Abstract
Aflatoxin contamination, primarily caused by Aspergillus flavus, poses a significant threat to peanut (Arachis hypogaea L.) production, food safety, and global trade. Despite extensive efforts, breeding for durable resistance remains difficult due to the polygenic and environmentally sensitive nature of resistance. Although germplasm such as J11 have shown partial resistance, none of the identified lines demonstrated stable or comprehensive protection across diverse environments. Resistance involves physical barriers, biochemical defenses, and suppression of toxin biosynthesis. However, these traits typically exhibit modest effects and are strongly influenced by genotype–environment interactions. A paradigm shift is underway with increasing focus on host susceptibility (S) genes, native peanut genes exploited by A. flavus to facilitate colonization or toxin production. Recent studies have identified promising S gene candidates such as AhS5H1/2, which suppress salicylic acid-mediated defense, and ABR1, a negative regulator of ABA signaling. Disrupting such genes through gene editing holds potential for broad-spectrum resistance. To advance resistance breeding, an integrated pipeline is essential. This includes phenotyping diverse germplasm under stress conditions, mapping resistance loci using QTL and GWAS, and applying multi-omics platforms to identify candidate genes. Functional validation using CRISPR/Cas9, Cas12a, base editors, and prime editing allows precise gene targeting. Validated genes can be introgressed into elite lines through breeding by marker-assisted and genomic selection, accelerating the breeding of aflatoxin-resistant peanut varieties. This review highlights recent advances in peanut aflatoxin resistance research, emphasizing susceptibility gene targeting and genome editing. Integrating conventional breeding with multi-omics and precision biotechnology offers a promising path toward developing aflatoxin-free peanut cultivars.
Keywords:
aflatoxin; Aspergillus flavus; susceptibility genes; Arachis hypogaea; CRISPR/Cas9 genome editing; host-induced gene silencing; GWAS; QTL; breeding; MAS Key Contribution:
This review summarizes recent advances in aflatoxin resistance in peanuts; with a particular focus on the identification and targeting of host susceptibility genes. It proposes an integrated breeding pipeline that combines traditional and modern tools; including multi-omics approaches and genome editing to accelerate the development of aflatoxin-free peanut cultivars.
1. Introduction
Aspergillus flavus is a fungal pathogen and the primary source of aflatoxin contamination in a wide range of crops, posing a constant threat to food safety and crop productivity worldwide [1]. Due to its lack of host specificity, A. flavus infects numerous plant species, but its impact is particularly severe in peanuts [2,3,4,5,6]. The fungus thrives in hot climates and becomes especially problematic in regions where peanuts are exposed to high temperatures, drought, and insect damage. These stressors not only weaken plant defenses but also create physical entry points that facilitate fungal invasion and aflatoxin production [7,8,9,10]. Following entry through wounds, A. flavus can penetrate the shell and seed coat and establish within the cotyledon, often resulting in reduced germination and seedling vigor [11]. The pathogen persists in infected plant debris such as conidia, sclerotia, or mycelia, enabling survival between growing seasons [12]. Under favorable conditions, sclerotia start germinating and lead to the production of mycelia and conidiophores [13,14]. These structures generate conidia, which can spread via air currents, water splashes, or insect vectors. Insects such as Helicoverpa armigera and Caryedon serratus play a significant role in dispersal and infection by creating entry points in plant tissues or directly transporting spores [15].
Ultimately, A. flavus infection leads to the accumulation of aflatoxins, highly toxic, carcinogenic secondary metabolites that pose serious health and trade risks. Aflatoxins are primarily produced by A. flavus and A. parasiticus, with key analogs including AFB1, AFB2, AFG1, AFG2, AFM1, and AFM2. Among these, AFB1 is the most prevalent and toxic in peanuts. It is a potent hepatocarcinogen that forms DNA adducts in the liver, contributing to mutations in tumor suppressor genes such as TP53 [16]. Chronic exposure in children has been linked to stunted growth, micronutrient deficiencies, and immune suppression [17,18]. In animals, ingestion of contaminated peanut meal reduces productivity and can result in the transfer of AFM1 into milk and dairy products [19,20].
Although improved irrigation, proper drying, and secure storage can reduce aflatoxin contamination to some extent, these measures alone are insufficient to fully address the problem. Genetic resistance remains the most cost-effective and sustainable long-term solution. Resistance to A. flavus infection and aflatoxin accumulation in peanut is now recognized as a complex, multigenic trait that is strongly influenced by environmental factors [10]. However, progress in breeding aflatoxin-resistant cultivars has been limited due to low levels of resistance in the available germplasm and the complex, multigenic nature of the resistance mechanisms [21]. Recent breeding strategies are shifting focus from the identification of major resistance (R) genes to targeting host susceptibility (S) genes. Disruption of such S genes can provide broad-spectrum, and durable resistance without the need for transgenic approaches. Advances in transcriptomics and proteomics have enabled the identification of hundreds of differentially expressed genes between resistant and susceptible peanut genotypes, many of which are involved in defense signaling, oxidative stress response, and producing secondary metabolites [22,23,24,25]. These findings provide a valuable foundation for pinpointing candidate S genes and deciphering the underlying molecular networks that govern resistance.
This review centers on peanut resistance mechanisms to A. flavus and reduced aflatoxin contamination, with an emphasis on recent insights into host susceptibility genes and pathways that could be manipulated to enhance resistance. We discuss advances in conventional breeding and QTL mapping, summarize new biotechnological approaches like host-induced gene silencing, and highlight emerging evidence for peanut S genes that could be targeted by modern gene editing tools to confer aflatoxin resistance. By integrating these diverse strategies, we aim to outline a path forward for developing peanut cultivars with enhanced and durable resistance to aflatoxin contamination.
2. Genetic and Molecular Regulation of Aflatoxin Biosynthesis
Producing aflatoxins in A. flavus is a developmentally coordinated and tightly regulated process governed by a complex network of genetic and environmental signals. The availability of its complete genome sequence, along with a comprehensive microarray platform, has accelerated insights into the molecular mechanisms driving aflatoxin biosynthesis [26].
2.1. Aflatoxin Biosynthesis
Aflatoxins are synthesized via a highly coordinated polyketide-derived biosynthetic pathway involving over two dozen enzymatic transformations [2,26,27,28,29]. The genes responsible for aflatoxin biosynthesis are clustered within a ~70-kilobase region near the telomeric end of chromosome 3 (Figure 1). This cluster includes about 30 co-regulated genes, designated with the prefix “afl”, ranging from aflA to aflY [2,26,30]. These genes encode enzymes responsible for specific transformations in the biosynthetic pathway. The pathway begins with the polymerization of acetate units into a polyketide backbone, followed by successive steps that yield intermediates such as norsolorinic acid (NOR), averantin (AVN), 5′-hydroxyaverantin (HAVN), oxoaverantin (OAVN), averufin (AVF), hydroxyversicolorone (HVN), versiconal hemiacetal acetate (VHA), versiconol acetate (VOAc), versiconal (VOH), versicolorin B (VERB), versicolorin A (VERA), demethylsterigmatocystin (DMST), sterigmatocystin (ST), O-methylsterigmatocystin (OMST), and 11-hydroxy-OMST, before culminating in AFB1 [28].
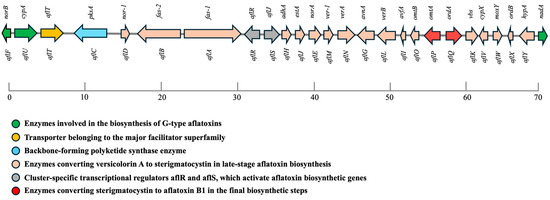
Figure 1.
Genomic organization of the aflatoxin biosynthetic gene cluster in A. flavus. The ~70 kb gene cluster contains structural, regulatory, transporter, and tailoring genes required for aflatoxin biosynthesis. Polyketide synthesis is initiated by aflC (pksA), followed by sequential action of enzymes synthesizing sterigmatocystin and other intermediates (peach), leading to B- and G-type aflatoxins. Green arrows indicate genes specific to G-type aflatoxin biosynthesis; red arrows represent enzymes finalizing aflatoxin formation. Major facilitator transporter genes (aflT) are highlighted in yellow, and regulatory elements (aflR, aflS) in gray. The spatial clustering of these genes underpins coordinated pathway activation during infection.
2.2. Transcriptional Regulation of Aflatoxins Biosynthetic Genes, aflR and aflS
Transcription of the aflatoxin cluster is mainly controlled by two key regulators: aflR and aflS. These genes are adjacent to one another in the gene cluster and are transcribed divergently from closely spaced promoters [29,31]. aflR encodes a zinc finger DNA-binding protein that serves as the principal transcriptional activator of the pathway. Its overexpression leads to a 50-fold increase in aflatoxin production, whereas its deletion nearly abolishes toxin biosynthesis [32,33]. Moreover, chemical inhibitors of aflatoxin production often exert their effects by downregulating aflR expression [34,35]. aflS plays a supporting role by enhancing aflR-mediated activation of biosynthetic genes. Overexpression of aflS in A. flavus has been shown to increase aflatoxin levels further [29,36], and in A. parasiticus, evidence suggests a physical interaction between aflS and aflR, potentially enhancing aflR’s promoter binding efficiency [31].
2.3. Developmental, Epigenetic, and Environmental Regulation of Aflatoxin Biosynthesis
Aflatoxin biosynthesis is intricately linked with fungal development and morphogenesis. Genes regulating conidiophore and sclerotial development also impact toxin production. For example, hbx1 deletion completely halts aflatoxin production and developmental differentiation, in part through downregulation of cluster genes including aflC, aflD, aflM, and aflR [37]. Additional transcriptional regulators such as NsdC and NsdD are essential for sclerotia formation and mycotoxin biosynthesis [38,39]. Other important factors include AfRafA, AfStuA, AflRsmA, AflSkn7, and Rum1, all of which modulate developmentally associated secondary metabolism [40,41,42,43,44]. Furthermore, disruption of genes such as hexA, sakA, aflPex5, and Aflndk has been shown to affect sporulation, stress response, and aflatoxin yield [43,45,46].
A central component of this regulatory network is the Velvet complex, comprising VeA and LaeA. This complex is a hub that integrates light signaling, developmental control, and secondary metabolism. Deletion of either veA or laeA leads to complete loss of both aflatoxin production and sclerotium formation [47,48,49]. Genes involved in hyphal fusion, such as hamF, hamG, hamH, and hamI, also play a role, with their deletion resulting in decreased aflatoxin production and developmental defects [50]. The kinetochore-associated protein Spc105, which physically interacts with LaeA, is also necessary for proper conidiophore and sclerotial development [51].
Beyond intrinsic genetic control, aflatoxin biosynthesis is strongly influenced by environmental cues. Factors such as temperature, pH, oxidative stress, and nutrient availability can activate signaling cascades that modulate the expression of aflatoxin biosynthetic genes [6,28]. These environmental stimuli not only affect growth and development but also integrate with global regulators such as LaeA and aflR to fine-tune the metabolic output of the fungus. A comprehensive understanding of these interactions is crucial for devising effective strategies to mitigate aflatoxin contamination in crops under variable field and storage conditions. Understanding the fungal genes that drive aflatoxin production also identifies molecular targets for interventions; for instance, host-induced gene silencing can target some of these fungal regulators. We next examine how peanuts defend themselves and how we can leverage both peanut and fungal genes for resistance.
3. Structural, Biochemical, and Induced Defenses in Peanut Against A. flavus Infection
Understanding the natural resistance mechanisms of peanut against A. flavus infection and subsequent aflatoxin contamination has been a major focus of research for decades. This resistance is multifaceted, involving structural barriers, biochemical pathways, and inducible defense responses that operate at different stages of the infection process. Resistance against A. flavus is typically classified into three main categories including resistance to seed colonization (IVSC) (the ability of seeds to restrict fungal growth), resistance to pre-harvest aflatoxin contamination (PAC) (limiting toxin accumulation in the field), and resistance to aflatoxin production (AP) (suppressing toxin biosynthesis even after infection) [21,24,52] (Figure 2). The genetic basis of resistance to A. flavus in peanut is complex and predominantly quantitative, involving the interplay of multiple genes [9]. This polygenic nature, coupled with strong gene-environment (G × E) interactions, poses significant challenges for breeding programs. The expression and effectiveness of resistance traits can vary widely among peanut genotypes and are often inconsistent across different environmental conditions [10,53]. Even when additive genetic effects are present, environmental variability can limit the stability and utility of resistant varieties, complicating their deployment in diverse agroecological zones [7,10].
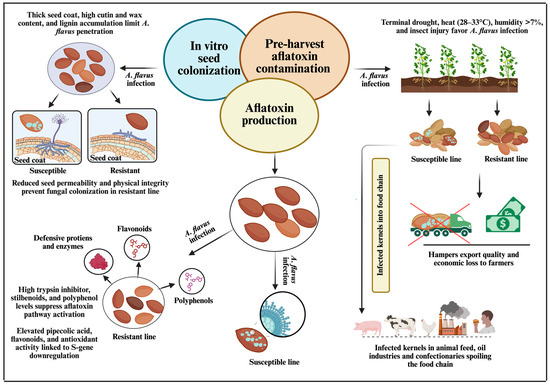
Figure 2.
Multifaceted resistance mechanism in peanut against A. flavus, typically classified into three main categories: resistance to seed colonization (IVSC), resistance to pre-harvest aflatoxin contamination (PAC), and resistance to aflatoxin production (AP).
3.1. Preformed Structural Barriers, Pod Shell, and Seed Coat Serve as Physical Barriers for A. flavus Infection
Peanuts employ robust structural defenses to mitigate A. flavus infection, with the pod shell and seed coat forming the first line of defense. The shell’s architecture, particularly its thickness, texture, and permeability, has been linked to reduced infection rates, especially in resistant cultivars [7,53,54]. These structural traits act as mechanical barriers that restrict fungal entry. Similarly, the seed coat, the outermost layer of the peanut kernel, serves a dual role as a physical and chemical barrier. Its resistance potential has been associated with morphological features such as thickness, density, and biochemical content [11,53]. These features, when absent or underdeveloped, may represent structural susceptibility factors that facilitate infection.
3.2. Biochemical Defenses—Lignin, Waxes, Phenolics, and Flavonoids
Specialized compounds in the pod and seed coat offer further protection. Lignin, abundant in wild peanut species, contributes to cell wall rigidity and resistance to mechanical and biological stress [55]. Earlier LaPrade (1973) proposed that resistance may correlate with seed coat thickness and structural complexity [56]. Wax and cutin layers add hydrophobicity, minimizing water retention and fungal adhesion, thereby acting as chemical barriers against A. flavus [57]. In addition to structural defenses, peanut tissues are enriched with antifungal phytochemicals. Phenolic compounds such as p-coumaric acid, ferulic acid, hydroxybenzoic acid, and chlorogenic acid have been identified in the seed coat, along with flavonoids like epicatechin, quercetin, and resveratrol [58,59]. These metabolites possess antioxidants and antimicrobial activity and are believed to contribute significantly to fungal resistance [60,61,62]. Among the most potent biochemical defenses are secondary metabolites and enzymatic inhibitors that directly interfere with fungal physiology. Tannins in the peanut testa have also been reported to inhibit A. flavus growth [11,63]. Likewise, specific antifungal molecules such as trypsin inhibitors and 5,7-dimethoxy isoflavone have been linked to enhanced resistance in certain peanut lines [64,65]. More recently, Sharma et al. (2021) identified pipecolic acid (Pip) as a resistance-associated metabolite, showing higher accumulation in A. flavus-challenged resistant genotypes, highlighting its emerging role in plant immunity [66]. Recent complementary profiling across drought-stressed and well-watered lines (e.g., Zhonghua 6 vs. Yuanza 9102) identified elevated resveratrol, cinnamic, coumaric and ferulic acids, and 13S-HPODE in resistant seeds, all correlating with reduced fungal colonization [67]. A recent study profiled peanut seed coats and identified phenylpropanoid-derived metabolites with antifungal activity, notably 2,5-dihydroxybenzaldehyde, which inhibited A. flavus growth by 98.7% and nearly eliminated aflatoxin contamination in vitro [11]. These findings highlight how enhanced metabolite synthesis in resistant varieties can mitigate fungal invasion and mycotoxin accumulation. Deficiency in such compounds may therefore be a biochemical susceptibility trait influencing breeding outcomes.
3.3. Induced Defense by Signaling—PTI, ETI, and MAPK Pathways
When A. flavus infects peanut plants, the host mounts an innate immune response composed of two interconnected layers: PAMP-triggered immunity (PTI) and effector-triggered immunity (ETI). PTI begins with the recognition of pathogen-associated molecular patterns (PAMPs) by pattern recognition receptors (PRRs) at the plant cell surface, which activate early defense responses such as reactive oxygen species (ROS) bursts, mitogen-activated protein kinase (MAPK) cascades, and transcription of resistance genes. In contrast, ETI is initiated by the direct recognition of pathogen effectors via intracellular nucleotide-binding site leucine-rich repeat (NBS-LRR) proteins, often leading to hypersensitive response (HR) and localized cell death [68]. Recent transcriptomic analyses have revealed that these immune responses are robust in certain peanut genotypes. Cui et al. (2022) reported significant upregulation of MAP kinases (Arahy.L410JY, Arahy.BC5GM2), cytochrome P450, and the PRR gene RPVOD7 as part of the PTI response [69]. Simultaneously, six NBS-LRR genes and a serine/threonine kinase (Arahy.D2YYPY) were highly expressed in resistant cultivars during A. flavus infection, indicating a coordinated PTI-ETI response [69,70]. These differences suggest immunological signaling loci represent molecular susceptibility checkpoints worth targeting.
3.4. Hormonal Crosstalk and Lipoxygenase (LOX) Pathway
The interplay between jasmonic acid (JA), salicylic acid (SA), and ethylene forms the core of the peanut’s hormonal defense network. These signaling molecules regulate key resistance genes and coordinate responses against biotic stress. This complex hormonal orchestration is fine-tuned by transcription factor (TF) families like WRKY, bZIP, ERF, MYB, MYC, and NAC, which modulate downstream defense mechanisms such as ROS production, synthesis of antifungal secondary metabolites, and expression of pathogenesis-related (PR) proteins [23,24,57,71,72]. Moreover, oxylipins, which are lipid-derived signals, add another regulatory layer. Fungal oxylipins enhance fungal colonization, while plant-derived oxylipins act antagonistically to suppress aflatoxin biosynthesis in A. flavus [73]. Peanut plants challenged by A. flavus infection, especially under drought stress, exhibit a pronounced oxidative burst marked by the accumulation of ROS. This response is intricately linked to increased lipoxygenase (LOX) pathway activity. LOX enzymes catalyze lipid peroxidation and generate signaling molecules that reinforce cell walls, promote phytoalexin biosynthesis, and contribute to hypersensitive cell death [74]. Their activity is also associated with producing defense-related fatty acids, including JA and methyl-JA, which further amplify immune responses [72,75,76]. LOX pathway deficiencies may therefore identify hormonal susceptibility points.
Notably, the SA and JA signaling pathways often act antagonistically, with activation of one typically suppressing the other [77,78]. SA-mediated defenses are more effective against biotrophic pathogens, whereas JA signaling is central to resistance against necrotrophs. A. flavus, a predominantly saprophytic soil fungus, can become parasitic by opportunistically invading developing seeds or senescent, stressed tissues, particularly under environmental stress such as drought or high temperature [79,80]. Consequently, JA is the dominant hormone mediating resistance to aflatoxin-producing fungi. Peanut genotypes with resistance to A. flavus show stronger induction of JA-responsive genes and LOX activity, while susceptible lines tend to display elevated SA signaling [24]. Similar trends have been observed in maize, where disruption of JA biosynthesis (e.g., ZmLOX3 knockouts) increased susceptibility to Aspergillus spp. and aflatoxin contamination [81].
3.5. Pathogenesis-Related Proteins and Phytoalexin Accumulation
Pathogenesis-related proteins and phytoalexins form a critical layer of the peanut’s defense system. Resistant cultivars accumulate higher levels of phenylalanine ammonia-lyase (PAL), which is pivotal for lignin and phytoalexin biosynthesis, and glutathione S-transferases (GSTs), which detoxify ROS and support stress resilience [82]. Enzymes like chitinases and β-1,3-glucanases are also more abundant in resistant lines such as GT-YY9 and GT-YY20, where they degrade fungal cell walls and release PAMPs, thereby enhancing immunity [83,84]. Among the phytoalexins, resveratrol has been documented for its antifungal activity, effectively inhibiting A. flavus spore germination and hyphal growth [85]. However, drought stress can compromise this defense by suppressing phytoalexin production [86]. Additional flavonoids, such as quercetin, are also involved in host defense, as they have been shown to downregulate aflatoxin biosynthesis genes in the pathogen [87]. Failure to induce effective chemical defenses may define chemical susceptibility, a critical target for breeding and editing efforts.
4. Genetic and Genomic Strategies for Enhancing Aflatoxin Resistance in Peanut
Breeding for resistance against A. flavus infection is considered a cost-effective and sustainable approach to mitigate aflatoxin contamination [72]. However, efforts have been hindered by the narrow genetic base of cultivated peanut, the complex, quantitative nature of resistance, which is heavily influenced by environmental interactions, and the lack of elite parental lines that consistently exhibit strong and stable resistance to infection [88,89]. Despite these challenges, screening of diverse germplasm has led to identifying resistant genotypes [9]. Assessing resistance typically involves a combination of phenotypic evaluations, such as the percent seed infection index, visual scoring of disease symptoms, and aflatoxin quantification, and molecular tools like QTL mapping and marker-assisted selection [90]. These methods, alongside functional genomics, have collectively expanded our understanding of the genes and pathways that govern peanut and A. flavus interactions.
4.1. Genomic Mapping of Resistance Loci Against A. flavus in Peanut
Quantitative trait loci (QTL) studies have identified several genomic regions associated with resistance traits. Liang et al. (2009) mapped six QTLs using three recombinant inbred line (RIL) populations, identifying key regions on chromosomes A01, A02, and B05, each explaining over 10% of phenotypic variation [91]. Similarly, Yu et al. (2019) detected 14 QTLs in a Zhonghua 10 × ICG 12,625 population, including qPSIIA10, which consistently explained 11.32–13% PVE [90]. For aflatoxin levels, overlapping QTLs qAFB1A07, qAFB1B06.1, qAFB2A07, and qAFB2B06 were reported, with individual PVE values reaching up to 21.02%. More recent studies continue to uncover stable and environment-specific QTLs. For example, Khan et al. (2020) reported qRAF-3-1 and qRAF-14-1 on chromosomes A03 and B04 [92], respectively, Jiang et al. (2021) identified six QTLs in a Zhonghua 16 × J11 population, with qPSIIB10 demonstrating consistency across four years [93]. Jin et al. (2023) found additional QTLs (qAFTB05.2, qAFTA05.1, qAFTB06.3) and used conditional mapping to reveal additive effects [94], while Yu et al. (2024) highlighted qAFTsA07.1, which consistently accounted for 13.39% PVE [95].
4.2. Multi-Omics Insights into Resistance Mechanisms
Functional genomics has complemented QTL studies by identifying differentially expressed genes (DEGs), proteins (DEPs), and metabolites linked to resistance. Wang et al. (2010) reported 12 DEPs between resistant YJ-1 and susceptible Yueyou 7 genotypes [96], and Guo et al. (2011) identified 62 upregulated genes, including lipoxygenase and PR10, using microarrays [22]. Wang et al. (2012) expanded the analysis by identifying 490 resistance-associated genes using a custom microarray, including 64 defense-related genes [25], while Nayak et al. (2017) and Korani et al. (2018) [23,24] found 4445 and 4272 DEGs in J11 vs. JL24 and ICG 1471 vs. Florida-07, respectively. Zhao et al. (2019) detected 663 DEGs and 314 DEPs in J11, further solidifying its resistance profile [97]. Additional layers of insight were provided by recent multi-omics studies revealing complex regulation at transcript, protein, and metabolite levels [67,69,98]. Across these datasets, several families of defense-related genes consistently appear. These include oxidative stress regulators such as glutathione S-transferases (GSTs), transcription factors like WRKY and MYB, and enzymes associated with pathogenesis-related (PR) responses, late embryogenesis abundant (LEA) proteins, defensins, and lipoxygenases, all of which are upregulated in resistant lines, indicating their central role in defense.
4.3. GWAS and SNP Marker Discovery
Genome-wide association studies (GWAS) have complemented QTL and transcriptomic approaches by uncovering specific single-nucleotide polymorphisms (SNPs) associated with resistance traits. Yu et al. (2020) performed GWAS on 99 accessions, identifying 60 SNPs linked to aflatoxin resistance, explaining 16.87–31.70% PVE [89]. Two resistant accessions, Zh.h0551 and Zh.h2150, were particularly noteworthy. Marker peaks were spread across 11 chromosomes, highlighting the polygenic nature of resistance. Ding et al. (2022) identified 16 SNPs associated with shell and seed infection indices (SLII, SDII) and aflatoxin content [99]. Candidate genes near these loci included MYB transcription factors such as Arahy.J7VJ5I and NLR-type resistance genes like Arahy.R1ATPI, suggesting a role for transcriptional regulation and innate immunity in peanut resistance.
4.4. Case Study—The J11 Genotype
The Indian peanut cultivar J11 has emerged as a model genotype for resistance to A. flavus. Known for its early maturity, drought tolerance, and seed coat-related traits, J11 has shown strong resistance since the 1980s, first identified through in vitro seed colonization (IVSC) assays. Transcriptomic and proteomic studies have since supported this resistance. Zhao et al. (2019) found 663 DEGs and 314 DEPs post-infection in J11 [97], while Nayak et al. (2017) reported 4445 DEGs [24]. QTL mapping studies by recent studies further demonstrated that J11 contributes major alleles for both infection resistance and aflatoxin suppression [93,94]. Its consistent performance makes J11 an invaluable resource for resistance breeding and gene discovery.
4.5. Integrating Omics for Improving Resistance
The combined application of QTL mapping, GWAS, transcriptomics, and proteomics highlights the complexity of peanut resistance to A. flavus. Resistance is clearly polygenic and modulated by both genetic background and environmental conditions. The consistent identification of stress-mitigation enzymes, transcription factors, and PR proteins in resistant genotypes reinforces their relevance as breeding targets. These insights support the development of marker-assisted selection and genomic prediction models and open avenues for precise genome editing to enhance aflatoxin resistance in elite peanut cultivars. While most conventional breeding efforts have concentrated on accumulating favorable resistance alleles from diverse germplasm, there is growing recognition that enhancing resistance durability may also require eliminating or modifying the plant’s own susceptibility factors. These susceptibility genes host loci that pathogens exploit to promote colonization or toxin production and represent promising new targets for genetic intervention. Genomic tools now enable not only the stacking of resistance QTLs, but also the precise knockout or suppression of S genes such as MLO, AhS5H1, and ABR1 using CRISPR-based editing. These genes, when inactivated, may confer broad-spectrum and stable resistance by disrupting pathogen compatibility at its source. The following sections explore these innovative strategies that bridge classical resistance breeding with targeted genome engineering to combat aflatoxin contamination more effectively [100,101,102].
5. Transgenic Strategies—Silencing Pathogen Genes and Boosting Host Defenses
Innovative gene silencing and overexpression approaches have emerged as powerful tools to enhance peanut resistance to A. flavus infection and suppress aflatoxin accumulation. Host-induced gene silencing (HIGS) and RNA interference (RNAi) have shown notable promise. RNA interference (RNAi) operates within the plant to silence its own genes via small interfering RNAs (siRNAs), while host-induced gene silencing (HIGS) is a transgenic approach that enables plants to produce dsRNAs targeting pathogenic genes, thereby triggering cross-kingdom gene silencing to suppress infection [103,104,105]. In a foundational study, Sharma et al. (2018) employed HIGS to target two crucial aflatoxin biosynthesis genes, aflM and aflP, substantially reducing aflatoxin levels [106]. Complementarily, the overexpression of antifungal defensins MsDef1 and MtDef4.2 in the susceptible peanut variety JL 24 significantly limited fungal colonization and toxin production. Strikingly, combining these two strategies, HIGS for metabolic suppression and defensin overexpression for fungal resistance, produced peanut lines with robust, cross-morphotype resistance, often achieving aflatoxin levels as low as 1–2 ppb or even undetectable in some events. Building on this success, Prasad et al. (2023) implemented a multiplexed HIGS system targeting multiple developmental and biosynthetic genes nsdC, veA, aflM, and aflR [107]. These transgenic peanut lines exhibited significantly reduced fungal colonization and aflatoxin biosynthesis. The proteomic analysis confirmed the suppression of fungal proteins central to toxin production and morphogenesis, including VelC, AflC, AflL, AflM, AflQ, AflR, AflS, AflV, AflW, VeA, and AflJ [107].
5.1. Targeting aflR, aflM, veA, and Other Key Regulators
RNAi strategies have further demonstrated success in silencing essential genes in the aflatoxin pathway. Two different studies effectively reduced aflatoxin accumulation by targeting aflR, aflS, aflC, pes1, and aflep in peanut [108,109]. In a RIL population derived from the cross between Xuhua13 (susceptible) and Zhonghua 6 (resistant), RNA-seq analysis led to the identification of AhAftr1, an NB-LRR-encoding gene featuring a unique structural variation in its LRR domain [95]. Functional studies validated AhAftr1 as a key player in aflatoxin resistance via the ETI pathway. Beyond peanuts, similar strategies have shown efficacy in maize. Targeted silencing of aflR, aflM, and aflC, along with developmental genes such as alk, amy1, and p2c, has led to reduced A. flavus growth and aflatoxin content in transgenic maize lines [39,110,111,112,113,114], demonstrating cross-crop potential for these gene-targeting strategies.
5.2. Overexpression of Defensins, Chitinases, and PR Proteins in Host
Parallel to silencing strategies, overexpressing host defense genes has provided another layer of protection. When expressed in peanuts, the rice-derived chitinase gene RChit enhanced resistance to A. flavus, offering an effective defense mechanism by degrading fungal cell walls [84]. Similarly, transgenic peanut lines overexpressing the ARAhPR10 gene, belonging to the PR10 family, showed reduced fungal colonization and lower aflatoxin content [115]. Sundaresha et al. (2010) demonstrated that introducing a tobacco β-1,3-glucanase gene further bolstered peanut defenses against fungal invasion [116]. Recent studies have identified AhS5H1 and AhS5H2 in peanut as salicylate 5-hydroxylases that convert SA into 2,5-dihydroxybenzoic acid (2,5-DHBA) [101]. Overexpression of these genes in Arabidopsis and Nicotiana benthamiana resulted in significantly reduced SA levels and enhanced susceptibility to Pseudomonas syringae pv. tomato DC3000 [101]. Conversely, maintaining higher SA levels by suppressing AhS5H expression correlates with improved resistance. These findings highlight AhS5H1 and AhS5H2 as susceptibility factors, where overexpression compromises SA-dependent defense and enhances vulnerability to SA-sensitive pathogens like P. syringae [101]. These genetic engineering successes demonstrate that both disabling fungal virulence factors and enhancing host defense genes can significantly reduce aflatoxin. Another complementary approach is to alter the host’s own S genes to create inherent resistance, which we discuss next. Numerous gene targets have been explored for transgenic and gene-silencing approaches to mitigate A. flavus infection and aflatoxin contamination. Table 1 summarizes the major host and fungal gene targets, associated outcomes, and experimental interventions that have been validated in peanut.

Table 1.
Host-induced gene silencing, RNA interference and overexpression studies in peanuts.
6. Exploiting Plant Susceptibility Genes (S) for Durable Resistance
Plant susceptibility (S) genes are native host genes that are co-opted by pathogens to facilitate infection, colonization, or toxin production [118]. Emerging evidence suggests that A. flavus relies on a subset of these host genes to weaken defense responses and promote aflatoxin biosynthesis. Unlike resistance (R) genes, which trigger immune responses, S genes act as vulnerability points in the plant’s defense network [119]. Disrupting or silencing these genes can lead to recessively inherited, durable resistance without the need to introduce external resistance alleles [120,121,122,123,124].
6.1. Identification and Functional Validation of S Genes
Traditional breeding has largely focused on dominant R genes, which recognize pathogen effectors and trigger defense responses. However, these resistances are often short-lived due to the high mutation rate of pathogen effectors, allowing them to evade host recognition. In response, gene stacking, introducing multiple R genes into one genotype, has emerged as a strategy to broaden and stabilize resistance [125]. Yet, a parallel and increasingly compelling approach targets S genes that pathogens exploit for successful colonization. Disrupting or silencing these genes can result in recessive, durable, and occasionally broad-spectrum resistance [102,118,121].
The discovery of S genes typically follows either forward or reverse genetics approaches. Forward genetics screens for mutants with altered susceptibility and has traditionally relied on model organisms like Arabidopsis thaliana [126]. Reverse genetics, by contrast, begins with candidate genes identified via gene expression profiling or pathogen effector studies and validates them using RNAi or HIGS techniques [121]. Another powerful method involves identifying host proteins targeted by pathogen effectors, often using yeast two-hybrid assays to detect key interactions [127]. Furthermore, the evolutionary conservation of S genes enables ortholog mining in crops. Comparative phylogenetic analyses, supported by transcriptome and genome sequencing, allow prediction and functional exploration of candidate S genes [126,128]. In peanuts, identifying and editing S genes represents a transformative strategy for mitigating aflatoxin contamination. Candidate genes such as MLO, AhS5H1, AhS5H2 (salicylic acid 5-hydroxylase genes), and ABR1 (an ABA-responsive transcription factor) have emerged as key players involved in host susceptibility to A. flavus infection and aflatoxin biosynthesis [100,101,102]. These genes are frequently associated with immune suppression or hormonal signaling pathways that pathogens exploit. In recent studies, these candidate genes have been proposed for functional validation based on consistent differential expression patterns between resistant and susceptible genotypes, functional annotations implicating them in hormone-regulated immunity, and sequence homology to characterized S genes in model plants such as Arabidopsis thaliana [100,101,102,121,126].
The S genes are functionally diverse and fall into three general categories [124]. The first category includes genes facilitating pathogen entry, such as the MLO (Mildew resistance locus O) gene family, whose inactivation provides strong resistance to powdery mildew in several crops [118,129]. The second comprises negative regulators of plant immunity, such as DMR6 in Arabidopsis thaliana, whose loss results in elevated salicylic acid (SA) levels and resistance to Hyaloperonospora parasitica [130]. The third category includes compatibility genes co-opted by pathogens for nutrition and proliferation, exemplified by rice’s SWEET sugar transporter family. OsSWEET11 is transcriptionally activated by TALEs from Xanthomonas oryzae pv. oryzae, promoting sugar efflux to nourish the pathogen [131,132]. Loss-of-function mutations in SWEET genes have been shown to confer broad-spectrum resistance against Xanthomonas in multiple crops. In rice, targeted editing of OsSWEET13 and OsSWEET14 disrupted TAL effector-mediated activation, providing resistance to various Xanthomonas oryzae strains and reducing susceptibility to brown planthopper [117,133]. Similar resistance phenotypes have been observed in cassava [134], cotton [135], and citrus, where CRISPR/Cas9-mediated knockout of CsSWEET15 significantly reduced disease symptoms caused by citrus canker and huanglongbing [120]. Disabling S genes can restrict pathogen survival, while comparative genomics enables the identification of conserved S gene homologs across plant species [118,121]. As our understanding of S gene functions deepens, targeting these genes offers a promising strategy for crop breeding to improve disease resistance. Table 2 provides a summary of identified and candidate S genes in peanut, their functional roles, and their potential as genome editing targets for durable resistance.

Table 2.
Candidate susceptibility genes in peanut for genome editing.
6.2. Genome Editing and Precision Breeding Tools for S Gene Manipulation
New precision breeding technologies, including genome editing platforms like ZFNs, TALENs, and especially CRISPR/Cas9, have revolutionized the efficient manipulation of S genes, offering high specificity with minimal off-target effects [136,137,138,139]. Among these, CRISPR/Cas9 has gained prominence for its simplicity, specificity, and versatility, enabling targeted gene knockouts, insertions, and fine-scale modulation of gene activity. Phogat et al. (2024) recently provided a comprehensive review detailing notable progress in peanut transformation efficiency, delivery technologies, and genome editing approaches [140]. Beyond the ubiquitous Cas9, alternative CRISPR systems like Cas12a (formerly Cpf1) are broadening the plant genome editing toolkit. Cas12a recognizes a distinct thymine-rich PAM sequence (5′-TTTV), allowing access to genomic regions less amenable to Cas9 targeting [141]. It also relies on a shorter CRISPR RNA and possesses intrinsic RNase activity for processing CRISPR arrays, enabling efficient multiplex editing from a single transcript. Importantly, Cas12a induces DNA breaks with staggered ends that often result in larger deletions, an advantage for knocking out susceptibility genes [141]. While not yet demonstrated in peanut, Cas12a represents a promising genome editing tool due to its high editing efficiency, T-rich PAM recognition, and multiplexing capability. It has been successfully applied in several crops such as rice, maize, and cotton, and thus holds potential as a future strategy for legume editing [142]. However, its efficacy in peanut remains to be experimentally validated. The expanding CRISPR nuclease repertoire provides breeders with greater flexibility in choosing optimal tools for specific targets and contexts.
Prime editing represents another leap in genome-editing technology, offering a versatile “search and replace” capability for targeted DNA modifications [143]. Prime editing employs a fusion of Cas9 nickase with a reverse transcriptase, guided by a specialized prime editing gRNA (pegRNA) that encodes the desired edit, to directly install small insertions, deletions, or base substitutions at the target site. Although still nascent in plant systems, prime editing has been successfully implemented in several crops (including rice, wheat, and tomato) and is being optimized for higher efficiency [144,145]. In legumes like peanut, initial experiments have shown promise. Biswas et al. (2022) reported the use of prime editing in peanut protoplasts to correct a mutant GFP reporter gene, achieving detectable but low editing efficiencies (~0.2–0.5%) [146]. These early results highlight both the potential and the current technical challenges of prime editing in peanut. The continued refinement of base and prime editors is poised to complement CRISPR/Cas9 and Cas12a, ultimately providing a spectrum of tools for fine-tuning the peanut genome for aflatoxin resistance. These precision tools offer breeders a robust toolkit to develop resistant crops by neutralizing pathogen susceptibility factors.
One of the most powerful aspects of genome editing lies in its capacity for multiplexing simultaneous editing of multiple loci within a single generation. This capability facilitates precision trait stacking and pyramiding of resistance alleles. For instance, concurrent disruption of several S genes or resistance-associated QTLs can lead to additive or synergistic effects, enhancing durability and breadth of resistance under diverse environmental conditions [112]. Various multiplexing strategies have been developed, such as polycistronic tRNA-sgRNA expression cassettes and exploitation of Cas12a’s ability to process CRISPR arrays, enabling simultaneous targeting of numerous genomic sites [141]. This allows researchers to pyramid multiple resistance mechanisms in a single genetic background, an approach that could significantly strengthen aflatoxin resistance in peanut. CRISPR/Cas9-based allele pyramiding can also be integrated with other technologies, such as RNAi and HIGS, enabling multi-tiered defense strategies targeting fungal infection and toxin production. The combination of these advanced molecular tools holds significant promise for developing peanut cultivars with broad-spectrum and long-lasting resistance to A. flavus and aflatoxin contamination.
Despite their promise, both CRISPR/Cas12a and prime editing face significant technical challenges in peanut. Cas12a has not yet been demonstrated in peanut, and its deployment is further hampered by transformation and regeneration inefficiencies across genotypes. These issues include the lack of robust tissue culture protocols optimized for Cas12a, as well as potential targeting limitations due to its T-rich PAM requirement (5′-TTTV). Broader application may require codon optimization and the development of PAM-relaxed Cas12a variants [147]. Prime editing has only been tested in peanut protoplasts, where a study reported the correction of a mutant GFP reporter gene, but at very low efficiencies (~0.2–0.5%) [148]. No prime edits have been stably transmitted to regenerated plants. These limitations stem from challenges in pegRNA design, delivery efficiency, and ineffective DNA repair pathways in transformed cells. Recent reviews of peanut genome editing technology have highlighted tissue culture-free delivery strategies including nanoparticle-mediated gene transfer, viral vectors, pollen magnetofection, pollen tube injection, node injection, and vacuum infiltration as promising avenues for overcoming genotype-dependent transformation and regeneration challenges in peanut [149].
6.3. Pleiotropic Effects and Trade-Offs
Despite their benefits, impairing S genes can sometimes cause undesirable pleiotropic effects. Since many S genes also serve critical roles in plant growth or development, their inactivation may lead to side effects such as dwarfism, early senescence, or reduced abiotic stress tolerance [126]. For instance, in barley, HvMLO mutants show premature leaf senescence under certain conditions [118]. Similarly, DND1 silencing in tomatoes caused severe growth defects, while in potatoes, its effects were mild and environmentally dependent [150]. In citrus, mutation of CsSWEET15 led to mild chlorosis and stunted growth over time, particularly in older plants and when combined with other SWEET gene mutations, likely due to impaired phloem loading and reduced sugar transport [120]. Nonetheless, careful allele selection and background breeding can mitigate these effects. In many cases, mild loss-of-function variants or context-specific editing offer resistance without compromising plant fitness. As understanding of gene function and regulatory networks deepens, the targeted use of S genes remains a viable and powerful avenue for durable resistance. Moreover, targeted editing of S genes using CRISPR/Cas9 can reduce susceptibility and boost resistance without transgene introduction, offering regulatory advantages in non-GMO breeding contexts. Additionally, editing of non-coding regions such as cis-regulatory motifs or promoter elements offers opportunities for fine-tuning gene expression, thereby achieving resistance while minimizing potential pleiotropic effects. Although such effects have not yet been reported in peanuts, current efforts to edit susceptibility genes remain largely at the proof-of-concept stage, and no studies have systematically evaluated the pleiotropic consequences of these edits. This remains a key consideration for future research to ensure that resistance traits do not compromise growth, yield, or abiotic stress tolerance.
6.4. Candidate S Genes in Peanut
Although S gene research in peanut is still emerging, several studies have identified promising candidates. Prasad et al. (2023) used HIGS to silence fungal genes (nsdC, veA, aflM, aflR) [107]. They observed the upregulation of host susceptibility-associated proteins (SAPs) like annexins, syntaxins, calmodulin, and MLO-family proteins in wild-type plants. These findings position SAPs as potential targets for gene editing in future resistance breeding efforts. In a genome-wide analysis, Traore et al. (2021) reported 25 AhMLO loci across 14 chromosomes, with two loci in Clade V associated with powdery mildew susceptibility in other crops highlighted as strong S gene candidates [102]. The presence of defense-related cis-elements such as TC-box and thymine-rich motifs in their promoters further supports their functional relevance [151,152]. Liang et al. (2023) identified AhS5H1 and AhS5H2, two SA hydroxylase genes whose overexpression reduced endogenous SA and increased 2,5-DHBA levels, heightening susceptibility to Pseudomonas syringae pv. tomato DC3000 [101]. These genes likely interfere with SA-mediated defenses and are strong candidates for functional knockouts. Finally, Clevenger et al. (2016) proposed the ethylene-responsive transcription factor ABR1 as a potential peanut S gene [100]. As a repressor of ABA signaling, ABR1 may influence pre-harvest aflatoxin susceptibility, acting as a molecular switch during fungal infection. These candidate genes lay a foundational roadmap for future peanut breeding efforts focused on loss-of-susceptibility traits. Their functional validation and precision editing hold great promises for developing peanut varieties with stable resistance to A. flavus and reduced aflatoxin contamination.
While empirical evidence on peanut S genes remains limited, the candidates discussed, such as AhS5H1/2, ABR1, and MLO-like proteins, offer compelling starting points for functional validation. Although homologs of other known susceptibility genes from model species, such as DMR6, DND1, or NPR1, are likely present in peanut, their roles in aflatoxin-related susceptibility remain speculative and unverified. As no peanut S gene has yet been intentionally knocked out using CRISPR/Cas systems, future research should prioritize functional screens through targeted gene editing or TILLING of these candidate genes to assess whether their loss-of-function confers enhanced resistance without adverse agronomic effects. Such efforts would not only confirm the causal roles of these loci but also open the door to practical applications in breeding for durable, non-transgenic aflatoxin resistance. Disabling functional S genes can prevent fungal colonization and aflatoxin accumulation, while several biotechnological tools are now available to precisely target these loci.
7. Future Roadmap and Conclusions
Over the past two decades, significant advances have been made in understanding the complex biology of aflatoxin contamination in peanuts, alongside the development of molecular and genomic strategies aimed at mitigating its impact. Despite these achievements, the development of peanut cultivars with stable, heritable, and field-effective resistance to aflatoxin remains a critical and largely unmet objective [99]. One of the major reasons for this is the quantitative nature of aflatoxin resistance, which is typically governed by multiple small-effect loci. These loci are often environmentally responsive, with resistance expression strongly influenced by abiotic and biotic stress factors, such as drought, elevated temperatures, and insect damage. These stressors collectively create favorable conditions for A. flavus colonization and aflatoxin biosynthesis [72]. In addition to the complex inheritance of resistance traits, the genomic architecture of peanut poses additional challenges. Cultivated peanut is an allotetraploid species, comprising two highly similar sub genomes (A and B), which exhibit substantial gene redundancy and the presence of homeologous gene pairs [153]. This polyploidy complicates both traditional QTL mapping and modern genome-editing efforts, where distinguishing between homeolog-specific gene copies and achieving simultaneous edits across multiple gene copies becomes particularly difficult. Moreover, peanut transformation protocols remain inefficient and genotype-dependent, limiting the routine deployment of transgenic or edited lines for research or commercial purposes [140].
To overcome these obstacles, an integrated pipeline is essential (Figure 3). The pipeline illustrates the need to integrate phenotyping, genetic mapping, omics technologies, gene editing, and precision breeding into a unified framework. The first step involves the strategic collection of genetically diverse peanut germplasm from global repositories and agroecological zones. Broadening the genetic base is critical for capturing naturally occurring variation in resistance traits, especially in wild relatives and landraces that may harbor valuable alleles absent in elite cultivars. Next, these accessions are subjected to high-throughput phenotyping under both controlled and stress-inducing field conditions. Artificial inoculation assays, including in vitro seed colonization and pre-harvest aflatoxin quantification, enable accurate classification of resistant and susceptible genotypes [99]. Once reliable phenotypic data are available, the next phase involves integrating these data with genetic mapping strategies. QTL mapping in biparental populations and GWAS in diverse panels allow researchers to identify genomic regions associated with resistance traits [90,91,93]. These analyses provide a foundation for marker development, which can be used for marker-assisted selection (MAS) and to anchor multi-omics investigations. Importantly, combining QTLs and GWAS results with meta-analysis and haplotype-based approaches enhances the identification of stable, environment-independent loci across diverse genetic backgrounds [154]. Identification of the key loci opens the door to a deeper understanding of underlying biological mechanisms through multi-omics approaches. Transcriptomics reveal differentially expressed genes during pathogen infection; proteomics uncovers post-translational regulation and protein interactions; metabolomics highlights resistance-associated biochemical signatures; epigenomics explores methylation and chromatin changes; and interactomics identify regulatory and protein–protein interaction networks. Integration of these omics layers provides a systems-level view of the peanut A. flavus interaction, enabling the prioritization of high-confidence candidate genes within mapped loci [155]. Functional validation of these candidates is the next crucial step. Genome editing tools such as CRISPR/Cas9, Cas12a (Cpf1), base editors, and prime editing systems offer precise and versatile platforms for dissecting gene functions in peanut. These technologies allow researchers to knock out susceptibility genes, introduce resistance alleles, or correct detrimental variants with minimal genomic disruption. Multiplex genome editing holds special promise in peanut, where edits in multiple homeologous gene copies may be required to observe a phenotypic effect. Despite the promise, off-target effects, efficiency of editing in polyploid contexts, and the regeneration of edited plants remain areas for further optimization [156]. Following validation, elite cultivars can be improved by introgressing beneficial alleles or edited loci through backcross breeding combined with MAS or genomic selection. Moreover, pyramiding of multiple resistance QTLs alongside susceptibility gene knockouts may offer broad-spectrum, durable resistance that is less likely to be overcome by pathogen adaptation. Validation of improved lines under greenhouse, confined field trials, and multi-location environments is essential to confirm trait stability, yield performance, and resistance durability [157]. In the future, the integration of machine learning models, predictive breeding algorithms, and digital phenotyping platforms could further streamline this pipeline. Real-time stress monitoring, remote sensing, and UAV-based imaging can provide non-destructive assessments of aflatoxin contamination risk, supporting proactive breeding and management decisions [158].
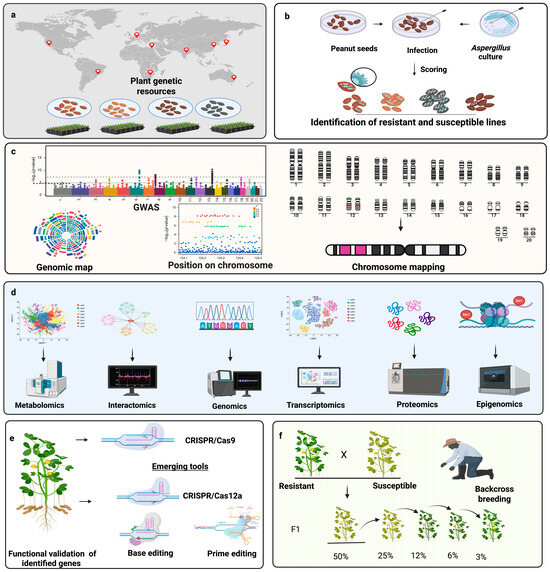
Figure 3.
Integrated pipeline for developing aflatoxin-resistant peanut cultivars. (a) Strategic collection of genetically diverse germplasm, including wild relatives, landraces, and elite lines from global locations to capture natural variation in resistance traits. (b) Artificial inoculation of seeds with Aspergillus flavus under controlled conditions, followed by phenotypic screening for resistance through fungal colonization and aflatoxin quantification. (c) Integration of phenotypic data using genome-wide association studies (GWAS) and quantitative trait loci (QTL) mapping to identify key loci. (d) Deployment of multi-omics platforms, including genomics, transcriptomics, proteomics, metabolomics, epigenomics, and interactomics to unravel resistance mechanisms and prioritize candidate genes. (e) Functional validation of candidate genes using advanced genome editing technologies. While CRISPR/Cas9 has been successfully applied in peanut, other systems such as Cas12a, base editors, and prime editors are considered promising tools but remain to be experimentally validated in this crop. (f) Introgression of validated resistance alleles or edited loci into elite cultivars using marker-assisted backcrossing and genomic selection to develop high-performing, aflatoxin-resistant lines.
In conclusion, the development of aflatoxin-resistant peanut cultivars requires a multidisciplinary and data-driven approach. This integrated strategy offers a realistic path forward to deliver aflatoxin-safe peanut cultivars that meet the demands of growers, processors, and consumers in both developed and developing regions.
Author Contributions
A.K.: conceptualization, writing, review, and editing; P.K.V., S.L. and N.P.: review and editing; A.K. and S.L.: Figures; M.R.J.: supervision, conceptualization, writing, review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We acknowledge the support from the Department of Plant and Soil Science at Texas Tech University for resources and infrastructure.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bhatnagar, D.; Cary, J.W.; Ehrlich, K.; Yu, J.; Cleveland, T.E. Understanding the genetics of regulation of aflatoxin production and Aspergillus flavus development. Mycopathologia 2006, 162, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Amaike, S.; Keller, N.P. Aspergillus flavus. Annu. Rev. Phytopathol. 2011, 49, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Cotty, P. Cottonseed losses mycotoxins. Compend. Cotton Dis. Part 2001, 1, 9–13. [Google Scholar]
- Horn, B.; Pitt, J. Yellow mold and aflatoxin. Compend. Peanut Dis. 1997, 2, 40–42. [Google Scholar]
- Leger, R.J.S.; Screen, S.E.; Shams-Pirzadeh, B. Lack of Host Specialization in Aspergillus flavus. Appl. Environ. Microbiol. 2000, 66, 320–324. [Google Scholar] [CrossRef]
- Klich, M.A. Environmental factors involved in preharvest Aspergillus flavus infection of cottonseed. JSM Mycotoxins 1988, 1988, 179–182. [Google Scholar] [CrossRef]
- Ncube, J.; Maphosa, M. Current state of knowledge on groundnut aflatoxins and their management from a plant breeding perspective: Lessons for Africa. Sci. Afr. 2020, 7, e00264. [Google Scholar] [CrossRef]
- Pal, K.K.; Dey, R.; Tilak, K. Fungal diseases of groundnut: Control and future challenges. In Future Challenges in Crop Protection Against Fungal Pathogens; Springer: New York, NY, USA, 2014; pp. 1–29. [Google Scholar]
- Pandey, M.K.; Kumar, R.; Pandey, A.K.; Soni, P.; Gangurde, S.S.; Sudini, H.K.; Fountain, J.C.; Liao, B.; Desmae, H.; Okori, P. Mitigating aflatoxin contamination in groundnut through a combination of genetic resistance and post-harvest management practices. Toxins 2019, 11, 315. [Google Scholar] [CrossRef] [PubMed]
- Soni, P.; Gangurde, S.S.; Ortega-Beltran, A.; Kumar, R.; Parmar, S.; Sudini, H.K.; Lei, Y.; Ni, X.; Huai, D.; Fountain, J.C. Functional biology and molecular mechanisms of host-pathogen interactions for aflatoxin contamination in groundnut (Arachis hypogaea L.) and maize (Zea mays L.). Front. Microbiol. 2020, 11, 227. [Google Scholar] [CrossRef]
- Commey, L.; Tengey, T.K.; Cobos, C.J.; Dampanaboina, L.; Dhillon, K.K.; Pandey, M.K.; Sudini, H.K.; Falalou, H.; Varshney, R.K.; Burow, M.D. Peanut seed coat acts as a physical and biochemical barrier against Aspergillus flavus infection. J. Fungi 2021, 7, 1000. [Google Scholar] [CrossRef] [PubMed]
- Wicklow, D.; Wilson, D.; Nelsen, T. Survival of Aspergillus flavus sclerotia and conidia buried in soil in Illinois or Georgia. Phytopathology 1993, 83, 1141. [Google Scholar] [CrossRef]
- Magan, N.; Aldred, D. Post-harvest control strategies: Minimizing mycotoxins in the food chain. Int. J. Food Microbiol. 2007, 119, 131–139. [Google Scholar] [CrossRef]
- Shabeer, S.; Asad, S.; Jamal, A.; Ali, A. Aflatoxin contamination, its impact and management strategies: An updated review. Toxins 2022, 14, 307. [Google Scholar] [CrossRef]
- Alam, T.; Anco, D.J.; Rustgi, S. Management of Aflatoxins in Peanut; Clemson University: Clemson, SC, USA, 2020. [Google Scholar]
- Groopman, J.D.; Kensler, T.W.; Wild, C.P. Protective interventions to prevent aflatoxin-induced carcinogenesis in developing countries. Annu. Rev. Public Health 2008, 29, 187–203. [Google Scholar] [CrossRef]
- Gong, Y.Y.; Cardwell, K.; Hounsa, A.; Egal, S.; Turner, P.C.; Hall, A.J.; Wild, C.P. Dietary aflatoxin exposure and impaired growth in young children from Benin and Togo: Cross sectional study. BMJ 2002, 325, 20–21. [Google Scholar] [CrossRef]
- Turner, P.C.; Moore, S.E.; Hall, A.J.; Prentice, A.M.; Wild, C.P. Modification of immune function through exposure to dietary aflatoxin in Gambian children. Environ. Health Perspect. 2003, 111, 217–220. [Google Scholar] [CrossRef]
- Guerre, P. Worldwide mycotoxins exposure in pig and poultry feed formulations. Toxins 2016, 8, 350. [Google Scholar] [CrossRef]
- Rustom, I.Y. Aflatoxin in food and feed: Occurrence, legislation and inactivation by physical methods. Food Chem. 1997, 59, 57–67. [Google Scholar] [CrossRef]
- Nigam, S.; Waliyar, F.; Aruna, R.; Reddy, S.; Kumar, P.L.; Craufurd, P.Q.; Diallo, A.; Ntare, B.; Upadhyaya, H.D. Breeding peanut for resistance to aflatoxin contamination at ICRISAT. Peanut Sci. 2009, 36, 42–49. [Google Scholar] [CrossRef]
- Guo, B.; Fedorova, N.D.; Chen, X.; Wan, C.-H.; Wang, W.; Nierman, W.C.; Bhatnagar, D.; Yu, J. Gene expression profiling and identification of resistance genes to Aspergillus flavus infection in peanut through EST and microarray strategies. Toxins 2011, 3, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Korani, W.; Chu, Y.; Holbrook, C.C.; Ozias-Akins, P. Insight into genes regulating postharvest aflatoxin contamination of tetraploid peanut from transcriptional profiling. Genetics 2018, 209, 143–156. [Google Scholar] [CrossRef]
- Nayak, S.N.; Agarwal, G.; Pandey, M.K.; Sudini, H.K.; Jayale, A.S.; Purohit, S.; Desai, A.; Wan, L.; Guo, B.; Liao, B. Aspergillus flavus infection triggered immune responses and host-pathogen cross-talks in groundnut during in-vitro seed colonization. Sci. Rep. 2017, 7, 9659. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, X.-P.; Li, H.-F.; Liu, H.-Y.; Hong, Y.-B.; Yang, Q.-L.; Chi, X.-Y.; Yang, Z.; Yu, S.-L.; Li, L. Transcriptome identification of the resistance-associated genes (RAGs) to Aspergillus flavus infection in pre-harvested peanut (Arachis hypogaea). Funct. Plant Biol. 2012, 40, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Tumukunde, E.; Xie, R.; Wang, S. Updates on the functions and molecular mechanisms of the genes involved in Aspergillus flavus development and biosynthesis of aflatoxins. J. Fungi 2021, 7, 666. [Google Scholar] [CrossRef]
- Birch, A. Biosynthesis of polyketides and related compounds. Science 1967, 156, 202–206. [Google Scholar] [CrossRef]
- Caceres, I.; Al Khoury, A.; El Khoury, R.; Lorber, S.; Oswald, P.I.; El Khoury, A.; Atoui, A.; Puel, O.; Bailly, J.-D. Aflatoxin biosynthesis and genetic regulation: A review. Toxins 2020, 12, 150. [Google Scholar] [CrossRef]
- Georgianna, D.R.; Payne, G.A. Genetic regulation of aflatoxin biosynthesis: From gene to genome. Fungal Genet. Biol. 2009, 46, 113–125. [Google Scholar] [CrossRef]
- Khan, R.; Ghazali, F.M.; Mahyudin, N.A.; Samsudin, N.I.P. Aflatoxin biosynthesis, genetic regulation, toxicity, and control strategies: A review. J. Fungi 2021, 7, 606. [Google Scholar] [CrossRef]
- Chang, P.-K. The Aspergillus parasiticus protein AFLJ interacts with the aflatoxin pathway-specific regulator AFLR. Mol. Genet. Genom. 2003, 268, 711–719. [Google Scholar] [CrossRef]
- Cary, J.; Ehrlich, K.; Wright, M.; Chang, P.-K.; Bhatnagar, D. Generation of aflR disruption mutants of Aspergillus parasiticus. Appl. Microbiol. Biotechnol. 2000, 53, 680–684. [Google Scholar] [CrossRef]
- Flaherty, J.E.; Payne, G. Overexpression of aflR leads to upregulation of pathway gene transcription and increased aflatoxin production in Aspergillus flavus. Appl. Environ. Microbiol. 1997, 63, 3995–4000. [Google Scholar] [CrossRef]
- Caceres, I.; El Khoury, R.; Medina, Á.; Lippi, Y.; Naylies, C.; Atoui, A.; El Khoury, A.; Oswald, I.P.; Bailly, J.-D.; Puel, O. Deciphering the anti-aflatoxinogenic properties of eugenol using a large-scale q-PCR approach. Toxins 2016, 8, 123. [Google Scholar] [CrossRef]
- Dhanamjayulu, P.; Boga, R.B.; Mehta, A. Inhibition of aflatoxin B1 biosynthesis and down regulation of aflR and aflB genes in presence of benzimidazole derivatives without impairing the growth of Aspergillus flavus. Toxicon 2019, 170, 60–67. [Google Scholar] [CrossRef]
- Du, W.; Obrian, G.; Payne, G. Function and regulation of aflJ in the accumulation of aflatoxin early pathway intermediate in Aspergillus flavus. Food Addit. Contam. 2007, 24, 1043–1050. [Google Scholar] [CrossRef]
- Cary, J.W.; Entwistle, S.; Satterlee, T.; Mack, B.M.; Gilbert, M.K.; Chang, P.K.; Scharfenstein, L.; Yin, Y.; Calvo, A.M. The transcriptional regulator Hbx1 affects the expression of thousands of genes in the aflatoxin-producing fungus Aspergillus flavus. G3: Genes Genomes Genet. 2019, 9, 167–178. [Google Scholar] [CrossRef]
- Cary, J.W.; Harris-Coward, P.Y.; Ehrlich, K.C.; Mack, B.M.; Kale, S.P.; Larey, C.; Calvo, A.M. NsdC and NsdD affect Aspergillus flavus morphogenesis and aflatoxin production. Eukaryot. Cell 2012, 11, 1104–1111. [Google Scholar] [CrossRef]
- Gilbert, M.K.; Majumdar, R.; Rajasekaran, K.; Chen, Z.-Y.; Wei, Q.; Sickler, C.M.; Lebar, M.D.; Cary, J.W.; Frame, B.R.; Wang, K. RNA interference-based silencing of the alpha-amylase (amy1) gene in Aspergillus flavus decreases fungal growth and aflatoxin production in maize kernels. Planta 2018, 247, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yang, G.; Zhang, D.; Liu, Y.; Li, Y.; Lin, G.; Guo, Z.; Wang, S.; Zhuang, Z. The PHD transcription factor Rum1 regulates morphogenesis and aflatoxin biosynthesis in Aspergillus flavus. Toxins 2018, 10, 301. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zha, W.; Liang, L.; Fasoyin, O.E.; Wu, L.; Wang, S. The bZIP transcription factor AflRsmA regulates aflatoxin B1 biosynthesis, oxidative stress response and sclerotium formation in Aspergillus flavus. Toxins 2020, 12, 271. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Zhang, F.; Nie, X.; Wang, X.; Yuan, J.; Zhuang, Z.; Wang, S. Essential APSES transcription factors for mycotoxin synthesis, fungal development, and pathogenicity in Aspergillus flavus. Front. Microbiol. 2017, 8, 2277. [Google Scholar] [CrossRef]
- Zhang, F.; Geng, L.; Huang, L.; Deng, J.; Fasoyin, O.E.; Yao, G.; Wang, S. Contribution of peroxisomal protein importer AflPex5 to development and pathogenesis in the fungus Aspergillus flavus. Curr. Genet. 2018, 64, 1335–1348. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, G.; Geng, L.; Lu, X.; Yang, K.; Yuan, J.; Nie, X.; Zhuang, Z.; Wang, S. The stress response regulator AflSkn7 influences morphological development, stress response, and pathogenicity in the fungus Aspergillus flavus. Toxins 2016, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Nie, X.; Yang, K.; Xu, P.; Wang, X.; Liu, M.; Yang, Y.; Chen, Z.; Wang, S. Molecular and structural basis of nucleoside diphosphate kinase–mediated regulation of spore and sclerotia development in the fungus Aspergillus flavus. J. Biol. Chem. 2019, 294, 12415–12431. [Google Scholar] [CrossRef]
- Yuan, J.; Li, D.; Qin, L.; Shen, J.; Guo, X.; Tumukunde, E.; Li, M.; Wang, S. HexA is required for growth, aflatoxin biosynthesis and virulence in Aspergillus flavus. BMC Mol. Biol. 2019, 20, 4. [Google Scholar] [CrossRef]
- Amaike, S.; Keller, N.P. Distinct roles for VeA and LaeA in development and pathogenesis of Aspergillus flavus. Eukaryot. Cell 2009, 8, 1051–1060. [Google Scholar] [CrossRef]
- Chang, P.-K.; Scharfenstein, L.L.; Ehrlich, K.C.; Wei, Q.; Bhatnagar, D.; Ingber, B.F. Effects of laeA deletion on Aspergillus flavus conidial development and hydrophobicity may contribute to loss of aflatoxin production. Fungal Biol. 2012, 116, 298–307. [Google Scholar] [CrossRef]
- Duran, R.M.; Cary, J.W.; Calvo, A.M. Production of cyclopiazonic acid, aflatrem, and aflatoxin by Aspergillus flavus is regulated by veA, a gene necessary for sclerotial formation. Appl. Microbiol. Biotechnol. 2007, 73, 1158–1168. [Google Scholar] [CrossRef]
- Zhao, X.; Spraker, J.E.; Bok, J.W.; Velk, T.; He, Z.-M.; Keller, N.P. A cellular fusion cascade regulated by LaeA is required for sclerotial development in Aspergillus flavus. Front. Microbiol. 2017, 8, 1925. [Google Scholar] [CrossRef]
- Zhi, Q.-Q.; He, L.; Li, J.-Y.; Li, J.; Wang, Z.-L.; He, G.-Y.; He, Z.-M. The kinetochore protein Spc105, a novel interaction partner of LaeA, regulates development and secondary metabolism in Aspergillus flavus. Front. Microbiol. 2019, 10, 1881. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, C.C.; Guo, B.; Wilson, D.; Timper, P. The US breeding program to develop peanut with drought tolerance and reduced aflatoxin contamination. Peanut Sci. 2009, 36, 50–53. [Google Scholar] [CrossRef]
- Upadhyaya, H.; Nigam, S.; Thakur, R. Genetic Enhancement for Resistance to Aflatoxin Contamination in Groundnut. In Proceedings of the Summary Proceedings of the Seventh ICRISAT Regional Groundnut Meeting for Western and Central Africa, Cotonu, Benin, 6–8 December 2000. [Google Scholar]
- Sudhakar, P.; Latha, P.; Babitha, M.; Reddy, P.; Naidu, P. Relationship of drought tolerance traits with aflatoxin contamination in groundnut. Indian J. Plant Physiol. 2007, 12, 261–265. [Google Scholar]
- Guimarães, P.M.; Brasileiro, A.C.; Morgante, C.V.; Martins, A.C.; Pappas, G.; Silva, O.B.; Togawa, R.; Leal-Bertioli, S.C.; Araujo, A.C.; Moretzsohn, M.C. Global transcriptome analysis of two wild relatives of peanut under drought and fungi infection. BMC Genom. 2012, 13, 387. [Google Scholar] [CrossRef]
- LaPrade, J. Correlation of peanut seed-coat surface wax accumulations with tolerance to colonization by Aspergillus flavus. J. Am. Peanut Res. 1973, 5, 89–94. [Google Scholar]
- Liang, X. Studies on the Mechanism and Inheritance of Resistance to Aspergillus flavus Invasion and Aflatoxin Production in Peanut (Arachis hypogaea L.); University of South China: Guangzhou, China, 2002. [Google Scholar]
- Sobolev, V.S. Localized production of phytoalexins by peanut (Arachis hypogaea) kernels in response to invasion by Aspergillus species. J. Agric. Food Chem. 2008, 56, 1949–1954. [Google Scholar] [CrossRef]
- Win, M.M.; Abdul-Hamid, A.; Baharin, B.S.; Anwar, F.; Saari, N. Effects of roasting on phenolics composition and antioxidant activity of peanut (Arachis hypogaea L.) kernel flour. Eur. Food Res. Technol. 2011, 233, 599–608. [Google Scholar] [CrossRef]
- Ahmad, M.M.; Qamar, F.; Saifi, M.; Abdin, M.Z. Natural inhibitors: A sustainable way to combat aflatoxins. Front. Microbiol. 2022, 13, 993834. [Google Scholar] [CrossRef]
- Hua, S.S.; Grosjean, O.K.; Baker, J. Inhibition of aflatoxin biosynthesis by phenolic compounds. Lett. Appl. Microbiol. 1999, 29, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Campbell, B.C.; Mahoney, N.E.; Chan, K.L.; Molyneux, R.J. Identification of phenolics for control of Aspergillus flavus using Saccharomyces cerevisiae in a model target-gene bioassay. J. Agric. Food Chem. 2004, 52, 7814–7821. [Google Scholar] [CrossRef]
- Lindsey, D.; Turner, R.B. Inhibition of growth of Aspergillus flavus and Trichoderma viride by peanut embryos. Mycopathologia 1975, 55, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Pan, R.; Zhou, G. Relationship of trypsin inhibitor in peanut seed and resistance to Aspergillus flavus invasion. Acta Agron. Sin. 2003, 29, 295–299. [Google Scholar]
- Turner, R.B.; Lindsey, D.; Davis, D.D.; Bishop, R.D. Isolation and identification of 5, 7-Dimethoxyisoflavone, an inhibitor of Aspergillus flavus from peanuts. Mycopathologia 1975, 57, 39–40. [Google Scholar] [CrossRef]
- Sharma, S.; Choudhary, B.; Yadav, S.; Mishra, A.; Mishra, V.K.; Chand, R.; Chen, C.; Pandey, S.P. Metabolite profiling identified pipecolic acid as an important component of peanut seed resistance against Aspergillus flavus infection. J. Hazard. Mater. 2021, 404, 124155. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, D.; Yin, H.; Wang, H.; Cao, C.; Wang, J.; Zheng, J.; Liu, J. Transcriptomic and metabolomic analyses of the response of resistant peanut seeds to Aspergillus flavus infection. Toxins 2023, 15, 414. [Google Scholar] [CrossRef]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Cui, M.; Han, S.; Wang, D.; Haider, M.S.; Guo, J.; Zhao, Q.; Du, P.; Sun, Z.; Qi, F.; Zheng, Z. Gene Co-expression Network Analysis of the Comparative Transcriptome Identifies Hub Genes Associated With Resistance to Aspergillus flavus L. in Cultivated Peanut (Arachis hypogaea L.). Front. Plant Sci. 2022, 13, 899177. [Google Scholar] [CrossRef]
- Song, H.; Wang, P.; Li, C.; Han, S.; Zhao, C.; Xia, H.; Bi, Y.; Guo, B.; Zhang, X.; Wang, X. Comparative analysis of NBS-LRR genes and their response to Aspergillus flavus in Arachis. PLoS ONE 2017, 12, e0171181. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, X.; Zhou, X.; Huang, L.; Yan, L.; Lei, Y.; Liao, B.; Huang, J.; Huang, S.; Wei, W. Dynamics in the resistant and susceptible peanut (Arachis hypogaea L.) root transcriptome on infection with the Ralstonia solanacearum. BMC Genom. 2014, 15, 1078. [Google Scholar] [CrossRef] [PubMed]
- Fountain, J.C.; Khera, P.; Yang, L.; Nayak, S.N.; Scully, B.T.; Lee, R.D.; Chen, Z.-Y.; Kemerait, R.C.; Varshney, R.K.; Guo, B. Resistance to Aspergillus flavus in maize and peanut: Molecular biology, breeding, environmental stress, and future perspectives. Crop J. 2015, 3, 229–237. [Google Scholar] [CrossRef]
- Jayaprakash, A.; Thanmalagan, R.R.; Roy, A.; Arunachalam, A.; Lakshmi, P. Strategies to understand Aspergillus flavus resistance mechanism in Arachis hypogaea L. Curr. Plant Biol. 2019, 20, 100123. [Google Scholar]
- Liang, X.; Luo, M.; Guo, B. Resistance mechanisms to Aspergillus flavus infection and aflatoxin contamination in peanut (Arachis hypogaea). Plant Pathol. J. 2006, 5, 115–124. [Google Scholar] [CrossRef]
- Burow, G.B.; Gardner, H.W.; Keller, N.P. A peanut seed lipoxygenase responsive to Aspergillus colonization. Plant Mol. Biol. 2000, 42, 689–701. [Google Scholar] [CrossRef]
- Hammond-Kosack, K.E.; Jones, J. Resistance gene-dependent plant defense responses. Plant Cell 1996, 8, 1773. [Google Scholar][Green Version]
- Derksen, H.; Rampitsch, C.; Daayf, F. Signaling cross-talk in plant disease resistance. Plant Sci. 2013, 207, 79–87. [Google Scholar] [CrossRef]
- Spoel, S.H.; Johnson, J.S.; Dong, X. Regulation of tradeoffs between plant defenses against pathogens with different lifestyles. Proc. Natl. Acad. Sci. USA 2007, 104, 18842–18847. [Google Scholar] [CrossRef]
- Rajasekaran, K.; Sayler, R.J.; Sickler, C.M.; Majumdar, R.; Jaynes, J.M.; Cary, J.W. Control of Aspergillus flavus growth and aflatoxin production in transgenic maize kernels expressing a tachyplesin-derived synthetic peptide, AGM182. Plant Sci. 2018, 270, 150–156. [Google Scholar] [CrossRef]
- Reverberi, M.; Punelli, M.; Scala, V.; Scarpari, M.; Uva, P.; Mentzen, W.I.; Dolezal, A.L.; Woloshuk, C.; Pinzari, F.; Fabbri, A.A. Genotypic and phenotypic versatility of Aspergillus flavus during maize exploitation. PLoS ONE 2013, 8, e68735. [Google Scholar] [CrossRef]
- Gao, X.; Brodhagen, M.; Isakeit, T.; Brown, S.H.; Göbel, C.; Betran, J.; Feussner, I.; Keller, N.P.; Kolomiets, M.V. Inactivation of the lipoxygenase ZmLOX3 increases susceptibility of maize to Aspergillus spp. Mol. Plant-Microbe Interact. 2009, 22, 222–231. [Google Scholar] [CrossRef]
- Xuanqiang, L.; Guiyuan, Z.; Ruichi, P. Changes of some biochemical substances in peanut seeds under infection of Aspergillus flavus and their role in resistance to seed invasion. Chin. J. Oil Crop Sci. 2001, 23, 26–30. [Google Scholar]
- Liang, X.; Holbrook, C.; Lynch, R.; Guo, B. β-1, 3-Glucanase activity in peanut seed (Arachis hypogaea) is induced by inoculation with Aspergillus flavus and copurifies with a conglutin-like protein. Phytopathology 2005, 95, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Bhatnagar-Mathur, P.; Waliyar, F.; Sharma, K.K. Overexpression of a chitinase gene in transgenic peanut confers enhanced resistance to major soil borne and foliar fungal pathogens. J. Plant Biochem. Biotechnol. 2013, 22, 222–233. [Google Scholar] [CrossRef]
- Wotton, H.R.; Strange*, R.N. Circumstantial evidence for phytoalexin involvement in the resistance of peanuts to Aspergillus flavus. Microbiology 1985, 131, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Strange, R. Phytoalexin accumulation in groundnuts in response to wounding. Plant Sci. 1991, 78, 157–163. [Google Scholar] [CrossRef]
- Li, X.-M.; Li, Z.-Y.; Wang, Y.-D.; Wang, J.-Q.; Yang, P.-L. Quercetin inhibits the proliferation and aflatoxins biosynthesis of Aspergillus flavus. Toxins 2019, 11, 154. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Zhuang, W. Obliging tactics to mitigate the intricate problem of aflatoxin contamination in peanut: A review. J. Food Sci. Technol. 2019, 4, 986–1005. [Google Scholar]
- Yu, B.; Jiang, H.; Pandey, M.K.; Huang, L.; Huai, D.; Zhou, X.; Kang, Y.; Varshney, R.K.; Sudini, H.K.; Ren, X. Identification of two novel peanut genotypes resistant to aflatoxin production and their SNP markers associated with resistance. Toxins 2020, 12, 156. [Google Scholar] [CrossRef]
- Yu, B.; Huai, D.; Huang, L.; Kang, Y.; Ren, X.; Chen, Y.; Zhou, X.; Luo, H.; Liu, N.; Chen, W.; et al. Identification of genomic regions and diagnostic markers for resistance to aflatoxin contamination in peanut (Arachis hypogaea L.). BMC Genet. 2019, 20, 32. [Google Scholar] [CrossRef]
- Liang, X.; Zhou, G.; Hong, Y.; Chen, X.; Liu, H.; Li, S. Overview of research progress on peanut (Arachis hypogaea L.) host resistance to aflatoxin contamination and genomics at the Guangdong Academy of Agricultural Sciences. Peanut Sci. 2009, 36, 29–34. [Google Scholar] [CrossRef]
- Khan, S.A.; Chen, H.; Deng, Y.; Chen, Y.; Zhang, C.; Cai, T.; Ali, N.; Mamadou, G.; Xie, D.; Guo, B. High-density SNP map facilitates fine mapping of QTLs and candidate genes discovery for Aspergillus flavus resistance in peanut (Arachis hypogaea). Theor. Appl. Genet. 2020, 133, 2239–2257. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, H.; Yu, B.; Ding, Y.; Kang, Y.; Huang, L.; Zhou, X.; Liu, N.; Chen, W.; Guo, J. High-density genetic linkage map construction using whole-genome resequencing for mapping QTLs of resistance to Aspergillus flavus infection in peanut. Front. Plant Sci. 2021, 12, 745408. [Google Scholar] [CrossRef]
- Jin, G.; Liu, N.; Yu, B.; Jiang, Y.; Luo, H.; Huang, L.; Zhou, X.; Yan, L.; Kang, Y.; Huai, D. Identification and pyramiding major QTL loci for simultaneously enhancing Aflatoxin resistance and yield components in peanut. Genes 2023, 14, 625. [Google Scholar] [CrossRef]
- Yu, B.; Liu, N.; Huang, L.; Luo, H.; Zhou, X.; Lei, Y.; Yan, L.; Wang, X.; Chen, W.; Kang, Y. Identification and application of a candidate gene AhAftr1 for aflatoxin production resistance in peanut seed (Arachis hypogaea L.). J. Adv. Res. 2024, 62, 15–26. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, E.; Chen, X.; Li, L.; Liang, X. Identification of seed proteins associated with resistance to pre-harvested aflatoxin contamination in peanut (Arachis hypogaea L). BMC Plant Biol. 2010, 10, 267. [Google Scholar] [CrossRef]
- Zhao, X.; Li, C.; Yan, C.; Wang, J.; Yuan, C.; Zhang, H.; Shan, S. Transcriptome and proteome analyses of resistant preharvest peanut seed coat in response to Aspergillus flavus infection. Electron. J. Biotechnol. 2019, 39, 82–90. [Google Scholar] [CrossRef]
- Jayaprakash, A.; Roy, A.; Thanmalagan, R.R.; Arunachalam, A.; Ptv, L. Immune response gene coexpression network analysis of Arachis hypogaea infected with Aspergillus flavus. Genomics 2021, 113, 2977–2988. [Google Scholar] [CrossRef]
- Ding, Y.; Qiu, X.; Luo, H.; Huang, L.; Guo, J.; Yu, B.; Sudini, H.; Pandey, M.; Kang, Y.; Liu, N. Comprehensive evaluation of Chinese peanut mini-mini core collection and QTL mapping for aflatoxin resistance. BMC Plant Biol. 2022, 22, 207. [Google Scholar] [CrossRef]
- Clevenger, J.; Marasigan, K.; Liakos, V.; Sobolev, V.; Vellidis, G.; Holbrook, C.; Ozias-Akins, P. RNA sequencing of contaminated seeds reveals the state of the seed permissive for pre-harvest aflatoxin contamination and points to a potential susceptibility factor. Toxins 2016, 8, 317. [Google Scholar] [CrossRef]
- Liang, B.; Bai, Y.; Zang, C.; Pei, X.; Xie, J.; Lin, Y.; Liu, X.; Ahsan, T.; Liang, C. Overexpression of the first peanut-susceptible gene, AhS5H1 or AhS5H2, enhanced susceptibility to Pst DC3000 in Arabidopsis. Int. J. Mol. Sci. 2023, 24, 14210. [Google Scholar] [CrossRef] [PubMed]
- Traore, S.M.; Han, S.; Binagwa, P.; Xu, W.; Chen, X.; Liu, F.; He, G. Genome-wide identification of mlo genes in the cultivated peanut (Arachis hypogaea L.). Euphytica 2021, 217, 61. [Google Scholar] [CrossRef]
- Fusaro, A.F.; Matthew, L.; Smith, N.A.; Curtin, S.J.; Dedic-Hagan, J.; Ellacott, G.A.; Watson, J.M.; Wang, M.B.; Brosnan, C.; Carroll, B.J. RNA interference-inducing hairpin RNAs in plants act through the viral defence pathway. EMBO Rep. 2006, 7, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Ghag, S.B.; Shekhawat, U.K.; Ganapathi, T.R. Host-induced post-transcriptional hairpin RNA-mediated gene silencing of vital fungal genes confers efficient resistance against F usarium wilt in banana. Plant Biotechnol. J. 2014, 12, 541–553. [Google Scholar] [CrossRef]
- Koch, A.; Wassenegger, M. Host-induced gene silencing–mechanisms and applications. New Phytol. 2021, 231, 54–59. [Google Scholar] [CrossRef]
- Sharma, K.K.; Pothana, A.; Prasad, K.; Shah, D.; Kaur, J.; Bhatnagar, D.; Chen, Z.Y.; Raruang, Y.; Cary, J.W.; Rajasekaran, K. Peanuts that keep aflatoxin at bay: A threshold that matters. Plant Biotechnol. J. 2018, 16, 1024–1033. [Google Scholar] [CrossRef]
- Prasad, K.; Yogendra, K.; Sanivarapu, H.; Rajasekaran, K.; Cary, J.W.; Sharma, K.K.; Bhatnagar-Mathur, P. Multiplexed host-induced gene silencing of Aspergillus flavus genes confers aflatoxin resistance in groundnut. Toxins 2023, 15, 319. [Google Scholar] [CrossRef]
- Arias, R.S.; Dang, P.M.; Sobolev, V.S. RNAi-mediated control of aflatoxins in peanut: Method to analyze mycotoxin production and transgene expression in the peanut/Aspergillus pathosystem. J. Vis. Exp. 2015, 21, 53398. [Google Scholar]
- Power, I.L.; Faustinelli, P.C.; Orner, V.A.; Sobolev, V.S.; Arias, R.S. Analysis of small RNA populations generated in peanut leaves after exogenous application of dsRNA and dsDNA targeting aflatoxin synthesis genes. Sci. Rep. 2020, 10, 13820. [Google Scholar] [CrossRef] [PubMed]
- Masanga, J.O.; Matheka, J.M.; Omer, R.A.; Ommeh, S.C.; Monda, E.O.; Alakonya, A.E. Downregulation of transcription factor aflR in Aspergillus flavus confers reduction to aflatoxin accumulation in transgenic maize with alteration of host plant architecture. Plant Cell Rep. 2015, 34, 1379–1387. [Google Scholar] [CrossRef]
- Omolehin, O.; Raruang, Y.; Hu, D.; Han, Z.-Q.; Wei, Q.; Wang, K.; Rajasekaran, K.; Cary, J.W.; Chen, Z.-Y. Resistance to aflatoxin accumulation in maize mediated by host-induced silencing of the Aspergillus flavus alkaline protease (alk) gene. J. Fungi 2021, 7, 904. [Google Scholar] [CrossRef]
- Raruang, Y.; Omolehin, O.; Hu, D.; Wei, Q.; Han, Z.-Q.; Rajasekaran, K.; Cary, J.W.; Wang, K.; Chen, Z.-Y. Host induced gene silencing targeting Aspergillus flavus aflM reduced aflatoxin contamination in transgenic maize under field conditions. Front. Microbiol. 2020, 11, 754. [Google Scholar] [CrossRef]
- Raruang, Y.; Omolehin, O.; Hu, D.; Wei, Q.; Promyou, S.; Parekattil, L.J.; Rajasekaran, K.; Cary, J.W.; Wang, K.; Chen, Z.-Y. Targeting the Aspergillus flavus p2c gene through host-induced gene silencing reduces A. flavus infection and aflatoxin contamination in transgenic maize. Front. Plant Sci. 2023, 14, 1150086. [Google Scholar] [CrossRef]
- Thakare, D.; Zhang, J.; Wing, R.A.; Cotty, P.J.; Schmidt, M.A. Aflatoxin-free transgenic maize using host-induced gene silencing. Sci. Adv. 2017, 3, e1602382. [Google Scholar] [CrossRef]
- Xie, C.; Wen, S.; Liu, H.; Chen, X.; Li, H.; Hong, Y.; Liang, X. Overexpression of ARAhPR10, a member of the PR10 family, decreases levels of Aspergillus flavus infection in peanut seeds. Am. J. Plant Sci. 2013, 4, 602–607. [Google Scholar] [CrossRef]
- Sundaresha, S.; Manoj Kumar, A.; Rohini, S.; Math, S.; Keshamma, E.; Chandrashekar, S.; Udayakumar, M. Enhanced protection against two major fungal pathogens of groundnut, Cercospora arachidicola and Aspergillus flavus in transgenic groundnut over-expressing a tobacco β 1–3 glucanase. Eur. J. Plant Pathol. 2010, 126, 497–508. [Google Scholar] [CrossRef]
- Yu, L.; Chen, Y.; Zeng, X.; Lou, Y.; Baldwin, I.T.; Li, R. Brown planthoppers manipulate rice sugar transporters to benefit their own feeding. Curr. Biol. 2024, 34, 2990–2996.e2994. [Google Scholar] [CrossRef]
- Garcia-Ruiz, H.; Szurek, B.; Van den Ackerveken, G. Stop helping pathogens: Engineering plant susceptibility genes for durable resistance. Curr. Opin. Biotechnol. 2021, 70, 187–195. [Google Scholar] [CrossRef]
- Wang, D.; Mandal, P.; Rahman, M.S.; Yang, L. Engineering tomato disease resistance by manipulating susceptibility genes. Front. Genome Ed. 2025, 7, 1537148. [Google Scholar] [CrossRef]
- Khadgi, A.; Zayed, O.; Sagawa, C.H.; Zhang, F.; Seymour, D.K.; Irish, V.F. Mutations in the SWEET15 Sugar Transporter Gene Affect Response of Citrus to Huanglongbing Disease and Citrus Canker. Mol. Plant Pathol. 2025, 26, e70094. [Google Scholar] [CrossRef]
- Pavan, S.; Jacobsen, E.; Visser, R.G.; Bai, Y. Loss of susceptibility as a novel breeding strategy for durable and broad-spectrum resistance. Mol. Breed. 2010, 25, 1–12. [Google Scholar] [CrossRef]
- Pessina, S.; Lenzi, L.; Perazzolli, M.; Campa, M.; Dalla Costa, L.; Urso, S.; Valè, G.; Salamini, F.; Velasco, R.; Malnoy, M. Knockdown of MLO genes reduces susceptibility to powdery mildew in grapevine. Hortic. Res. 2016, 3, 16016. [Google Scholar] [CrossRef]
- Tapia, R.R.; Barbey, C.R.; Chandra, S.; Folta, K.M.; Whitaker, V.M.; Lee, S. Evolution of the MLO gene families in octoploid strawberry (Fragaria× ananassa) and progenitor diploid species identified potential genes for strawberry powdery mildew resistance. Hortic. Res. 2021, 8, 153. [Google Scholar] [CrossRef] [PubMed]
- van Schie, C.C.; Takken, F.L. Susceptibility genes 101: How to be a good host. Annu. Rev. Phytopathol. 2014, 52, 551–581. [Google Scholar] [CrossRef] [PubMed]
- Bent, A.F.; Mackey, D. Elicitors, effectors, and R genes: The new paradigm and a lifetime supply of questions. Annu. Rev. Phytopathol. 2007, 45, 399–436. [Google Scholar] [CrossRef] [PubMed]
- Koseoglou, E.; van der Wolf, J.M.; Visser, R.G.; Bai, Y. Susceptibility reversed: Modified plant susceptibility genes for resistance to bacteria. Trends Plant Sci. 2022, 27, 69–79. [Google Scholar] [CrossRef]
- Galletta, B.J.; Rusan, N.M. A yeast two-hybrid approach for probing protein–protein interactions at the centrosome. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 129, pp. 251–277. [Google Scholar]
- Gibriel, H.A.; Thomma, B.P.; Seidl, M.F. The age of effectors: Genome-based discovery and applications. Phytopathology 2016, 106, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Kusch, S.; Panstruga, R. mlo-based resistance: An apparently universal “weapon” to defeat powdery mildew disease. Mol. Plant-Microbe Interact. 2017, 30, 179–189. [Google Scholar] [CrossRef]
- Van Damme, M.; Huibers, R.P.; Elberse, J.; Van den Ackerveken, G. Arabidopsis DMR6 encodes a putative 2OG-Fe (II) oxygenase that is defense-associated but required for susceptibility to downy mildew. Plant J. 2008, 54, 785–793. [Google Scholar] [CrossRef]
- Chen, L.Q. SWEET sugar transporters for phloem transport and pathogen nutrition. New Phytol. 2014, 201, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Streubel, J.; Pesce, C.; Hutin, M.; Koebnik, R.; Boch, J.; Szurek, B. Five phylogenetically close rice SWEET genes confer TAL effector-mediated susceptibility to Xanthomonas oryzae pv. oryzae. New Phytol. 2013, 200, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Oliva, R.; Ji, C.; Atienza-Grande, G.; Huguet-Tapia, J.C.; Perez-Quintero, A.; Li, T.; Eom, J.-S.; Li, C.; Nguyen, H.; Liu, B. Broad-spectrum resistance to bacterial blight in rice using genome editing. Nat. Biotechnol. 2019, 37, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Elliott, K.; Veley, K.M.; Jensen, G.; Gilbert, K.B.; Norton, J.; Kambic, L.; Yoder, M.; Weil, A.; Motomura-Wages, S.; Bart, R.S. CRISPR/Cas9-generated mutations in a sugar transporter gene reduce cassava susceptibility to bacterial blight. Plant Physiol. 2024, 195, 2566. [Google Scholar] [CrossRef]
- Cox, K.L.; Meng, F.; Wilkins, K.E.; Li, F.; Wang, P.; Booher, N.J.; Carpenter, S.C.; Chen, L.-Q.; Zheng, H.; Gao, X. TAL effector driven induction of a SWEET gene confers susceptibility to bacterial blight of cotton. Nat. Commun. 2017, 8, 15588. [Google Scholar] [CrossRef]
- Barka, G.D.; Lee, J. Advances in S gene targeted genome-editing and its applicability to disease resistance breeding in selected Solanaceae crop plants. Bioengineered 2022, 13, 14646–14666. [Google Scholar] [CrossRef] [PubMed]
- Kamburova, V.S.; Nikitina, E.V.; Shermatov, S.E.; Buriev, Z.T.; Kumpatla, S.P.; Emani, C.; Abdurakhmonov, I.Y. Genome editing in plants: An overview of tools and applications. Int. J. Agron. 2017, 2017, 7315351. [Google Scholar] [CrossRef]
- Mores, A.; Borrelli, G.M.; Laidò, G.; Petruzzino, G.; Pecchioni, N.; Amoroso, L.G.M.; Desiderio, F.; Mazzucotelli, E.; Mastrangelo, A.M.; Marone, D. Genomic approaches to identify molecular bases of crop resistance to diseases and to develop future breeding strategies. Int. J. Mol. Sci. 2021, 22, 5423. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.S.; Mukhtar, M.S.; Mansoor, S. Genome editing: Targeting susceptibility genes for plant disease resistance. Trends Biotechnol. 2018, 36, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Phogat, S.; Lankireddy, S.V.; Lekkala, S.; Anche, V.C.; Sripathi, V.R.; Patil, G.B.; Puppala, N.; Janga, M.R. Progress in genetic engineering and genome editing of peanuts: Revealing the future of crop improvement. Physiol. Mol. Biol. Plants 2024, 30, 1759–1775. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, Q.; Tang, X.; Liu, S.; Malzahn, A.A.; Zhou, J.; Wang, J.; Yin, D.; Pan, C.; Yuan, M. Expanding the scope of plant genome engineering with Cas12a orthologs and highly multiplexable editing systems. Nat. Commun. 2021, 12, 1944. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Kancharla, N.; Javalkote, V.S.; Dasgupta, S.; Brutnell, T.P. CRISPR-Cas12a (Cpf1): A versatile tool in the plant genome editing tool box for agricultural advancement. Front. Plant Sci. 2020, 11, 584151. [Google Scholar] [CrossRef]
- Anzalone, A.V.; Randolph, P.B.; Davis, J.R.; Sousa, A.A.; Koblan, L.W.; Levy, J.M.; Chen, P.J.; Wilson, C.; Newby, G.A.; Raguram, A. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 2019, 576, 149–157. [Google Scholar] [CrossRef]
- Lin, Q.; Zong, Y.; Xue, C.; Wang, S.; Jin, S.; Zhu, Z.; Wang, Y.; Anzalone, A.V.; Raguram, A.; Doman, J.L. Prime genome editing in rice and wheat. Nat. Biotechnol. 2020, 38, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Tian, Y.; Shen, R.; Yao, Q.; Zhong, D.; Zhang, X.; Zhu, J.K. Precise genome modification in tomato using an improved prime editing system. Plant Biotechnol. J. 2020, 19, 415. [Google Scholar] [CrossRef]
- Biswas, S.; Bridgeland, A.; Irum, S.; Thomson, M.J.; Septiningsih, E.M. Optimization of prime editing in rice, peanut, chickpea, and cowpea protoplasts by restoration of GFP activity. Int. J. Mol. Sci. 2022, 23, 9809. [Google Scholar] [CrossRef] [PubMed]
- Anjanappa, R.B.; Gruissem, W. Current progress and challenges in crop genetic transformation. J. Plant Physiol. 2021, 261, 153411. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S. Gene Editing in Rice and Peanut: Multiplex Editing, Protoplast Validation, and Prime Editing. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 2022. [Google Scholar]
- Alam, T. Advances in Tissue Culture-Free Genetic Engineering and Genome Editing of Peanut. Mol. Biotechnol. 2025, 67. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; van Tuinen, A.; van Kan, J.A.; Wolters, A.-M.A.; Jacobsen, E.; Visser, R.G.; Bai, Y. Silencing of DND1 in potato and tomato impedes conidial germination, attachment and hyphal growth of Botrytis cinerea. BMC Plant Biol. 2017, 17, 235. [Google Scholar] [CrossRef] [PubMed]
- Andolfo, G.; Iovieno, P.; Ricciardi, L.; Lotti, C.; Filippone, E.; Pavan, S.; Ercolano, M.R. Evolutionary conservation of MLO gene promoter signatures. BMC Plant Biol. 2019, 19, 150. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Zhao, J.; Du, C.; Xiang, Y.; Cao, J.; Qin, X. Genome-scale identification of MLO domain-containing genes in soybean (Glycine max L. Merr.). Genes Genet. Syst. 2012, 87, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.S.; Shirasawa, K.; Sharma, V.; Isobe, S.N.; Hirakawa, H.; Kuwata, C.; Pandey, M.K.; Varshney, R.K.; Gowda, M.C. Population genomics of peanut. In Population Genomics: Crop Plants; Springer: Berlin/Heidelberg, Germany, 2021; pp. 793–835. [Google Scholar]
- Gangurde, S.S.; Pasupuleti, J.; Parmar, S.; Variath, M.T.; Bomireddy, D.; Manohar, S.S.; Varshney, R.K.; Singam, P.; Guo, B.; Pandey, M.K. Genetic mapping identifies genomic regions and candidate genes for seed weight and shelling percentage in groundnut. Front. Genet. 2023, 14, 1128182. [Google Scholar] [CrossRef] [PubMed]
- Roychowdhury, R.; Das, S.P.; Gupta, A.; Parihar, P.; Chandrasekhar, K.; Sarker, U.; Kumar, A.; Ramrao, D.P.; Sudhakar, C. Multi-omics pipeline and omics-integration approach to decipher plant’s abiotic stress tolerance responses. Genes 2023, 14, 1281. [Google Scholar] [CrossRef]
- Paul, N.C.; Park, S.-W.; Liu, H.; Choi, S.; Ma, J.; MacCready, J.S.; Chilvers, M.I.; Sang, H. Plant and fungal genome editing to enhance plant disease resistance using the CRISPR/Cas9 system. Front. Plant Sci. 2021, 12, 700925. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, R.Z.; Mahmood, M.A.; Mansoor, S.; Amin, I.; Asif, M. Omics-driven exploration and mining of key functional genes for the improvement of food and fiber crops. Front. Plant Sci. 2024, 14, 1273859. [Google Scholar] [CrossRef]
- Asif, M.; Rayamajhi, A.; Mahmud, M.S. Technological Progress Toward Peanut Disease Management: A Review. Sensors 2025, 25, 1255. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).