berdalet, E.; Fleming, L.E.; Gowen, R.; Davidson, K.; Hess, P.; Backer, L.C.; Moore, S.K.; Hoagland, P.; Enevoldsen, H. Marine harmful algal blooms, human health and wellbeing: Challenges and opportunities in the 21st century. J. Mar. Biol. Assoc. United Kingd. 2016, 96, 61–91. [Google Scholar] [CrossRef]
- Gaillard, S.; Small, H.J.; Ayache, N.; Tanniou, S.; Hess, P.; Réveillon, D.; Harris, C.M.; Harris, T.M.; Scott, G.P.; MacIntyre, A. Investigating the role of allelochemicals in the interaction between Alexandrium monilatum and other phytoplankton species. Harmful Algae 2024, 139, 102706. [Google Scholar] [CrossRef]
- Long, M. Allelochemical Interactions Between the Dinoflagellate Alexandrium minutum and the Diatom Chaetoceros muelleri. Ph.D. Thesis, Université de Bretagne Occidentale, Brest, France, University of Wollongong, Wollongong, Australia, 2018. [Google Scholar]
- Long, M.; Peltekis, A.; González-Fernández, C.; Hegaret, H.; Bailleul, B. Allelochemicals of Alexandrium minutum: Kinetics of membrane disruption and photosynthesis inhibition in a co-occurring diatom. Harmful Algae 2021, 103, 101997. [Google Scholar] [CrossRef]
- Anderson, D.M. Physiology and bloom dynamics of toxic Alexandrium species, with emphasis on life cycle transitions. Nato Asi Ser. G Ecol. Sci. 1998, 41, 29–48. [Google Scholar]
- Bravo, I.; Garcés, E.; Diogène, J.; Fraga, S.; Sampedro, N.; Figueroa, R.I. Resting cysts of the toxigenic dinoflagellate genus Alexandrium in recent sediments from the Western Mediterranean coast, including the first description of cysts of A. kutnerae and A. peruvianum. Eur. J. Phycol. 2006, 41, 293–302. [Google Scholar] [CrossRef]
- John, U.; Litaker, R.W.; Montresor, M.; Murray, S.; Brosnahan, M.L.; Anderson, D.M. Formal Revision of the Alexandrium tamarense Species Complex (Dinophyceae) Taxonomy: The Introduction of Five Species with Emphasis on Molecular-based (rDNA) Classification. Protist 2014, 165, 779–804. [Google Scholar] [CrossRef] [PubMed]
- Bolch, C.; Blackburn, S.; Cannon, J.; Hallegraeff, G. The resting cyst of the red-tide dinoflagellate Alexandrium minutum (Dinophyceae). Phycologia 1991, 30, 215–219. [Google Scholar] [CrossRef]
- Park, T.G.; Lim, W.A.; Park, Y.T.; Lee, C.K.; Jeong, H.J. Economic impact, management and mitigation of red tides in Korea. Harmful Algae 2013, 30, S131–S143. [Google Scholar] [CrossRef]
- Sellner, K.G.; Rensel, J.E. Prevention, control, and mitigation of harmful algal bloom impacts on fish, shellfish, and human consumers. In Harmful Algal Blooms; John Wiley & Sons, Incorporated: Hoboken, NJ, USA, 2018; pp. 435–492. [Google Scholar] [CrossRef]
- Suddleson, M.; Hoagland, P. Proceedings of the Workshop on the Socio-Economic Effects of Harmful Algal Blooms in the United States. In Proceedings of the Workshop on the Socio-economic Effects of Harmful Algal Blooms in the United States, Woods Hole, MA, USA, 27 July–5 August 2020. [Google Scholar]
- Willis, C.; Papathanasopoulou, E.; Russel, D.; Artioli, Y. Harmful algal blooms: The impacts on cultural ecosystem services and human well-being in a case study setting, Cornwall, UK. Mar. Policy 2018, 97, 232–238. [Google Scholar] [CrossRef]
- Anderson, L.M.; Scrimshaw, S.C.; Fullilove, M.T.; Fielding, J.E. Task Force on Community Preventive Services The Community Guide’s model for linking the social environment to health. Am. J. Prev. Med. 2003, 24, 12–20. [Google Scholar] [CrossRef]
- Lee, H.-G.; Kim, H.M.; Min, J.; Park, C.; Jeong, H.J.; Lee, K.; Kim, K.Y. Quantification of the paralytic shellfish poisoning dinoflagellate Alexandrium species using a digital PCR. Harmful Algae 2020, 92, 101726. [Google Scholar] [CrossRef] [PubMed]
- Kim, H. Mitigation and controls of HABs. In Ecology of Harmful Algae; Springer: Berlin/Heidelberg, Germany, 2006; pp. 327–338. [Google Scholar]
- Condie, S.A.; Oliver, E.C.; Hallegraeff, G.M. Environmental drivers of unprecedented Alexandrium catenella dinoflagellate blooms off eastern Tasmania, 2012–2018. Harmful Algae 2019, 87, 101628. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO), World Health Organization (WHO). Technical Paper on Toxicity Equivalency Factors for Marine Biotoxins Associated with Bivalve Molluscs; FAO/WHO: Rome, Italy, 2016; p. 108. [Google Scholar]
- Trayler, K.; Cosgrove, J. Blooming surprise: Toxic algal blooms in Perth rivers. Landscope 2021, 36, 50–53. [Google Scholar]
- Atkins, R.; Rose, T.; Brown, R.; Robb, M. The microcystis cyanobacteria bloom in the Swan River-February 2000. Water Sci. Technol. 2001, 43, 107–114. [Google Scholar] [CrossRef]
- Shirota, A. Red tide problem and countermeasures. II. Int. J. Aqua. Fish. Technol. 1989, 1, 195–223. [Google Scholar]
- Yu, Z.; Song, X.; Cao, X.; Liu, Y. Mitigation of harmful algal blooms using modified clays: Theory, mechanisms, and applications. Harmful Algae 2017, 69, 48–64. [Google Scholar] [CrossRef]
- Balaji-Prasath, B.; Wang, Y.; Su, Y.P.; Hamilton, D.P.; Lin, H.; Zheng, L.; Zhang, Y. Methods to control harmful algal blooms: A review. Environ. Chem. Lett. 2022, 20, 3133–3152. [Google Scholar] [CrossRef]
- Lu, G.; Song, X.; Yu, Z.; Cao, X.; Yuan, Y. Environmental effects of modified clay flocculation on Alexandrium tamarense and paralytic shellfish poisoning toxins (PSTs). Chemosphere 2015, 127, 188–194. [Google Scholar] [CrossRef]
- Lu, G.; Song, X.; Yu, Z.; Cao, X.; Yuan, Y. Effects of modified clay flocculation on major nutrients and diatom aggregation during Skeletonema costatum blooms in the laboratory. Chin. J. Oceanol. Limnol. 2015, 33, 1007–1019. [Google Scholar] [CrossRef]
- Song, W.; Song, X.; Li, J.; Zhang, Y.; Shen, H.; Zhang, P.; Yu, Z. Toxin remained in residual Alexandrium pacificum after flocculation with modified clay. Oceanol. Limnol. Sin. 2021, 52, 917–924. [Google Scholar]
- Song, W.; Song, X.; Shen, H.; Ding, Y.; Cheng, R.; Yu, Z. Degradation of paralytic shellfish toxins during flocculation of Alexandrium pacificum by an oxidized modified clay: A laboratory experiment. Ecotoxicol. Environ. Saf. 2023, 253, 114667. [Google Scholar] [CrossRef]
- Burson, A.; Matthijs, H.C.P.; de Bruijne, W.; Talens, R.; Hoogenboom, R.; Gerssen, A.; Visser, P.M.; Stomp, M.; Steur, K.; van Scheppingen, Y.; et al. Termination of a toxic Alexandrium bloom with hydrogen peroxide. Harmful Algae 2014, 31, 125–135. [Google Scholar] [CrossRef]
- Chen, X.; Wang, D.; Wang, Y.; Sun, P.; Ma, S.; Chen, T. Algicidal effects of a high-efficiency algicidal bacterium shewanella Y1 on the toxic bloom-causing dinoflagellate Alexandrium pacificum. Mar. Drugs 2022, 20, 239. [Google Scholar] [CrossRef]
- Sengco, M.R.; Hagström, J.A.; Granéli, E.; Anderson, D.M. Removal of Prymnesium parvum (Haptophyceae) and its toxins using clay minerals. Harmful Algae 2005, 4, 261–274. [Google Scholar] [CrossRef]
- Seger, A.; Hallegraeff, G. Application of clay minerals to remove extracellular ichthyotoxins produced by the dinoflagellates Karlodinium veneficum and Karenia mikimotoi. Harmful Algae 2022, 111, 102151. [Google Scholar] [CrossRef]
- Seger, A.; Dorantes-Aranda, J.J.; Müller, M.N.; Body, A.; Peristyy, A.; Place, A.R.; Park, T.G.; Hallegraeff, G. Mitigating fish-killing Prymnesium parvum algal blooms in aquaculture ponds with clay: The importance of pH and clay type. J. Mar. Sci. Eng. 2015, 3, 154–174. [Google Scholar] [CrossRef]
- Han, M.Y.; Kim, W. A theoretical consideration of algae removal with clays. Microchem. J. 2001, 68, 157–161. [Google Scholar] [CrossRef]
- Sengco, M.R. The Aggregation of Clay Minerals and Marine Microalgal Cells: Physicochemical Theory and Implications for Controlling Harmful Algal Blooms. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, UK, 2001. [Google Scholar]
- Kim, S.-J. Removal of Red Tide Organisms-2. Flocculation of Red Tide Organisms by Using Loess. Korean J. Fish. Aquat. Sci. 2000, 33, 455–462. [Google Scholar]
- Mkpuma, V.O.; Moheimani, N.R.; Ennaceri, H. Commercial paper as a promising carrier for biofilm cultivation of Chlorella sp. for the treatment of anaerobic digestate food effluent (ADFE): Effect on the photosynthetic efficiency. Sci. Total Environ. 2023, 898, 165439. [Google Scholar] [CrossRef] [PubMed]
- Ennaceri, H.; Nwoba, E.G.; Ogbonna, C.N.; Bahri, P.A.; Moheimani, N.R. Progress of non-destructive hydrocarbon extraction technology of Botryococcus braunii. Algal Res. 2023, 73, 103156. [Google Scholar] [CrossRef]
- Mkpuma, V.O.; Ishika, T.; Moheimani, N.R.; Ennaceri, H. The potential of coupling wastewater treatment with hydrocarbon production using Botryococcus braunii. Algal Res. 2023, 74, 103214. [Google Scholar] [CrossRef]
- Nazloo, E.K.; Danesh, M.; Sarrafzadeh, M.-H.; Moheimani, N.R.; Ennaceri, H. Biomass and hydrocarbon production from Botryococcus braunii: A review focusing on cultivation methods. Sci. Total Env. 2024, 926, 171734. [Google Scholar] [CrossRef]
- Mkpuma, V.O.; Moheimani, N.R.; Ennaceri, H. Effect of light intensity on Chlorella sp. biofilm growth on anaerobically digested food effluents (ADFE). J. Environ. Manag. 2024, 371, 123015. [Google Scholar] [CrossRef]
- Ennaceri, H.; Mkpuma, V.O.; Moheimani, N.R. Nano-clay modified membranes: A promising green strategy for microalgal antifouling filtration. Sci. Total Env. 2023, 902, 166479. [Google Scholar] [CrossRef]
- Baraka, A.E.; Ennaceri, H.; Ennaoui, A.; Ghennioui, A.; Jorio, A.; Khaldoun, A. A novel approach to evaluate soiling adhesion on the surface of CSP reflectors via extended DLVO theory. Appl. physics. A Mater. Sci. Process. 2019, 125, 1–8. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X.; Yu, Z.; Song, X.; Qiu, L. Flocculation of harmful algal cells using modified clay: Effects of the properties of the clay suspension. J. Appl. Phycol. 2016, 28, 1623–1633. [Google Scholar] [CrossRef]
- Ennaceri, H.; Alami, H.E.; Brik, H.; Mokssit, O.; Khaldoun, A. Lotus effect and super-hydrophobic coatings for concentrated solar power systems (CSP). In Proceedings of the 2014 International Conference on Composite Materials & Renewable Energy Applications (ICCMREA), Sousse, Tunisia, 22–24 January 2014; pp. 1–4. [Google Scholar]
- Abdulwahid, K.D. Phytoremediation of cadmium pollutants in wastewater by using Ceratophyllum demersum L. as an aquatic macrophytes. Water Conserv. Manag. 2023, 7, 83–88. [Google Scholar] [CrossRef]
- Peng, C.; Fu, X.; Niu, Z.; Sun, W.; Yue, T. Protonation behavior study of the active sites on typical sulfide minerals surface using surface complexation model. Colloids Surf. A Physicochem. Eng. Asp. 2025, 710, 136307. [Google Scholar] [CrossRef]
- Wu, T.; Yan, X.; Cai, X.; Tan, S.; Li, H.; Liu, J.; Yang, W. Removal of Chattonella marina with clay minerals modified with a gemini surfactant. Appl. Clay Sci. 2010, 50, 604–607. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X.; Yu, Z.; Song, X.; Qiu, L. Controlling harmful algae blooms using aluminum-modified clay. Mar. Pollut. Bull. 2016, 103, 211–219. [Google Scholar] [CrossRef]
- Yu, Z.; Sengco, M.R.; Anderson, D.M. Flocculation and removal of the brown tide organism, Aureococcus anophagefferens (Chrysophyceae), using clays. J. Appl. Phycol. 2004, 16, 101–110. [Google Scholar] [CrossRef]
- Jiang, W.; Yu, Z.; Cao, X.; Jiang, K.; Yuan, Y.; Anderson, D.M.; Song, X. Effects of soluble organics on the settling rate of modified clay and development of improved clay formulations for harmful algal bloom control. Environ. Pollut. 2021, 289, 117964. [Google Scholar] [CrossRef]
- Devillier, V.M.; Hall, E.R.; Lovko, V.; Pierce, R.; Anderson, D.M.; Lewis, K.A. Mesocosm study of PAC-modified clay effects on Karenia brevis cells and toxins, chemical dynamics, and benthic invertebrate physiology. Harmful Algae 2024, 134, 102609. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Yu, Z.; Jiang, W.; Song, X.; Cao, X. Dosage-effectiveness of modified clay flocculating red tide organisms: Mechanical mechanism and mathematical model. Sep. Purif. Technol. 2023, 305, 122422. [Google Scholar] [CrossRef]
- Devillier, V.M.; Hall, E.R.; Anderson, D.M.; Lewis, K.A. Exposure of blue crab (Callinectes sapidus) to modified clay treatment of Karenia brevis as a bloom control strategy. Harmful Algae 2023, 128, 102492. [Google Scholar] [CrossRef]
- Beaulieu, S.E.; Sengco, M.R.; Anderson, D.M. Using clay to control harmful algal blooms: Deposition and resuspension of clay/algal flocs. Harmful Algae 2005, 4, 123–138. [Google Scholar] [CrossRef]
- Gallardo-Rodríguez, J.J.; Astuya-Villalón, A.; Llanos-Rivera, A.; Avello-Fontalba, V.; Ulloa-Jofré, V. A critical review on control methods for harmful algal blooms. Rev. Aquac. 2019, 11, 661–684. [Google Scholar] [CrossRef]
- Sengco, M.R.; Li, A.; Tugend, K.; Kulis, D.; Anderson, D.M. Removal of red-and brown-tide cells using clay flocculation. I. Laboratory culture experiments with Gymnodinium breve and Aureococcus anophagefferens. Mar. Ecol. Prog. Ser. 2001, 210, 41–53. [Google Scholar] [CrossRef]
- Pan, G.; Chen, J.; Anderson, D.M. Modified local sands for the mitigation of harmful algal blooms. Harmful Algae 2011, 10, 381–387. [Google Scholar] [CrossRef]
- Yu, Z.; Zou, J.; Ma, X. A more effective clay for removing red tide organisms. J. Nat. Disasters 1994, 3, 105–109. [Google Scholar]
- Padilla, L.V.; San Diego-McGlone, M.L.; Azanza, R.V. Exploring the potential of clay in mitigating Pyrodinium bahamense var. compressum and other harmful algal species in the Philippines. J. Appl. Phycol. 2010, 22, 761–768. [Google Scholar] [CrossRef]
- Seto, D.S.; Karp-Boss, L.; Wells, M.L. Effects of increasing temperature and acidification on the growth and competitive success of Alexandrium catenella from the Gulf of Maine. Harmful Algae 2019, 89, 101670. [Google Scholar] [CrossRef]
- Anderson, D.M.; Andersen, P.; Bricelj, V.M.; Cullen, J.J.; Rensel, J.J. Monitoring and Management Strategies for Harmful Algal Blooms in Coastal Waters; Unesco: Paris, France, 2001. [Google Scholar]
- Nabweteme, R.; Yoo, M.; Kwon, H.-S.; Kim, Y.J.; Hwang, G.; Lee, C.-H.; Ahn, I.-S. Application of the extended DLVO approach to mechanistically study the algal flocculation. J. Ind. Eng. Chem. 2015, 30, 289–294. [Google Scholar] [CrossRef]
- Lu, G.; Song, X.; Yu, Z.; Cao, X. Application of PAC-modified kaolin to mitigate Prorocentrum donghaiense: Effects on cell removal and phosphorus cycling in a laboratory setting. J. Appl. Phycol. 2017, 29, 917–928. [Google Scholar] [CrossRef]
- Genovesi, B.; Mouillot, D.; Laugier, T.; Fiandrino, A.; Laabir, M.; Vaquer, A.; Grzebyk, D. Influences of sedimentation and hydrodynamics on the spatial distribution of Alexandrium catenella/tamarense resting cysts in a shellfish farming lagoon impacted by toxic blooms. Harmful Algae 2013, 25, 15–25. [Google Scholar] [CrossRef]
- Genovesi, B.; Shin-Grzebyk, M.-S.; Grzebyk, D.; Laabir, M.; Gagnaire, P.-A.; Vaquer, A.; Pastoureaud, A.; Lasserre, B.; Collos, Y.; Berrebi, P. Assessment of cryptic species diversity within blooms and cyst bank of the Alexandrium tamarense complex (Dinophyceae) in a Mediterranean lagoon facilitated by semi-multiplex PCR. J. Plankton Res. 2011, 33, 405–414. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, Z.; Song, X.; Cao, X.; Han, X. Effects of modified clay on cysts of Scrippsiella trochoidea for harmful algal bloom control. Chin. J. Oceanol. Limnol. 2014, 32, 1373–1382. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Z.; Song, X.; Yuan, Y.; Cao, X. Effects of modified clay used for the control of harmful algal blooms on Alexandrium pacificum cysts. Harmful Algae 2018, 72, 36–45. [Google Scholar] [CrossRef]
- Lewis, M.A.; Dantin, D.D.; Walker, C.C.; Kurtz, J.C.; Greene, R.M. Toxicity of clay flocculation of the toxic dinoflagellate, Karenia brevis, to estuarine invertebrates and fish. Harmful Algae 2003, 2, 235–246. [Google Scholar] [CrossRef]
- Song, X.; Yu, Z.; Gao, Y. Removal of different species of red tide organisms with an effective clay-complex system. Ying Yong Sheng Tai Xue Bao = J. Appl. Ecol. 2003, 14, 1165–1168. [Google Scholar]
- Gao, Y.; Yu, Z.; Song, X. Impact of modified clays on the infant oyster (Crassostrea gigas). Mar. Sci. Bull.-Tianjin-Chin. Ed.-Bull. 2007, 26, 53. [Google Scholar]
- Anderson, D.M.; Greene, R.M.; Bricelj, V.M.; Lewis, M.; Pierce, R. ECOHAB: Control of Harmful Algal Blooms Using Clay; Woods Hole Oceanographic Institution: Woods Hole, MA, USA, 2022. [Google Scholar]
- Jin, D.; Thunberg, E.; Hoagland, P. Economic impact of the 2005 red tide event on commercial shellfish fisheries in New England. Ocean. Coast. Manag. 2008, 51, 420–429. [Google Scholar] [CrossRef]
- Vila, M.; Giacobbe, M.G.; Masó, M.; Gangemi, E.; Penna, A.; Sampedro, N.; Azzaro, F.; Camp, J.; Galluzzi, L. A comparative study on recurrent blooms of Alexandrium minutum in two Mediterranean coastal areas. Harmful Algae 2005, 4, 673–695. [Google Scholar] [CrossRef]
- Oh, C.-O.; Ditton, R.B. A time series approach to estimating the economic impacts of exogenous events on recreational fishing. Hum. Dimens. Wildl. 2008, 13, 348–360. [Google Scholar] [CrossRef]
- Ranston, E.R.; Webber, D.F. Phytoplankton distribution in a highly eutrophic estuarine bay, Hunts Bay, Kingston Harbour, Jamaica. Bull. Mar. Sci. 2003, 73, 307–324. [Google Scholar]
- Ranston, E.R.; Webber, D.F.; Larsen, J. The first description of the potentially toxic dinoflagellate, Alexandrium minutum in Hunts Bay, Kingston Harbour, Jamaica. Harmful Algae 2007, 6, 29–47. [Google Scholar] [CrossRef]
- Armi, Z.; Milandri, A.; Turki, S.; Hajjem, B. Alexandrium catenella and Alexandrium tamarense in the North Lake of Tunis: Bloom characteristics and the occurrence of paralytic shellfish toxin. Afr. J. Aquat. Sci. 2011, 36, 47–56. [Google Scholar] [CrossRef]
- Beaumais, O.; Appéré, G. Recreational shellfish harvesting and health risks: A pseudo-panel approach combining revealed and stated preference data with correction for on-site sampling. Ecol. Econ. 2010, 69, 2315–2322. [Google Scholar] [CrossRef]
- Larkin, S.L.; Adams, C.M. Harmful algal blooms and coastal business: Economic consequences in Florida. Soc. Nat. Resour. 2007, 20, 849–859. [Google Scholar] [CrossRef]
- Hoagland, P.; Scatasta, S. The Economic Effects of Harmful Algal Blooms, Ecology of Harmful Algae; Springer: Berlin/Heidelberg, Germany, 2006; pp. 391–402. [Google Scholar]
- Blackburn, S.I.; Bolch, C.J.S.; Haskard, K.A.; Hallegraeff, G.M. Reproductive compatibility among four global populations of the toxic dinoflagellate Gymnodinium catenatum (Dinophyceae). Phycologia 2001, 40, 78–87. [Google Scholar] [CrossRef]
- Moheimani, N.R.; Borowitzka, M.A.; Isdepsky, A.; Sing, S.F. Standard Methods for Measuring Growth of Algae and Their Composition; Springer: Dordrecht, The Netherlands, 2012; pp. 265–284. [Google Scholar]
- DBCA. Annual Swan Canning Estuarine Data Report 2020-21; Department of Biodiversity, Conservation and Attractions: Perth, Australia, 2024.
- Silburn, B.; Kröger, S.; Parker, E.; Sivyer, D.; Hicks, N.; Powell, C.; Johnson, M.; Greenwood, N. Benthic pH gradients across a range of shelf sea sediment types linked to sediment characteristics and seasonal variability. Biogeochemistry 2017, 135, 69–88. [Google Scholar] [CrossRef] [PubMed]

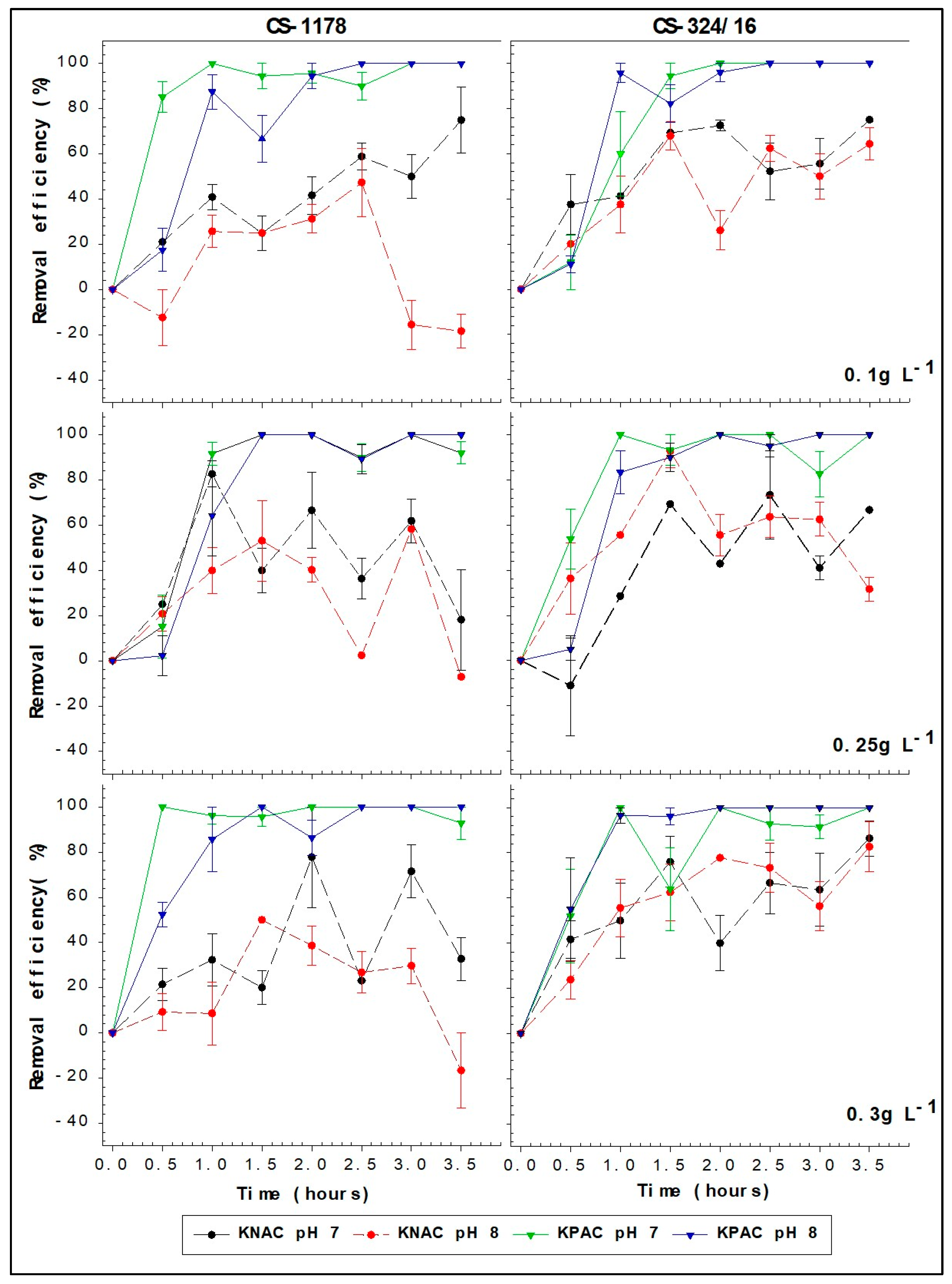
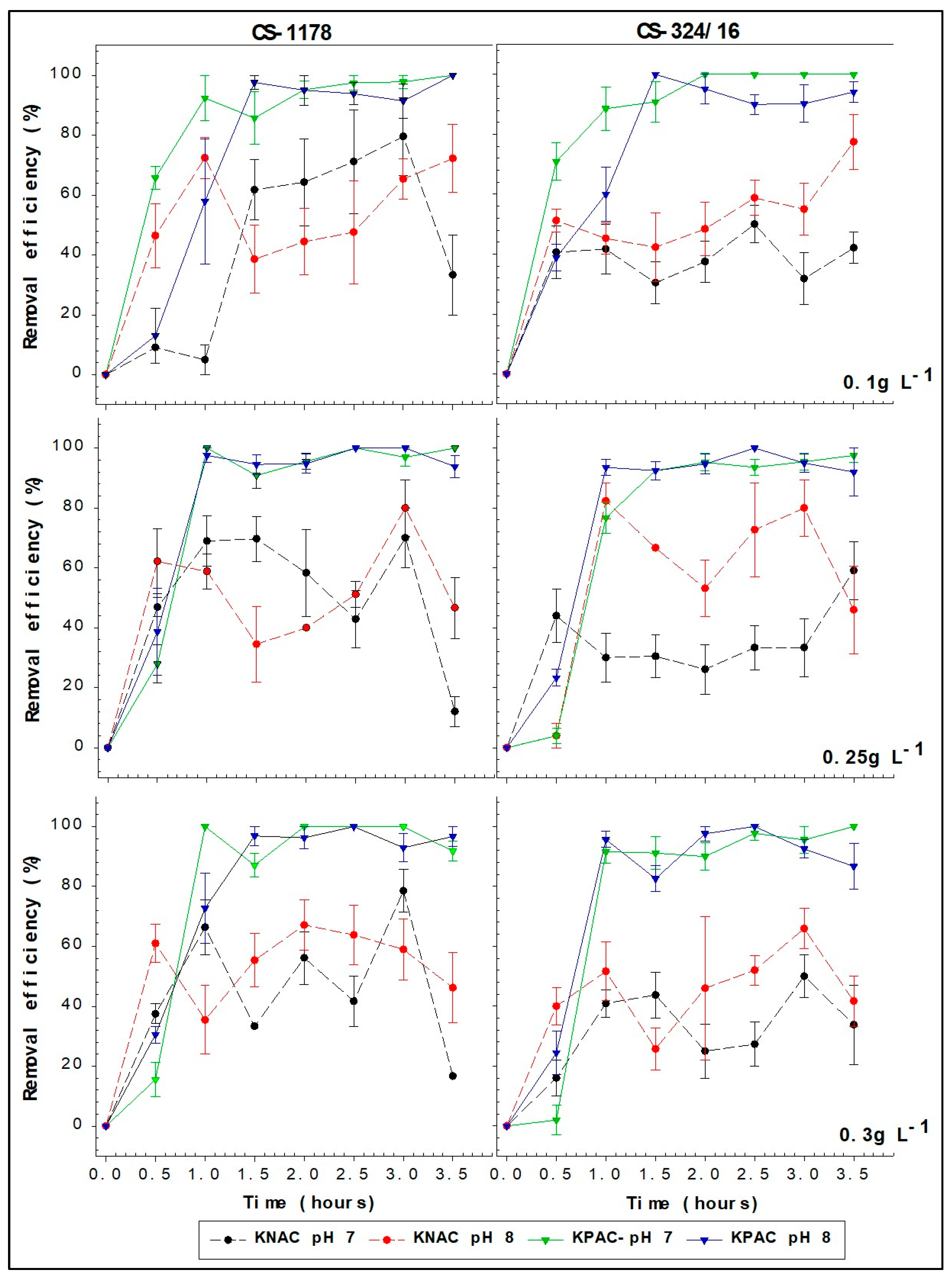

| A. minutum Species | Cell Density | pH | 0.1 g L−1 | 0.25 g L−1 | 0.3 g L−1 |
|---|---|---|---|---|---|
| Time (Hours) | |||||
| CS-1178 | 1.0 × 107 cells L−1 | 7 | 0.62 ± 0.04 b | 1.50 ± 0.22 | 0.05 ± 0.04 a |
| 8 | 2.58 ± 0.80 y | 2.42 ± 0.78 x | 1.53 ± 0.22 | ||
| 2.0 × 107 cells L−1 | 7 | 1.31 ± 0.16 | 2.04 ± 0.45 | 2.36 ± 0.55 | |
| 8 | 2.15 ± 0.64 | 2.10 ± 0.36 | 2.04 ± 0.37 | ||
| CS-324/16 | 1.0 × 107 cells L−1 | 7 | 2.03 ± 0.80 | 1.23 ± 0.17 | 0.88 ± 0.13 |
| 8 | 0.71 ± 0.06 c | 2.27 ± 0.60 | 0.71 ± 0.06 c | ||
| 2.0 × 107 cells L−1 | 7 | 1.65 ± 0.29 | 1.57 ± 0.24 | 2.10 ± 0.56 | |
| 8 | 1.37 ± 0.15 | 2.70 ± 2.89 z | 2.21 ± 0.15 | ||
| Kaolinite PAC Clay (KPAC—140.8 kg ha−1) | Kaolinite Natural Clay (Yellow Loess—3840 kg ha−1) | |
|---|---|---|
| USD Per ha 1 | ||
| Labor 2 | 46.20 | 211.23 |
| Clay 3 | 11.26 | 614.00 |
| PAC 4 | 20.49 | - |
| Storage 5 | 4.44 | 60.48 |
| Transport 5 | 48.29 | 1232.85 |
| Boat per day 6 | 185.85 | 311.85 |
| Operational costs | 316.53 | 2430.41 |
| Dispenser 7 | 4300 | 4300 |
| Operational + fixed costs | 4616.53 | 6730.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwambai, C.S.; Ennaceri, H.; Lymbery, A.J.; Laird, D.W.; Cosgrove, J.; Moheimani, N.R. Effectiveness of Kaolinite with and Without Polyaluminum Chloride (PAC) in Removing Toxic Alexandrium minutum. Toxins 2025, 17, 395. https://doi.org/10.3390/toxins17080395
Kwambai CS, Ennaceri H, Lymbery AJ, Laird DW, Cosgrove J, Moheimani NR. Effectiveness of Kaolinite with and Without Polyaluminum Chloride (PAC) in Removing Toxic Alexandrium minutum. Toxins. 2025; 17(8):395. https://doi.org/10.3390/toxins17080395
Chicago/Turabian StyleKwambai, Cherono Sheilah, Houda Ennaceri, Alan J. Lymbery, Damian W. Laird, Jeff Cosgrove, and Navid Reza Moheimani. 2025. "Effectiveness of Kaolinite with and Without Polyaluminum Chloride (PAC) in Removing Toxic Alexandrium minutum" Toxins 17, no. 8: 395. https://doi.org/10.3390/toxins17080395
APA StyleKwambai, C. S., Ennaceri, H., Lymbery, A. J., Laird, D. W., Cosgrove, J., & Moheimani, N. R. (2025). Effectiveness of Kaolinite with and Without Polyaluminum Chloride (PAC) in Removing Toxic Alexandrium minutum. Toxins, 17(8), 395. https://doi.org/10.3390/toxins17080395






