Heatwave-Induced Thermal Stratification Shaping Microbial-Algal Communities Under Different Climate Scenarios as Revealed by Long-Read Sequencing and Imaging Flow Cytometry
Abstract
1. Introduction
2. Results
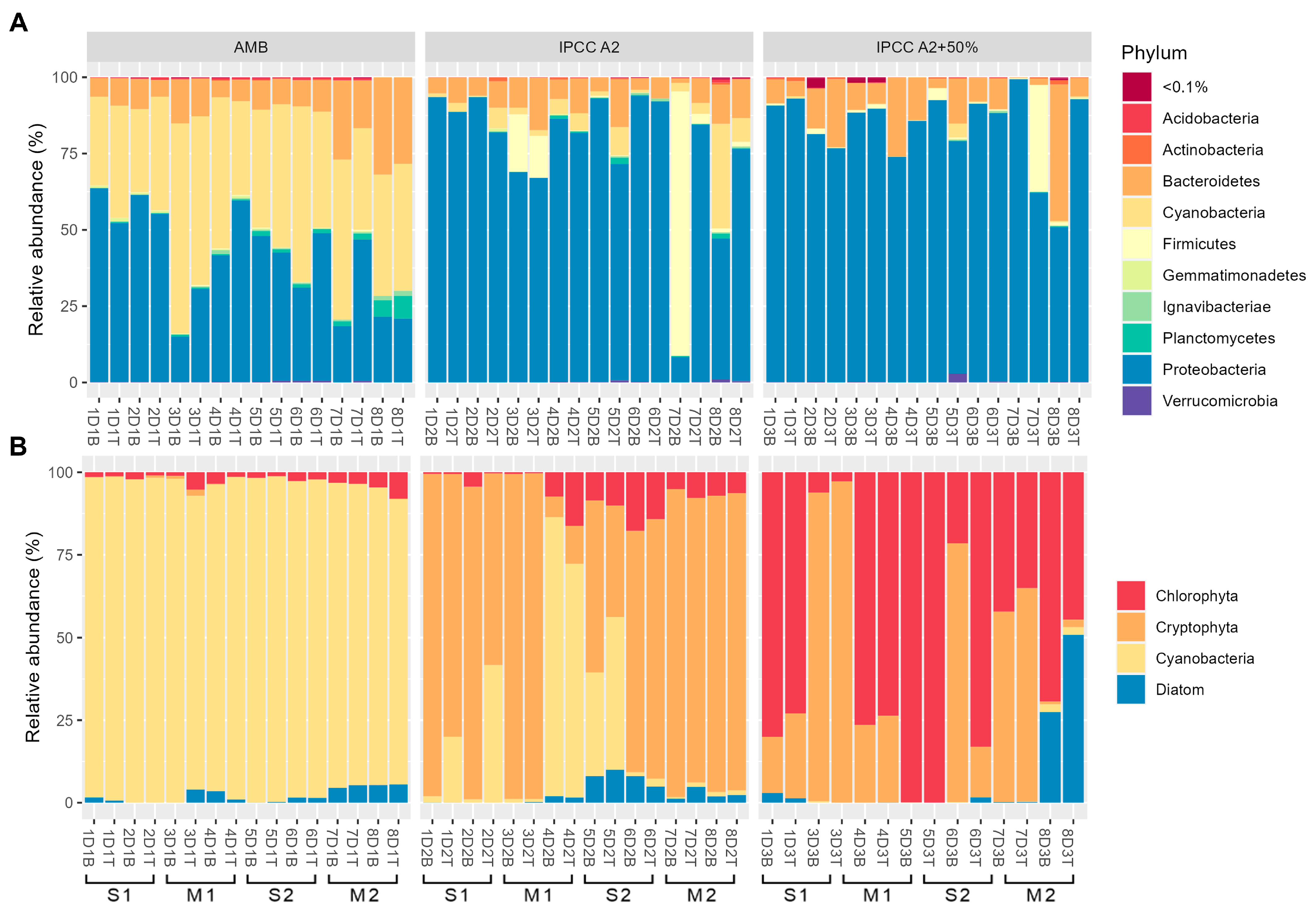
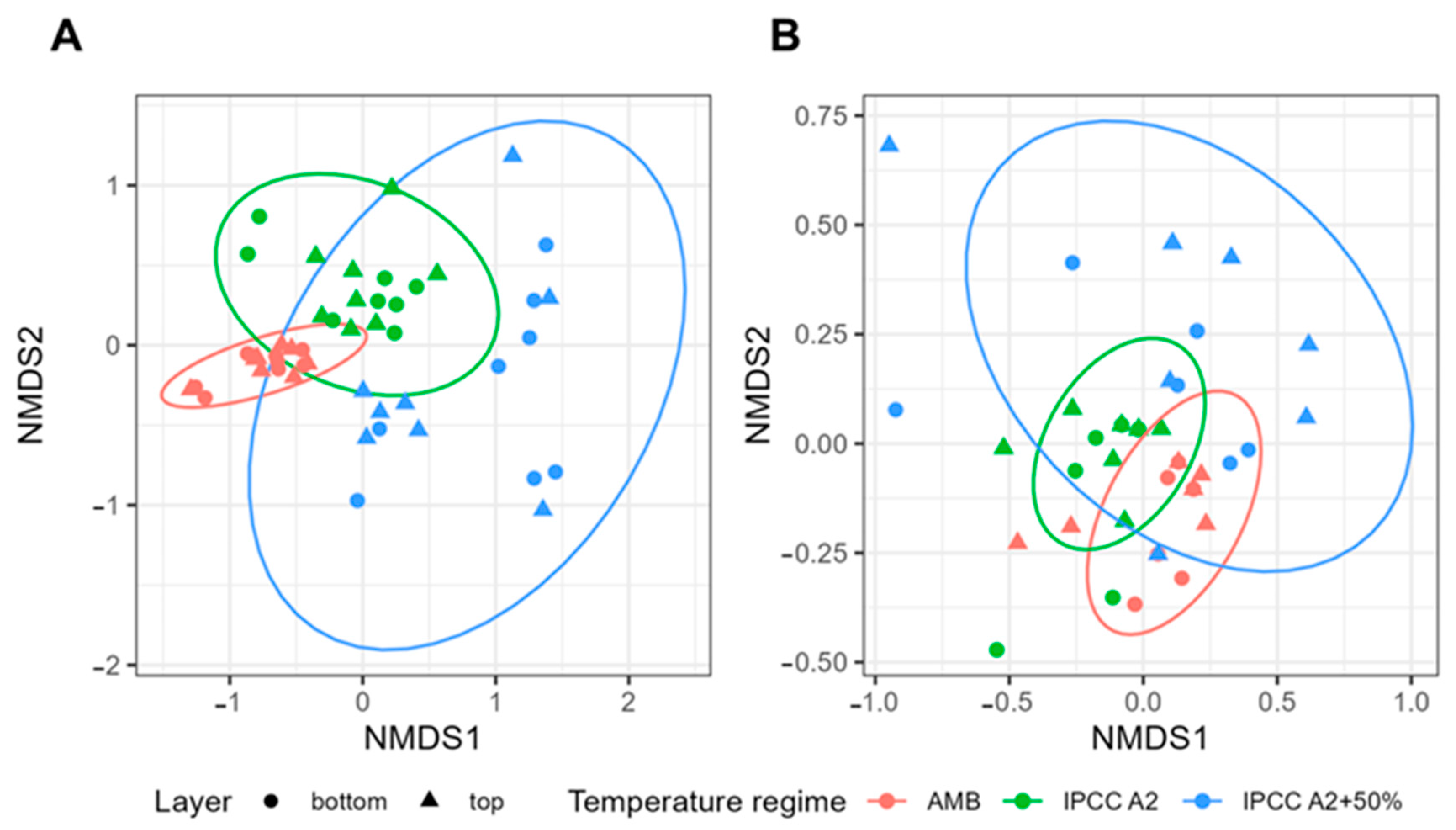
2.1. Microbial and Phytoplankton Community Composition Is Influenced by Temperature
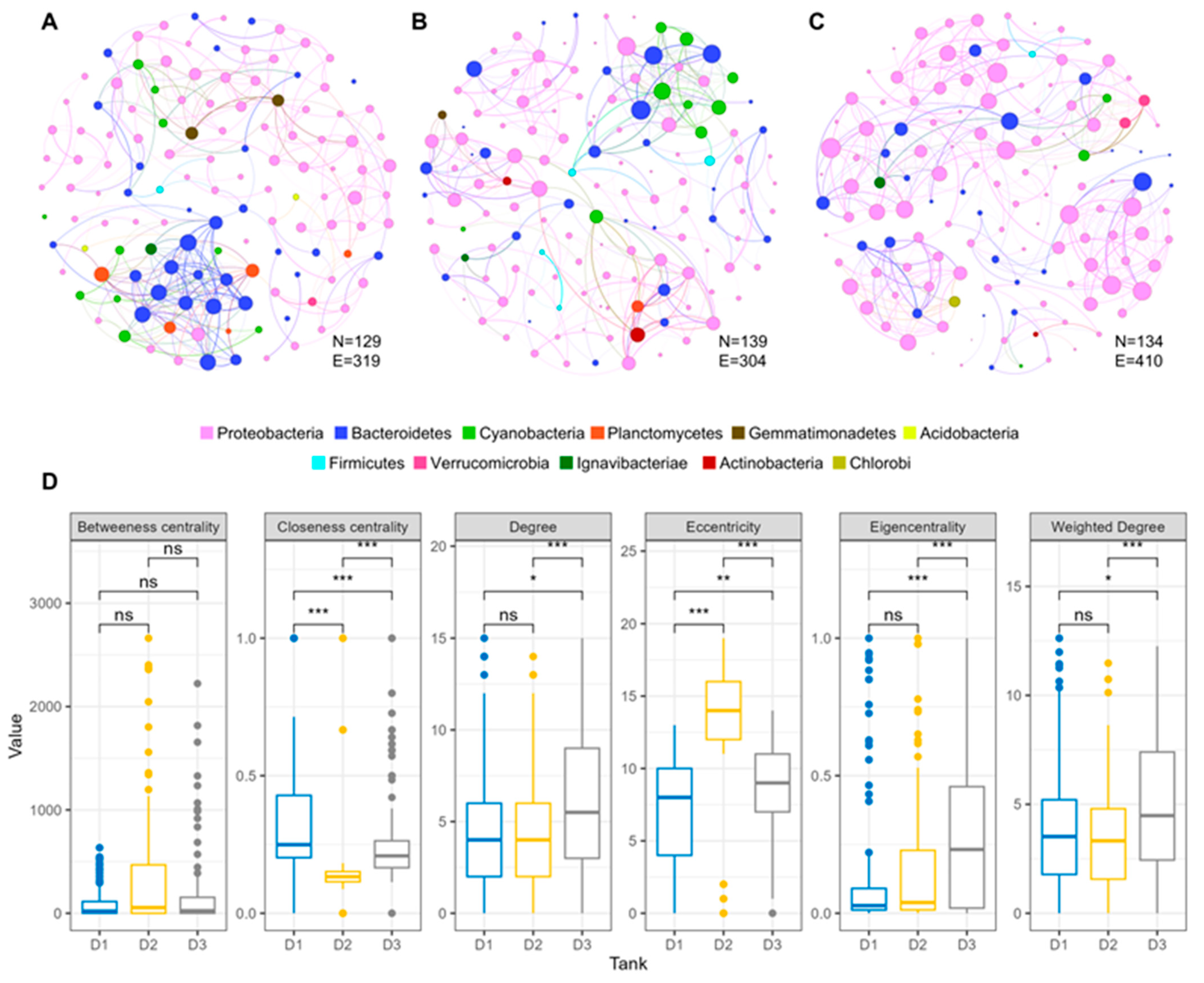
2.2. Network Analysis of Microbial Communities Across Varying Temperature Regimes
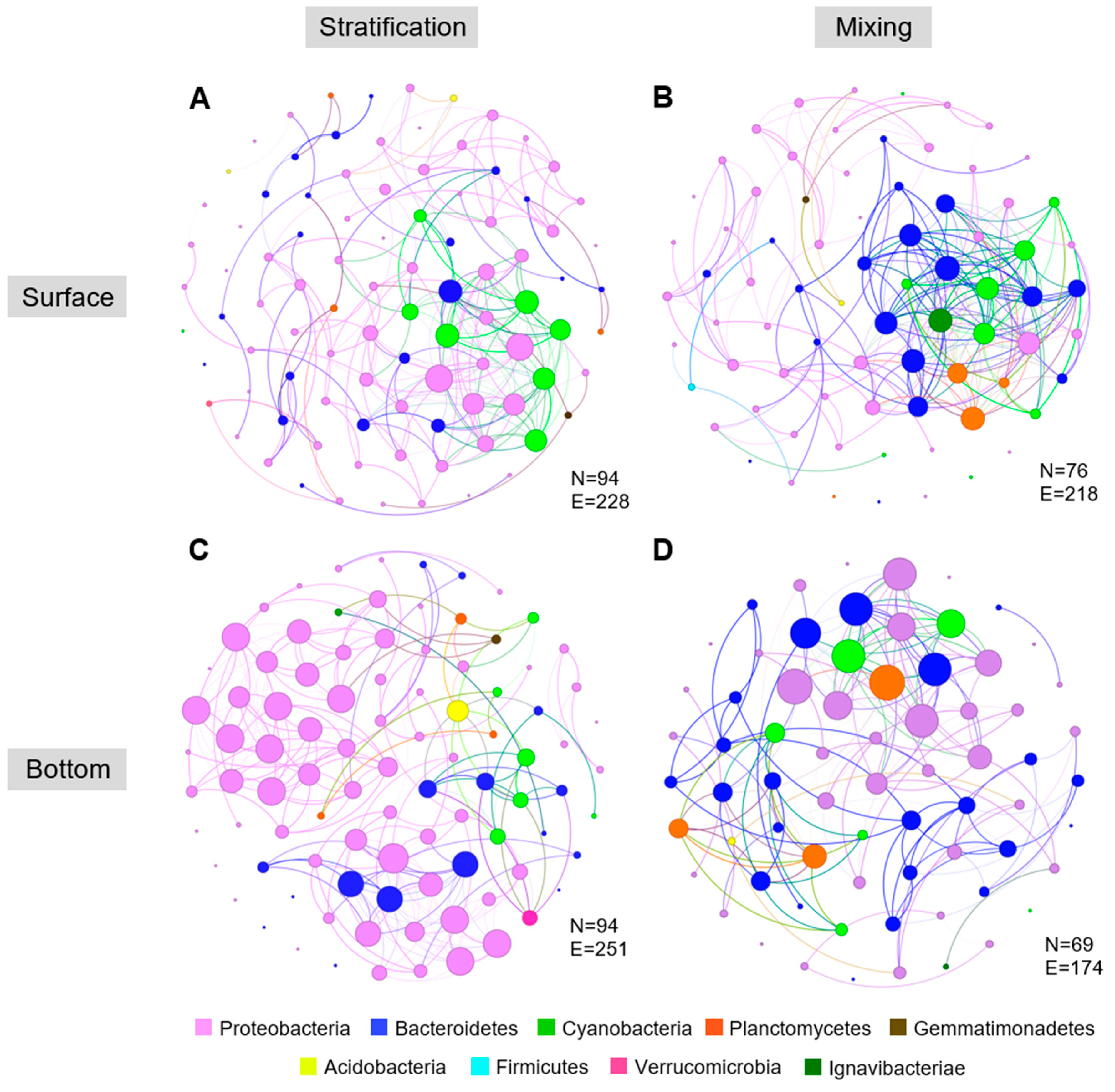
2.3. Temporary Stratification Impacts Microbial Community Composition
2.4. Environmental Drivers and Phytoplankton-Associated Microbial Communities
2.4.1. CCA of Environmental Gradients
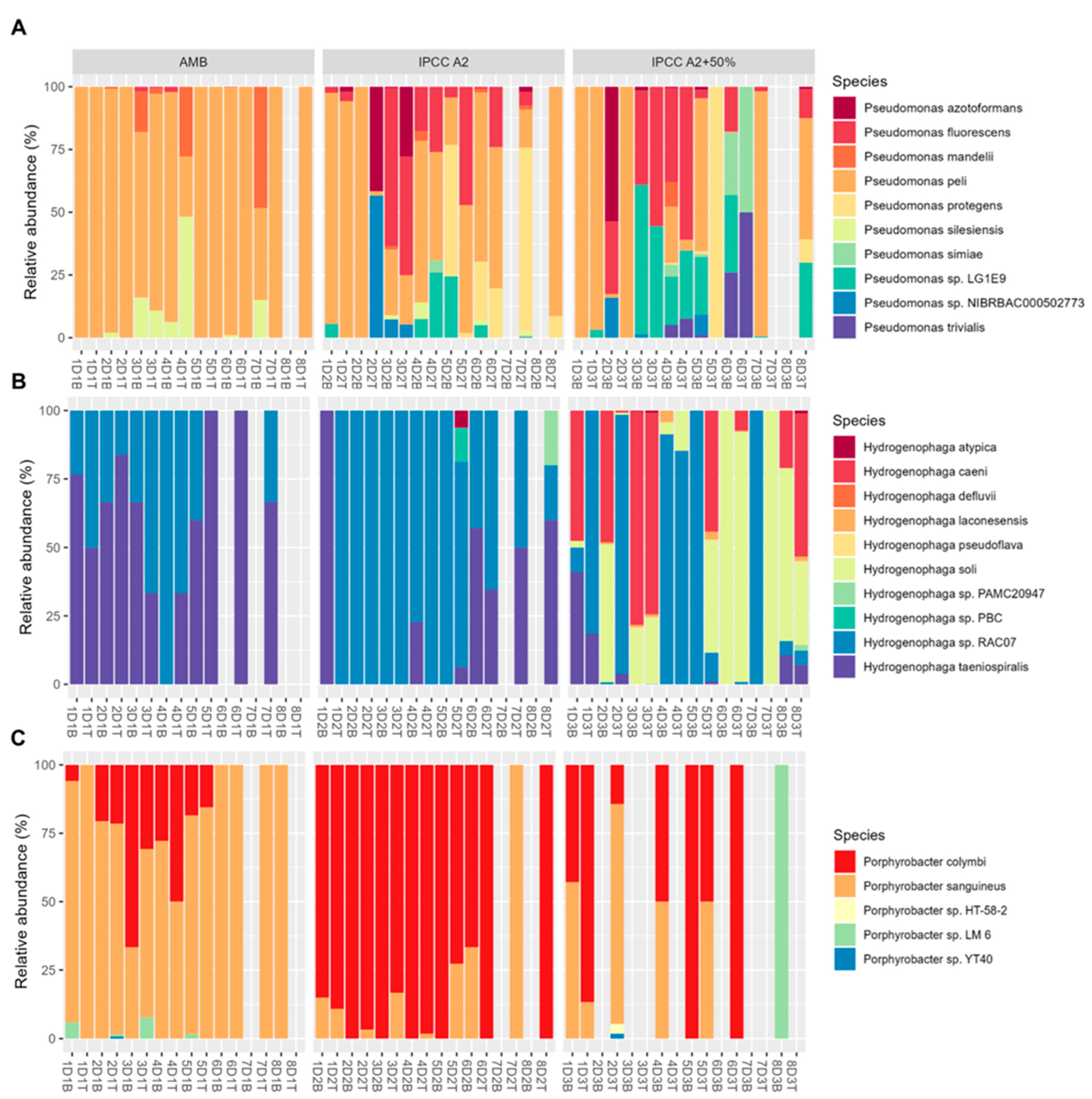
2.4.2. Species-Level Patterns in Dominant Bacterial Genera
2.4.3. Cryptophyta-Associated Microbial Clusters
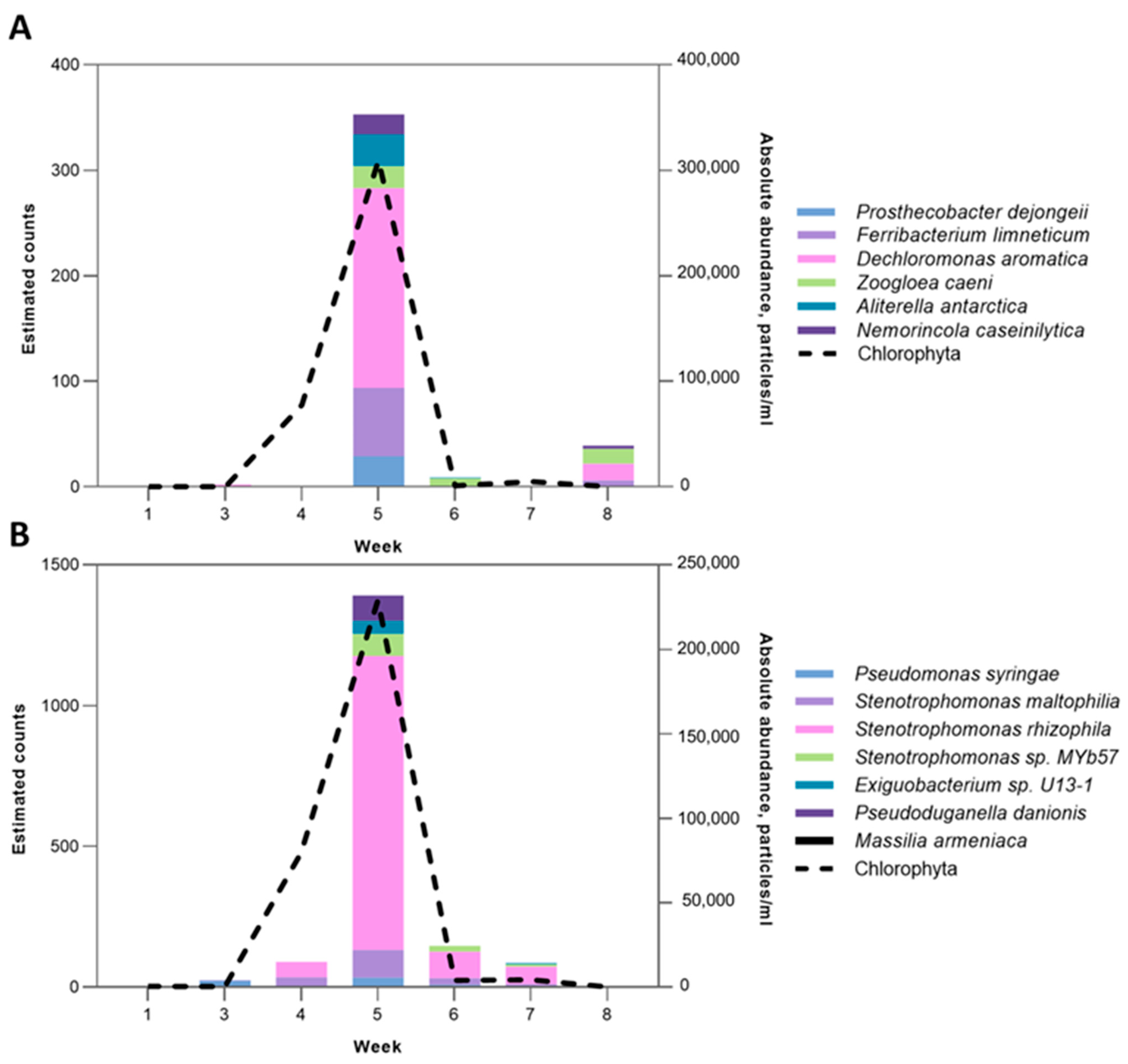
2.4.4. Chlorophyta-Associated Microbial Clusters
2.5. Microcystis-Associated Microbial Clusters
3. Discussion
3.1. Microcystis-Associated Microbiomes
3.2. Cryptophyta- and Chlorophyta-Associated Microbiomes
3.3. Associated Microbiomes and Environmental Parameters
4. Conclusions
5. Materials and Methods
5.1. Collection of Mesocosm Samples
5.2. Environmental DNA Extraction and Sequencing Library Preparation
5.3. Bioinformatic Analysis
5.4. Imaging Flow Cytometry-Based Analysis of Phytoplankton Community Composition
5.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DNA | Deoxyribonucleic acid |
| Std Dev | Standard deviation |
| SRA | Sequence read archive |
| rRNA | Ribosomal ribonucleic acid |
| PCR | Polymerase chain reaction |
| OTU(s) | Operational taxonomic unit(s) |
| ONT | Oxford nanopore technologies |
| NMDS | Non-metric multidimensional scaling |
| NGS | Next-generation sequencing |
| NCSC | Non-colonial small clusters |
| mNGS-IFC | Microbial NGS combined with imaging cytometry |
| IPCC | Intergovernmental Panel on Climate Change |
| IFC | Imaging flow cytometry |
| HAB | Harmful algal bloom |
| FCM | Flow cytometry |
| CCA | Canonical correspondence analysis |
| ANOSIM | Analysis of Similarities |
| AMB | Ambient temperature regime |
References
- Rabalais, N.; Diaz, R.J.; Levin, L.; Turner, R.E.; Gilbert, D.; Zhang, J. Dynamics and Distribution of Natural and Human-Caused Hypoxia. Biogeosciences 2010, 7, 585–619. [Google Scholar] [CrossRef]
- Scheffer, M.; Hosper, S.H.; Meijer, M.L.; Moss, B.; Jeppesen, E. Alternative Equilibria in Shallow Lakes. Trends Ecol. Evol. 1993, 8, 275–279. [Google Scholar] [CrossRef]
- Carmichael, W.W. Health Effects of Toxin-Producing Cyanobacteria: “The CyanoHABs”. Hum. Ecol. Risk Assess. 2001, 7, 1393–1407. [Google Scholar] [CrossRef]
- Nugumanova, G.; Ponomarev, E.D.; Askarova, S.; Fasler-Kan, E.; Barteneva, N.S. Freshwater cyanobacterial toxins, cyanopeptides and neurodegenerative diseases. Toxins 2023, 15, 233. [Google Scholar] [CrossRef]
- Carmichael, W. A World Overview—One-Hundred-Twenty-Seven Years of Research on Toxic Cyanobacteria—Where Do We Go from Here? In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Springer: Berlin/Heidelberg, Germany, 2008; pp. 105–125. [Google Scholar]
- Paerl, H.W.; Otten, T.G. Duelling ‘CyanoHABs’: Unravelling the Environmental Drivers Controlling Dominance and Succession among Diazotrophic and non-N2-fixing Harmful Cyanobacteria. Environ. Microbiol. 2016, 18, 316–324. [Google Scholar] [CrossRef]
- Cubasch, U.; Meehl, G.A.; Boer, G.J.; Stouffer, R.J.; Dix, M.; Noda, A.; Senior, C.A.; Raper, S.U.; Yap, K.S. Projections of future climate change. In Climate Change 2001: The Scientific Basis. Contribution of WG1 to the Third Assessment Report of the IPCC (TAR); Cambridge University Press: Cambridge, UK, 2001; pp. 525–582. [Google Scholar]
- Jeppesen, E.; Pierson, D.; Jennings, E. Effect of extreme climate events on lake ecosystems. Water 2021, 13, 282. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Y.; Zhang, Y.; Shi, K.; Qian, H.; Yang, H.; Niu, Y.; Qin, B.; Zhu, G.; Woolway, R.I.; et al. The unprecedented 2022 extreme summer heatwaves increased harmful cyanobacteria blooms. Sci. Total Environ. 2023, 896, 165312. [Google Scholar] [CrossRef]
- Urrutia-Cordero, P.; Zhang, H.; Chaguaceda, F.; Geng, H.; Hansson, L. Climate Warming and Heat Waves Alter Harmful Cyanobacterial Blooms along the Benthic-Pelagic Interface. Ecology 2020, 101, e03025. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Y.; Jarin, M.; Xie, X. Increasingly Frequent Extreme Weather Events Urge the Development of Point-of-Use Water Treatment Systems. npj Clean Water 2022, 5, 36. [Google Scholar] [CrossRef]
- Harke, M.J.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A.; Paerl, H.W. A Review of the Global Ecology, Genomics, and Biogeography of the Toxic Cyanobacterium, Microcystis spp. Harmful Algae 2016, 54, 4–20. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef]
- Cook, K.V.; Li, C.; Cai, H.; Krumholz, L.R.; Hambright, K.D.; Paerl, H.W.; Steffen, M.M.; Wilson, A.E.; Burford, M.A.; Grossart, H. The Global Microcystis Interactome. Limnol. Oceanogr. 2020, 65, S194–S207. [Google Scholar] [CrossRef]
- Reinl, K.L.; Brookes, J.D.; Carey, C.C.; Harris, T.D.; Ibelings, B.W.; Morales-Williams, A.M.; Domis, L.N.D.S.; Atkins, K.S.; Isles, P.D.; Mesman, J.P.; et al. Cyanobacterial blooms in oligotrophic lakes: Shifting the high-nutrient paradigm. Freshwater Biol. 2021, 66, 1846–1859. [Google Scholar] [CrossRef]
- Rossi, F.; De Philippis, R. Role of Cyanobacterial Exopolysaccharides in Phototrophic Biofilms and in Complex Microbial Mats. Life 2015, 5, 1218–1238. [Google Scholar] [CrossRef]
- Havens, K.E. Cyanobacteria Blooms: Effects on Aquatic Ecosystems. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Springer: Berlin/Heidelberg, Germany, 2008; pp. 733–747. [Google Scholar]
- van Hannen, E.J.; Mooij, W.; van Agterveld, M.P.; Gons, H.J.; Laanbroek, H.J. Detritus-Dependent Development of the Microbial Community in an Experimental System: Qualitative Analysis by Denaturing Gradient Gel Electrophoresis. Appl. Environ. Microbiol. 1999, 65, 2478–2484. [Google Scholar] [CrossRef]
- Croft, M.T.; Lawrence, A.D.; Raux-Deery, E.; Warren, M.J.; Smith, A.G. Algae Acquire Vitamin B12 through a Symbiotic Relationship with Bacteria. Nature 2005, 438, 90–93. [Google Scholar] [CrossRef]
- Droop, M.R. Vitamins, Phytoplankton and Bacteria: Symbiosis or Scavenging? J. Plankton Res. 2007, 29, 107–113. [Google Scholar] [CrossRef]
- Sokolovskaya, O.M.; Shelton, A.N.; Taga, M.E. Sharing Vitamins: Cobamides Unveil Microbial Interactions. Science 2020, 369, eaba0165. [Google Scholar] [CrossRef]
- Wang, H.; Tomasch, J.; Jarek, M.; Wagner-Döbler, I. A Dual-Species Co-Cultivation System to Study the Interactions between Roseobacters and Dinoflagellates. Front. Microbiol. 2014, 5, 311. [Google Scholar] [CrossRef]
- Durham, B.P.; Sharma, S.; Luo, H.; Smith, C.B.; Amin, S.A.; Bender, S.J.; Dearth, S.P.; Van Mooy, B.A.; Campagna, S.R.; Kujawinski, E.B. Cryptic Carbon and Sulfur Cycling between Surface Ocean Plankton. Proc. Natl. Acad. Sci. USA 2015, 112, 453–457. [Google Scholar] [CrossRef]
- Cooper, M.B.; Kazamia, E.; Helliwell, K.E.; Kudahl, U.J.; Sayer, A.; Wheeler, G.L.; Smith, A.G. Cross-Exchange of B-Vitamins Underpins a Mutualistic Interaction between Ostreococcus Tauri and Dinoroseobacter Shibae. ISME J. 2019, 13, 334–345. [Google Scholar] [CrossRef]
- Amin, S.A.; Green, D.H.; Hart, M.C.; Küpper, F.C.; Sunda, W.G.; Carrano, C.J. Photolysis of Iron-Siderophore Chelates Promotes Bacterial-Algal Mutualism. Proc. Natl. Acad. Sci. USA 2024, 106, 17071–17076. [Google Scholar] [CrossRef]
- Amin, S.A.; Green, D.H.; Gärdes, A.; Romano, A.; Trimble, L.; Carrano, C.J. Siderophore-Mediated Iron Uptake in Two Clades of Marinobacter Spp. Associated with Phytoplankton: The Role of Light. Biometals 2012, 25, 181–192. [Google Scholar] [CrossRef]
- Thompson, A.W.; Foster, R.A.; Krupke, A.; Carter, B.J.; Musat, N.; Vaulot, D.; Kuypers, M.M.; Zehr, J.P. Unicellular Cyanobacterium Symbiotic with a Single-Celled Eukaryotic Alga. Science 2012, 337, 1546–1550. [Google Scholar] [CrossRef]
- Christie-Oleza, J.A.; Sousoni, D.; Lloyd, M.; Armengaud, J.; Scanlan, D.J. Nutrient Recycling Facilitates Long-Term Stability of Marine Microbial Phototroph-Heterotroph Interactions. Nat. Microbiol. 2017, 2, 1–10. [Google Scholar] [CrossRef]
- Segev, E.; Wyche, T.P.; Kim, K.H.; Petersen, J.; Ellebrandt, C.; Vlamakis, H.; Barteneva, N.; Paulson, J.N.; Chai, L.; Clardy, J. Dynamic Metabolic Exchange Governs a Marine Algal-Bacterial Interaction. Elife 2016, 5, e17473. [Google Scholar] [CrossRef]
- Zhang, Z.; Nair, S.; Tang, L.; Zhao, H.; Hu, Z.; Chen, M.; Zhang, Y.; Kao, S.-J.; Jiao, N.; Zhang, Y. Long-Term Survival of Synechococcus and Heterotrophic Bacteria without External Nutrient Supply after Changes in Their Relationship from Antagonism to Mutualism. MBio 2021, 12, 10–1128. [Google Scholar] [CrossRef]
- Kent, A.D.; Yannarell, A.C.; Rusak, J.A.; Triplett, E.W.; McMahon, K.D. Synchrony in Aquatic Microbial Community Dynamics. ISME J. 2007, 1, 38–47. [Google Scholar] [CrossRef]
- Schiaffino, M.R.; Huber, P.; Sagua, M.; Sabio y García, C.A.; Reissig, M. Covariation Patterns of Phytoplankton and Bacterioplankton in Hypertrophic Shallow Lakes. FEMS Microbiol. Ecol. 2020, 96, fiaa161. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Ahn, C.-Y.; Kim, H.-S.; Oh, H.-M. Cyanobactericidal Effect of Rhodococcus Sp. Isolated from Eutrophic Lake on Microcystis sp. Biotechnol. Lett. 2010, 32, 1673–1678. [Google Scholar]
- Barteneva, N.S.; Meirkhanova, A.; Malashenkov, D.; Vorobjev, I.A. To Die or Not to Die—Regulated cell death and survival in cyanobacteria. Microorganisms 2022, 10, 1657. [Google Scholar] [CrossRef]
- Schär, C.; Vidale, P.L.; Lüthi, D.; Frei, C.; Häberli, C.; Liniger, M.A.; Appenzeller, C. The Role of Increasing Temperature Variability in European Summer Heatwaves. Nature 2004, 427, 332–336. [Google Scholar] [CrossRef]
- Viergutz, C.; Kathol, M.; Norf, H.; Arndt, H.; Weitere, M. Control of Microbial Communities by the Macrofauna: A Sensitive Interaction in the Context of Extreme Summer Temperatures? Oecologia 2007, 151, 115–124. [Google Scholar] [CrossRef]
- Woolway, R.I.; Jennings, E.; Shatwell, T.; Golub, M.; Pierson, D.C.; Maberly, S.C. Lake Heatwaves under Climate Change. Nature 2021, 589, 402–407. [Google Scholar] [CrossRef]
- Brussaard, C.; Mari, X.; Van Bleijswijk, J.; Veldhuis, M. A Mesocosm Study of Phaeocystis Globosa (Prymnesiophyceae) Population Dynamics: II. Significance for the Microbial Community. Harmful Algae 2005, 4, 875–893. [Google Scholar] [CrossRef]
- Passow, U.; Christina, L.; Arnosti, C.; Grossart, H.-P.; Murray, A.E.; Engel, A. Microbial Dynamics in Autotrophic and Heterotrophic Seawater Mesocosms. I. Effect of Phytoplankton on the Microbial Loop. Aquat. Microb. Ecol. 2007, 49, 109–121. [Google Scholar] [CrossRef][Green Version]
- Bergen, B.; Endres, S.; Engel, A.; Zark, M.; Dittmar, T.; Sommer, U.; Jürgens, K. Acidification and Warming Affect Prominent Bacteria in Two Seasonal Phytoplankton Bloom Mesocosms. Environ. Microbiol. 2016, 18, 4579–4595. [Google Scholar] [CrossRef]
- Karayanni, H.; Kormas, K.A.; Moustaka-Gouni, M.; Sommer, U. Changes in Heterotrophic Picoplankton Community Structure after Induction of a Phytoplankton Bloom under Different Light Regimes. Diversity 2019, 11, 195. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O. Vaginal Microbiome of Reproductive-Age Women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef]
- Lamb, C.E.; Watts, J.E. Microbiome Species Diversity and Seasonal Stability of Two Temperate Marine Sponges Hymeniacidon Perlevis and Suberites Massa. Environ. Microbiome 2023, 18, 52. [Google Scholar] [CrossRef]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H. Structure and Function of the Global Topsoil Microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef]
- Shu, D.; Guo, J.; Zhang, B.; He, Y.; Wei, G. rDNA-and rRNA-Derived Communities Present Divergent Assemblage Patterns and Functional Traits throughout Full-Scale Landfill Leachate Treatment Process Trains. Sci. Total Environ. 2019, 646, 1069–1079. [Google Scholar] [CrossRef]
- Dove, N.C.; Taş, N.; Hart, S.C. Ecological and Genomic Responses of Soil Microbiomes to High-Severity Wildfire: Linking Community Assembly to Functional Potential. ISME J. 2022, 16, 1853–1863. [Google Scholar] [CrossRef]
- Iftikhar, S.; Riaz, M.A.; Majeed, M.Z.; Afzal, M.; Ali, A.; Saadia, M.; Ali, Z.; Ahmed, S. Isolation, Characterization and Larvicidal Potential of Indigenous Soil Inhabiting Bacteria against Larvae of Southern House Mosquito (Culex Quinquefasciatus Say). Inter. J. Trop. Insect Sci. 2023, 43, 781–791. [Google Scholar] [CrossRef]
- Eloe-Fadrosh, E.A.; Ivanova, N.N.; Woyke, T.; Kyrpides, N.C. Metagenomics Uncovers Gaps in Amplicon-Based Detection of Microbial Diversity. Nat. Microbiol. 2016, 1, 1–4. [Google Scholar] [CrossRef]
- Nakano, K.; Shiroma, A.; Shimoji, M.; Tamotsu, H.; Ashimine, N.; Ohki, S.; Shinzato, M.; Minami, M.; Nakanishi, T.; Teruya, K. Advantages of Genome Sequencing by Long-Read Sequencer Using SMRT Technology in Medical Area. Hum. Cell 2017, 30, 149–161. [Google Scholar] [CrossRef]
- Jain, M.; Koren, S.; Miga, K.H.; Quick, J.; Rand, A.C.; Sasani, T.A.; Tyson, J.R.; Beggs, A.D.; Dilthey, A.T.; Fiddes, I.T. Nanopore Sequencing and Assembly of a Human Genome with Ultra-Long Reads. Nat. Biotech. 2018, 36, 338–345. [Google Scholar] [CrossRef]
- Tedersoo, L.; Albertsen, M.; Anslan, S.; Callahan, B. Perspectives and Benefits of High-Throughput Long-Read Sequencing in Microbial Ecology. Appl. Environ. Microbiol. 2021, 87, e00626-21. [Google Scholar] [CrossRef]
- Rosenqvist, T.; Danielsson, M.; Schleich, C.; Ahlinder, J.; Brindefalk, B.; Pullerits, K.; Dacklin, I.; Salomonsson, E.N.; Sundell, D.; Forsman, M. Succession of Bacterial Biofilm Communities Following Removal of Chloramine from a Full-Scale Drinking Water Distribution System. npj Clean Water 2023, 6, 41. [Google Scholar] [CrossRef]
- Cai, H.; McLimans, C.J.; Beyer, J.E.; Krumholz, L.R.; Hambright, K.D. Microcystis Pangenome Reveals Cryptic Diversity within and across Morphospecies. Sci. Adv. 2023, 9, eadd3783. [Google Scholar] [CrossRef]
- Malashenkov, D.; Dashkova, V.; Vorobjev, I.A.; Barteneva, N.S. Optimizing FlowCam Imaging Flow Cytometry Operation for Classification and Quantification of Microcystis Morphospecies. In Spectral and Imaging Cytometry: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2023; pp. 245–258. [Google Scholar]
- Zhumakhanova, A.; Mirasbekov, Y.; Meirkhanova, A.; Malashenkov, D.V.; Davidson, T.A.; Levi, E.E.; Jeppesen, E.; Barteneva, N.S. From Colonial Clusters to Colonial Sheaths: Imaging Flow Cytometry Analysis of Microcystis Morphospecies Dynamics in Mesocosm and Links to CyanoHABs Management. Ecol. Indic. 2024, 163, 112100. [Google Scholar] [CrossRef]
- Guedes, I.A.; Rachid, C.T.; Rangel, L.M.; Silva, L.H.; Bisch, P.M.; Azevedo, S.M.; Pacheco, A.B. Close link between harmful cyanobacterial dominance and associated bacterioplankton in a tropical eutrophic reservoir. Front. Microb. 2018, 9, 424. [Google Scholar] [CrossRef]
- González-Camejo, J.; Aparicio, S.; Pachés, M.; Borrás, L.; Seco, A. Comprehensive assessment of the microalgae-nitrifying bacteria competition in microalgae-based wastewater treatment systems: Relevant factors, evaluation methods and control strategies. Algal Res. 2022, 61, 102563. [Google Scholar] [CrossRef]
- Parveen, B.; Ravet, V.; Djediat, C.; Mary, I.; Quiblier, C.; Debroas, D.; Humbert, J. Bacterial Communities Associated with Microcystis Colonies Differ from Free-living Communities Living in the Same Ecosystem. Environ. Microbiol. Rep. 2013, 5, 716–724. [Google Scholar] [CrossRef]
- Su, X.; Steinman, A.D.; Tang, X.; Xue, Q.; Zhao, Y.; Xie, L. Response of Bacterial Communities to Cyanobacterial Harmful Algal Blooms in Lake Taihu, China. Harmful Algae 2017, 68, 168–177. [Google Scholar] [CrossRef]
- Zhao, D.; Cao, X.; Huang, R.; Zeng, J.; Wu, Q.L. Variation of Bacterial Communities in Water and Sediments during the Decomposition of Microcystis Biomass. PLoS ONE 2017, 12, e0176397. [Google Scholar] [CrossRef]
- Pineda-Mendoza, R.M.; Briones-Roblero, C.I.; Gonzalez-Escobedo, R.; Rivera-Orduña, F.N.; Martínez-Jerónimo, F.; Zúñiga, G. Seasonal Changes in the Bacterial Community Structure of Three Eutrophicated Urban Lakes in Mexico City, with Emphasis on Microcystis spp. Toxicon 2020, 179, 8–20. [Google Scholar] [CrossRef]
- Jankowiak, J.G.; Gobler, C.J. The Composition and Function of Microbiomes within Microcystis Colonies Are Significantly Different than Native Bacterial Assemblages in Two North American Lakes. Front. Microbiol. 2020, 11, 1016. [Google Scholar] [CrossRef]
- Zhou, S.; Yin, H.; Tang, S.; Peng, H.; Yin, D.; Yang, Y.; Liu, Z.; Dang, Z. Physiological Responses of Microcystis Aeruginosa against the Algicidal Bacterium Pseudomonas Aeruginosa. Ecotoxicol. Environ. Saf. 2016, 127, 214–221. [Google Scholar] [CrossRef]
- Mankiewicz-Boczek, J.; Morón-López, J.; Serwecińska, L.; Font-Najera, A.; Gałęzowska, G.; Jurczak, T.; Kokociński, M.; Wolska, L. Algicidal Activity of Morganella Morganii against Axenic and Environmental Strains of Microcystis Aeruginosa: Compound Combination Effects. Chemosphere 2022, 309, 136609. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, Y.; Wen, X.; Liu, H.; Zhang, Y.; Zhang, Z. The Effect of Algicidal and Denitrifying Bacteria on the Vertical Distribution of Cyanobacteria and Nutrients. Water 2022, 14, 2129. [Google Scholar] [CrossRef]
- Mohan, R.; Pillai, S.S.; Purushothaman, A.; Thomas, L.C.; Padmakumar, K. Phylogenic Diversity of Bacteria Associated with Potentially Toxic Cyanobacteria Microcystis Aeruginosa: A Synthesis on Its Bloom Dynamics. Folia Microbiol. 2024, 69, 677–691. [Google Scholar] [CrossRef]
- Ashraf, N.; Ahmad, F.; Lu, Y. Synergy between Microalgae and Microbiome in Polluted Waters. Trends Microbiol. 2023, 31, 9–21. [Google Scholar] [CrossRef]
- Pang, Q.; Zhao, G.; Wang, D.; Zhu, X.; Xie, L.; Zuo, D.; Wang, L.; Tian, L.; Peng, F.; Xu, B.; et al. Water periods impact the structure and metabolic potential of the nitrogen-cycling microbial communities in rivers of arid and semi-arid regions. Water Res. 2024, 267, 122472. [Google Scholar] [CrossRef]
- Jezberová, J.; Jezbera, J.; Znachor, P.; Nedoma, J.; Kasalický, V.; Šimek, K. The Limnohabitans Genus Harbors Generalistic and Opportunistic Subtypes: Evidence from Spatiotemporal Succession in a Canyon-Shaped Reservoir. Appl. Environ. Microbiol. 2017, 83, e01530-17. [Google Scholar] [CrossRef]
- Cole, J.J.; Pace, M.L.; Carpenter, S.R.; Kitchell, J.F. Persistence of Net Heterotrophy in Lakes during Nutrient Addition and Food Web Manipulations. Limnol. Oceanogr. 2000, 45, 1718–1730. [Google Scholar] [CrossRef]
- Tromas, N.; Fortin, N.; Bedrani, L.; Terrat, Y.; Cardoso, P.; Bird, D.; Greer, C.W.; Shapiro, B.J. Characterising and Predicting Cyanobacterial Blooms in an 8-Year Amplicon Sequencing Time Course. ISME J. 2017, 11, 1746–1763. [Google Scholar] [CrossRef]
- Osman, O.A.; Beier, S.; Grabherr, M.; Bertilsson, S. Interactions of Freshwater Cyanobacteria with Bacterial Antagonists. Appl. Environ. Microbiol. 2017, 83, e02634-e16. [Google Scholar] [CrossRef]
- Teeling, H.; Fuchs, B.M.; Bennke, C.M.; Krüger, K.; Chafee, M.; Kappelmann, L.; Reintjes, G.; Waldmann, J.; Quast, C.; Glöckner, F.O. Recurring Patterns in Bacterioplankton Dynamics during Coastal Spring Algae Blooms. eLife 2016, 5, e11888. [Google Scholar] [CrossRef]
- Needham, D.M.; Fuhrman, J.A. Pronounced Daily Succession of Phytoplankton, Archaea and Bacteria Following a Spring Bloom. Nat. Mcrobiol. 2016, 1, 1–7. [Google Scholar] [CrossRef]
- Warnecke, F.; Amann, R.; Pernthaler, J. Actinobacterial 16S rRNA Genes from Freshwater Habitats Cluster in Four Distinct Lineages. Environ. Microbiol. 2004, 6, 242–253. [Google Scholar] [CrossRef]
- David, G.M.; López-García, P.; Moreira, D.; Alric, B.; Deschamps, P.; Bertolino, P.; Restoux, G.; Rochelle-Newall, E.; Thébault, E.; Simon, M. Small Freshwater Ecosystems with Dissimilar Microbial Communities Exhibit Similar Temporal Patterns. Mol. Ecol. 2021, 30, 2162–2177. [Google Scholar] [CrossRef]
- Newton, R.J.; Jones, S.E.; Eiler, A.; McMahon, K.D.; Bertilsson, S. A Guide to the Natural History of Freshwater Lake Bacteria. Microbiol. Mol. Biol. Rev. 2011, 75, 14–49. [Google Scholar] [CrossRef]
- Kara, E.L.; Hanson, P.C.; Hu, Y.H.; Winslow, L.; McMahon, K.D. A Decade of Seasonal Dynamics and Co-Occurrences within Freshwater Bacterioplankton Communities from Eutrophic Lake Mendota, WI, USA. ISME J. 2013, 7, 680–684. [Google Scholar] [CrossRef]
- Steele, J.A.; Countway, P.D.; Xia, L.; Vigil, P.D.; Beman, J.M.; Kim, D.Y.; Chow, C.-E.T.; Sachdeva, R.; Jones, A.C.; Schwalbach, M.S. Marine Bacterial, Archaeal and Protistan Association Networks Reveal Ecological Linkages. ISME J. 2011, 5, 1414–1425. [Google Scholar] [CrossRef]
- Biller, S.J.; Berube, P.M.; Dooley, K.; Williams, M.; Satinsky, B.M.; Hackl, T.; Hogle, S.L.; Coe, A.; Bergauer, K.; Bouman, H.A. Marine Microbial Metagenomes Sampled across Space and Time. Sci. Data 2018, 5, 1–7. [Google Scholar] [CrossRef]
- Obertegger, U.; Pindo, M.; Flaim, G. Multifaceted Aspects of Synchrony between Freshwater Prokaryotes and Protists. Mol. Ecol. 2019, 28, 4500–4512. [Google Scholar] [CrossRef]
- Mikhailov, I.S.; Galachyants, Y.P.; Bukin, Y.S.; Petrova, D.P.; Bashenkhaeva, M.V.; Sakirko, M.V.; Blinov, V.V.; Titova, L.A.; Zakharova, Y.R.; Likhoshway, Y.V. Seasonal Succession and Coherence among Bacteria and Microeukaryotes in Lake Baikal. Microb. Ecol. 2022, 84, 404–422. [Google Scholar] [CrossRef]
- Faust, K.; Raes, J. Microbial Interactions: From Networks to Models. Nat. Rev. Microbiol. 2012, 10, 538–550. [Google Scholar] [CrossRef]
- Weiss, S.; Van Treuren, W.; Lozupone, C.; Faust, K.; Friedman, J.; Deng, Y.; Xia, L.C.; Xu, Z.Z.; Ursell, L.; Alm, E.J. Correlation Detection Strategies in Microbial Data Sets Vary Widely in Sensitivity and Precision. ISME J. 2016, 10, 1669–1681. [Google Scholar] [CrossRef]
- Li, Q.; Lin, F.; Yang, C.; Wang, J.; Lin, Y.; Shen, M.; Park, M.S.; Li, T.; Zhao, J. A Large-Scale Comparative Metagenomic Study Reveals the Functional Interactions in Six Bloom-Forming Microcystis-Epibiont Communities. Front. Microbiol. 2018, 9, 746. [Google Scholar] [CrossRef]
- Eiler, A.; Bertilsson, S. Composition of Freshwater Bacterial Communities Associated with Cyanobacterial Blooms in Four Swedish Lakes. Environ. Microbiol. 2004, 6, 1228–1243. [Google Scholar] [CrossRef]
- Steffen, M.M.; Li, Z.; Effler, T.C.; Hauser, L.J.; Boyer, G.L.; Wilhelm, S.W. Comparative Metagenomics of Toxic Freshwater Cyanobacteria Bloom Communities on Two Continents. PLoS ONE 2012, 7, e44002. [Google Scholar] [CrossRef]
- Johnson, J.S.; Spakowicz, D.J.; Hong, B.-Y.; Petersen, L.M.; Demkowicz, P.; Chen, L.; Leopold, S.R.; Hanson, B.M.; Agresta, H.O.; Gerstein, M. Evaluation of 16S rRNA Gene Sequencing for Species and Strain-Level Microbiome Analysis. Nat. Comm. 2019, 10, 5029. [Google Scholar] [CrossRef]
- Urban, L.; Holzer, A.; Baronas, J.J.; Hall, M.B.; Braeuninger-Weimer, P.; Scherm, M.J.; Kunz, D.J.; Perera, S.N.; Martin-Herranz, D.E.; Tipper, E.T. Freshwater Monitoring by Nanopore Sequencing. eLife 2021, 10, e61504. [Google Scholar] [CrossRef]
- Komárek, J.; Komárková, J. Review of the European Microcystis Morphospecies (Cyanoprokaryotes) from Nature. Fottea 2002, 2, 1–24. [Google Scholar]
- Xiao, M.; Li, M.; Reynolds, C.S. Colony Formation in the Cyanobacterium Microcystis. Biol. Rev. 2018, 93, 1399–1420. [Google Scholar] [CrossRef]
- Otsuka, S.; Suda, S.; Shibata, S.; Oyaizu, H.; Matsumoto, S.; Watanabe, M.M. A Proposal for the Unification of Five Species of the Cyanobacterial Genus Microcystis Kützing Ex Lemmermann 1907 under the Rules of the Bacteriological Code. Int. J. Syst. Evol. Microbiol. 2001, 51, 873–879. [Google Scholar] [CrossRef]
- Schweitzer-Natan, O.; Ofek-Lalzar, M.; Sher, D.; Sukenik, A. The Microbial Community Spatially Varies during a Microcystis Bloom Event in Lake Kinneret. Freshw. Biol. 2023, 68, 349–363. [Google Scholar] [CrossRef]
- Gutierrez, T. Aerobic Hydrocarbon-Degrading Gammaproteobacteria: Xanthomonadales. In Taxonomy, Genomics and Ecophysiology of Hydrocarbon-Degrading Microbes; Springer: Berlin/Heidelberg, Germany, 2019; pp. 191–205. [Google Scholar]
- Manage, P.M.; Kawabata, Z.; Nakano, S. Algicidal Effect of the Bacterium Alcaligenes Denitrificans on Microcystis spp. Aquat. Microb. Ecol. 2000, 22, 111–117. [Google Scholar] [CrossRef]
- Manage, P.M.; Kawabata, Z.; Nakano, S. Dynamics of Cyanophage-like Particles and Algicidal Bacteria Causing Microcystis Aeruginosa Mortality. Limnology 2001, 2, 73–78. [Google Scholar] [CrossRef]
- Van Wichelen, J.; Vanormelingen, P.; Codd, G.A.; Vyverman, W. The Common Bloom-Forming Cyanobacterium Microcystis Is Prone to a Wide Array of Microbial Antagonists. Harmful Algae 2016, 55, 97–111. [Google Scholar] [CrossRef]
- Rashidan, K.; Bird, D. Role of Predatory Bacteria in the Termination of a Cyanobacterial Bloom. Microb. Ecol. 2001, 41, 97–105. [Google Scholar] [CrossRef]
- Manage, P.M. Seasonal Changes in the Abundance of Biological Agents Killing Microcystis Aeruginosa in a Hypereutrophic Pond. Vidyodaya J. Sci. 2009, 14, 85–101. [Google Scholar]
- Krug, L.; Erlacher, A.; Markut, K.; Berg, G.; Cernava, T. The Microbiome of Alpine Snow Algae Shows a Specific Inter-Kingdom Connectivity and Algae-Bacteria Interactions with Supportive Capacities. ISME J. 2020, 14, 2197–2210. [Google Scholar] [CrossRef]
- Pernthaler, J.; Posch, T.; Šimek, K.; Vrba, J.; Pernthaler, A.; Glöckner, F.O.; Nübel, U.; Psenner, R.; Amann, R. Predator-Specific Enrichment of Actinobacteria from a Cosmopolitan Freshwater Clade in Mixed Continuous Culture. Appl. Environ. Microbiol. 2001, 67, 2145–2155. [Google Scholar] [CrossRef]
- Šimek, K.; Kasalický, V.; Zapomělová, E.; Horňák, K. Alga-Derived Substrates Select for Distinct Betaproteobacterial Lineages and Contribute to Niche Separation in Limnohabitans Strains. Appl. Environ. Microbiol. 2011, 77, 7307–7315. [Google Scholar] [CrossRef]
- Salcher, M.M.; Ewert, C.; Šimek, K.; Kasalický, V.; Posch, T. Interspecific Competition and Protistan Grazing Affect the Coexistence of Freshwater Betaproteobacterial Strains. FEMS Microbiol. Ecol. 2016, 92, fiv156. [Google Scholar] [CrossRef]
- Avila, M.P.; Brandão, L.P.; Brighenti, L.S.; Tonetta, D.; Reis, M.P.; Stæhr, P.A.; Asmala, E.; Amado, A.M.; Barbosa, F.A.; Bezerra-Neto, J.F. Linking Shifts in Bacterial Community with Changes in Dissolved Organic Matter Pool in a Tropical Lake. Sci.Total Environ. 2019, 672, 990–1003. [Google Scholar] [CrossRef]
- Ma, Y.; Li, P.; Zhong, H.; He, M.; Wang, B.; Mou, X.; Wu, L. The Ecological Differentiation of Particle-Attached and Free-Living Bacterial Communities in a Seasonal Flooding Lake—The Poyang Lake. Microb. Ecol. 2023, 86, 795–809. [Google Scholar] [CrossRef]
- Siddiqi, M.Z.; Im, W.-T. Pseudobacter Ginsenosidimutans Gen. Nov., Sp. Nov., Isolated from Ginseng Cultivating Soil. Int. J. Syst. Evolution. Microbiol. 2016, 66, 3449–3455. [Google Scholar] [CrossRef]
- Dziallas, C.; Grossart, H. Temperature and Biotic Factors Influence Bacterial Communities Associated with the Cyanobacterium Microcystis sp. Environ. Microbiol. 2011, 13, 1632–1641. [Google Scholar]
- Smith, D.J.; Tan, J.Y.; Powers, M.A.; Lin, X.N.; Davis, T.W.; Dick, G.J. Individual Microcystis Colonies Harbour Distinct Bacterial Communities That Differ by Microcystis Oligotype and with Time. Environ. Microbiol. 2021, 23, 3020–3036. [Google Scholar] [CrossRef]
- Joehnk, K.D.; Huisman, J.; Sharples, J.; Sommeijer, B.; Visser, P.M.; Stroom, J.M. Summer Heatwaves Promote Blooms of Harmful Cyanobacteria. Glob. Change Biol. 2008, 14, 495–512. [Google Scholar] [CrossRef]
- Wilk-Woźniak, E.; Krztoń, W.; Budziak, M.; Walusiak, E.; Žutinič, P.; Udovič, M.G.; Koreivienė, J.; Karosienė, J.; Kasperovičienė, J.; Kobos, J. Harmful Blooms across a Longitudinal Gradient in Central Europe during Heatwave: Cyanobacteria Biomass, Cyanotoxins, and Nutrients. Ecol. Indic. 2024, 160, 111929. [Google Scholar] [CrossRef]
- Gobler, C.J.; Jankowiak, J.G. Dynamic Responses of Endosymbiotic Microbial Communities within Microcystis Colonies in North American Lakes to Altered Nitrogen, Phosphorus, and Temperature Levels. Front. Microbiol. 2022, 12, 781500. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Chisholm, S.W.; Krebs, C.J.; Schindler, D.W.; Wright, R.F. Ecosystem Experiments. Science 1995, 269, 324–327. [Google Scholar] [CrossRef]
- Schindler, D.W.; Hecky, R.E.; Findlay, D.; Stainton, M.; Parker, B.; Paterson, M.; Beaty, K.; Lyng, M.; Kasian, S. Eutrophication of Lakes Cannot Be Controlled by Reducing Nitrogen Input: Results of a 37-Year Whole-Ecosystem Experiment. Proc. Natl. Acad. Sci. USA 2008, 105, 11254–11258. [Google Scholar] [CrossRef]
- Spivak, A.C.; Vanni, M.J.; Mette, E.M. Moving on up: Can Results from Simple Aquatic Mesocosm Experiments Be Applied across Broad Spatial Scales? Freshw. Biol. 2008, 56, 279–291. [Google Scholar] [CrossRef]
- Stewart, R.I.; Dossena, M.; Bohan, D.A.; Jeppesen, E.; Kordas, R.L.; Ledger, M.E.; Meerhoff, M.; Moss, B.; Mulder, C.; Shurin, J.B. Mesocosm Experiments as a Tool for Ecological Climate-Change Research. Adv. Ecol. Res. 2013, 48, 71–181. [Google Scholar]
- Liboriussen, L.; Landkildehus, F.; Meerhoff, M.; Bramm, M.E.; Søndergaard, M.; Christoffersen, K.; Richardson, K.; Søndergaard, M.; Lauridsen, T.L.; Jeppesen, E. Global Warming: Design of a Flow-through Shallow Lake Mesocosm Climate Experiment. Limnol. Oceanogr. Methods 2005, 3, 1–9. [Google Scholar] [CrossRef]
- Abreu-Silva, J.; Ribeirinho-Soares, S.; Oliveira-Inocêncio, I.; Pedrosa, M.; Silva, A.M.; Nunes, O.C.; Manaia, C.M. Performance of Polycarbonate, Cellulose Nitrate and Polyethersulfone Filtering Membranes for Culture-Independent Microbiota Analysis of Clean Waters. J. Environ. Chem. Engineer. 2023, 11, 109132. [Google Scholar] [CrossRef]
- Curry, K.D.; Wang, Q.; Nute, M.G.; Tyshaieva, A.; Reeves, E.; Soriano, S.; Wu, Q.; Graeber, E.; Finzer, P.; Mendling, W. Emu: Species-Level Microbial Community Profiling of Full-Length 16S rRNA Oxford Nanopore Sequencing Data. Nat. Methods 2022, 19, 845–853. [Google Scholar] [CrossRef]
- Len, P.; Meirkhanova, A.; Nugumanova, G.; Cestaro, A.; Jeppesen, E.; Vorobjev, I.A.; Donati, C.; Barteneva, N.S. Species-Level Classification Provides New Insights into the Biogeographical Patterns of Microbial Communities in Shallow Saline Lakes. bioRxiv 2023, 12, 570325. [Google Scholar] [CrossRef]
- Romette, A. Multivariate analyses in microbial ecology. FEMS Microb. Ecol. 2007, 62, 142–160. [Google Scholar] [CrossRef]
- Cáceres, M.D.; Legendre, P. Associations between Species and Groups of Sites: Indices and Statistical Inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- De Cáceres, M.; Sol, D.; Lapiedra, O.; Legendre, P. A Framework for Estimating Niche Metrics Using the Resemblance between Qualitative Resources. Oikos 2011, 120, 1341–1350. [Google Scholar] [CrossRef]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An open-source software for exploring and manipulating networks. In Proceedings of the International AAAI Conference on Web and Social Media, San Jose, CA, USA, 17–20 May 2009; Volume 3, pp. 361–362. [Google Scholar]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meirkhanova, A.; Zhumakhanova, A.; Len, P.; Schoenbach, C.; Levi, E.E.; Jeppesen, E.; Davidson, T.A.; Barteneva, N.S. Heatwave-Induced Thermal Stratification Shaping Microbial-Algal Communities Under Different Climate Scenarios as Revealed by Long-Read Sequencing and Imaging Flow Cytometry. Toxins 2025, 17, 370. https://doi.org/10.3390/toxins17080370
Meirkhanova A, Zhumakhanova A, Len P, Schoenbach C, Levi EE, Jeppesen E, Davidson TA, Barteneva NS. Heatwave-Induced Thermal Stratification Shaping Microbial-Algal Communities Under Different Climate Scenarios as Revealed by Long-Read Sequencing and Imaging Flow Cytometry. Toxins. 2025; 17(8):370. https://doi.org/10.3390/toxins17080370
Chicago/Turabian StyleMeirkhanova, Ayagoz, Adina Zhumakhanova, Polina Len, Christian Schoenbach, Eti Ester Levi, Erik Jeppesen, Thomas A. Davidson, and Natasha S. Barteneva. 2025. "Heatwave-Induced Thermal Stratification Shaping Microbial-Algal Communities Under Different Climate Scenarios as Revealed by Long-Read Sequencing and Imaging Flow Cytometry" Toxins 17, no. 8: 370. https://doi.org/10.3390/toxins17080370
APA StyleMeirkhanova, A., Zhumakhanova, A., Len, P., Schoenbach, C., Levi, E. E., Jeppesen, E., Davidson, T. A., & Barteneva, N. S. (2025). Heatwave-Induced Thermal Stratification Shaping Microbial-Algal Communities Under Different Climate Scenarios as Revealed by Long-Read Sequencing and Imaging Flow Cytometry. Toxins, 17(8), 370. https://doi.org/10.3390/toxins17080370





