Engineered Metal Nanoparticles: A Possible Small Solution to Big Problems Associated with Toxigenic Fungi and Mycotoxins
Abstract
1. Introduction
2. Engineered Nanomaterials Against Toxigenic Fungi and Mycotoxin Production
2.1. Definition of Nanoparticle
2.2. Engineered Nanoparticles as Antifungal Systems
3. Synthesis of MNPs
3.1. Top-Down Methods
3.1.1. Mechanical Ball Milling
3.1.2. Laser Ablation
3.1.3. Sputtering
3.1.4. Spray Pyrolysis
3.1.5. Electrospray
3.2. Bottom-Up Methods
3.2.1. Physical Methods
Physical Vapor Deposition (PVD)
3.2.2. Chemical Methods
Sol–Gel Method
Chemical Vapor Deposition (CVD)
Atomic Layer Deposition (ALD)
Electrochemical Reduction
Chemical Reduction
3.2.3. Biological or “Green” Synthesis
4. Antifungal Mechanisms of Metal Nanoparticles
- (a)
- The fungal cell wall undergoes changes and damage, including surface shrinkage, cell aggregation, pit and pore formation, and general deformation. Internalization of the NPs into fungal cells occurs through three principal mechanisms: (i) direct penetration of NPs through the cell wall, (ii) specific receptor-mediated adsorption followed by internalization, and (iii) uptake through ion transport proteins. During adsorption, NPs can embed within fungal cell walls, which induces morphological and functional changes [250]. Additionally, the NPs can release metal ions from the extracellular space. These ions can enter the fungal cell, thereby disrupting its biological processes [191].
- (b)
- The metal ions will contribute to the formation of NPs intracellularly through reduction processes by cellular organic compounds [251]. The fungal cell wall plays a critical role in various processes, including fungal growth and defense, morphogenesis, and biofilm formation. The primary functions of the cell include buffering fluctuations in osmotic pressure, sensing external stimuli, and protecting against detrimental conditions such as dryness, heat, and toxic molecules. The cell wall plays a pivotal role in the pathogenicity and virulence of pathogenic fungi, aiding in their invasion while protecting the fungus from host defense mechanisms [191]. The cell wall, a structural component of cells, appears to be a rigid structure; nevertheless, it is dynamic and is subject to constant remodeling due to several factors. These include fungal growth, which encompasses processes such as expansion, sporulation, and branching, as well as environmental challenges. The process of binary fission, also known as the expansion of hyphae, is contingent upon the concurrent activity of anabolic and catabolic enzymes. Therefore, based on the composition of the fungal cell wall, it can be concluded that the structure provides an optimal target for antifungal MNPs [191].
- (c)
- The disruption of the cell membrane is a consequence of the interaction between MNPs and the fungal cell membrane, leading to structural damage. As Slavin’s hypothesis states [191], positively charged metal-based NPs establish a robust bond with cell membranes, thereby increasing membrane permeability. This process facilitates the diffusion of essential ions and molecules, ultimately resulting in the demise of fungal cells.
- (d)
- The internal membranes are distorted, and there is an alteration in the organelle disposition. This phenomenon is evidenced by an increase in the intracellular vesicle and vacuole count and a decrease in cytoplasmic content. The loss of intracellular structure results in the accumulation of cytoplasm within the cell, accompanied by an apparent absence of organelles. This complicates the process of distinguishing between the cytoplasm, plasma membrane, and cell wall boundaries after exposure to NPs.
- (e)
- The underlying mechanism of this complication is the alteration of these structures by NPs, thereby obscuring the boundaries [185,190,251,252,253]. The generation of reactive oxygen species (ROS) is an inherent process within the human body. In the cell, the presence of metal ions or NPs has been observed to trigger the generation of ROS, which includes superoxide radicals and hydrogen peroxide. It has been demonstrated that ROS play a critical role in the antifungal activity mechanism of NPs. These substances have been demonstrated to induce oxidative stress in fungal cells. The oxidative stress can suppress the antioxidant defense mechanism of the fungus against ROS. Subsequently, these metal ions have been demonstrated to interact with cellular structures, thereby inducing damage to cellular components such as proteins, lipids, and DNA, which ultimately results in cell death [254,255].
- (f)
- Interaction with the fungal DNA is indicated herein. MNPs have been observed to penetrate fungal cells and interact with the DNA. The NPs can bind to the genetic material (which is negatively charged), resulting in structural damage, DNA fragmentation, or hindrance to DNA replication and transcription. This, in turn, disrupts the ability of the fungi to proliferate [191]. In addition, the NPs can induce mitochondrial DNA fragmentation, ribosome depolymerization, cellular dysfunction, and apoptosis [191]. The inhibition of enzyme activity is a consequence of the presence of MNPs within the fungal cell, thereby interfering with the function of the enzymes contained within. MNPs can bind to sulfhydryl groups on enzymes, thereby inhibiting their normal function and resulting in metabolic disruptions [118,256]. Metal ions have been observed to form strong coordination bonds with N, O, or S atoms. These atoms are found in abundance in organic compounds and biomolecules. Given the non-specific nature of the bond between metal ions and biomolecules, metal-based NPs typically demonstrate a broad spectrum of activities. It has been demonstrated that AgNPs exhibit reduced chemical reactivity in comparison to Ag+ ions. The interaction of Ag+ ions with a diverse array of biomolecules within the cell has been well documented, including nucleic acids, components of the cell wall, sulfhydryl groups of metabolic enzymes, and sulfur-containing cell components [257].
- (g)
5. Main Metal and Metal Oxide NPs Tested Against Toxigenic Fungi and Mycotoxin Production
5.1. Silver Nanoparticles
| Nanoparticle Properties | Antifungal Properties | |||||
|---|---|---|---|---|---|---|
| Synthesis Method | Size (nm) | Shape | Fungal Species | Methodology | Growth Reduction/(%)/Treatment | Ref. |
| Chemical | 14–100 (30) | Spherical | F. graminearum, F. culmorum, F. sporotrichioides, F. langsethiae, F. poae, F. oxysporum, F. proliferatum, F. verticillioides | Medium: Maize-based medium. Inoculum: From a spore suspension (1 × 105 spores/mL) previously treated with AgNPs for 2, 10, 20, and 30 h. AgNP concentration: 2, 5, 10, 15, 30, and 45 ppm. Incubation: 28–25 °C, 10 days. Fungal growth record: Spore viability (sv) and colony diameter (cd). | 100% (sv, cd)/2.0 ppm (30 h), 100% (sv, cd)/15.0 ppm (30 h), 100% (sv, cd)/10.0 ppm (30 h), 100% (sv, cd)/2.0 ppm (30 h), 100% (sv, cd)/2.0 ppm (30 h), 100% (sv, cd)/45.0 ppm (30 h), 100% (sv, cd)/30.0 ppm (30 h), 100% (sv, cd)/30.0 ppm (30 h). For each species, respectively | [275] |
| Chemical | 14–100 (30) | Spherical | A. flavus, A. parasiticus, A. carbonarius, A. niger, A. ochraceus, A. steynii, A. westerdijkiae, P. verrucosum | Medium: Maize-based medium. Inoculum: From a spore suspension (1 × 105 spores/mL) previously treated with AgNPs for 2, 10, 20, and 30 h. AgNP concentration: 2, 5, 10, 15, 30, and 45 ppm. Incubation: 20–37 °C, 10 days. Fungal growth record: Spore viability (sv) and colony diameter (cd). | 100% (sv, cd)/15.0 ppm (30 h), 100% (sv, cd)/30.0 ppm (30 h), 100% (sv, cd)/10.0 ppm (30 h), 100% (sv, cd)/15.0 ppm (30 h), 100% (sv, cd)/5.0 ppm (30 h), 100% (sv, cd)/5.0 ppm (30 h), 100% (sv, cd)/5.0 ppm (30 h), 100% (sv, cd)/15.0 ppm (30 h). For each species, respectively | [238] |
| Chemical | 20 | Spherical | A. parasiticus | Medium: Potato Dextrose Agar (PDA), Czapeck Dox Agar (CZA), Potato Dextrose Broth (PDB), and Czapeck Dox Broth (CZB). Inoculum: From a spore suspension (1 × 105 spores/mL) previously treated with AgNPs for 2, 10, 20, and 30 h. AgNP concentration: 25, 50, 100, and 200 ppm. Incubation: 28 °C, 7 days. Fungal growth record: Colony diameter (cd) and mycelium weight (mw). | 34% and 41% (cd)/200 ppm (in PDA and CZA, respectively) 92% (mw)/250 ppm (in both PDB and CZB) | [276] |
| Chemical | ∼7.5 | Spherical | Gibberella fujikuroi | Medium: PDA. Inoculum: From a spore suspension (7 × 104 spores/mL) previously treated with AgNPs for 1, 10, and 20 min. AgNP concentration: 0.00015, 0.0015, 0.015, 0.15, 1.5, 15, and 150 ppm. Incubation: 25 °C, 3 days. Fungal growth record: CFU. Medium: PDA. Inoculum: Rice seeds previously dipped in a spore suspension (5 × 105 spores/mL). AgNP concentration: Solution of 150 ppm (rice seeds previously infected with fungal spores are immersed in this solution for 1/6, 1/3, 1/2, 1, 3, 6, or 24 h). Incubation: 25 °C, 3 days. Fungal growth record: CFU (on seed surface). | 50% CFU/0.15–1.5 ppm (1 min) 100% CFU/≥ 1.5 ppm (1 min) 96.2% CFU/150 ppm (≥10 min) | [286] |
| Chemical | 25 ± 2.6 | Spherical | P. digitatum, P. italicum | Medium: PDA. Inoculum: From a spore suspension (1 × 106 spores/mL) previously treated with AgNPs for 24 h. AgNP concentration: 1–10 ppm. Incubation: 22 ± 1 °C, 4 days. Fungal growth record: CFU. Medium: Lemons. Inoculum: From a spore suspension (1 × 106 spores/mL) previously treated with AgNPs for 24 h. Concentration of AgNPs: 1–10 ppm. Incubation: 20 °C, 95% RH, 5–14 days. Fungal growth record: Lemon disease incidence. | 100% CFU/10 ppm (24 h) 100%/10 ppm/24 h) | [287] |
| Chemical | 17 ± 1.5 | Spherical | F. verticillioides | Medium: Nutrient Broth (according to CLSI for filamentous fungi). Inoculum: From a spore suspension (2 × 104 spores/mL). AgNP concentration: 5–200 ppm. Incubation: 25 °C, 48 h. Fungal growth record: MIC. | 100% (MIC)/75 ppm | [285] |
| Chemical | 12 ± 4 | F. avenaceum, F. equiseti | Medium: PDA. Inoculum: From a spore suspension (—) previously treated with AgNPs for 24–240 h. AgNP concentration: 2.5, 5, and 10 ppm. Incubation: 21 °C, 168 h. Fungal growth record: Colony diameter. | ∼25%/10 ppm (168 h) ∼43%/10 ppm (168 h) For each species, respectively | [270] | |
| Biological (Penicillium chrysogenum, Fusarium chlamydosporum) | 9–17.5 6–26 | Spherical Spherical | A. flavus, A. ochraceus | Medium: CZA. Inoculum: From a spore suspension (2 × 106 spores/mL). AgNP concentration: 10–50 ppm. Incubation: 30 °C, 16 h. Fungal growth record: Spore germination. | 100%/45–48 ppm 100%/47–51 ppm For each species, respectively | [284] |
| Biological (Aspergillus terreus) | 5–30 | Spherical | A. flavus (5 strains) | Medium: PDA. Inoculum: Agar plugs (6 mm) from a fungal culture. AgNP concentration: 50, 100, and 150 ppm. Incubation: 25 ± 2 °C, 7 days. Fungal growth record: Colony diameter. | 8.9–21.1%/50 ppm 43.3–54.8%/100 ppm 71.1–86.3%/150 ppm | [277] |
| Biological (Fusarium oxysporum) | 93 ± 11 | Spherical | A. flavus, A. melleus, A. nomius, A. ochraceus, A. parasiticus | Medium: Nutrient Broth and Sabouraud Dextrose Agar (SDA). Inoculum: From a spore suspension (1 × 105 spores/mL). AgNP concentration: 0.26–135 ppm. Incubation: 30 °C, 72 h. Fungal growth record: MIC and MFC (<3 colonies/plate). | MIC and MFC/8 and 64 ppm, MIC and MFC/4 and 16 ppm, MIC and MFC/8 and 32 ppm, MIC and MFC/4 and 16 ppm, MIC and MFC/8 and 32 ppm For each species, respectively | [288] |
| Biological (Malva parviflora L.) | 50.6 | Spherical | F. solani, F. oxysporum, A. alternata | Medium: PDA. Inoculum: Agar plugs (6 mm) from a fungal culture. AgNP concentration: 50,000 ppm. Incubation: 25 ± 2 °C, 7–9 days. Fungal growth record: Colony diameter. | 81.1%/50,000 ppm, 80.7%/50,000 ppm, 83.0%/50,000 ppm, For each species, respectively | [289] |
| Biological (Honeybee) | — | Cubic | F. avenaceum, F. culmorum, F. graminearum, F. oxysporum, F. poae, F. proliferatum, F. pseudograminearum, F. sambucinum, F. semitectum, F. sporotrichioides, F. verticillioides | Medium: PDA. Inoculum: From a spore suspension (2.5 × 103 spores/mL) (well diffusion method). AgNP concentration: 5, 25, 50, 75, and 100 ppm. Incubation: 25 ± 2 °C, 7 days. Fungal growth record: Growth reduction (%). | 3.3 ± 0.0–31.2 ± 0.0%/5–100 ppm, 2.2 ± 0.0–36.8 ± 0.3%/5–100 ppm, 2.2 ± 0.0–25.8 ± 0.1%/5–100 ppm, 1.1 ± 0.0–16.8 ± 0.2%/5–100 ppm, 2.2 ± 0.1–26.8 ± 0.2%/5–100 ppm, 2.2 ± 0.1–23.4 ± 0.2%/5–100 ppm, 4.4 ± 0.2–27.8 ± 0.1%/5–100 ppm, 4.4 ± 0.2–31.2 ± 0.2%/5–100 ppm, 3.3 ± 0.1–25.6 ± 0.2%/5–100 ppm, 2.2 ± 0.1–18.9 ± 0.3%/5–100 ppm, 2.2 ± 0.0–28.9 ±0.4%/5–100 ppm, For each species, respectively | [278] |
| Biological (Chaetomium globosum) | 11–14 | Spherical | F. oxysporum | Medium: PDA, Corn Meal Agar (CMA), and Malt Extract Agar (MEA). Inoculum: —. AgNP concentration: 50, 100, and 500 ppm. Incubation: 28–30 °C, 7 days. Fungal growth record: Colony diameter and CFU. Medium: Tomato seeds. Inoculum: —. AgNP concentration: 500 ppm. Incubation: 28–30 °C, 4 h. Fungal growth record: Seedlings wilt. | 88% (cd)/500 ppm 100% (CFU)/500 ppm Complete inhibition of seedlings wilt/500 ppm. | [290] |
| Biological (Epifocal nigrum) | 1–22 | Spherical | A. flavus | Medium: Nutrient Broth (documents M27-A, M38-A). Inoculum: From a spore suspension (2.5–5 ×103 spores/mL). AgNP concentration: 0.125–64 ppm. Incubation: 25 °C, 48 h. Fungal growth record: MIC (reduction of fungal growth by 80%). | 80%/5 ppm | [291] |
| Biological (Tropaeolum majus) | — | Spherical | A. niger | Medium: Nutrient Broth + resazurin indicator solution. Inoculum: From a spore suspension (5 × 106 spores/mL). AgNP concentration: 1–500 ppm. Incubation: —. Fungal growth record: At a glance. | 100%/≥ 31.2 ppm | [292] |
| Biological (Trichoderma harzianum) | — | — | F. moniliforme | Medium: PDA, CZA, Yeast Dextrose Agar (YES). Water Agar (WA). Inoculum: Agar plugs (5 mm) from a fungal culture. Concentrations of AgNPs: 0–800 ppm. Incubation: 25 ± 2 °C, 5 days. Fungal growth record: Colony diameter. | 0–60.04%/0–800 ppm | [293] |
| Biological (Geranium leaves) | 38.5 ± 18.5 | Spherical | F. oxysporum f. sp. lycopersici | Medium: PDA. Inoculum: Agar plugs (6 mm) from a fungal culture. AgNP concentration: 10, 20, 40, 75, and 150 ppm. Incubation: 27 ± 2 °C, 7 days. Fungal growth record: Colony diameter. Medium: PDB. Inoculum: From a spore suspension (1 × 104 spores/mL). AgNP concentration: 10, 20, 40, 75, and 150 ppm. Incubation: 27 ± 2 °C, 72 h. Fungal growth record: MIC. Medium: Tomatoes. Inoculum: From a spore suspension (1.5 × 105 spores/mL). AgNP concentration: 10, 20, 40, 75, and 150 ppm. Incubation: 27 ± 2 °C, 6 days. Fungal growth record: Tomato disease incidence. | 94.6%/150 ppm 100%/75 ppm 100%/10–100 ppm | [294] |
| Biological (Althaea officinalis, Thymus vulgaris, Mentha pulegium) | 50 50 50 | Spherical Spherical Spherical | A. flavus, P. chrysogenum | Medium: PDA. Inoculum: From a spore suspension (1 × 105 spores/mL, disk diffusion method). AgNP concentration: suspension 1 mM. Incubation: 26–27 °C, 60 h. Fungal growth record: Diameter inhibition zone. | 35–36 mm/1 mM 36–37 mm/1 mM For each species, respectively | [295] |
| Biological (Arthroderma fulvum) | 15.5 ± 2.5 | Spherical | A. flavus, A. terreus, F. solani, F. moniliforme, F. oxysporum | Medium: Nutrient broth (document M38-A2). Inoculum: From a spore suspension (1.0–2.0 × 104 spores/mL). AgNP concentration: 0.125–64 ppm. Incubation: 28 °C, 48 h. Fungal growth record: MIC (reduction of fungal growth by 80%). | 80%/2.00 ppm, 80%/1.00 ppm, 80%/2.00 ppm, 80%/4.00 ppm, 80%/2.00 ppm, For each species, respectively | [296] |
| Biological (Amaranthus retroflexus) | 10–32 | Spherical | A. alternata, F. oxysporum | Medium: PDA. Inoculum: Agar plugs (5 mm) from a fungal culture. AgNP concentration: 50, 100, 200, and 400 ppm. Incubation: 25 °C, 5 days. Fungal growth record: Colony diameter. | 50%/337.09 ± 19.72 ppm, 50%/328.05 ± 13.29 ppm, For each species, respectively | [297] |
| Biological (Phyllanthus urinaria, Pouzolzia zeylanica, Scoparia dulcis) | 28.3 26.7 <26.7 | Spherical Spherical, hexagonal, triangle, etc. Spherical | A. niger, A. flavus, F. oxysporum | Medium: PDA. Inoculum: Colonies from fungal cultures. AgNP concentration: 15, 30, and 45 ppm. Incubation: Room temperature, 4 days. Fungal growth record: Colony diameter. | 40–60%/45 ppm Depending on the fungal species | [298] |
| Biological (Aspergillus terreus) | 15–29 | Spherical | F. solani, A. alternata, A. flavus, A. ochraceus | Medium: CZA. Inoculum: –. AgNP concentration: 1 mM, 2 mM, 5 mM, 10 mM, and 20 mM (wells, 7 mm). Incubation: 28 °C, 3–5 days. Fungal growth record: Diameter inhibition zone. | 3–13 mm/0.84–1.68 ppm | [299] |
| Biological (Trichoderma longibrachiatum) | 5–30 (10) | Spherical | A. alternata, F. verticillioides, F. moniliforme, A. flavus, P. brevicompactum | Medium: PDA. Inoculum: From a spore suspension treated with 0.5 mM AgNPs. AgNP concentration: 0.5 mM. Incubation: 28 °C, 24 h. Fungal growth record: CFU. | 93.0%/0.5 mM, 96.4%/0.5 mM, 93.6%/0.5 mM, 86.7%/0.5 mM, 92.9%/0.5 mM, For each species, respectively | [300] |
| Biological (Momordica charantia, Psidium guajava) | 5–29 (17) 5–53 (25.7) | Spherical Spherical | A. niger, A. flavus, F. oxysporum | Medium: PDA. Inoculum: –. AgNP concentration: 20 and 40 ppm. Incubation: 30 °C, 24–96 h. Fungal growth record: Colony diameter. | <50%/40 ppm (96 h) | [301] |
| Biological (Cryptococcus laurentii, Rhodotorula glutinis) | 15–400 | Spherical | P. expansum, A. niger, Alternaria sp. | Medium: PDA. Inoculum: From a spore suspension (2.0 × 106 spores/mL) (wells of 3 mm). AgNP concentration: 3 ppm. Incubation: 28 ± 4 °C, 7 days. Fungal growth record: Diameter inhibition zone. | 11.1 ± 1.4 mm/3 ppm, 14.8 ± 2.2 mm/3 ppm, 11.3 ± 1.6 mm/3 ppm, For each species, respectively | [302] |
| Biological (Pseudomonas poae) | 19.8–44.9 | Spherical | F. graminearum | Medium: PDA and PDB. Inoculum: Agar plugs (5 mm) from a fungal culture. AgNP concentration: 5, 10, 15, and 20 ppm. Incubation: 28 °C, 5 days. Fungal growth record: Colony diameter in PDA and mycelium growth in PDB. | PDA: 45.56%/5 ppm 62.22%/10 ppm 72.78%/15 ppm 80.56%/20 ppm PDB: 48.56%/5 ppm 65.11%/10 ppm 75.50%/15 ppm 85.78%/20 ppm | [279] |
| Biological (Alternaria sp.) | 3–10 | Spherical | F. oxysporum, F. moniliforme, F. tricinctum, Alternaria sp. | Medium: PDA. AgNP concentration: 1000 ppm (25, 50, and 100 µL (wells 8 mm). Inoculum: —. Incubation: 28 ± 1 °C, 5 days. Fungal growth record: Diameter inhibition zone. | 14.7–21.3 mm/1000 ppm, 9–21.6 mm/1000 ppm, 11–21.2 mm/1000 ppm, 17.1–21.6 mm/1000 ppm, For each species, respectively | [303] |
| Biological (Penicillium verrucosum) | 10–12 | Spherical | A. flavus, F. chlamydosporum | Medium: PDA. Inoculum: Agar plugs (3 mm) from a fungal culture. AgNP concentration: 50, 100, 150, and 200 ppm. Incubation: 27 ± 2 °C, 7 days. Fungal growth record: Colony diameter. | 59.13%/200 ppm, 56.67%/200 ppm, For each species, respectively | [304] |
| Biological (Rhizoctonia solani, Cladosporium cladosporioides) | 80–100 | Spherical | A. flavus, P. citrinum, F. oxysporum | Medium: Sabouraud Dextrose Agar (SDA). Inoculum: —. AgNP concentration: 5000, 10,000, 15,000 ppm (wells 5 mm). Incubation: —. Fungal growth record: Diameter inhibition zone. | 15–21 mm/5000–15,000 ppm, 8–17 mm/5000–15,000 ppm, 8.33–13.33/5000–15,000 ppm, For each species, respectively | [305] |
| Biological (Trigonella foenum-graecum) | 20–25 | Spherical and cubic | A. alternata | Medium: PDA. Concentration of AgNPs: 100 ppm. Inoculum: —. Incubation: —. Fungal growth record: Colony diameter. | 40–50%/100 ppm | [306] |
| Biological (Nigrospora oryzae) | 3–13 | Spherical | F. sporotrichioides, F. oxysporum, F. moniliforme, F. solani, F. anthophilium | Medium: CZA and PDA. Inoculum: Agar plugs (4 mm) from a fungal culture. AgNP concentration: 50, 100, 150, and 200 ppm. Incubation. 28 ± 2 °C, 5 days. Fungal growth record: Colony diameter. | 50–70%/200 ppm Depending on the species | [307] |
| Biological (Allium cepa, Zingiber officinale, Allium sativum) | 1–9 1–6 2–10 | Spherical | F. graminearum, F. avenaceum, F. culmorum | Medium: PDB. Inoculum: —. AgNP concentration: 10, 30, 50, 70, 90, 110, 130, and 150 ppm. Incubation: 28 °C, 2 days. Fungal growth record: MIC. | 100%/90–110 ppm, 100%/90–110 ppm, 100%/110 ppm, For each species, respectively | [308] |
| Biological (Honey) | 9.9 | Spherical | A. parasiticus, A. ochraceus | Medium: PDA. Inoculum: From a spore suspension (1 × 106 spores/mL). AgNP concentration: 10, 20, 30, and 40 µg/well (well diffusion technique). Incubation: 28 °C, 3 days. Fungal growth record: Diameter inhibition zone. | 24.2 ± 0.77 mm/40 µg, 28.2 ± 1.04 mm/40 µg For each species, respectively | [280] |
| Biological (Penicillium expansum, Aspergillus terreus) | 14–25 10–18 | Spherical Spherical | A. ochraceus, A. parasiticus, A. niger | Medium: PDA. Inoculum: From a spore suspension (1 × 106 spores/mL). AgNP concentration: 3, 6, and 9 μg/well (5 mm). Incubation: 28 °C, 3 days. Fungal growth record: Diameter inhibition zone. | 16.33 ± 96 mm/9 μg/well, No detected, 10.1–11.3 mm/6–9 μg/well For each species, respectively | [281] |
| Biological (Viola odorata) | 18 | Spherical | F. oxysporum f. sp. radicis-lycopersici | Medium PDA. Inoculum: Agar plugs (5 mm) from a fungal culture. AgNP concentration: 600 ppm. Incubation: 28 °C, 7 days. Fungal growth record: Colony diameter. Medium: Tomato plants. Inoculum: From a spore suspension (1.5 × 104 spores/mL) (30 mL/plant). AgNP concentration: 60 ppm. Incubation: 24 °C ± 5 °C and photoperiod, 7 days. Fungal growth record: Percentage of plants that survive. | ∼50%/600 ppm 80%/solution of 60 ppm | [309] |
| Commercial | — | — | A. parasiticus | Medium: PDB. Inoculum: From a spore suspension (1 × 106 spores/mL). AgNP concentration: 60, 80, 100, 120, 140, 160, 180, and 200 ppm. Incubation: 28 °C, 96 h, 130 rpm. Fungal growth record: At a glance. | 100%/180 ppm | [282] |
| Commercial | 200– ≤0.65 | Spherical | P. verrucosum | Medium: Malt Extract Broth. Inoculum: From a spore suspension (1 × 106 spores/mL). AgNP concentration: 1–100 ppm. Incubation: 25 °C, 7 days. Fungal growth record: GNU Image Analysis Program GIMP 2.8.10. | 100% spore germination/>2 ppm (AgNPs 0.65 nm) 100% fungal growth/>5 ppm (AgNPs 5 nm) | [283] |
| Commercial | 2.0 | — | F. graminearum | Medium: PDA. Inoculum: Agar plugs (5 mm) from a fungal culture. AgNP concentration: 1, 1.5, 2, 3, 6, 8, and 10 ppm. Incubation: 25 °C, 2–3 days. Fungal growth record: Colony diameter. | 50%/1.88 ppm 90%/1.15 ppm | [186] |
| Commercial Argovit-1220 Argovit-1221 Argovit-C | 8–80 8.5 ± 3.3 14.95 ± 10.1 | Spherical, Pyramidal Spherical, Spherical | F. oxysporum f. sp. cubense | Medium: Mueller Hinton Broth + resazurin. Inoculum: From a spore suspension (1 × 104 spores/mL). AgNP concentration: 0.8, 1.6, 3.1, 6.3, 12.5, 25, 50, and 100 ppm. Incubation: 28 °C, 3 days. Fungal growth record: Mycelial growth. | 50%/3.1–6.3 ppm >90%/25–50 ppm Depending on commercial AgNPs | [310] |
| Commercial | 7–25 | A. alternata, F. oxysporum f. sp. cucumerinum, F. oxysporum f. sp. lycopersici, F. oxysporum, F. solani | Medium: PDA, MEA, and CMA (Corn Meal Agar). AgNP concentration: 10, 25, 50, and 100 ppm. Inoculum: Agar plugs (8 mm) from fungal cultures. Incubation: 28 ± 2 °C, 14 days. Fungal growth record: Colony diameter. | PDA 81.1%/100 ppm, 59.5%/100 ppm, 89.6%/100 ppm, 84.0%/100 ppm, 80.7%/100 ppm, For each strain, respectively MEA 65.35%/100 ppm, 36.5%/100 ppm, 26.5%/100 ppm, 21.2%/100 ppm, 52.9%/100 ppm, For each strain, respectively CMA 77.6%/100 ppm, 76.5%/100 ppm, 80.0%/100 ppm, 68.2%/100 ppm, 81.2%/100 ppm, For each strain, respectively | [311] | |
| Commercial | — | — | F. oxysporum | Medium: PDA. Inoculum: Agar plugs (3 mm) from a fungal culture. AgNP concentration: 5, 15, 25, and 35 ppm. Incubation: 22 °C, 14 days. Fungal growth record: Colony diameter. | 32.6%/35 ppm | [312] |
| Nanoparticle Properties | Anti-Mycotoxin Properties | |||||
|---|---|---|---|---|---|---|
| Synthesis Method | Size (nm) | Shape | Fungal Species (Mycotoxin Production) | Methodology | Mycotoxin/Reduction (%)/Treatment | Ref. |
| Chemical | 14–100 (30) | Spherical | F. graminearum (DON, 3-AcDON, ZEA), F. culmorum (DON, 3-AcDON, ZEA), F. sporotrichioides (T-2, HT-2), F. langsethiae (T-2, HT-2), F. poae (NIV), F. proliferatum (FB1, FB2), F. verticillioides (FB1, FB2) | Medium: Maize-based medium. Inoculum: From a spore suspension (1 × 105 spores/mL) previously treated with AgNPs for 2, 10, 20, and 30 h. AgNP concentration: 2, 5, 10, 15, 30, and 45 ppm. Incubation: 25–28 °C, 10 days. Mycotoxins analysis: UPLC-MS/MS. | DON/100%/2–15 ppm, 30 h, 3 AcDON/100%/2–15 ppm, 30 h, ZEA/100%/2–15 ppm. 30 h, T-2/100%/2 ppm, 30 h, HT-2/100%/2 ppm, 30 h, NIV/100%/2 ppm, 30 h, FB1/100%/30 ppm, 30 h, FB2/100%/30 ppm, 30 h, Depending on species and mycotoxin | [275] |
| Chemical | 14–100 (30) | Spherical | A. flavus (AFB1, AFB2), A. parasiticus (AFB1, AFB2, AFG1, AFG2), A. carbonarius (OTA), A. niger (OTA), A. ochraceus (OTA), A. steynii (OTA), A. westerdijkiae (OTA), P. verrucosum (OTA) | Medium: Maize-based medium. Inoculum: From a spore suspension (1 × 105 spores/mL) previously treated with AgNPs for 2, 10, 20, and 30 h. AgNP concentration: 2, 5, 10, 15, 30, and 45 ppm. Incubation: 20–37 °C, 10 days. Mycotoxins analysis: UPLC-MS/MS. | AFB1/100%/15–30 ppm, 30 h, AFB2/100%/15–30 ppm, 30 h, AFG1/100%/30 ppm, 30 h, AFG2/100%/30 ppm, 30 h, OTA/100%/5–15 ppm, 30 h, Depending on species and mycotoxin | [238] |
| Chemical | 20 | Spherical | A. parasiticus (AFB1, AFB2, AFG1, AFG2) | Medium: YES. Inoculum: From a spore suspension (1 × 105 spores/mL). AgNP concentration: 25, 50, 100, and 200 ppm. Incubation: 28–30 °C, 14 days. Aflatoxin analysis: HPLC. | AFB1/6%/200 ppm, AFB2/57.3%/200 ppm, AFG1/20.4%/200 ppm, AFG1/95.8%/200 ppm | [276] |
| Chemical | 17 ± 1.5 | Spherical | F. verticillioides (FB1) | Medium: Nutrient Broth (according to CLSI for filamentous fungi). Inoculum: From a spore suspension (2 × 104 spores/mL). AgNP concentration: 5–200 ppm. Incubation: 28 °C, 14 days. FB1 analysis: HPLC. | FB1/100%/75 ppm | [285] |
| Biological (Aspergillus terreus) | 5–30 | Spherical | A. flavus (AFB1) (5 strains) | Medium: SMKY broth. Inoculum: Agar plugs (6 mm) from a fungal culture. AgNP concentration: 50, 100, and 150 ppm. Incubation: 25 ± 2 °C, 20 days. Aflatoxin analysis: –. | AFB1/48.2–61.8%/50 ppm, AFB1/64.1–82.2%/100 ppm, AFB1/75.9–100%/150 ppm, Depending on the strain | [277] |
| Biological (Honeybee) | 20–60 | Cubic | F. avenaceum (DON), F. proliferatum (DON), F. sambucinum (DON), F. verticilliodes (DON), F. semitectum (DON), | Medium: SMKY broth. Inoculum: From a spore suspension (2.5 × 103 spores/mL). AgNP concentration: 5, 25, 50, 75, and 100 ppm. Incubation: 25 ± 2 °C, 10 days. DON analysis: ELISA. | DON/0.03–22%/5 ppm, DON/5.61–8.46%%/25 ppm, DON/25.40–34.44%/50 ppm, DON/25.75–34.60%/75 ppm, DON/25.49–34.89%/100 ppm, For each species, respectively | [278] |
| Biological (Pseudomonas poae) | 19.8–44.9 | Spherical | F. graminearum (DON) | Medium: GYEP. Inoculum: From a spore suspension (1 × 106 spores/mL). AgNP concentration: 5, 10, 15, and 20 ppm. Incubation: 28 °C, 7 days. DON analysis: ELISA. | DON/33%/5 ppm, DON/53%/10 ppm, DON/73%/15 ppm, DON/83%/20 ppm | [279] |
| Biological (Honey) | 9.9 | Spherical | A. parasiticus (AFB1, AFB2, AFG1, AFG2), A. ochraceus (OTA) | Medium: Yeast Extract Sucrose (YES). Inoculum: From a spore suspension (1 × 106 spores/mL). AgNP concentration: 10, 20, and 30 ppm. Incubation: 28 °C, 14 days. Aflatoxins B1, B2, G1 and G2, and OTA. analysis: HPLC. | AFB1/58.76%/30 ppm, AFB2/66.56%/30 ppm, AFG1/77.55%/30 ppm, AFG2/62.91%/30 ppm, OTA/79.85%/30 ppm | [280] |
| Biological (Penicillium expansum, Aspergillus terreus) | 14–25 10–18 | A. ochraceus (OTA) | Medium: YES Broth. Inoculum: From a spore suspension (1 × 106 spores/mL). AgNP concentration: 0.5, 1.1, and 2.2 ppm. Incubation: 28 °C, 14 days. OTA analysis: HPLC. | OTA/58.87–52·18%/2.2 ppm | [281] | |
| Biological synthesis (Green and black teas) | 10–20 | Spherical | A. flavus (Aflatoxins) A. parasiticus (Aflatoxins) | Medium: (CZA) Czapek’s Agar. Inoculum: From a spore suspension (1 × 106 spores/mL). AgNP concentration: 10, 25, 50, 100 ppm. Incubation: 25 ± 2 °C, 15 days. Aflatoxin analysis: HPLC. | Aflatoxins/100%/100 ppm | [313] |
| Biological synthesis (Syzygium cumini) | 11–19 | Spherical | A. flavus (Aflatoxins) A. parasiticus (Aflatoxins) | Medium: Czapek Dox Broth (CZB). Inoculum: From a spore suspension (1 × 106 spores/mL). AgNP concentration: 25, 50, 100 ppm. Incubation: 25 ± 2 °C, 15 days. Aflatoxin analysis: HPLC. | Aflatoxins/100%/100 ppm | [314] |
| Commercial | — | — | A. parasiticus (AFB1) | Medium: PDB. Inoculum: From a spore suspension (1 × 106 spores/mL). AgNP concentration: 45, 90, 100, 135, ppm. Incubation: 28 °C, 7 days, 130 rpm. AFB1 analysis: HPLC. | AFB1/50%/90 ppm AFB1/100%/100 ppm | [282] |
| Commercial | 200– ≤0.65 | Spherical | P. verrucosum (OTA, CIT) | Medium: MEA-liquid-medium. Inoculum: From a spore suspension (1 × 106 spores/mL). AgNP concentration: 1–100 ppm. Incubation: 25 °C, 7 days. OTA and CIT analysis: HPLC. | DON/100%/5 ppm (AgNPs 5 nm) CIT/100%/5 ppm (AgNPs 5 nm) | [283] |
| Commercial | 2.0 | — | F. graminearum (DON) | Medium: Toxin biosynthesis inducing (TBI) broth medium. Inoculum: Agar plugs (5 mm) from a fungal culture. AgNP concentration: 1, 1.5, 2, 3, 6, 8, and 10 ppm. Incubation: 25 °C, 2 + 6 days. DON analysis: kit Wis008 (Wise Science, Zhenjiang, China). | DON/Increase 50%/1.15 ppm DON/Increase > 50%/1.88 ppm | [185] |
5.2. Copper Nanoparticles
| Nanoparticle Properties | Antifungal Properties | |||||
|---|---|---|---|---|---|---|
| Synthesis Method | Size (nm) | Shape | Fungal Species | Methodology | Growth Reduction (%)/Treatment | Ref. |
| Chemical | 3–10 | Spherical | A. alternata, F. oxysporum | Medium: PDA. Inoculum: —. CuNP concentration: 20 μg/disc (disc diffusion method. Incubation: 28 °C, 2–3 days. Fungal growth record: Diameter inhibition zone. | 18 ± 1.1 mm/20 μg/disc, 24 ± 0.5 mm/20 μg/disc, For each species, respectively | [326] |
| Chemical | 100–500 | Flower | A. niger, F. moniliforme, F. culmorum, F. oxysporum, F. tricinctum | Medium: PDA. Inoculum: —. CuNP concentration: 100 mM (disc diffusion method). Incubation: 28 ± 2 °C, 2–3 days. Fungal growth record: Diameter inhibition zone. | ∼0–30 mm/100 mM Depending on the fungal species | [327] |
| Chemical | 14–37 | Truncated, octahedrons | F. oxysporum | Medium: PDA. Inoculum: From a spore suspension (1 × 106 spores/mL). CuNP concentration: 100, 250, and 500 ppm. Incubation: 29 °C, 6 days. Fungal growth record: Colony diameter. | 90–100%/500 ppm | [328] |
| Chemical | 50 | Spherical | A. flavus, P. chrysogenum | Medium: PDA. Inoculum: From a spore suspension (1 × 105 spores/mL). CuNP concentration: 1 mM. Incubation: 26–27 °C, 60 h. Fungal growth record: Diameter inhibition zone. | 24–27 mm/1 mM | [295] |
| Chemical | 20–50 | Spherical | Fusarium sp. | Medium: PDA (+ chloramphenicol). Inoculum: —. CuNP concentration: 300, 380, and 450 ppm. Incubation: —, 9 days. Fungal growth record: Colony diameter. | 67.38%/450 ppm (3 days) 93.98%/450 ppm (9 days) | [329] |
| Chemical | 200–500 | — | F. oxysporum f. sp. lycopersici | Medium: PDA. Inoculum: From a spore suspension (1 × 106 spores/mL). CuNP concentration 100, 250, 500, 750, and 1000 ppm. Incubation: 29 °C, 7 days. Fungal growth record: Colony diameter. Medium: Tomato plants. Inoculum: From a spore suspension (1 × 106 spores/mL). CuNP concentration: 500 ppm. Incubation: room temperature (June-August), 60 days. Fungal growth record: Ratio of leaves with symptoms/total leaves. | >80%/1000 ppm 70%/500 ppm | [325] |
| Chemical | 14 ± 2 | Spherical | A. niger, A. oryzae | Medium: PDA. Inoculum: From a spore suspension (—). CuNP concentration: 0.2, 0.4, 0.6, and 0.8 ppm (well diffusion method). Incubation: 28 ± 4 °C, 2 days. Fungal growth record: Diameter inhibition zone. | 17–24 mm/0.2–0.8 ppm. 15–20 mm/0.2–0.8 ppm. For each species, respectively | [330] |
| Chemical | 8 | Spherical | P. chrysogenum, A. alternata, F. solani, A. flavus | Medium: PDA. Inoculum: From a spore suspension (1 × 104 spores/mL). CuNP concentration: 20, 40, 60, 80, and 100 ppm. Incubation: 25 °C, 6 days. Fungal growth record: MIC. | 100%/40 ppm, 100%/60 ppm, 100%/60 ppm, 100%/80 ppm, For each species, respectively | [331] |
| Chemical | 53–174 | Spherical | F. oxysporum | Medium: PDA. Inoculum: — (well 4 mm). CuNP concentration: 5, 10, and 20 ppm. Incubation: 30 °C, 2–5 days. Fungal growth record: Diameter inhibition zone. | 49–72%/20 ppm | [332] |
| Chemical | 3–30 | Spherical | F. culmorum, F. oxysporum, F. equiseti | Medium: PDA. Inoculum: From a spore suspension (—). CuNP concentration: —. Incubation: 28 ± 2 °C, 3 days. Fungal growth record: Diameter inhibition zone. | 19 mm/—, 20 mm/—, 25 mm/—, For each species, respectively | [333] |
| Chemical | 200–500 | — | F. solani, F. oxysporum | Medium: PDA. Inoculum: From a spore suspension (1 × 106 spores/mL). CuNP concentration: 100, 250, 500, 750, and 1000 ppm. Incubation: 29 °C, 6 days. Fungal growth record: Colony diameter. | 95–97%/500 ppm | [334] |
| Chemical | 2.5 ± 0.3 | Spherical | F. verticillioides | Medium: Nutrient Broth (according to CLSI for filamentous fungi). Inoculum: From a spore suspension (2 × 104 spores/mL). CuNP concentration: 5–200 ppm. Incubation: 25 °C, 48 h. Fungal growth record: MIC. | 100%/125 ppm | [285] |
| Chemical | — | — | A. niger | Medium: PDA. Inoculum: —. CuNP concentration: 0.5, 1, and 1.5%. Incubation: 30 °C, 3 days. Fungal growth record: Colony diameter. | ∼35–100%/0.5–1.5% | [335] |
| Biological (Streptomyces capillispiralis Ca-1) | 3.6–59 | Spherical | Alternaria spp., A. niger, Fusarium spp. | Medium: PDA. Inoculum: Agar plugs (4 mm) from a fungal culture. CuNP Concentration: 5, 10, 15, and 20 mM. Incubation: 30 °C, 7 days. Fungal growth record: Colony diameter. | 57.14%/20 mM, 63.81%/20 mM, 42.61%/20 mM, For each species, respectively | [35] |
| Biological (Citrus medica Linn) | 10–60 (33) | Spherical | F. culmorum, F. oxysporum, F. graminearum | Medium: PDA. Inoculum: From a spore suspension (—). CuNP concentration: 2.18 × 108 particles/mL Incubation: 25 ± 2 °C, 52 h. Fungal growth record: Diameter inhibition zone. | ∼20–33 mm/2.18 × 108 particles/mL Depending on the fungal species | [336] |
| Biological (Talaromyces pinophilus) | 9 | Spherical | A. niger, A. terreus, A. fumigatus | Medium: Malt Extract Agar (MEA). Inoculum: From a spore suspension (1 × 107 spores/mL). CuNP concentration: 2000 ppm (wells 7.5 mm). Incubation: 30 °C, 72 h. Fungal growth record: Diameter inhibition zone. | 21.3 ± 0.58 mm/2000 ppm, 20.2 ± 1.26 mm/2000 ppm, 22.3 ± 1.53 mm/2000 ppm, For each species, respectively | [337] |
| Biological (Celastrus paniculatus leaves) | 2–10 | Spherical | F. oxysporum | Medium: PDA. Inoculum: Agar plugs (—) from a fungal culture. CuNP concentration: 0.12, 0.18, and 0.24%, w/v. Incubation: —. Fungal growth record: Colony diameter. | 76.29 ± 1.52%/0.24% 73.70 ± 1.52%/0.18% 59.25 ± 0.57%/0.12% | [338] |
| Commercial | 25 | — | A. alternata, F. solani, F. oxysporum f. sp. radicis lycopersici | Medium: PDA. Inoculum: Agar plug (5 mm) from a fungal culture. CuNP concentration: 0, 1, 10, 100, 500, 1000 ppm. Incubation: 25 °C, 4 days. Fungal growth record: Colony diameter. Medium: PDA. Inoculum: From a spore suspension (1 × 103 spores/mL). CuNP concentration: 0, 1, 2.5, 5, 10, 20, 50, 100 ppm. Incubation: 25 °C, 2 days. Fungal growth record: CFU. | 50%/296.56 ± 8.72 ppm 50%/261.16 ± 12.54 ppm 50%/328.12 ± 20.30 ppm For each species, respectively 50%/7.69 ± 1.00 ppm, 50%/18.84 ± 2.44 ppm, 50%/29.04 ± 4.32 ppm, For each species, respectively | [339] |
| Nanoparticle Properties | Antifungal Properties | |||||
|---|---|---|---|---|---|---|
| Synthesis Method | Size (nm) | Shape | Fungal Species | Methodology | Growth Reduction (%)/Treatment | Ref. |
| Biological (Morinda citrifolia) | 20–50 (29) | Spherical | A. flavus, A. niger | Medium: Sabouraud Dextrose agar (SDA). Inoculum: From a spore suspension (—). CuONP concentration: — (wells 5 mm). Incubation: 37 °C, 24 h. Fungal growth record: Diameter inhibition zone. | 7.6–13.1 mm/—, 9.2–14.7 mm/—, For each species, respectively | [340] |
| Biological (Penicillium chrysogenum) | 10.5–59.7 | Spherical | F. solani, F. oxysporum, A. terreus | Medium: PDA. Inoculum: —. CuONP concentration: 10,000 ppm (discs 7 mm). Incubation: 30 °C, 5 days. Fungal growth record: Diameter inhibition zone. | 31.66 ± 0.88 mm/10,000 ppm, 22.66 ± 0.66 mm/10,000 ppm, 28.66 ± 1.76 mm/10,000 ppm, For each species, respectively | [341] |
| Biological (Cissus quadrangularis) | 30 ± 2 | Spherical | A. niger, A. flavus | Medium: PDB. Inoculum: —. CuONP concentration: 500 and 1000 ppm. Incubation: —, 7 days. Fungal growth record: Fungal biomass. | 86%/1000 ppm, 85%/1000 ppm, For each species, respectively | [342] |
| Biological (Bougainvillea flower) | 5–20 (12 ± 4) | Spherical | A. niger | Medium: PDA. Inoculum: From a spore suspension (—). CuONP concentration: 5000 ppm. Incubation: 30 °C, 1 day. Fungal growth record: Diameter inhibition zone. | 80%/5000 ppm | [343] |
| Biological (Eichhornia crassipes) | 28 ± 4 | Spherical | F. culmorum, A. niger, A. flavus, F. oxysporum | Medium: PDA. Inoculum: —. CuONP concentration: 25, 50, 75, and 100 ppm. Incubation: Room temperature, 2 days. Fungal growth record: Diameter inhibition zone. | 21.26 ± 1 mm/100 ppm, 18.33 ± 1 mm/100 ppm, 16–18 mm/100 ppm, 15–17 mm/100 ppm, For each species, respectively | [344] |
| Biological (Aloe vera) | 11 ± 0.5 | Spherical | P. digitatum, P. italicum | Medium: PDA. Inoculum: From a spore suspension (1 × 106 spores/mL) (treated with CuONPs for 24 h). CuONP concentration: 100–1000 ppm. Incubation: 22 ± 1 °C, 4 days. Fungal growth record: CFU. Medium: Lemons. Inoculum: Steel rod previously immersed in conidial suspensions (treated with CuONPs for 24 h). CuONP concentration: 100–1000 ppm. Incubation: 20 °C, 95% RH, 5–14 days. Fungal growth record: Lemon disease incidence. | 100%/1000 ppm 100%/1000 ppm | [287] |
| Biological (Lemon peels extract) | 16.8 | Rounded, elongated spherical | A. citri | Medium: PDA. Inoculum: From a spore suspension (—). CuONP concentration: 10–100 ppm. Incubation: 28 °C, 5 days. Fungal growth record: Diameter inhibition zone. | 18.5–50 mm/10–100 ppm | [345] |
| Biological (Penicillium chrysogenum) | 9.7 | — | F. oxysporum, A. solani, A. niger, P. citrinum | Medium: Sabouraud Dextrose Agar. Inoculum: —. CuONP concentration: 250 ppm. Incubation: 28 °C, 5 days. Fungal growth record: Diameter inhibition zone. | 37.0 mm/250 ppm, 28.0 mm/250 ppm, 26.5 mm/250 ppm, 20.7 mm/250 ppm, For each species, respectively | [346] |
| Commercial | 46 | Spherical | F. oxysporum, A. solani | Medium: PDA. Inoculum: Agar plugs (5 mm) from a fungal culture. CuONP concentration: 100, 250, 500, 700, and 1000 ppm. Incubation: 25 ± 2 °C, 7–11 days. Fungal growth record: Colony diameter. | 31.48–95.57%/100–1000 ppm, 10.69–95.4%/100–1000 ppm, For each species, respectively | [347] |
| Nanoparticle Properties | Antifungal Properties | |||||
|---|---|---|---|---|---|---|
| Synthesis Method | Size (nm) | Shape | Fungal Species | Methodology | Mycotoxin/Reduction (%)/Treatment | Ref. |
| Chemical | 2.5 ± 0.3 | Spherical | F. verticillioides (FB1) | Medium: Nutrient broth (according to CLSI for filamentous fungi). Inoculum: From a spore suspension (2 × 104 spores/mL). CuNP Concentration: 5–200 ppm. Incubation: 28 °C, 14 days. Fumonisin B1 analysis: HPLC. | FB1/100%/100 ppm | [285] |
| Biological (Green and black tea leaves) | 26–40 | Spherical | A. flavus (Aflatoxins) A. parasiticus (Aflatoxins) | Medium: Czapek Dox agar Inoculum: From a spore suspension (1 × 106 spores/mL) CuNP Concentration: 10, 25, 50, and 100 ppm Incubation: 25 ± 2 °C, 15 days Aflatoxin analysis: HPLC | AFs/11.3 ± 1.2–83.1 ± 2.9/10–100 ppm | [313] |
| Biological (Syzygium cumini leaves) | 28−35 | Spherical | A. flavus (Aflatoxins) A. parasiticus (Aflatoxins) | Medium: Czapek Dox Liquid Inoculum: From a spore suspension (1 × 106 spores/mL) CuNP Concentration: 25, 50, and 100 ppm Incubation: 25 ± 2 °C, 15 days Aflatoxin analysis: HPLC | AFs/75.7± 3.2/100 ppm 80.0 ± 2.1/100 ppm | [314] |
5.3. Zinc Nanoparticles
5.4. Other Metal Oxide Nanoparticles
6. Engineered Metal and Metal Oxide NP as Antifungal Additives in Food
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hawksworth, D.L.; Lücking, R. Fungal Diversity Revisited: 2.2 to 3.8 Million Species. Microbiol. Spectr. 2017, 5, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, M. The fungi: 1, 2, 3 ... 5.1 million species? Am. J. Bot. 2011, 98, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and multi-national prevalence of fungal diseases—Estimate precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Thambugala, K.M.; Daranagama, D.A.; Tennakoon, D.S.; Jayatunga, D.P.W.; Hongsanan, S.; Xie, N. Humans vs. Fungi: An Overview of Fungal Pathogens against Humans. Pathogens 2024, 13, 426. [Google Scholar] [CrossRef]
- Bastos, R.W.; Rossato, L.; Goldman, G.H.; Santos, D.A. Fungicide effects on human fungal pathogens: Cross-resistance to medical drugs and beyond. PLoS Pathog. 2021, 17, e1010073. [Google Scholar] [CrossRef]
- Denning, D.W. Global Incidence and Mortality of Severe Fungal Disease. Lancet Infect. Dis. 2024, 24, e428–e438. [Google Scholar] [CrossRef]
- Rokas, A. Evolution of the human pathogenic lifestyle in fungi. Nat. Microbiol. 2022, 7, 607–619. [Google Scholar] [CrossRef]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef]
- Pangga, I.B.; Salvacion, A.R.; Cumagun, C.J.R. Climate Change and Plant Diseases Caused by Mycotoxigenic Fungi: Implications for Food Security. In Climate Change and Mycotoxins; Botana, L.M., Sainz, M.J., Eds.; De Gruyter: Berlin, Germany, 2015; pp. 1–28. [Google Scholar]
- Pitt, J.I.; Miller, D. A Concise History of Mycotoxin Research. J. Agric. Food Chem. 2017, 65, 7021–7033. [Google Scholar] [CrossRef]
- Stoev, S.D. Food security, underestimated hazard of joint mycotoxin exposure and management of the risk of mycotoxin contamination. Food Control 2024, 159, 110235. [Google Scholar] [CrossRef]
- Bezerra da Rocha, M.E.; Oliveira Freire, F.C.; Feitosa Maia, F.E.; Florindo Guedes, M.I.; Rondina, D. Mycotoxins and their effects on human and animal health. Food Control 2014, 36, 159–165. [Google Scholar] [CrossRef]
- Madalena, M.; Sobral, C.; Faria, M.A.; Cunha, S.C.; Ferreira, I. Toxicological interactions between mycotoxins from ubiquitous fungi: Impact on hepatic and intestinal human epithelial cells. Chemosphere 2018, 202, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Dellafiora, L.; Dall’Asta, C.; Galaverna, G. Toxicodynamics of Mycotoxins in the Framework of Food Risk Assessment: An In Silico Perspective. Toxins 2018, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Freire, L.; Sant’Ana, A.S. Modified mycotoxins: An updated review on their formation, detection, occurrence, and toxic effects. Food Chem. Toxicol. 2018, 111, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Maragos, C.M.; Dall’Asta, C. Introduction to masked mycotoxins. In Masked Mycotoxins in Food: Formation, Occurrence and Toxicological Relevance; Dall’Asta, C., Berthiller, F., Eds.; Royal Society of Chemistry: London, UK, 2016; pp. 1–13. [Google Scholar]
- García-Esparza, M.Á.; Mateo, E.M.; Robles, J.A.; Capoferri, M.; Jiménez, M.; Soria, J.M. Unveiling the Neurotoxic Effects of Ochratoxin A and Its Impact on Neuroinflammation. Toxins 2025, 17, 264. [Google Scholar] [CrossRef]
- Moretti, A.T.; Logrieco, A.F.; Susca, A. Mycotoxin: An Underhand Food Problem. In Mycotoxigenic Fungi: Methods and Protocols; Moretti, A., Susca, A., Eds.; Humana Press: New York, NY, USA, 2017; pp. 3–12. [Google Scholar]
- Luo, S.; Du, H.; Kebede, H.; Liu, Y.; Xing, F. Contamination status of major mycotoxins in agricultural products and foodstuffs in Europe. Food Control 2021, 127, 108120. [Google Scholar] [CrossRef]
- Pandey, A.K.; Samota, M.K.; Kumar, A.; Silva, A.S.; Dubey, N.K. Fungal Mycotoxins in Food Commodities: Present Status and Future Concerns. Front. Sustain. Food Syst. 2023, 7, 1162595. [Google Scholar] [CrossRef]
- Johns, L.E.; Bebber, D.P.; Gurr, S.J.; Brown, N.A. Emerging health threat and cost of Fusarium mycotoxins in European wheat. Nat. Food 2022, 3, 1014–1019. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajslova, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens—The IARC Monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef]
- Kamle, M.; Mahato, D.K.; Devi, S.; Lee, K.E.; Kang, S.G.; Kumar, P. Fumonisins: Impact on agriculture, food, and human health and their management strategies. Toxins 2019, 11, 328. [Google Scholar] [CrossRef]
- Leslie, J.F.; Moretti, A.; Mesterházy, Á.; Ameye, M.; Audenaert, K.; Singh, P.K.; Richard-Forget, F.; Chulze, S.N.; Ponte, E.M.D.; Chala, A.; et al. Key Global Actions for Mycotoxin Management in Wheat and Other Small Grains. Toxins 2021, 13, 725. [Google Scholar] [CrossRef]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A Global Concern for Food Safety, Human Health and Their Management. Front. Microbiol. 2017, 7, 2170. [Google Scholar] [CrossRef] [PubMed]
- Sarmast, E.; Fallah, A.A.; Jafari, T.; Khaneghah, A.M. Occurrence and fate of mycotoxins in cereals and cereal-based products: A narrative review of systematic reviews and meta-analyses studies. Curr. Opin. Food Sci. 2021, 39, 68–75. [Google Scholar] [CrossRef]
- Yu, J.; Pedroso, I.R. Mycotoxins in Cereal-Based Products and Their Impacts on the Health of Humans, Livestock Animals and Pets. Toxins 2023, 15, 480. [Google Scholar] [CrossRef]
- Kolawole, O.; Siri-Anusornsak, W.; Petchkongkaew, A.; Elliott, C. A systematic review of global occurrence of emerging mycotoxins in crops and animal feeds, and their toxicity in livestock. Emerg. Contam. 2024, 10, 100305. [Google Scholar] [CrossRef]
- Khaneghah, A.M.; Fakhri, Y.; Gahruie, H.H.; Niakousari, M.; Sant’Ana, A.S. Mycotoxins in cereal-based products during 24 years (1983–2017): A global systematic review. Trends Food Sci. Technol. 2019, 91, 95–105. [Google Scholar] [CrossRef]
- Wang, J.; Sufar, E.K.; Bernhoft, A.; Seal, C.; Rempelos, L.; Hasanaliyeva, G.; Zhao, B.; Iversen, P.O.; Baranski, M.; Volakakis, N.; et al. Mycotoxin Contamination in Organic and Conventional Cereal Grain and Products: A Systematic Literature Review and Meta-Analysis. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13363. [Google Scholar] [CrossRef]
- Sá, S.V.M.; Monteiro, C.; Fernandes, J.O.; Pinto, E.; Faria, M.A.; Cunha, S.C. Emerging mycotoxins in infant and children foods: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 1707–1721. [Google Scholar] [CrossRef]
- Hassan, S.E.-D.; Salem, S.S.; Fouda, A.; Awad, M.A.; El-Gamal, M.S.; Abdo, A. New Approach for Antimicrobial Activity and Bio-Control of Various Pathogens by Biosynthesized Copper Nanoparticles Using Endophytic Actinomycetes. J. Radiat. Res. Appl. Sci. 2018, 11, 262–270. [Google Scholar] [CrossRef]
- Schabo, D.C.; Alvarenga, V.O.; Schaffner, D.W.; Magnani, M. A worldwide systematic review, meta-analysis, and health risk assessment study of mycotoxins in beers. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1351–1368. [Google Scholar] [CrossRef] [PubMed]
- Narváez, A.; Rodríguez-Carrasco, Y.; Castaldo, L.; Izzo, L.; Graziani, G.; Ritieni, A. Occurrence and Exposure Assessment of Mycotoxins in Ready-to-Eat Tree Nut Products through Ultra-High Performance Liquid Chromatography Coupled with High Resolution Q-Orbitrap Mass Spectrometry. Metabolites 2020, 10, 344. [Google Scholar] [CrossRef] [PubMed]
- González-Curbelo, M.Á.; Kabak, B. Occurrence of mycotoxins in dried fruits worldwide, with a focus on aflatoxins and ochratoxin A: A review. Toxins 2023, 15, 576. [Google Scholar] [CrossRef] [PubMed]
- Azaiez, I.; Font, G.; Mañes, J.; Fernández-Franzón, M. Survey of mycotoxins in dates and dried fruits from Tunisian and Spanish markets. Food Control 2015, 51, 340–346. [Google Scholar] [CrossRef]
- Abdallah, M.F.; Krska, R.; Sulyok, M. Occurrence of ochratoxins, fumonisin B2, aflatoxins (B1 and B2), and other secondary fungal metabolites in dried date palm fruits from Egypt: A mini survey. J. Food Sci. 2018, 83, 559–564. [Google Scholar] [CrossRef]
- Palumbo, J.D.; O’Keeffe, T.L.; Ho, Y.S.; Santillan, C.J. Occurrence of Ochratoxin a Contamination and Detection of Ochratoxigenic Aspergillus Species in Retail Samples of Dried Fruits and Nuts. J. Food Prot. 2015, 78, 836–842. [Google Scholar] [CrossRef]
- Rahimi, E.; Shakerian, A. Ochratoxin A in Dried Figs, Raisins, Apricots, and Dates on Iranian Retail Market. Health 2013, 5, 2077–2080. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Mehmood, Z.; Asi, M.R.; Shahid, M.; Sehar, M.; Malik, N. Co-occurrence of aflatoxins and ochratoxin A in nuts, dry fruits, and nutty products. J. Food Saf. 2018, 38, e12462. [Google Scholar] [CrossRef]
- Calderari, T.O.; Iamanaka, B.T.; Frisvad, J.C.; Pitt, J.I.; Sartori, D.; Pereira, J.L.; Fungaro, M.H.P.; Taniwaki, M.H. The Biodiversity of Aspergillus Section Flavi in Brazil Nuts: From Rainforest to Consumer. Int. J. Food Microbiol. 2013, 160, 267–272. [Google Scholar] [CrossRef]
- Russell, R.; Paterson, M.; Lima, N.; Taniwak, M.H. Coffee, mycotoxins and climate change. Food Res. Int. 2014, 61, 1–15. [Google Scholar] [CrossRef]
- Galarce-Bustos, O.; Alvarado, M.; Vega, M.; Aranda, M. Occurrence of ochratoxin A in roasted and instant coffees in Chilean market. Food Control 2014, 46, 102–107. [Google Scholar] [CrossRef]
- Vieira, T.; Cunha, S.; Casal, S. Mycotoxins in Coffee. Chapter 25. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: London, UK, 2015; pp. 225–233. [Google Scholar]
- Bessaire, T.; Perrin, I.; Tarres, A.; Bebius, A.; Reding, F.; Theurillat, V. Mycotoxins in green coffee: Occurrence and risk assessment. Food Control 2019, 96, 59–67. [Google Scholar] [CrossRef]
- Vecchio, A.; Mineo, V.; Planeta, D. Ochratoxin A in Instant Coffee in Italy. Food Control 2012, 28, 220–223. [Google Scholar] [CrossRef]
- Al Attiya, W.; Ul Hassan, Z.; Al-Thani, R.; Jaoua, S. Prevalence of toxigenic fungi and mycotoxins in Arabic coffee (Coffea arabica): Protective role of traditional coffee roasting, brewing and bacterial volatiles. PLoS ONE 2021, 16, e0259302. [Google Scholar] [CrossRef]
- Copetti, M.V.; Iamanaka, B.T.; Pitt, J.I.; Taniwaki, M.H. Fungi and Mycotoxins in Cocoa: From Farm to Chocolate. Int. J. Food Microbiol. 2014, 178, 13–20. [Google Scholar] [CrossRef]
- Pickova, D.; Ostry, V.; Malir, J.; Toman, J.; Malir, F.A. Review on Mycotoxins and Microfungi in Spices in the Light of the Last Five Years. Toxins 2020, 12, 789. [Google Scholar] [CrossRef]
- Kabak, B.; Dobson, A.D.W. Mycotoxins in spices and herbs—An update. Crit. Rev. Food Sci. Nutr. 2017, 57, 18–34. [Google Scholar] [CrossRef]
- El Darra, N.; Gambacorta, L.; Solfrizzo, M. Multimycotoxins Occurrence in Spices and Herbs Commercialized in Lebanon. Food Control 2019, 95, 63–70. [Google Scholar] [CrossRef]
- Cighir, A.; Curticăpean, A.; Mare, A.D.; Cighir, T.; Gabor, M.R.; Toma, F.; Man, A. Fungal and Mycotoxin Contamination of Green Leaf Spices Commercialized in Romania: A Food Choice Perspective. Sustainability 2023, 15, 16437. [Google Scholar] [CrossRef]
- Benkerroum, N. Mycotoxins in dairy products: A review. Int. Dairy J. 2016, 62, 63–75. [Google Scholar] [CrossRef]
- Flores-Flores, M.E.; Lizarraga, E.; López de Cerain, A.; González-Peñas, E. Presence of mycotoxins in animal milk: A review. Food Control 2015, 53, 163–176. [Google Scholar] [CrossRef]
- Leite, M.; Freitas, A.; Barbosa, J.; Ramos, F. Regulated and Emerging Mycotoxins in Bulk Raw Milk: What Is the Human Risk? Toxins 2023, 15, 605. [Google Scholar] [CrossRef]
- Becker-Algeri, T.A.; Castagnaro, D.; de Bortoli, K.; de Souza, C.; Drunkler, D.A.; Badiale-Furlong, E. Mycotoxins in bovine milk and dairy products: A review. J. Food Sci. 2016, 81, R544–R552. [Google Scholar] [CrossRef]
- Hocking, A.D.; Leong, S.L.; Kazi, B.A.; Emmett, R.W.; Scott, E.S. Fungi and Mycotoxins in Vineyards and Grape Products. Int. J. Food Microbiol. 2007, 119, 84–88. [Google Scholar] [CrossRef]
- Kollia, E.; Kanapitsas, A.; Markaki, P. Occurrence of aflatoxin B1 and ochratoxin A in dried vine fruits from Greek market. Food Addit. Contam. Part B 2014, 7, 11–16. [Google Scholar] [CrossRef]
- Paterson, R.R.M.; Venâncio, A.; Lima, N.; Guilloux-Bénatier, M.; Rousseaux, S. Predominant Mycotoxins, Mycotoxigenic Fungi and Climate Change Related to Wine. Food Res. Int. 2018, 103, 478–491. [Google Scholar] [CrossRef]
- Mateo, F.; Tarazona, A.; Gavara, R.; Mateo, E.M. Bioactive Films with Essential Oils and Machine Learning for Controlling Aspergillus niger Growth and Fumonisin B2 Production In Vitro. Int. J. Food Microbiol. 2025, 439, 111251. [Google Scholar] [CrossRef]
- Rodrigues, P.; Silva, D.; Costa, P.; Abrunhosa, L.; Venâncio, A.; Teixeira, A. Mycobiota and mycotoxins in Portuguese pork, goat and sheep dry-cured hams. Mycotoxin Res. 2019, 35, 405–412. [Google Scholar] [CrossRef]
- Merla, C.; Andreoli, G.; Garino, C.; Vicari, N.; Tosi, G.; Guglielminetti, M.L.; Moretti, A.; Biancardi, A.; Arlorio, M.; Fabbi, M. Monitoring of Ochratoxin A and Ochratoxin-Producing Fungi in Traditional Salami Manufactured in Northern Italy. Mycotoxin Res. 2018, 34, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Tawab, A.A.; El-Diasty, E.M.; Khater, D.F.; Al-baaly, Y.M. Mycological identification of some fungi isolated from meat products and spices with molecular identification of some Penicillium isolates. Adv. Anim. Vet. Sci. 2020, 8, 124–129. [Google Scholar] [CrossRef]
- Toman, J.; Pickova, D.; Rejman, L.; Ostry, V.; Malir, F. Investigation of ochratoxin A in air-dry-cured hams. Meat Sci. 2024, 217, 109605. [Google Scholar] [CrossRef] [PubMed]
- Lešić, T.; Zadravec, M.; Zdolec, N.; Vulić, A.; Perković, I.; Škrivanko, M.; Kudumija, N.; Jakopović, Ž.; Pleadin, J. Mycobiota and Mycotoxin Contamination of Traditional and Industrial Dry-Fermented Sausage Kulen. Toxins 2021, 13, 798. [Google Scholar] [CrossRef]
- Alkuwari, A.; Hassan, Z.U.; Zeidan, R.; Al-Thani, R.; Jaoua, S. Occurrence of mycotoxins and toxigenic fungi in cereals and application of yeast volatiles for their biological control. Toxins 2022, 14, 404. [Google Scholar] [CrossRef]
- Hassan, Z.U.; Al-Thani, R.F.; Migheli, Q.; Jaoua, S. Detection of Toxigenic Mycobiota and Mycotoxins in Cereal Feed Market. Food Control 2018, 84, 389–394. [Google Scholar] [CrossRef]
- Susca, A.; Villani, A.; Moretti, A.; Stea, G.; Logrieco, A. Identification of toxigenic fungal species associated with maize ear rot: Calmodulin as a single informative gene. Int. J. Food Microbiol. 2020, 319, 108491. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006. Off. J. Eur. Union 2023, L 119, 103–157.
- Commission Regulation (EU) 2024/1022 of 8 April 2024 amending Regulation (EU) 2023/915 as regards maximum levels of deoxynivalenol in food. Off. J. Eur. Union 2024, L 1022, 1–4.
- Commission Regulation (EU) 2024/1038 of 9 April 2024 amending Regulation (EU) 2023/915 as regards maximum levels of T-2 and HT-2 toxins in food. Off. J. Eur. Union 2024, L 10, 1–5.
- Gruber-Dorninger, C.; Novak, B.; Nagl, V.; Berthiller, F. Emerging Mycotoxins: Beyond Traditionally Determined Food Contaminants. J. Agric. Food Chem. 2017, 65, 7052–7070. [Google Scholar] [CrossRef]
- Perrone, G.; Ferrara, M.; Medina, A.; Pascale, M.; Magan, N. Toxigenic Fungi and Mycotoxins in a Climate Change Scenario: Ecology, Genomics, Distribution, Prediction and Prevention of the Risk. Microorganisms 2020, 8, 1496. [Google Scholar] [CrossRef] [PubMed]
- Moretti, A.; Pascale, M.; Logrieco, A.F. Mycotoxin Risks under a Climate Change Scenario in Europe. Trends Food Sci. Technol. 2019, 84, 38–40. [Google Scholar] [CrossRef]
- Casu, A.; Leggieri, M.C.; Toscano, P.; Battilani, P. Changing Climate, Shifting Mycotoxins: A Comprehensive Review of Climate Change Impact on Mycotoxin Contamination. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13323. [Google Scholar] [CrossRef] [PubMed]
- Battilani, P.; Toscano, P.; Van Der Fels-Klerx, H.J.; Moretti, A.; Leggieri, M.C.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328. [Google Scholar] [CrossRef]
- Lv, C.; Jin, J.; Wang, P.; Dai, X.; Liu, Y.; Zheng, M.; Xing, F. Interaction of water activity and temperature on the growth, gene expression, and aflatoxin production by Aspergillus flavus on paddy and polished rice. Food Chem. 2019, 293, 472–478. [Google Scholar] [CrossRef]
- Al-Zaban, M.I. Impacts of temperature and water activity interactions on growth, aflatoxin B1 production and expression of major biosynthetic genes of AFB1 in Aspergillus flavus isolates. Microorganisms 2023, 11, 1199. [Google Scholar] [CrossRef]
- Kifer, D.; Jakšić, D.; Šegvić Klarić, M. Assessing the effect of mycotoxin combinations: Which mathematical model is (the most) appropriate? Toxins 2020, 12, 153. [Google Scholar] [CrossRef]
- Smith, M.C.; Madec, S.; Coton, E.; Hymery, N. Natural Co-occurrence of Mycotoxins in Foods and Feeds and their in vitro Combined Toxicological Effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef]
- Peijers, G.J.A.; Speijers, M.H.M. Combined Toxic Effects of Mycotoxins. Toxicol. Lett. 2004, 153, 91–98. [Google Scholar] [CrossRef]
- Venkatesh, N.; Keller, N.P. Mycotoxins in Conversation with Bacteria and Fungi. Front. Microbiol. 2019, 10, 403. [Google Scholar] [CrossRef]
- Assunção, R.; Silva, M.J.; Alvito, P. Challenges in risk assessment of multiple mycotoxins in food. World Mycotoxin J. 2016, 9, 791–811. [Google Scholar] [CrossRef]
- Nji, Q.N.; Babalola, O.O.; Ekwomadu, T.I.; Nleya, N.; Mwanza, M. Six Main Contributing Factors to High Levels of Mycotoxin Contamination in African Foods. Toxins 2022, 14, 318. [Google Scholar] [CrossRef] [PubMed]
- Hamad, G.M.; Mehany, T.; Simal-Gandara, J.; Abou-Alella, S.; Esua, O.J.; Abdel-Wahhab, M.A.; Hafez, E.E. A Review of Recent Innovative Strategies for Controlling Mycotoxins in Foods. Food Control 2023, 144, 109350. [Google Scholar] [CrossRef]
- Kabak, B. The fate of mycotoxins during thermal food processing. J. Sci. Food Agric. 2009, 89, 549–554. [Google Scholar] [CrossRef]
- Inglis, A.; Parnell, A.C.; Subramani, N.; Doohan, F.M. Machine Learning Applied to the Detection of Mycotoxin in Food: A Systematic Review. Toxins 2024, 16, 268. [Google Scholar] [CrossRef]
- Tarazona, A.; Mateo, E.M.; Gómez, J.V.; Romera, D.; Mateo, F. Potential use of machine learning methods in assessment of Fusarium culmorum and Fusarium proliferatum growth and mycotoxin production in treatments with antifungal agents. Fungal Biol. 2021, 125, 123–133. [Google Scholar] [CrossRef]
- Tarazona, A.; Mateo, E.M.; Gómez, J.V.; Gavara, R.; Jiménez, M.; Mateo, F. Machine learning approach for predicting Fusarium culmorum and F. proliferatum growth and mycotoxin production in treatments with ethylene-vinyl alcohol copolymer films containing pure components of essential oils. Int. J. Food Microbiol. 2021, 338, 109012. [Google Scholar] [CrossRef]
- Mateo, E.M.; Gómez, J.V.; Tarazona, A.; García-Esparza, M.A.; Mateo, F. Comparative Analysis of Machine Learning Methods to Predict Growth of F. sporotrichioides and Production of T-2 and HT-2 Toxins in Treatments with Ethylene-Vinyl Alcohol Films Containing Pure Components of Essential Oils. Toxins 2021, 13, 545. [Google Scholar] [CrossRef]
- Mateo, E.M.; Tarazona, A.; Jiménez, M.; Mateo, F. Lactic Acid Bacteria as Potential Agents for Biocontrol of Aflatoxigenic and Ochratoxigenic Fungi. Toxins 2022, 14, 807. [Google Scholar] [CrossRef]
- Mateo, E.M.; Tarazona, A.; Aznar, R.; Mateo, F. Exploring the Impact of Lactic Acid Bacteria on the Biocontrol of Toxigenic Fusarium spp. and Their Main Mycotoxins. Int. J. Food Microbiol. 2023, 387, 110054. [Google Scholar] [CrossRef] [PubMed]
- Mateo, F.; Gadea, R.; Mateo, E.M.; Jiménez, M. Multilayer Perceptron Neural Networks and Radial-Basis Function Networks as Tools to Forecast Accumulation of Deoxynivalenol in Barley Seeds Contaminated with Fusarium culmorum. Food Control 2011, 22, 88–95. [Google Scholar] [CrossRef]
- Wang, X.; Liu, C.; van der Fels-Klerx, H.J. Regional Prediction of Multi-Mycotoxin Contamination of Wheat in Europe Using Machine Learning. Food Res. Int. 2022, 159, 111588. [Google Scholar] [CrossRef]
- Aggarwal, A.; Mishra, A.; Tabassum, N.; Kim, Y.-M.; Khan, F. Detection of mycotoxin contamination in foods using artificial intelligence: A review. Foods 2024, 13, 3339. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2023: Sixth Assessment Report (AR6). The Synthesis Report Is Based on the Content of the Three Working Groups Assessment Reports: WGI–The Physical Science Basis, WGII—Impacts, Adaptation and Vulnerability, WGIII—Mitigation of Climate Change. Geneva, Switzerland. Available online: https://www.ipcc.ch/report/sixth-assessment-report-cycle (accessed on 3 May 2025).
- Waheed, A.; Haxim, Y.; Islam, W.; Ahmad, M.; Muhammad, M.; Alqahtani, F.M.; Hashem, M.; Salih, H.; Zhang, D. Climate Change Reshaping Plant-Fungal Interaction. Environ. Res. 2023, 238 Pt 2, 117282. [Google Scholar] [CrossRef]
- Lahlali, R.; Taoussi, M.; Laasli, S.E.; Gachara, G.; Ezzouggari, R.; Belabess, Z.; Aberkani, K.; Assouguem, A.; Meddich, A.; El Jarroudi, M.; et al. Effects of Climate Change on Plant Pathogens and Host-Pathogen Interactions. Crop Environ. 2024, 3, 159–170. [Google Scholar] [CrossRef]
- Liu, Y.; Yamdeu, J.H.G.; Gong, Y.Y.; Orfila, C. A review of postharvest approaches to reduce fungal and mycotoxin contamination of foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1521–1560. [Google Scholar] [CrossRef]
- Mateo, F.; Mateo, E.M.; Tarazona, A.; García-Esparza, M.Á.; Soria, J.M.; Jiménez, M. New Strategies and Artificial Intelligence Methods for the Mitigation of Toxigenic Fungi and Mycotoxins in Foods. Toxins 2025, 17, 231. [Google Scholar] [CrossRef]
- Brauer, V.S.; Rezende, C.P.; Pessoni, A.M.; De Paula, R.G.; Rangappa, K.S.; Nayaka, S.C.; Gupta, V.K.; Almeida, F. Antifungal Agents in Agriculture: Friends and Foes of Public Health. Biomolecules 2019, 9, 521. [Google Scholar] [CrossRef]
- Marín, P.; de Ory, A.; Cruz, A.; Magan, N.; González-Jaén, M.T. Potential effects of environmental conditions on the efficiency of the antifungal tebuconazole controlling Fusarium verticillioides and Fusarium proliferatum growth rate and fumonisin biosynthesis. Int. J. Food Microbiol. 2013, 165, 251–258. [Google Scholar] [CrossRef]
- Mateo, E.M.; Valle-Algarra, F.M.; Mateo, R.; Jiménez, M.; Magan, N. Effect of Fenpropimorph, Prochloraz and Tebuconazole on Growth and Production of T-2 and HT-2 Toxins by Fusarium langsethiae in Oat-Based Medium. Int. J. Food Microbiol. 2011, 151, 289–298. [Google Scholar] [CrossRef]
- Vitiello, A.; Ferrara, F.; Boccellino, M.; Ponzo, A.; Cimmino, C.; Comberiati, E.; Zovi, A.; Clemente, S.; Sabbatucci, M. Antifungal Drug Resistance: An Emergent Health Threat. Biomedicines 2023, 11, 1063. [Google Scholar] [CrossRef]
- Cui, X.; Wang, L.; Lü, Y.; Yue, C. Development and Research Progress of Anti-Drug Resistant Fungal Drugs. J. Infect. Public Health 2022, 15, 986–1000. [Google Scholar] [CrossRef]
- Hahn, M. The Rising Threat of Fungicide Resistance in Plant Pathogenic Fungi: Botrytis as a Case Study. J. Chem. Biol. 2014, 7, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.D.R.E.; Spolti, P.; Del Ponte, E.M.; Donato, K.Z.; Schrekker, H.; Fuentefria, A.M. Is the emergence of fungal resistance to medical triazoles related to their use in the agroecosystems? Braz. J. Microbiol. 2016, 47, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Robbins, N.; Caplan, T.; Cowen, L.E. Molecular evolution of antifungal drug resistance. Annu. Rev. Microbiol. 2017, 71, 753–775. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC); European Chemicals Agency (ECHA); European Environment Agency (EEA); European Medicines Agency (EMA); European Commission’s Joint Research Centre (JRC). Impact of the use of azole fungicides, other than as human medicines, on the development of azole-resistant Aspergillus spp. EFSA J. 2025, 23, e9200. [Google Scholar]
- Revie, N.M.; Iyer, K.R.; Robbins, N.; Cowen, L.E. Antifungal drug resistance: Evolution, mechanisms and impact. Curr. Opin. Microbiol. 2018, 45, 70–76. [Google Scholar] [CrossRef]
- Hokken, M.W.; Zwaan, B.J.; Melchers, W.J.; Verweij, P.E. Facilitators of Adaptation and Antifungal Resistance Mechanisms in Clinically Relevant Fungi. Fungal Genet. Biol. 2019, 132, 103254. [Google Scholar] [CrossRef]
- Habschied, K.; Krstanović, V.; Zdunić, Z.; Babić, J.; Mastanjević, K.; Šarić, G.K. Mycotoxins Biocontrol Methods for Healthier Crops and Stored Products. J. Fungi 2021, 7, 348. [Google Scholar] [CrossRef]
- Spanic, V.; Zdunić, Z.; Drezner, G.; Sarkanj, B. The Pressure of Fusarium Disease and Its Relation with Mycotoxins in the Wheat Grain and Malt. Toxins 2019, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.S.d.L.P.B.; Weber, S.H.; Luciano, F.B. Resistance of Transgenic Maize Cultivars to Mycotoxin Production—Systematic Review and Meta-Analysis. Toxins 2024, 16, 373. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Li, X.; Maier, M.; O’Brien-Simpson, N.M.; Heath, D.E.; O’Connor, A.J. Using Inorganic Nanoparticles to Fight Fungal Infections in the Antimicrobial Resistant Era. Acta Biomater. 2023, 158, 56–79. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.; Brandhoff, P.; Weigel, S.; Marvin, H.; Bouwmeester, H.; Aschberger, K.; Rauscher, H.; Amenta, V.; Arena, M.; Moniz, F.B.; et al. Inventory of Nanotechnology Applications in the Agricultural, Feed and Food Sector. EFSA Support. Publ. 2014, 11, 621E. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Y.; Zhang, X.; Liu, J.; Gong, P.; Su, Z.; Fan, L.; Li, G. Emerging Nanoparticles in Food: Sources, Application, and Safety. J. Agric. Food Chem. 2023, 71, 3564–3582. [Google Scholar] [CrossRef]
- Singh, S.; Chaurasia, P.K.; Bharat, S.L. Functional roles of essential oils as an effective alternative of synthetic food preservatives: A review. Bharat J. Food Process Preserv. 2022, 46, e16804. [Google Scholar] [CrossRef]
- Atanda, S.A.; Shaibu, R.O.; Agunbiade, F.O. Nanoparticles in agriculture: Balancing food security and environmental sustainability. Discov. Agric. 2025, 3, 26. [Google Scholar] [CrossRef]
- Dangi, K.; Verma, A.K. Efficient and Eco-friendly Smart Nano-pesticides: Emerging Prospects for Agriculture. Mater. Today Proc. 2021, 45, 3819–3824. [Google Scholar]
- Kopittke, P.M.; Lombi, E.; Wang, P.; Schjoerring, J.K.; Husted, S. Nanomaterials as fertilizers for improving plant mineral nutrition and environmental outcomes. Environ. Sci. 2019, 6, 3513–3524. [Google Scholar] [CrossRef]
- Santás-Miguel, V.; Arias-Estévez, M.; Rodríguez-Seijo, A.; Arenas-Lago, D. Use of metal nanoparticles in agriculture. A review on the effects on plant germination. Environ. Pollut. 2023, 334, 122222. [Google Scholar] [CrossRef]
- Mansoor, S.; Zahoor, I.; Baba, T.R.; Padder, S.A.; Bhat, Z.A.; Koul, A.M.; Jiang, L. Fabrication of silver nanoparticles against fungal pathogens. Front. Nanotechnol. 2021, 3, 679358. [Google Scholar] [CrossRef]
- Kaningini, A.G.; Nelwamondo, A.M.; Azizi, S.; Maaza, M.; Mohale, K.C. Metal nanoparticles in agriculture: A review of possible use. Coatings 2022, 12, 1586. [Google Scholar] [CrossRef]
- Jaskulski, D.; Jaskulska, I.; Majewska, J.; Radziemska, M.; Bilgin, A.; Brtnicky, M. Silver nanoparticles (AgNPs) in urea solution in laboratory tests and field experiments with crops and vegetables. Materials 2022, 15, 870. [Google Scholar] [CrossRef]
- Zahra, Z.; Habib, Z.; Chung, S.; Badshah, M.A. Exposure Route of TiO2 NPs from Industrial Applications to Wastewater Treatment and Their Impacts on the Agro-Environment. Nanomaterials 2020, 10, 1469. [Google Scholar] [CrossRef]
- Dey, S.; Ghosh, N.; Nath, S.; Gopal, G.; Paul, S.; Mukherjee, A.; Paul, S.; Kundu, R. Application of multi-metallic nanoparticles in agriculture: The more, the better? Biocatal. Agric. Biotechnol. 2024, 58, 103238. [Google Scholar] [CrossRef]
- Singh, R.P.; Handa, R.; Manchanda, G. Nanoparticles in sustainable agriculture: An emerging opportunity. J. Control. Release 2021, 329, 1234–1248. [Google Scholar] [CrossRef]
- Mgadi, K.; Ndaba, B.; Roopnarain, A.; Rama, H.; Adeleke, R. Nanoparticle Applications in Agriculture: Overview and Response of Plant-Associated Microorganisms. Front. Microbiol. 2024, 15, 1354440. [Google Scholar] [CrossRef]
- Su, C.; Chen, A.; Liang, W.; Xie, W.; Xu, X.; Zhan, X.; Zhang, W.; Peng, C. Copper-based nanomaterials: Opportunities for sustainable agriculture. Sci. Total Environ. 2024, 926, 171948. [Google Scholar] [CrossRef]
- Cruz-Luna, A.R.; Cruz-Martínez, H.; Vásquez-López, A.; Medina, D.I. Metal Nanoparticles as Novel Antifungal Agents for Sustainable Agriculture: Current Advances and Future Directions. J. Fungi 2021, 7, 1033. [Google Scholar] [CrossRef]
- Cruz-Luna, A.R.; Vásquez-López, A.; Rojas-Chávez, H.; Valdés-Madrigal, M.A.; Cruz-Martínez, H.; Medina, D.I. Engineered Metal Oxide Nanoparticles as Fungicides for Plant Disease Control. Plants 2023, 12, 2461. [Google Scholar] [CrossRef]
- Kutawa, A.B.; Ahmad, K.; Ali, A.; Hussein, M.Z.; Abdul Wahab, M.A.; Adamu, A.; Ismaila, A.A.; Gunasena, M.T.; Rahman, M.Z.; Hossain, M.I. Trends in Nanotechnology and Its Potentialities to Control Plant Pathogenic Fungi: A Review. Biology 2021, 10, 881. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Nguyen, N.T.; Nguyen, P.T.; Phan, Q.N.; Le, T.L.; Do, H.D.K. Current and Emerging Nanotechnology for Sustainable Development of Agriculture: Implementation Design Strategy and Application. Heliyon 2024, 10, e31503. [Google Scholar] [CrossRef]
- Baker, S.; Volova, T.; Prudnikova, S.V.; Satish, S.; Prasad, M.N.N. Nanoagroparticles emerging trends and future prospect in modern agriculture system. Environ. Toxicol. Pharmacol. 2017, 53, 10–17. [Google Scholar] [CrossRef]
- Adisa, I.O.; Pullagurala, V.L.R.; Peralta-Videa, J.R.; Dimkpa, C.O.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Recent advances in nano-enabled fertilizers and pesticides: A critical review of mechanisms of action. Environ. Sci. Nano 2019, 6, 2002–2030. [Google Scholar] [CrossRef]
- Agrimonti, C.; Lauro, M.; Visioli, G. Smart agriculture for food quality: Facing climate change in the 21st century. Crit. Rev. Food Sci. Nutr. 2021, 61, 971–981. [Google Scholar] [CrossRef]
- Castro-Mayorga, J.L.; Cabrera-Villamizar, L.; Balcucho-Escalante, J.; Fabra, M.J.; López-Rubio, A. Applications of Nanotechnology in Agri-Food Productions. In Nanotoxicity; Rajendran, S., Mukherjee, A., Nguyen, T.A., Godugu, C., Shukla, R.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 319–340. [Google Scholar]
- Mishra, S.; Keswani, C.; Abhilash, P.C.; Fraceto, L.F.; Singh, H.B. Integrated Approach of Agri-nanotechnology: Challenges and Future Trends. Front. Plant Sci. 2017, 8, 471. [Google Scholar] [CrossRef]
- Tanwar, A.; Role, S. Role and Effects of Nanotechnology Used in Pesticides and Agriculture Field. AIP Conf. Proc. 2019, 2142, 5122581. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C. Gold nanoparticles for drug delivery and cancer therapy. Appl. Sci. 2020, 10, 3824. [Google Scholar] [CrossRef]
- Puttasiddaiah, R.; Basavegowda, N.; Lakshmanagowda, N.K.; Raghavendra, V.B.; Sagar, N.; Sridhar, K.; Dikkala, P.K.; Bhaswant, M.; Baek, K.-H.; Sharma, M. Emerging Nanoparticle-Based Diagnostics and Therapeutics for Cancer: Innovations and Challenges. Pharmaceutics 2025, 17, 70. [Google Scholar] [CrossRef]
- Islam, F.; Shohag, S.; Uddin, M.J.; Islam, M.R.; Nafady, M.H.; Akter, A.; Mitra, S.; Roy, A.; Emran, T.B.; Cavalu, S. Exploring the journey of zinc oxide nanoparticles (ZnO-NPs) toward biomedical applications. Materials 2022, 15, 2160. [Google Scholar] [CrossRef]
- Zhu, C.; Ji, Z.; Ma, J.; Ding, Z.; Shen, J.; Wang, Q. Recent Advances of Nanotechnology-Facilitated Bacteria-Based Drug and Gene Delivery Systems for Cancer Treatment. Pharmaceutics 2021, 13, 940. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef]
- Hofmann-Amtenbrink, M.; Grainger, D.W.; Hofmann, H. Nanoparticles in Medicine: Current Challenges Facing Inorganic Nanoparticle Toxicity Assessments and Standardizations. Nanomedicine 2015, 11, 1689–1694. [Google Scholar] [CrossRef]
- Lam, E.; Luong, J.H. Carbon Materials as Catalyst Supports and Catalysts in the Transformation of Biomass to Fuels and Chemicals. ACS Catal. 2014, 4, 3393–3410. [Google Scholar] [CrossRef]
- Rassaei, L.; Marken, F.; Sillanpää, M.; Amiri, M.; Cirtiu, C.M.; Sillanpää, M. Nanoparticles in electrochemical sensors for environmental monitoring. TrAC Trends Anal. Chem. 2011, 30, 1704–1715. [Google Scholar] [CrossRef]
- Khan, F.; Shahid, A.; Zhu, H.; Wang, N.; Javed, M.R.; Ahmad, N.; Xu, J.; Alam, M.A.; Mehmood, M.A. Prospects of algae-based green synthesis of nanoparticles for environmental applications. Chemosphere 2022, 293, 133571. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Li, C.; Nabeel, F.; Khalid, M.; Iqbal, H.M.N. Catalytic Potential of Bio-Synthesized Silver Nanoparticles Using Convolvulus arvensis Extract for the Degradation of Environmental Pollutants. J. Photochem. Photobiol. B Biol. 2018, 181, 44–52. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, W.; Wen, Q.; Xu, D.; Ren, J.; Lin, Q. Aptamer-Engineered Nanomaterials to Aid in Mycotoxin Determination. Food Control 2022, 135, 108661. [Google Scholar] [CrossRef]
- Rai, P.K.; Kumar, V.; Lee, S.S.; Raza, N.; Kim, K.-H.; Ok, Y.S.; Tsang, D.C.W. Nanoparticle-Plant Interaction: Implications in Energy, Environment, and Agriculture. Environ. Int. 2018, 119, 1–19. [Google Scholar] [CrossRef]
- Altammar, K.A. A review on nanoparticles: Characteristics, synthesis, applications, and challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef]
- Wasilewska, A.; Bielicka, M.; Klekotka, U.; Kalska-Szostko, B. Nanoparticle Applications in Food—A Review. Food Funct. 2023, 14, 2544–2567. [Google Scholar] [CrossRef]
- Horky, P.; Skalickova, S.; Baholet, D.; Skladanka, J. Nanoparticles as a Solution for Eliminating the Risk of Mycotoxins. Nanomaterials 2018, 8, 727. [Google Scholar] [CrossRef]
- Boholm, M.; Arvidsson, R. A definition framework for the terms nanomaterial and nanoparticle. Nanoethics 2016, 10, 25–40. [Google Scholar] [CrossRef]
- Zain, M.; Yasmeen, H.; Yadav, S.S.; Amir, S.; Bilal, M.; Shahid, A.; Khurshid, M. Applications of Nanotechnology in Biological Systems and Medicine. In Micro and Nano Technologies, Nanotechnology for Hematology, Blood Transfusion, and Artificial Blood; Denizli, A., Nguyen, T.A., Rajan, M., Alam, M.F., Rahman, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 215–235. [Google Scholar]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.M.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Commission Recommendation of 10 June 2022 on the definition of nanomaterial (Text with EEA relevance) (2022/C 229/01) (C/2022/3689). Off. J. Eur. Union 2022, C 229, 1–5.
- Commission Recommendation of 18 October 2011 on the definition of nanomaterial (Text with EEA relevance) (2011/696/EU). Off. J. Eur. Union 2011, L 275, 38–40.
- Commission Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 and repealing Regulation (EC) No 258/97 and Commission Regulation (EC) No 1852/2001. Off. J. Eur. Union 2015, L 327, 1–22.
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef]
- Erdoğan, N.; Akkın, S.; Bilensoy, E. Nanocapsules for Drug Delivery: An Updated Review of the Last Decade. Recent Pat. Drug Deliv. Formul. 2019, 12, 252–266. [Google Scholar] [CrossRef]
- Ozuna-Valencia, K.H.; Moreno-Vásquez, M.J.; Graciano-Verdugo, A.Z.; Rodríguez-Félix, F.; Robles-García, M.Á.; Barreras-Urbina, C.G.; Quintero-Reyes, I.E.; Cornejo-Ramírez, Y.I.; Tapia-Hernández, J.A. The Application of Organic and Inorganic Nanoparticles Incorporated in Edible Coatings and Their Effect on the Physicochemical and Microbiological Properties of Seafood. Processes 2024, 12, 1889. [Google Scholar] [CrossRef]
- Dhaka, A.; Mali, S.C.; Sharma, S.; Trivedi, R.A. Review on Biological Synthesis of Silver Nanoparticles and Their Potential Applications. Results Chem. 2023, 6, 101108. [Google Scholar] [CrossRef]
- Nami, S.; Aghebati-Maleki, A.; Aghebati-Maleki, L. Current Applications and Prospects of Nanoparticles for Antifungal Drug Delivery. EXCLI J. 2021, 20, 562–584. [Google Scholar]
- Ealia, S.A.; Saravanakumar, M. A Review on the Classification, Characterization, Synthesis of Nanoparticles and Their Application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar] [CrossRef]
- Rajput, V.D.; Singh, A.; Minkina, T.; Rawat, S.; Mandzhieva, S.; Sushkova, S.; Shuvaeva, V.; Nazarenko, O.; Rajput, P.; Komariah; et al. Nano-Enabled Products: Challenges and Opportunities for Sustainable Agriculture. Plants 2021, 10, 2727. [Google Scholar] [CrossRef]
- Abd-Elsalam, K.A.; Hashim, A.F.; Alghuthaymi, M.A.; Said-Galiev, E. Nanobiotechnological strategies for toxigenic fungi and mycotoxin control. In Nanotechnology in the Agri-Food Industry. Food Preservation; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 337–364. [Google Scholar]
- Kheiri, A.; Moosawi Jorf, S.A.; Malihipour, A.; Saremi, H.; Nikkhah, M. Synthesis and characterization of chitosan nanoparticles and their effect on Fusarium head blight and oxidative activity in wheat. Int. J. Biol. Macromol. 2017, 102, 526–533. [Google Scholar] [CrossRef]
- Hassanein, M.M.M.; Abdel-Razek, A.G.; Al-Amrousi, E.F.; Badr, A.N. Application of lime peel oil composite nanoemulsion to prevent toxigenic fungi in nuts. Heliyon 2023, 9, e18620. [Google Scholar] [CrossRef]
- Almawash, S. Solid lipid nanoparticles, an effective carrier for classical antifungal drugs. Saudi Pharm. J. 2023, 31, 1167–1180. [Google Scholar] [CrossRef]
- Vogel, T.; Kohlmann, S.; Abboud, Z.; Thusek, S.; Fella, F.; Teßmar, J.; Sekimizu, K.; Miyashita, A.; Beilhack, A.; Groll, J.; et al. Beyond the Charge: Interplay of Nanogels’ Functional Group and Zeta-Potential for Antifungal Drug Delivery to Human Pathogenic Fungus Aspergillus fumigatus. Macromol. Biosci. 2024, 24, e2400082. [Google Scholar] [CrossRef]
- Mosallam, S.; Albash, R.; Abdelbari, M.A. Advanced Vesicular Systems for Antifungal Drug Delivery. AAPS PharmSciTech 2022, 23, 206. [Google Scholar] [CrossRef]
- Soe, H.M.S.H.; Maw, P.D.; Loftsson, T.; Jansook, P. A Current Overview of Cyclodextrin-Based Nanocarriers for Enhanced Antifungal Delivery. Pharmaceuticals 2022, 15, 1447. [Google Scholar] [CrossRef]
- Dariusz, T.; Młynarczyk, D.T.; Długaszewska, J.; Kałużna-Młynarczyk, A.; Gosliński, T. Dendrimers against fungi—A state of the art review. J. Control. Release 2021, 330, 599–617. [Google Scholar]
- Wei, H.; Mao, J.; Sun, D.; Zhang, Q.; Cheng, L.; Yang, X.; Li, P. Strategies to control mycotoxins and toxigenic fungi contamination by nano-semiconductor in food and agro-food: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 12488–12512. [Google Scholar] [CrossRef]
- Hu, X.; Li, H.; Yang, J.; Wen, X.; Wang, S.; Pan, M. Nanoscale Materials Applying for the Detection of Mycotoxins in Foods. Foods 2023, 12, 3448. [Google Scholar] [CrossRef]
- Thirugnanasambandan, T.; Gopinath, S.C.B. Nanomaterials in Food Industry for the Protection from Mycotoxins: An Update. Biotech 2023, 13, 64. [Google Scholar] [CrossRef]
- Mali, R.; Patil, J. Nanoparticles: A novel antifungal drug delivery system. Mater. Proc. 2023, 14, 61. [Google Scholar]
- Irshad, M.A.; Hussain, A.; Nasim, I.; Nawaz, R.; Azeem, S.; Al-Mutairi, A.A.; Al-Hussain, S.A.; Zaki, M.E.A. Exploring the antifungal activities of green nanoparticles for sustainable agriculture: A research update. Chem. Biol. Technol. Agric. 2024, 11, 133. [Google Scholar] [CrossRef]
- Chen, J.; Wu, L.; Lu, M.; Lu, S.; Li, Z.; Ding, W. Comparative Study on the Fungicidal Activity of Metallic MgO Nanoparticles and Macroscale MgO Against Soilborne Fungal Phytopathogens. Front. Microbiol. 2020, 11, 365. [Google Scholar] [CrossRef]
- Jian, Y.; Chen, X.; Ahmed, T.; Shang, Q.; Zhang, S.; Ma, Z.; Yin, Y. Toxicity and action mechanisms of silver nanoparticles against the mycotoxin-producing fungus Fusarium graminearum. J. Adv. Res. 2021, 38, 1–12. [Google Scholar] [CrossRef]
- Kandi, V.; Kandi, S. Antimicrobial properties of nanomolecules: Potential candidates as antibiotics in the era of multi-drug resistance. Epidemiol. Health 2015, 37, e2015020. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- De la Rosa-García, S.C.; Martínez-Torres, P.; Gómez-Cornelio, S.; Corral-Aguado, M.A.; Quintana, P.; Gómez-Ortíz, N.M. Antifungal Activity of ZnO and MgO Nanomaterials and Their Mixtures Against Colletotrichum gloeosporioides Strains from Tropical Fruit. J. Nanomater. 2018, 2018, e3498527. [Google Scholar] [CrossRef]
- Arciniegas-Grijalba, P.A.; Patiño-Portela, M.C.; Mosquera-Sánchez, L.P.; Guerrero-Vargas, J.A.; Rodríguez-Páez, J.E. ZnO nanoparticles (ZnO-NPs) and their antifungal activity against coffee fungus Erythricium salmonicolor. Appl. Nanosci. 2017, 7, 225–241. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Bach, H. Mechanisms of Antifungal Properties of Metal Nanoparticles. Nanomaterials 2022, 12, 4470. [Google Scholar] [CrossRef]
- Abd-Elsalam, K.A.; El-Naggar, M.A.; Ghannouchi, A.; Bouqellah, N.A. Nanomaterials and ozonation: Safe strategies for mycotoxin management. In Nanomycotoxicology; Rai, M., Abd-Elsalam, K.A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 285–308. [Google Scholar]
- Thipe, V.C.; Batista, J.G.S.; Lugão, A.B. Copper Nanomaterials for Eliminating the Risk of Mycotoxins. In Nanobiotechnology for Plant Protection: Copper Nanostructures: Next-Generation of Agrochemicals for Sustainable Agroecosystems; Abd-Elsalam, K.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 243–262. [Google Scholar]
- Bakshi, M.; Kumar, A. Applications of copper nanoparticles in plant protection and pollution sensing: Toward promoting sustainable agriculture. In Nanobiotechnology for Plant Protection, Copper Nanostructures: Next-Generation of Agrochemicals for Sustainable Agroecosystems; Abd-Elsalam, K.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 393–413. [Google Scholar]
- Dimitrijevic, M.; Karabasil, N.; Boskovic, M.; Teodorovic, V.; Vasilev, D.; Djordjevic, V.; Kilibarda, N.; Čobanović, N. Safety Aspects of Nanotechnology Applications in Food Packaging. Procedia Food Sci. 2015, 5, 57–60. [Google Scholar] [CrossRef]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review. Adv. Colloid Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef]
- Yadav, P.T.; Yadav, R.M.; Singh, D.P. Mechanical Milling: A Top Down Approach for the Synthesis of Nanomaterials and Nanocomposites. Nanosci. Nanotechnol. 2012, 2, 22–48. [Google Scholar] [CrossRef]
- Otis, G.; Ejgenberg, M.; Mastai, Y. Solvent-Free Mechanochemical Synthesis of ZnO Nanoparticles by High-Energy Ball Milling of ?-Zn(OH)2 Crystals. Nanomaterials 2021, 11, 238. [Google Scholar] [CrossRef]
- Venkatesh, R.; Karthi, N.; Kawin, N.; Prakash, T.; Kannan, C.; Karthigairajan, M.; Bobe, K. Synthesis and adsorbent performance of modified biochar with Ag/MgO nanocomposites for heat storage application. Adsorpt. Sci. Technol. 2022, 2022, 1–13. [Google Scholar] [CrossRef]
- Kim, M.; Osone, S.; Kim, T.; Higashi, H.; Seto, T. Synthesis of Nanoparticles by Laser Ablation: A Review. KONA Powder Partic. J. 2017, 34, 80–90. [Google Scholar] [CrossRef]
- Nikolov, A.S.; Stankova, N.E.; Karashanova, D.B.; Nedyalkov, N.N.; Pavlov, E.L.; Koev, K.T.; Najdenski, H.; Kussovski, V.; Avramov, L.A.; Ristoscu, C.; et al. Synergistic effect in a two-phase laser procedure for production of silver nanoparticles colloids applicable in ophthalmology. Opt. Laser Technol. 2021, 138, 106850. [Google Scholar] [CrossRef]
- Chugh, H.; Sood, D.; Chandra, I.; Tomar, V.; Dhawan, G.; Chandra, R. Role of Gold and Silver Nanoparticles in Cancer Nano-Medicine. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1210–1220. [Google Scholar] [CrossRef]
- Fares, A.; Mahdy, A.; Ahmed, G. Unraveling the mysteries of silver nanoparticles: Synthesis, characterization, antimicrobial effects and uptake translocation in plant-a review. Planta 2024, 260, 7. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Deng, L.; Yonezawa, T. Control of nanoparticles synthesized via vacuum sputter deposition onto liquids: A review. Soft Matter 2022, 18, 19–47. [Google Scholar] [CrossRef]
- Wender, H.; Migowski, P.; Feil, A.F.; Teixeira, S.R.; Dupont, J. Sputtering deposition of nanoparticles onto liquid substrates: Recent advances and future trends. Coord. Chem. Rev. 2013, 257, 2468–2483. [Google Scholar] [CrossRef]
- Sinha, R.; Lavrijsen, R.; Verheijen, M.A.; Zoethout, E.; Genuit, H.; van de Sanden, M.C.M.; Bieberle-Hütter, A. Electrochemistry of Sputtered Hematite Photoanodes: A Comparison of Metallic DC versus Reactive RF Sputtering. ACS Omega 2019, 4, 9262–9270. [Google Scholar] [CrossRef]
- Sergievskaya, A.; Chauvin, A.; Konstantinidis, S. Sputtering onto liquids: A critical review. Beilstein J. Nanotechnol. 2022, 13, 10–53. [Google Scholar] [CrossRef]
- Sergievskaya, A.; Absil, R.; Chauvin, A.; Yusenko, K.V.; Veselý, J.; Godfroid, T.; Konstantinidis, S. Sputtering onto liquids: How does the liquid viscosity affect the formation of nanoparticles and metal films? Phys. Chem. Chem. Phys. 2023, 25, 2803–2809. [Google Scholar] [CrossRef]
- Leng, J.; Wang, Z.; Wang, J.; Wu, H.-H.; Yan, G.; Li, X.; Guo, H.; Liu, Y.; Zhang, Q.; Guo, Z. Advances in Nanostructures Fabricated via Spray Pyrolysis and Their Applications in Energy Storage and Conversion. Chem. Soc. Rev. 2019, 48, 3015–3072. [Google Scholar] [CrossRef]
- Majerič, P.; Rudolf, R. Advances in Ultrasonic Spray Pyrolysis Processing of Noble Metal Nanoparticles-Review. Materials 2020, 13, 3485. [Google Scholar] [CrossRef]
- Debecker, D.P.; le Bras, S.; Boissière, C.; Chaumonnot, A.; Sánchez, C. Aerosol processing: A wind of innovation in the field of advanced heterogeneous catalysts. Chem. Soc. Rev. 2018, 47, 4112–4155. [Google Scholar] [CrossRef]
- Ko, Y.N.; Park, S.B.; Jung, K.Y.; Kang, Y.C. One-Pot Facile Synthesis of Ant-Cave-Structured Metal Oxide–Carbon Microballs by Continuous Process for Use as Anode Materials in Li-Ion Batteries. Nano Lett. 2013, 13, 5462–5466. [Google Scholar] [CrossRef]
- Workie, A.B.; Ningsih, H.S.; Shih, S.-J. A Comprehensive Review on the Spray Pyrolysis Technique: Historical Context, Operational Factors, Classifications, and Product Applications. J. Anal. Appl. Pyrolysis 2023, 170, 105915. [Google Scholar] [CrossRef]
- Ghaffarian, H.R.; Saiedi, M.; Sayyadnejad, M.A.; Rashidi, A.M. Synthesis of ZnO nanoparticles by spray pyrolysis method. Iran. J. Chem. Chem. Eng. (Int. Engl. Ed.) 2011, 30, 1–6. [Google Scholar]
- Allag, N.; Bouafia, A.; Chemsa, B.; Ben Mya, O.; Chala, A.; Siad, C.; Alam, M.W. Effect of precursors on structural, optical and surface properties of ZnO thin film prepared by spray pyrolysis method: Efficient removal of Cu (II) from wastewater. Transit. Met. Chem. 2024, 49, 39–51. [Google Scholar] [CrossRef]
- Sudha, M.; Madhan, A.B.; Geethac, E.; Satheeskumard, R. Synthesis of zinc oxide thin films by spray pyrolysis technique. J. Ovonic Res. 2024, 20, 267–272. [Google Scholar] [CrossRef]
- Ozcelik, B.K.; Ergun, C. Synthesis of ZnO nanoparticles by an aerosol process. Ceram. Int. 2014, 40, 7107–7116. [Google Scholar] [CrossRef]
- Tanhaei, A.; Mohammadi, M.; Hamishehkar, H.; Hamblin, M.R. Electrospraying as a novel method of particle engineering for drug delivery vehicles. J. Control. Release 2021, 330, 851–865. [Google Scholar] [CrossRef]
- Kim, M.J.; Song, J.Y.; Hwang, S.H.; Park, D.Y.; Park, S.M. Electrospray mode discrimination with current signal using deep convolutional neural network and class activation map. Sci. Rep. 2022, 12, 16281. [Google Scholar] [CrossRef]
- Patel, P.R.; Haemmerich, D. Review on Electrospray Nanoparticles for Drug Delivery: Exploring Applications. Polym. Adv. Technol. 2024, 35, e6507. [Google Scholar] [CrossRef]
- Burlec, A.F.; Corciova, A.; Boev, M.; Batir-Marin, D.; Mircea, C.; Cioanca, O.; Danila, G.; Danila, M.; Bucur, A.F.; Hancianu, M. Current Overview of Metal Nanoparticles’ Synthesis, Characterization, and Biomedical Applications, with a Focus on Silver and Gold Nanoparticles. Pharmaceuticals 2023, 16, 1410. [Google Scholar] [CrossRef]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emergent Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef]
- Förster, H.; Wolfrum, C.; Peukert, W. Experimental study of metal nanoparticle synthesis by an arc evaporation/condensation process. J. Nanopart. Res. 2012, 14, 926. [Google Scholar] [CrossRef]
- Markov, A.N.; Kapinos, A.A.; Petukhov, A.N.; Dokin, E.S.; Emelyanov, A.V.; Abarbanel, N.V.; Zarubin, D.M.; Golovacheva, A.A.; Suvorov, S.S.; Barysheva, A.V.; et al. Synthesis of Zinc Nanoparticles by the Gas Condensation Method in a Non-Contact Crucible and Their Physical-Chemical Characterization. Nanomaterials 2024, 14, 163. [Google Scholar] [CrossRef]
- Raffi, M.; Rumaiz, A.K.; Hasan, M.M.; Shah, S.I. Studies of the growth parameters for silver nanoparticle synthesis by inert gas condensation. J. Mater. Res. 2007, 22, 3378–3384. [Google Scholar] [CrossRef]
- Bokov, D.; Jalil, A.T.; Chupradit, S.; Suksatan, W.; Ansari, M.J.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021, 2021, 10201. [Google Scholar] [CrossRef]
- Parashar, M.; Shukla, V.K.; Singh, R. Metal oxides nanoparticles via sol–gel method: A review on synthesis, characterization and applications. J. Mater. Sci. Mater. Electron. 2020, 31, 3729–3749. [Google Scholar] [CrossRef]
- Chandekar, K.V.; Shkir, M.; Khan, A.; AlFaify, S. Novel magnetic materials preparation, characterizations and their applications. In Woodhead Publishing Series in Electronic and Optical Materials, Fundamentals and Industrial Applications of Magnetic Nanoparticles; Hussain, C.M., Patankar, K.K., Eds.; Woodhead Publishing: Sawston, UK, 2022; pp. 67–116. [Google Scholar]
- Manawi, Y.M.; Ihsanullah; Samara, A.; Al-Ansari, T.; Atieh, M.A. A Review of Carbon Nanomaterials’ Synthesis via the Chemical Vapor Deposition (CVD) Method. Materials 2018, 11, 822. [Google Scholar] [CrossRef]
- Piszczek, P.; Radtke, A. Silver Nanoparticles Fabricated Using Chemical Vapor Deposition and Atomic Layer Deposition Techniques: Properties, Applications and Perspectives: Review. In Noble and Precious Metals—Properties, Nanoscale Effects and Applications; Seehra, M.S., Bristow, A.D., Eds.; InTech: London, UK, 2018. [Google Scholar]
- Vikulova, E.S.; Dorovskikh, S.I.; Basova, T.V.; Zheravin, A.A.; Morozova, N.B. Silver CVD and ALD Precursors: Synthesis, Properties, and Application in Deposition Processes. Molecules 2024, 29, 5705. [Google Scholar] [CrossRef]
- Golrokhi, Z.; Chalker, S.; Sutcliffe, C.J.; Potter, R.J. Self-Limiting Atomic Layer Deposition of Conformal Nanostructured Silver Films. Appl. Surf. Sci. 2016, 364, 789–797. [Google Scholar] [CrossRef]
- Singaravelan, R.; Bangaru Sudarsan Alwar, S. Electrochemical synthesis, characterisation and phytogenic properties of silver nanoparticles. Appl. Nanosci. 2015, 5, 983–991. [Google Scholar] [CrossRef]
- Nasretdinova, G.R.; Fazleeva, R.R.; Mukhitova, R.K.; Nizameev, I.R.; Kadirov, M.K.; Ziganshina, A.Y.; Yanilkin, V.V. Electrochemical synthesis of silver nanoparticles in solution. Electrochem. Commun. 2015, 50, 69–72. [Google Scholar] [CrossRef]
- Anand, V.; Harshavardhan; Srivastava, V.C. Synthesis and Characterization of Copper Nanoparticles by Electrochemical Method: Effect of pH. J. Nano Res. 2015, 31, 81–92. [Google Scholar] [CrossRef]
- Nur, S.U.; Anung, P.; Enny, L.; Endang, S.; Hotman, L.; Triani, W.; Siska, F. Critical parameters of silver nanoparticles (AgNPs) synthesized by sodium borohydride reduction. Res. J. Chem. Environ. 2018, 22, 179–183. [Google Scholar]
- Nzekwe, I.T.; Agubata, C.O.; Umeyor, C.E.; Okoye, I.E.; Ogwueleka, C.B. Synthesis of Silver Nanoparticles by Sodium Borohydride Reduction Method: Optimization of Conditions for High Anti-staphylococcal Activity. J. Pharm. Res. Int. 2017, 14, 1–9. [Google Scholar] [CrossRef]
- Gómez, J.V.; Tarazona, A.; Mateo, F.; Jiménez, M.; Mateo, E.M. Potential impact of engineered silver nanoparticles in the control of aflatoxins, ochratoxin A and the main aflatoxigenic and ochratoxigenic species affecting foods. Food Control 2019, 101, 58–68. [Google Scholar] [CrossRef]
- Siddiqui, T.; Zia, M.K.; Muaz, M.; Ahsan, H.; Khan, F.H. Synthesis and Characterization of Silver Nanoparticles (AgNPs) using Chemico-physical Methods. Ind. J. Chem. Anal. 2023, 6, 124–132. [Google Scholar] [CrossRef]
- Nocerino, V.; Miranda, B.; Dardano, P.; Sanità, G.; Esposito, E.; De Stefano, L. Protocol for synthesis of spherical silver nanoparticles with stable optical properties and characterization by transmission electron microscopy. STAR Protoc. 2024, 5, 102920. [Google Scholar] [CrossRef]
- Mateo, E.M.; Jiménez, M. Silver Nanoparticle-Based Therapy: Can It Be Useful to Combat Multi-Drug Resistant Bacteria? Antibiotics 2022, 11, 1205. [Google Scholar] [CrossRef]
- Kirubakaran, D.; Abdul Wahid, J.B.; Karmegam, N.; Jeevika, R.; Sellapillai, L.; Rajkumar, M.; SenthilKumar, K.J. A Comprehensive Review on the Green Synthesis of Nanoparticles: Advancements in Biomedical and Environmental Applications. Biomed. Mater. Devices 2025. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Alharbi, N.S.; Alsubhi, N.S.; Felimban, A.I. Green Synthesis of Silver Nanoparticles Using Medicinal Plants: Characterization and Application. J. Radiat. Res. Appl. Sci. 2022, 15, 109–124. [Google Scholar] [CrossRef]
- Patil, P.V.; Nerlekar, N.A.; Kuldeep, A.R.; Patil, P.P.; Dandge, P.B.; Dongale, T.D.; Dandge, P.B.; Rashinkar, G.S. Terminalia bellirica (Gaertn.) Roxb. Extract-Mediated Green Synthesis of Magnesium Oxide Nanoparticles for Multifunctional Applications. Plant Nano Biol. 2024, 8, 100069. [Google Scholar] [CrossRef]
- Kaur, M.; Gautam, A.; Guleria, P.; Singh, K.; Kumar, V. Green synthesis of metal nanoparticles and their environmental applications. Curr. Opin. Environ. Sci. Health 2022, 29, 100390. [Google Scholar] [CrossRef]
- Hussain, I.; Singh, N.B.; Singh, A.; Singh, H.; Singh, S.C. Green synthesis of nanoparticles and its potential application. Biotechnol. Lett. 2016, 38, 545–560. [Google Scholar] [CrossRef]
- Saxena, R.; Kotnala, S.; Bhatt, S.C.; Uniyal, M.; Rawat, B.S.; Negi, P.; Riyal, M.K. A Review on Green Synthesis of Nanoparticles Toward Sustainable Environment. Sustain. Chem. Clim. Action 2025, 6, 100071. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green Synthesis of Nanoparticles: Current Developments and Limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Alghuthaymi, M.A.; Rajkuberan, C.; Rajiv, P.; Kalia, A.; Bhardwaj, K.; Bhardwaj, P.; Abd-Elsalam, K.A.; Valis, M.; Kuca, K. Nanohybrid Antifungals for Control of Plant Diseases: Current Status and Future Perspectives. J. Fungi 2021, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Muñoz, R.; Avalos-Borja, M.; Castro-Longoria, E. Ultrastructural Analysis of Candida albicans When Exposed to Silver Nanoparticles. PLoS ONE 2014, 9, e108876. [Google Scholar] [CrossRef]
- Selvaraj, M.; Pandurangan, P.; Ramasami, N.; Rajendran, S.B.; Sangilimuthu, S.N.; Perumal, P. Highly potential antifungal activity of quantum-sized silver nanoparticles against Candida albicans. Appl. Biochem. Biotechnol. 2014, 173, 55–66. [Google Scholar] [CrossRef]
- Athie-García, M.S.; Piñón-Castillo, H.A.; Muñoz-Castellanos, L.N.; Ulloa-Ogaz, A.L.; Martínez-Varela, P.I.; Quintero-Ramos, A.; Duran, R.; Murillo-Ramirez, J.G.; Orrantia-Borunda, E. Cell wall damage and oxidative stress in Candida albicans ATCC 10231 and Aspergillus niger caused by palladium nanoparticles. Toxicol. In Vitro 2018, 48, 111–120. [Google Scholar] [CrossRef]
- Hwang, I.; Lee, J.; Hwang, J.H.; Kim, K.J.; Lee, D.G. Silver Nanoparticles Induce Apoptotic Cell Death in Candida albicans through the Increase of Hydroxyl Radicals: Silver Nanoparticles Induce Apoptotic Cell Death. FEBS J. 2012, 279, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.M.; Choi, H.Y.; Cho, S.G. The role of reactive oxygen species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef] [PubMed]
- Carmo, P.H.F.d.; Garcia, M.T.; Figueiredo-Godoi, L.M.A.; Lage, A.C.P.; Silva, N.S.d.; Junqueira, J.C. Metal Nanoparticles to Combat Candida albicans Infections: An Update. Microorganisms 2023, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Liu, F.; Jia, M.; Ni, H.; Han, Y.; Chen, J.; Wang, H.; Gu, H.; Chen, Y.; Lin, Y.; et al. An Overview of the Direct Interaction of Synthesized Silver Nanostructures and Enzymes. Int. J. Biol. Macromol. 2024, 279, 135154. [Google Scholar] [CrossRef]
- Ribeiro, A.I.; Dias, A.M.; Zille, A. Synergistic effects between metal nanoparticles and commercial antimicrobial agents: A review. ACS Appl. Nano Mater. 2022, 5, 3030–3064. [Google Scholar] [CrossRef]
- Souza, J.A.S.; Alves, M.M.; Barbosa, D.B.; Lopes, M.M.; Pinto, E.; Figueiral, M.H.; Delbem, A.C.B.; Mira, N.P. Study of the activity of Punica granatum-mediated silver nanoparticles against Candida albicans and Candida glabrata, alone or in combination with azoles or polyenes. Med. Mycol. 2020, 58, 564–567. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018, 16, 14. [Google Scholar] [CrossRef]
- Kim, T.-H.; Kim, M.; Park, H.-S.; Shin, U.S.; Gong, M.-S.; Kim, H.-W. Size-dependent cellular toxicity of silver nanoparticles. J. Biomed. Mater. Res. Part A 2012, 100, 1033–1043. [Google Scholar] [CrossRef]
- Kittler, S.; Greulich, C.; Diendorf, J.; Koller, M.; Epple, M. Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions. Chem. Mater. 2010, 22, 4548–4554. [Google Scholar] [CrossRef]
- Meher, A.; Tandi, A.; Moharana, S.; Chakroborty, S.; Mohapatra, S.S.; Mondal, A.; Dey, S.; Chandra, P. Silver nanoparticle for biomedical applications: A review. Hybrid Adv. 2024, 6, 100184. [Google Scholar] [CrossRef]
- Kong, I.C.; Ko, K.-S.; Koh, D.-C. Evaluation of the effects of particle sizes of silver nanoparticles on various biological systems. Int. J. Mol. Sci. 2020, 21, 8465. [Google Scholar] [CrossRef]
- Gibała, A.; Zeliszewska, P.; Gosiewski, T.; Krawczyk, A.; Duraczyńska, D.; Szaleniec, J.; Szaleniec, M.; Oćwieja, M. Antibacterial and Antifungal Properties of Silver Nanoparticles—Effect of a Surface-Stabilizing Agent. Biomolecules 2021, 11, 1481. [Google Scholar] [CrossRef]
- Manosalva, N.; Tortella, G.; Cristina Diez, M.; Schalchli, H.; Seabra, A.B.; Durán, N.; Rubilar, O. Green synthesis of silver nanoparticles: Effect of synthesis reaction parameters on antimicrobial activity. World J. Microbiol. Biotechnol. 2019, 35, 88. [Google Scholar] [CrossRef]
- Oćwieja, M.; Barbasz, A. Sodium Hexametaphosphate–Induced Enhancement of Silver Nanoparticle Toxicity towards Leukemia Cells. J. Nanopart. Res. 2020, 22, 167. [Google Scholar] [CrossRef]
- Tomak, A.; Yilancioglu, B.; Winkler, D.; Karakus, C.O. Protein corona formation on silver nanoparticles under different conditions. Colloids Surf. A Physicochem. Eng. Aspects 2022, 651, 129666. [Google Scholar] [CrossRef]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [PubMed]
- Matras, E.; Gorczyca, A.; Przemieniecki, S.W.; Oćwieja, M. Surface properties-dependent antifungal activity of silver nanoparticles. Sci. Rep. 2022, 12, 18046. [Google Scholar] [CrossRef]
- Rai, M.; Kon, K.; Ingle, A.; Duran, N.; Galdiero, S.; Galdiero, M. Broad-Spectrum Bioactivities of Silver Nanoparticles: The Emerging Trends and Future Prospects. Appl. Microbiol. Biotechnol. 2014, 98, 1951–1961. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Batista, J.G.S.; Rodrigues, M.Á.V.; Thipe, V.C.; Minarini, L.A.R.; Lopes, P.S.; Lugão, A.B. Advances in silver nanoparticles: A comprehensive review on their potential as antimicrobial agents and their mechanisms of action elucidated by proteomics. Front. Microbiol. 2024, 15, 1440065. [Google Scholar] [CrossRef]
- Madkhali, O.A. A Comprehensive Review on Potential Applications of Metallic Nanoparticles as Antifungal Therapies to Combat Human Fungal Diseases. Saudi Pharm. J. 2023, 31, 101733. [Google Scholar] [CrossRef]
- Dell’Annunziata, F.; Mosidze, E.; Folliero, V.; Lamparelli, E.P.; Lopardo, V.; Pagliano, P.; Della Porta, G.; Galdiero, M.; Bakuridze, A.D.; Franci, G. Eco-friendly synthesis of silver nanoparticles from peel and juice C. limon and their antiviral efficacy against HSV-1 and SARS-CoV-2. Virus Res. 2024, 349, 199455. [Google Scholar] [CrossRef]
- Tarazona, A.; Gómez, J.V.; Mateo, E.M.; Jiménez, M.; Mateo, F. Antifungal effect of engineered silver nanoparticles on phytopathogenic and toxigenic Fusarium spp. and their impact on mycotoxin accumulation. Int. J. Food Microbiol. 2019, 306, 108259. [Google Scholar] [CrossRef]
- Sedaghati, E.; Molaei, S.; Molaei, M.; Doraki, N. An evaluation of antifungal and antitoxigenicity effects of Ag/Zn and Ag nanoparticles on Aspergillus parasiticus growth and aflatoxin production. PHJ 2018, 1, 34–43. [Google Scholar]
- Al-Othman, M.R.; Abd El-Aziz, A.R.M.; Mahmoud, M.A.; Eifan, S.A.; El-Shikh, M.S.; Majrashi, M. Application of silver nanoparticles as antifungal and antiaflatoxin B1 produced by Aspergillus flavus. Dig. J. Nanomater. Biostruct. 2014, 9, 151–157. [Google Scholar]
- El-Naggar, M.A.; Alrajhi, A.M.; Fouda, M.M.; Abdelkareem, E.M.; Thabit, T.M.; Bouqellah, N.A. Effect of Silver Nanoparticles on Toxigenic Fusarium spp. and Deoxynivalenol Secretion in Some Grains. J. AOAC Int. 2018, 101, 1534–1541. [Google Scholar] [CrossRef]
- Ibrahim, E.; Zhang, M.; Zhang, Y.; Hossain, A.; Qiu, W.; Chen, Y.; Wang, Y.; Wu, W.; Sun, G.; Li, B. Green-Synthesization of Silver Nanoparticles Using Endophytic Bacteria Isolated from Garlic and Its Antifungal Activity Against Wheat Fusarium Head Blight Pathogen Fusarium graminearum. Nanomaterials 2020, 10, 219. [Google Scholar] [CrossRef]
- El-Desouky, T.A.; Ammar, H.A.M. Honey Mediated Silver Nanoparticles and Their Inhibitory Effect on Aflatoxins and Ochratoxin A. J. Appl. Pharm. Sci. 2016, 6, 83–90. [Google Scholar] [CrossRef]
- Ammar, H.A.M.; El-Desouky, T.A. Green synthesis of nanosilver particles by Aspergillus terreus HA1N and Penicillium expansum HA2N and its antifungal activity against mycotoxigenic fungi. J. Appl. Microbiol. 2016, 121, 89–100. [Google Scholar] [CrossRef]
- Mousavi, S.A.A.; Pourtalebi, S. Inhibitory Effects of Silver Nanoparticles on Growth and Aflatoxin B1 Production by Aspergillus parasiticus. Iran. J. Med. Sci. 2015, 40, 501–506. [Google Scholar]
- Kotzybik, K.; Gräf, V.; Kugler, L.; Stoll, D.A.; Greiner, R.; Geisen, R.; Schmidt-Heydt, M. Influence of different nanomaterials on growth and mycotoxin production of Penicillium verrucosum. PLoS ONE 2016, 11, e0150855. [Google Scholar] [CrossRef]
- Khalil, N.M.; Abd El-Ghany, M.N.; Rodríguez-Couto, S. Antifungal and anti-mycotoxin efficacy of biogenic silver nanoparticles produced by Fusarium chlamydosporum and Penicillium chrysogenum at non-cytotoxic doses. Chemosphere 2019, 218, 477–486. [Google Scholar] [CrossRef]
- Pérez-de León, A.; Plasencia, J.; Vázquez-Durán, A.; Méndez-Albores, A. Comparison of the In Vitro Antifungal and Anti-Fumonigenic Activities of Copper and Silver Nanoparticles Against Fusarium verticillioides. J. Clust. Sci. 2020, 31, 213–220. [Google Scholar] [CrossRef]
- Jo, Y.-K.; Cromwell, W.; Jeong, H.-K.; Thorkelson, J.; Roh, J.-H.; Shin, D.-B. Use of silver nanoparticles for managing Gibberella fujikuroi on rice seedlings. Crop Prot. 2015, 74, 65–69. [Google Scholar] [CrossRef]
- Baigorria, C.G.; Cerioni, L.; Debes, M.A.; Ledesma, A.E.; Alastuey, P.; Tirado, M.; Volentini, S.I.; Rapisarda, V.A. Antifungal action of metallic nanoparticles against fungicide-resistant pathogens causing main postharvest lemon diseases. J. Fungi 2024, 10, 782. [Google Scholar] [CrossRef]
- Bocate, K.P.; Reis, G.F.; de Souza, P.C.; Oliveira Junior, A.G.; Durán, N.; Nakazato, G.; Furlaneto, M.C.; de Almeida, R.S.; Panagio, L.A. Antifungal activity of silver nanoparticles and simvastatin against toxigenic species of Aspergillus. Int. J. Food Microbiol. 2019, 291, 79–86. [Google Scholar] [CrossRef]
- Al-Otibi, F.; Perveen, K.; Al-Saif, N.A.; Alharbi, R.I.; Bokhari, N.A.; Albasher, G.; Al-Otaibi, R.M.; Al-Mosa, M.A. Biosynthesis of silver nanoparticles using Malva parviflora and their antifungal activity. Saudi J. Biol. Sci. 2021, 28, 2229–2235. [Google Scholar] [CrossRef]
- Madbouly, A.K.; Abdel-Aziz, M.S.; Abdel-Wahhab, M.A. Biosynthesis of nanosilver using Chaetomium globosum and its application to control Fusarium wilt of tomato in the greenhouse. IET Nanobiotechnol. 2017, 11, 702–708. [Google Scholar] [CrossRef]
- Qian, Y.; Yu, H.; He, D.; Yang, H.; Wang, W.; Wan, X.; Wang, L. Biosynthesis of Silver Nanoparticles by the Endophytic Fungus Epicoccum nigrum and Their Activity against Pathogenic Fungi. Bioprocess Biosyst. Eng. 2013, 36, 1613–1619. [Google Scholar] [CrossRef]
- Valsalam, S.; Agastian, P.; Arasu, M.V.; Al-Dhabi, N.A.; Ghilan, A.-K.M.; Kaviyarasu, K.; Ravindran, B.; Chang, S.W.; Arokiyaraj, S. Rapid Biosynthesis and Characterization of Silver Nanoparticles from the Leaf Extract of Tropaeolum majus L. and Its Enhanced In-Vitro Antibacterial, Antifungal, Antioxidant and Anticancer Properties. J. Photochem. Photobiol. B Biol. 2018, 191, 65–74. [Google Scholar] [CrossRef]
- Amrinder, K.; Jaspal, K.; Anu, K.; Narinder, S. Effect of media composition on extent of antimycotic activity of silver nanoparticles against plant pathogenic fungus Fusarium moniliforme. Plant Dis. Res. 2016, 31, 1–5. [Google Scholar]
- Macías Sánchez, K.L.; González Martínez, H.D.R.; Carrera Cerritos, R.; Martínez Espinosa, J.C. In vitro evaluation of the antifungal effect of AgNPs on Fusarium oxysporum f. sp. lycopersici. Nanomaterials 2023, 13, 1274. [Google Scholar] [CrossRef]
- Jafari, A.; Pourakbar, L.; Farhadi, K.; Mohamadgolizad, L.; Goosta, Y. Biological synthesis of silver nanoparticles and evaluation of antibacterial and antifungal properties of silver and copper nanoparticles. Turk. J. Biol. 2015, 39, 556–561. [Google Scholar] [CrossRef]
- Xue, B.; He, D.; Gao, S.; Wang, D.; Yokoyama, K.; Wang, L. Biosynthesis of Silver Nanoparticles by the Fungus Arthroderma fulvum and Its Antifungal Activity Against Genera of Candida, Aspergillus and Fusarium. Int. J. Nanomed. 2016, 11, 1899–1906. [Google Scholar] [CrossRef]
- Bahrami-Teimoori, B.; Nikparast, Y.; Hojatianfar, M.; Akhlaghi, M.; Ghorbani, R.; Pourianfar, H.R. Characterisation and antifungal activity of silver nanoparticles biologically synthesised by Amaranthus retroflexus leaf extract. J. Exp. Nanosci. 2017, 12, 129–139. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Lee, J.S.; Park, K.D.; Ching, Y.C.; Nguyen, X.T.; Phan, V.G.; Thi, T.T.H. Green Silver Nanoparticles Formed by Phyllanthus urinaria, Pouzolzia zeylanica, and Scoparia dulcis Leaf Extracts and the Antifungal Activity. Nanomaterials 2020, 10, 542. [Google Scholar] [CrossRef]
- Abdel-Hadi, A.M.; Awad, M.F.; Abo-Dahab, N.F.; ElKady, M.F. Extracellular synthesis of silver nanoparticles by Aspergillus terreus: Biosynthesis, characterization, and biological activity. Biosci. Biotechnol. Res. Asia 2014, 11, 1179–1186. [Google Scholar] [CrossRef]
- Elamawi, R.M.; Al-Harbi, R.E.; Hendi, A.A. Biosynthesis and Characterization of Silver Nanoparticles Using Trichoderma longibrachiatum and Their Effect on Phytopathogenic Fungi. Egypt J. Biol. Pest Control 2018, 28, 28. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Vo, T.N.N.; Nguyen, N.T.; Ching, Y.C.; Thi, T.T.H. Comparison of Biogenic Silver Nanoparticles Formed by Momordica charantia and Psidium guajava Leaf Extract and Antifungal Evaluation. PLoS ONE 2020, 15, e0239360. [Google Scholar] [CrossRef]
- Fernández, J.G.; Fernández-Baldo, M.A.; Berni, E.; Camí, G.; Durán, N.; Raba, J.; Sanz, M.I. Production of silver nanoparticles using yeasts and evaluation of their antifungal activity against phytopathogenic fungi. Process Biochem. 2016, 51, 1306–1313. [Google Scholar] [CrossRef]
- Win, T.T.; Khan, S.; Fu, P. Fungus (Alternaria sp.) Mediated Silver Nanoparticles Synthesis, Characterization, and Screening of Antifungal Activity against some Phytopathogens. J. Nanotechnol. 2020, 2020, 828878. [Google Scholar] [CrossRef]
- Yassin, M.A.; Elgorban, A.M.; El-Samawaty, A.E.-R.M.; Almunqedhi, B.M. Biosynthesis of Silver Nanoparticles Using Penicillium verrucosum and Analysis of Their Antifungal Activity. Saudi J. Biol. Sci. 2021, 28, 2123–2127. [Google Scholar] [CrossRef]
- Malik, M.; Wani, W.A.; Bhat, M.A.; Siddiqui, M.A.; Alamri, S.; Alrumman, S.A. Fungal-Mediated Synthesis of Silver Nanoparticles: A Novel Strategy for Plant Disease Management. Toxins 2024, 16, 199455. [Google Scholar] [CrossRef]
- Khan, A.U.; Khan, M.; Khan, M.M. Antifungal and antibacterial assay by silver nanoparticles synthesized from aqueous leaf extract of Trigonella foenum-graecum. BioNanoScience 2019, 9, 597–602. [Google Scholar] [CrossRef]
- Dawoud, T.M.; Yassin, M.A.; El-Samawaty, A.R.M.; Elgorban, A.M. Silver Nanoparticles Synthesized by Nigrospora oryzae Showed Antifungal Activity. Saudi J. Biol. Sci. 2021, 28, 1847–1852. [Google Scholar] [CrossRef]
- Gautam, N.; Salaria, N.; Thakur, K.; Bhardwaj, A.; Awasthi, A.; Kumar, V. Green Silver Nanoparticles for Phytopathogen Control. Proc. Natl. Acad. Sci. USA India Sect. B Biol. Sci. 2020, 90, 439–446. [Google Scholar] [CrossRef]
- Tabassum, R.Z.; Mehmood, A.; Khalid, A.U.R.; Ahmad, K.S.; Khan, M.A.R.; Amjad, M.S.; Raffi, M.; Khan, G.-e.-L.; Mustafa, A. Green synthesis of silver nanoparticles for antifungal activity against tomato fusarium wilt caused by Fusarium oxysporum. Biocatal. Agric. Biotechnol. 2024, 61, 103376. [Google Scholar] [CrossRef]
- Mendoza, N.V.; Yánez, P.; Magdama, F.; Pacheco, R.; Vielma, J.; Vanegas, M.E.; Bogdanchikova, N.; Pestryakov, A.; Chong, P. Inhibition of Fusarium oxysporum Growth in Banana by Silver Nanoparticles: In Vitro and In Vivo Assays. PLoS ONE 2025, 20, e0308200. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Jung, J.H.; Lamsal, K.; Kim, Y.S.; Min, J.S.; Lee, Y.S. Antifungal effects of silver nanoparticles (AgNPs) against various plant pathogenic fungi. Mycobiology 2012, 40, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrowicz-Trzcińska, M.; Szaniawski, A.; Olchowik, J.; Drozdowski, S. Effects of copper and silver nanoparticles on growth of selected species of pathogenic and wood-decay fungi in vitro. For. Chron. 2018, 94, 109–116. [Google Scholar] [CrossRef]
- Asghar, M.A.; Zahir, E.; Shahid, S.M.; Khan, M.N.; Iqbal, J.; Walker, G. Iron, copper and silver nanoparticles: Green synthesis using green and black tea leaves extracts and evaluation of antibacterial, antifungal and aflatoxin B1 adsorption activity. LWT 2018, 90, 98–107. [Google Scholar] [CrossRef]
- Asghar, M.A.; Zahir, E.; Asghar, M.A.; Iqbal, J.; Rehman, A.A. Facile, one-pot biosynthesis and characterization of iron, copper and silver nanoparticles using Syzygium cumini leaf extract: As an effective antimicrobial and aflatoxin B1 adsorption agents. PLoS ONE 2020, 15, e0234964. [Google Scholar] [CrossRef] [PubMed]
- Dananjaya, S.H.S.; Erandani, W.K.C.U.; Kim, C.-H.; Nikapitiya, C.; Lee, J.; De Zoysa, M. Comparative study on antifungal activities of chitosan nanoparticles and chitosan silver nanocomposites against Fusarium oxysporum species complex. Int. J. Biol. Macromol. 2017, 105, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Yassin, M.A.; El-Samawaty, A.E.M.A.; Dawoud, T.M.; Abd-Elkader, O.H.; Al Maary, K.S.; Hatamleh, A.A.; Elgorban, A.M. Characterization and Anti-Aspergillus flavus Impact of Nanoparticles Synthesized by Penicillium citrinum. Saudi J. Biol. Sci. 2017, 24, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Xu, M.; Luo, J.; Zhao, L.; Ye, G.; Shi, F.; Lv, C.; Chen, H.; Wang, Y.; Li, Y. Liver toxicity assessments in rats following sub-chronic oral exposure to copper nanoparticles. Environ. Sci. Eur. 2019, 31, 44. [Google Scholar] [CrossRef]
- He, X.; Deng, H.; Hwang, H.M. The Current Application of Nanotechnology in Food and Agriculture. J. Food Drug Anal. 2019, 27, 1–21. [Google Scholar] [CrossRef]
- Khamis, Y.; Hashim, A.F.; Margarita, R.; Alghuthaymi, M.A.; Abd-Elsalam, K.A. Fungicidal efficacy of chemically-produced copper nanoparticles against Penicillium digitatum and Fusarium solani on citrus fruit. Philipp. Agric. Sci. 2017, 100, 69–78. [Google Scholar]
- Nath, A.; Molnár, M.A.; Albert, K.; Das, A.; Bánvölgyi, S.; Márki, E.; Vatai, G. Agrochemicals from Nanomaterials—Synthesis, Mechanisms of Biochemical Activities and Applications. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; Volume 84, pp. 263–312. [Google Scholar]
- Sidhu, A.; Barmota, H.; Bala, A. Antifungal evaluation studies of copper sulfide nano-aquaformulations and its impact on seed quality of rice (Oryzae sativa). Appl. Nanosci. 2017, 7, 681–689. [Google Scholar] [CrossRef]
- Wang, P.; Yuan, Y.; Xu, K.; Zhong, H.; Yang, Y.; Jin, S.; Yang, K.; Qi, X. Biological applications of copper-containing materials. Bioact. Mater. 2021, 6, 916–927. [Google Scholar] [CrossRef]
- Konappa, N.; Krishnamurthy, S.; Arakere, U.C.; Chowdappa, S.; Akbarbasha, R.; Ramachandrappa, N.S. Nanofertilizers and Nanopesticides: Recent Trends, Future Prospects in Agriculture. In Woodhead Publishing Series in Food Science, Technology and Nutrition; Jogaiah, S., Singh, H.B., Fraceto, L.F., de Lima, R., Eds.; Woodhead Publishing: Cambridge, UK, 2021; pp. 281–330. [Google Scholar]
- Tegenaw, A.; Tolaymat, T.; Al-Abed, S.; El Badawy, A.; Luxton, T.; Sorial, G.; Genaidy, A. Characterization and Potential Environmental Implications of Select Cu-Based Fungicides and Bactericides Employed in U.S. Markets. Environ. Sci. Technol. 2015, 49, 1294–1302. [Google Scholar] [CrossRef]
- López-Lima, D.; Mtz-Enriquez, A.I.; Carrión, G.; Basurto-Cereceda, S.; Pariona, N. The bifunctional role of copper nanoparticles in tomato: Effective treatment for Fusarium wilt and plant growth promoter. Sci. Hortic. 2020, 277, 109810. [Google Scholar] [CrossRef]
- Kanhed, P.; Birla, S.; Gaikwad, S.; Gade, A.; Seabra, A.B.; Rubilar, O.; Durán, N.; Rai, M. In vitro antifungal efficacy of copper nanoparticles against selected crop pathogenic fungi. Mater. Lett. 2014, 115, 13–17. [Google Scholar] [CrossRef]
- Ingle, A.P.; Rai, M. Copper Nanoflowers as Effective Antifungal Agents for Plant Pathogenic Fungi. IET Nanobiotechnol. 2017, 11, 546–551. [Google Scholar] [CrossRef]
- Hermida-Montero, L.; Pariona, N.; Mtz-Enriquez, A.I.; Carrión, G.; Paraguay-Delgado, F.; Rosas-Saito, G. Aqueous-Phase Synthesis of Nanoparticles of Copper/Copper Oxides and Their Antifungal Effect Against Fusarium oxysporum. J. Hazard. Mater. 2019, 380, 120850. [Google Scholar] [CrossRef] [PubMed]
- Van Viet, P.; Nguyen, H.T.; Cao, T.M.; Van Hieu, L.; Pham, V. Fusarium Antifungal Activities of Copper Nanoparticles Synthesized by a Chemical Reduction Method. J. Nanomater. 2016, 2016, 1957612. [Google Scholar] [CrossRef]
- Seku, K.; Reddy, G.B.; Pejjai, B.; Kotu, G.M.; Narasimha, G. Hydrothermal Synthesis of Copper Nanoparticles, Characterization and Their Biological Applications. Int. J. Nano Dimens. 2018, 9, 7–14. [Google Scholar]
- Ghasemian, E.; Naghoni, A.; Tabaraie, B.; Tabaraie, T. In Vitro Susceptibility of Filamentous Fungi to Copper Nanoparticles Assessed by Rapid XTT Colorimetry and Agar Dilution Method. J. Mycol. Méd. 2012, 22, 322–328. [Google Scholar] [CrossRef]
- Pham, N.-D.; Duong, M.-M.; Le, M.-V.; Hoang, H.A.; Pham, L.-K. Preparation and Characterization of Antifungal Colloidal Copper Nanoparticles and Their Antifungal Activity against Fusarium oxysporum and Phytophthora capsici. C. R. Chim. 2019, 22, 786–793. [Google Scholar] [CrossRef]
- Bramhanwade, K.; Shende, S.; Bonde, S.; Gade, A.; Rai, M. Fungicidal activity of Cu nanoparticles against Fusarium causing crop diseases. Environ. Chem. Lett. 2016, 14, 229–235. [Google Scholar] [CrossRef]
- Pariona, N.; Mtz-Enriquez, A.I.; Sánchez-Rangel, D.; Carrión, G.; Paraguay-Delgado, F.; Rosas-Saito, G. Green-Synthesized Copper Nanoparticles as a Potential Antifungal against Plant Pathogens. RSC Adv. 2019, 9, 18835–18843. [Google Scholar] [CrossRef]
- Maqsood, S.; Qadir, S.; Hussain, A.; Asghar, A.; Saleem, R.; Zaheer, S.; Nayyar, N. Antifungal properties of copper nanoparticles against Aspergillus niger. Sch. Int. J. Biochem. 2020, 3, 87–91. [Google Scholar] [CrossRef]
- Shende, S.; Ingle, A.P.; Gade, A.; Rai, M. Green Synthesis of Copper Nanoparticles by Citrus medica Linn. (Idilimbu) Juice and Its Antimicrobial Activity. World J. Microbiol. Biotechnol. 2015, 31, 865–873. [Google Scholar] [CrossRef]
- Hasanin, M.; Al Abboud, M.A.; Alawlaqi, M.M.; Abdelghany, T.M.; Hashem, A.H. Ecofriendly Synthesis of Biosynthesized Copper Nanoparticles with Starch-Based Nanocomposite: Antimicrobial, Antioxidant, and Anticancer Activities. Biol. Trace Elem. Res. 2022, 200, 2099–2112. [Google Scholar] [CrossRef]
- Mali, S.C.; Dhaka, A.; Githala, C.K.; Trivedi, R. Green synthesis of copper nanoparticles using Celastrus paniculatus Willd. leaf extract and their photocatalytic and antifungal properties. Biotechnol. Rep. 2020, 27, e00518. [Google Scholar] [CrossRef] [PubMed]
- Malandrakis, A.A.; Kavroulakis, N.; Chrysikopoulos, C.V. Use of Copper, Silver and Zinc Nanoparticles against Foliar and Soil-Borne Plant Pathogens. Sci. Total Environ. 2019, 670, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Priya, M.; Venkatesan, R.; Deepa, S.; Babu, S.; Kavitha, S.; Sekar, M.; Chen, W.C.; Chen, J.H. Green synthesis, characterization, antibacterial, and antifungal activity of copper oxide nanoparticles derived from Morinda citrifolia leaf extract. Sci. Rep. 2023, 13, 18838. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.A.; Abu-Elghait, M.; Ahmed, N.E.; Salem, S.S. Eco-Friendly Mycogenic Synthesis of ZnO and CuO Nanoparticles for In Vitro Antibacterial, Antibiofilm, and Antifungal Applications. Biol. Trace Elem. Res. 2021, 199, 2788–2799. [Google Scholar] [CrossRef]
- Devipriya, D.; Roopan, S.M. Cissus quadrangularis Mediated Ecofriendly Synthesis of Copper Oxide Nanoparticles and Its Antifungal Studies Against Aspergillus niger and Aspergillus flavus. Mater. Sci. Eng. C 2017, 80, 38–44. [Google Scholar] [CrossRef]
- Shammout, M.W.; Awwad, A.M. A novel route for the synthesis of copper oxide nanoparticles using Bougainvillea plant flowers extract and antifungal activity evaluation. Chem. Int. 2021, 7, 71–78. [Google Scholar]
- Vanathi, P.; Rajiv, P.; Sivaraj, R. Synthesis and Characterization of Eichhornia-Mediated Copper Oxide Nanoparticles and Assessing Their Antifungal Activity Against Plant Pathogens. Bull. Mater. Sci. 2016, 39, 1165–1170. [Google Scholar] [CrossRef]
- Sardar, M.; Ahmed, W.; Al Ayoubi, S.; Nisa, S.; Bibi, Y.; Sabir, M.; Khan, M.M.; Ahmed, W.; Qayyum, A. Fungicidal synergistic effect of biogenically synthesized zinc oxide and copper oxide nanoparticles against Alternaria citri causing citrus black rot disease. Saudi J. Biol. Sci. 2022, 29, 88–95. [Google Scholar] [CrossRef]
- El-Batal, A.I.; El-Sayyad, G.S.; Mosallam, F.M.; Fathy, R.M. Penicillium chrysogenum-Mediated Mycogenic Synthesis of Copper Oxide Nanoparticles Using Gamma Rays for In Vitro Antimicrobial Activity Against Some Plant Pathogens. J. Clust. Sci. 2020, 31, 79–90. [Google Scholar] [CrossRef]
- Vera-Reyes, I.; Esparza-Arredondo, I.J.E.; Lira-Saldivar, R.H.; Granados-Echegoyen, C.A.; Alvarez-Roman, R.; Vásquez-López, A.; Díaz-Barriga Castro, E. In Vitro Antimicrobial Effect of Metallic Nanoparticles on Phytopathogenic Strains of Crop Plants. J. Phytopathol. 2019, 167, 461–469. [Google Scholar] [CrossRef]
- Cuajungco, M.P.; Ramirez, M.S.; Tolmasky, M.E. Zinc: Multidimensional Effects on Living Organisms. Biomedicines 2021, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Rani, R.; Singh, S. Biogenic zinc oxide nanoparticles and their biomedical applications: A review. J. Inorg. Organomet. Polym. Mater. 2023, 33, 1437–1452. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Bjørk, P.K.; Kolte, M.V.; Poulsen, E.; Dedic, E.; Drace, T.; Andersen, S.U.; Nadzieja, M.; Liu, H.; Castillo-Michel, H.; et al. Zinc mediates control of nitrogen fixation via transcription factor filamentation. Nature 2024, 631, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Raha, S.; Ahmaruzzaman, M. ZnO Nanostructured Materials and Their Potential Applications: Progress, Challenges and Perspectives. Nanoscale Adv. 2022, 4, 1868–1925. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Microbial Mediated Synthesis of Zinc Oxide Nanoparticles, Characterization and Multifaceted Applications. J. Inorg. Organomet. Polym. 2022, 32, 4114–4132. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; ur Rahman, A.; Tajuddin, A.; Husen, A.; Al-Warthan, A.; Al-Muhtaseb, A.H. Properties of Zinc Oxide Nanoparticles and Their Activity Against Microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef]
- Mirzaei, H.; Darroudi, M. Synthesis and Characterization of Zinc Oxide Nanoparticles: A Review. Ceram. Int. 2017, 43, 907–914. [Google Scholar] [CrossRef]
- Rehman, A.; Khan, S.; Sun, F.; Peng, Z.; Feng, K.; Wang, N.; Jia, Y.; Pan, Z.; He, S.; Wang, L.; et al. Exploring the nano-wonders: Unveiling the role of nanoparticles in enhancing salinity and drought tolerance in plants. Front. Plant Sci. 2024, 14, 1324176. [Google Scholar] [CrossRef]
- Smaoui, S.; Chérif, I.; Ben Hlima, H.; Khan, M.U.; Rebezov, M.; Thiruvengadam, M.; Sarkar, T.; Shariati, M.A.; Lorenzo, J.M. Zinc Oxide Nanoparticles in Meat Packaging: A Systematic Review of Recent Literature. Food Packag. Shelf Life 2023, 36, 101045. [Google Scholar] [CrossRef]
- Gökmen, G.G.; Mirsafi, F.S.; Leissner, T.; Akan, T.; Mishra, Y.K.; Kışla, D. Zinc Oxide Nanomaterials: Safeguarding Food Quality and Sustainability. Compr. Rev. Food Sci. Food Saf. 2024, 23, 45. [Google Scholar] [CrossRef] [PubMed]
- Ferri, M.; Papchenko, K.; Degli Esposti, M.; Tondi, G.; De Angelis, M.G.; Morselli, D.; Fabbri, P. Emerging Trends for ZnO Nanoparticles and Their Applications in Food Packaging. ACS Food Sci. Technol. 2022, 2, 763–781. [Google Scholar] [CrossRef]
- Dey, S.; Mohanty, D.L.; Mohanty, D.; Divya, N.; Bakshi, V.; Mohanty, A.; Rath, D.; Das, S.; Mondal, A.; Roy, S.; et al. A Critical Review on Zinc Oxide Nanoparticles: Synthesis, Properties and Biomedical Applications. Int. Pharm. 2024, 3, 53–70. [Google Scholar] [CrossRef]
- Hussain, I.; Malik, F.; Shah, S.; Al-Kahtani, M.A.; Almaghasla, M.I.; Al-Dosary, M.; Al-Shehri, M.; Al-Rashdi, A.S.; Shah, T.; Al-Kahtani, J.; et al. Efficacy of Biogenic Zinc Oxide Nanoparticles in Treating Wastewater for Sustainable Wheat Cultivation. Agronomy 2022, 12, 3058. [Google Scholar]
- Hussain, R.T.; Hossain, M.S.; Shariffuddin, J.H. Green Synthesis and Photocatalytic Insights: A Review of Zinc Oxide Nanoparticles in Wastewater Treatment. Mater. Today Sustain. 2024, 26, 100764. [Google Scholar] [CrossRef]
- Dimapilis, E.A.S.; Hsu, C.S.; Mendoza, R.M.O.; Lu, M.C. Zinc Oxide Nanoparticles for Water Disinfection. Sustain. Environ. Res. 2018, 28, 47–56. [Google Scholar] [CrossRef]
- Nawaz, A.; Farhan, A.; Maqbool, F.; Ahmad, H.; Qayyum, W.; Ghazy, E.; Rahdar, A.; Díez-Pascual, A.M.; Fathi-Karkan, S. Zinc Oxide Nanoparticles: Pathways to Micropollutant Adsorption, Dye Removal, and Antibacterial Actions—A Study of Mechanisms, Challenges, and Future Prospects. J. Mol. Struct. 2024, 1312, 138545. [Google Scholar] [CrossRef]
- Sun, Q.; Li, J.; Le, T. Zinc Oxide Nanoparticle as a Novel Class of Antifungal Agents: Current Advances and Future Perspectives. J. Agric. Food Chem. 2018, 66, 11209–11220. [Google Scholar] [CrossRef]
- Pariona, N.; Paraguay-Delgado, F.; Basurto-Cereceda, S.; Morales-Mendoza, J.E.; Hermida-Montero, L.A.; Mtz-Enriquez, A.I. Shape-Dependent Antifungal Activity of ZnO Particles against Phytopathogenic Fungi. Appl. Nanosci. 2020, 10, 435–443. [Google Scholar] [CrossRef]
- Savi, G.D.; Bortoluzzi, A.J.; Scussel, V.M. Antifungal properties of Zinc-compounds against toxigenic fungi and mycotoxin. Int. J. Food Sci. Technol. 2013, 48, 1786–1793. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Mustapha, A.; Lin, M. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol. Res. 2011, 166, 207–215. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Ghosh, S.; Gryzenhout, M.; Conradie, J. One-pot synthesis of zinc oxide nanoparticles via chemical precipitation for bromophenol blue adsorption and the antifungal activity against filamentous fungi. Sci. Rep. 2021, 11, 8305. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.H.; Shah, M.A. A Unique and Profound Effect of MgO and ZnO Nanoparticles on Some Plant Pathogenic Fungi. J. Appl. Pharm. Sci. 2012, 2, 40–44. [Google Scholar]
- Hassan, A.A.; Howayda, M.E.; Mahmoud, H.H. Effect of Zinc Oxide Nanoparticles on the Growth of Mycotoxigenic Mould. J. Stud. Chem. Process Technol. 2013, 1, 6–25. [Google Scholar]
- Zaki, S.A.; Ouf, S.A.; Albarakaty, F.M.; Habeb, M.M.; Aly, A.A.; Abd-Elsalam, K.A. Trichoderma harzianum-Mediated ZnO Nanoparticles: A Green Tool for Controlling Soil-Borne Pathogens in Cotton. J. Fungi 2021, 7, 952. [Google Scholar] [CrossRef]
- Zhu, W.; Hu, C.; Ren, Y.; Lu, Y.; Song, Y.; Ji, Y.; He, J. Green Synthesis of Zinc Oxide Nanoparticles Using Cinnamomum camphora (L.) Presl Leaf Extracts and Its Antifungal Activity. J. Environ. Chem. Eng. 2021, 9, 106659. [Google Scholar] [CrossRef]
- Jayaseelan, C.; Rahuman, A.A.; Kirthi, A.V.; Marimuthu, S.; Santhoshkumar, T.; Bagavan, A.; Gaurav, K.; Karthik, L.; Rao, K.V.B. Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 90, 78–84. [Google Scholar] [CrossRef]
- Rajiv, P.; Rajeshwari, S.; Venckatesh, R. Bio-Fabrication of Zinc Oxide Nanoparticles Using Leaf Extract of Parthenium hysterophorus L. and Its Size-Dependent Antifungal Activity against Plant Fungal Pathogens. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 112, 384–387. [Google Scholar] [CrossRef]
- Jamdagni, P.; Khatri, P.; Rana, J.S. Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity. J. King Saud Univ. Sci. 2018, 30, 168–175. [Google Scholar] [CrossRef]
- Kamal, A.; Saba, M.; Kamal, A.; Batool, M.; Asif, M.; Al-Mohaimeed, A.M.; Al Farraj, D.A.; Habib, D.; Ahmad, S. Bioinspired green synthesis of bimetallic iron and zinc oxide nanoparticles using mushroom extract and use against Aspergillus niger; the most devastating fungi of the green world. Catalysts 2023, 13, 400. [Google Scholar] [CrossRef]
- Alhazmi, N.M.; Sharaf, E.M. Fungicidal activity of zinc oxide nanoparticles against azole-resistant Aspergillus flavus isolated from yellow and white maize. Molecules 2023, 28, 711. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, R.; Singh, R.R.; Kumari, A. Evaluation of biogenic zinc oxide nanoparticles from Tinospora cordifolia stem extract for photocatalytic, anti-microbial, and antifungal activities. Mater. Chem. Phys. 2023, 297, 127382. [Google Scholar] [CrossRef]
- Jain, D.; Shivani; Bhojiya, A.A.; Singh, H.; Daima, H.K.; Singh, M.; Mohanty, S.R.; Stephen, B.J.; Singh, A. Microbial fabrication of zinc oxide nanoparticles and evaluation of their antimicrobial and photocatalytic properties. Front. Chem. 2020, 8, 778. [Google Scholar] [CrossRef] [PubMed]
- Karkhane, M.; Lashgarian, H.E.; Mirzaei, S.Z.; Ghaffarizadeh, A.; Sepahvand, A.; Marzban, A. Antifungal, antioxidant and photocatalytic activities of zinc nanoparticles synthesized by Sargassum vulgare extract. Biocatal. Agric. Biotechnol. 2020, 29, 101791. [Google Scholar] [CrossRef]
- Lakshmeesha, T.R.; Murali, M.; Ansari, M.A.; Udayashankar, A.C.; Alzohairy, M.A.; Almatroudi, A.; Niranjana, S.R. Biofabrication of Zinc Oxide Nanoparticles from Melia azedarach and Its Potential in Controlling Soybean Seed-Borne Phytopathogenic Fungi. Saudi J. Biol. Sci. 2020, 27, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Madhumitha, G.; Fowsiya, J.; Gupta, N.; Kumar, A.; Singh, M. Green synthesis, characterization, and antifungal and photocatalytic activity of Pithecellobium dulce peel–mediated ZnO nanoparticles. J. Phys. Chem. Solids 2019, 127, 43–51. [Google Scholar] [CrossRef]
- Kolahalam, L.A.; Prasad, K.R.S.; Krishna, P.M.; Supraja, N. Saussurea lappa plant rhizome extract-based zinc oxide nanoparticles: Synthesis, characterization and its antibacterial, antifungal activities and cytotoxic studies against Chinese hamster ovary (CHO) cell lines. Heliyon 2021, 7, e07265. [Google Scholar] [CrossRef]
- Sardella, D.; Gatt, R.; Valdramidis, V.P. Physiological effects and mode of action of ZnO nanoparticles against postharvest fungal contaminants. Food Res. Int. 2017, 101, 274–279. [Google Scholar] [CrossRef]
- Malandrakis, A.A.; Kavroulakis, N.; Chrysikopoulos, C.V. Zinc nanoparticles: Mode of action and efficacy against boscalid-resistant Alternaria alternata isolates. Sci. Total Environ. 2022, 829, 154638. [Google Scholar] [CrossRef]
- Yehia, R.S.; Ahmed, O.F. In Vitro Study of the Antifungal Efficacy of Zinc Oxide Nanoparticles Against Fusarium oxysporum and Penicillium expansum. Afr. J. Microbiol. Res. 2013, 19, 1917–1923. [Google Scholar]
- Zudyte, B.; Luksiene, Z. Visible Light-Activated ZnO Nanoparticles for Microbial Control of Wheat Crop. J. Photochem. Photobiol. B Biol. 2021, 219, 112206. [Google Scholar] [CrossRef]
- Koka, J.A.; Wani, A.H.; Bhat, M.Y. Evaluation of antifungal activity of magnesium oxide (MgO) and iron oxide (FeO) nanoparticles on rot causing fungi. J. Drug Deliv. Ther. 2019, 9, 173–292. [Google Scholar] [CrossRef]
- Silvestri, L.; Pettinato, M.; Furiosi, V.; Bavuso Volpe, L.; Nai, A.; Pagani, A. Managing the dual nature of iron to preserve health. Int. J. Mol. Sci. 2023, 24, 3995. [Google Scholar] [CrossRef] [PubMed]
- Rolić, T.; Yazdani, M.; Mandić, S.; Distante, S. Iron metabolism, calcium, magnesium and trace elements: A review. A review. Biol. Trace Elem. Res. 2024, 203, 2216–2225. [Google Scholar] [CrossRef] [PubMed]
- Al Alawi, A.M.; Majoni, S.W.; Falhammar, H. Magnesium and Human Health: Perspectives and Research Directions. Int. J. Endocrinol. 2018, 2018, 9041694. [Google Scholar] [CrossRef] [PubMed]
- Ahari, H.; Lahijani, L.K. Migration of Silver and Copper Nanoparticles from Food Coating. Coatings 2021, 11, 380. [Google Scholar] [CrossRef]
- Störmer, A.; Bott, J.; Kemmer, D.; Franz, R. Critical review of the migration potential of nanoparticles in food contact plastics. Trends Food Sci. Technol. 2017, 63, 39–50. [Google Scholar] [CrossRef]
- Ahari, H.; Jafari, A.; Ozdal, T.; Moradi, S.; Bahari, H.R.; Wu, Q.; Eş, I.; Khaneghah, A.M. Recent innovations in metal-based nanoparticles for food packaging: A focus on safety and environmental impact. Appl. Food Res. 2025, 5, 100860. [Google Scholar] [CrossRef]
- Rothen-Rutishauser, B.; Bogdanovich, M.; Harter, R.; Milosevic, A.; Petri-Fink, A. Use of nanoparticles in food industry: Current legislation, health risk discussions and public perception with a focus on Switzerland. Toxicol. Environ. Chem. 2021, 103, 423–437. [Google Scholar] [CrossRef]
- Rao, M.V.M.; Mohammad, N.; Banerjee, S.; Khanna, P.K. Synthesis and Food Packaging Application of Silver Nanoparticles: A Review. Hybrid Adv. 2024, 6, 100230. [Google Scholar] [CrossRef]
- Zhang, W.; Roy, S.; Rhim, J.W. Copper-Based Nanoparticles for Biopolymer-Based Functional Films in Food Packaging Applications. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1933–1952. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, K.; Sathishkumar, G.; Xu, L. An Overview of the Copper Oxide Nanofillers Integrated in Food Packaging Systems. Coatings 2024, 14, 81. [Google Scholar] [CrossRef]
- Herrera-Rivera, M.D.R.; Torres-Arellanes, S.P.; Cortés-Martínez, C.I.; Navarro-Ibarra, D.C.; Hernández-Sánchez, L.; Solis-Pomar, F.; Pérez-Tijerina, E.; Román-Doval, R. Nanotechnology in Food Packaging Materials: Role and Application of Nanoparticles. RSC Adv. 2024, 14, 21832–21858. [Google Scholar] [CrossRef]
- Joshi, N.C.; Negi, P.B.; Gururani, P.A. Review on Metal/Metal Oxide Nanoparticles in Food Processing and Packaging. Food Sci. Biotechnol. 2024, 33, 1307–1322. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Fawole, O.A. Metal-Based Nanoparticles in Food Packaging and Coating Technologies: A Review. Biomolecules 2023, 13, 1092. [Google Scholar] [CrossRef]

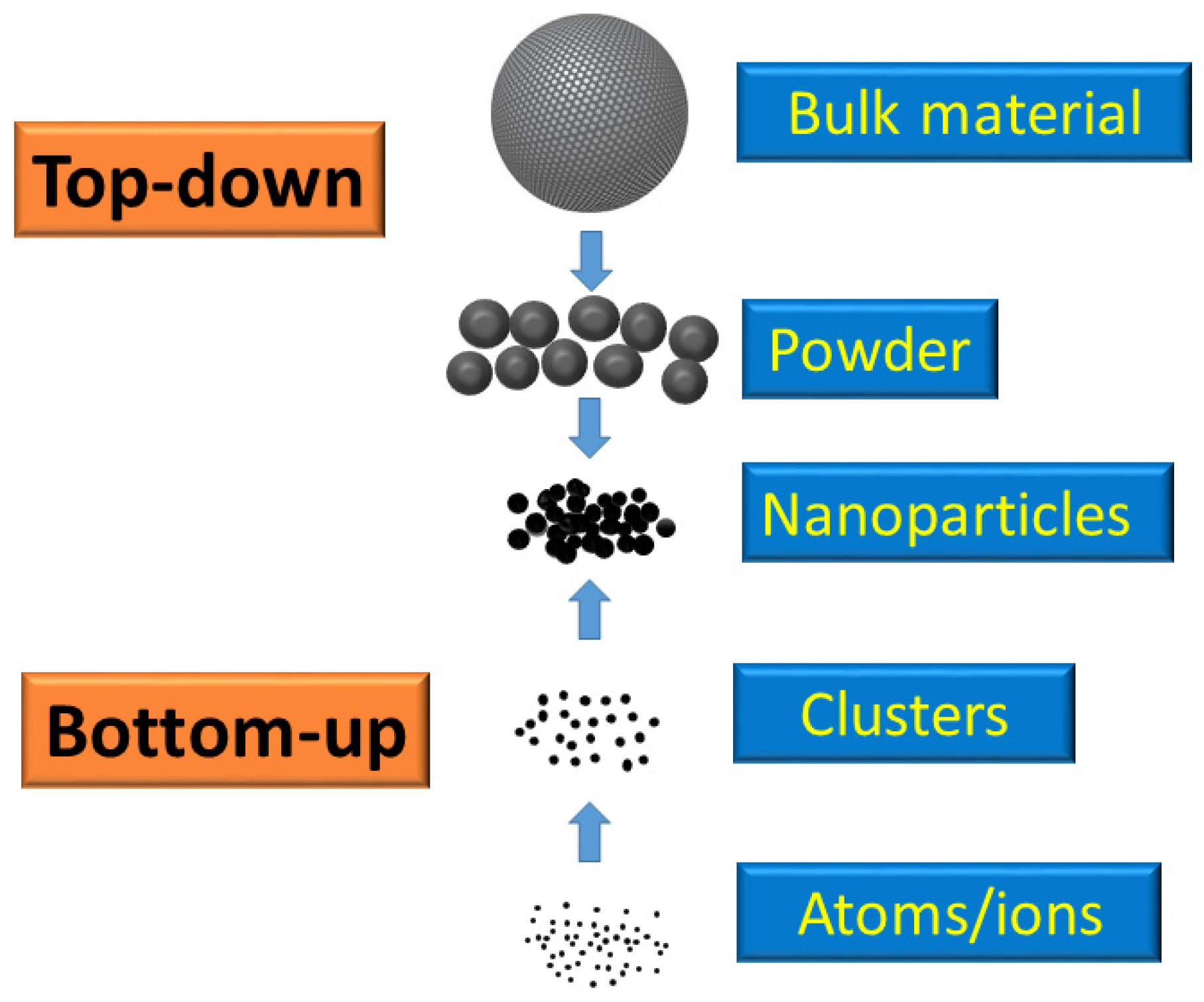
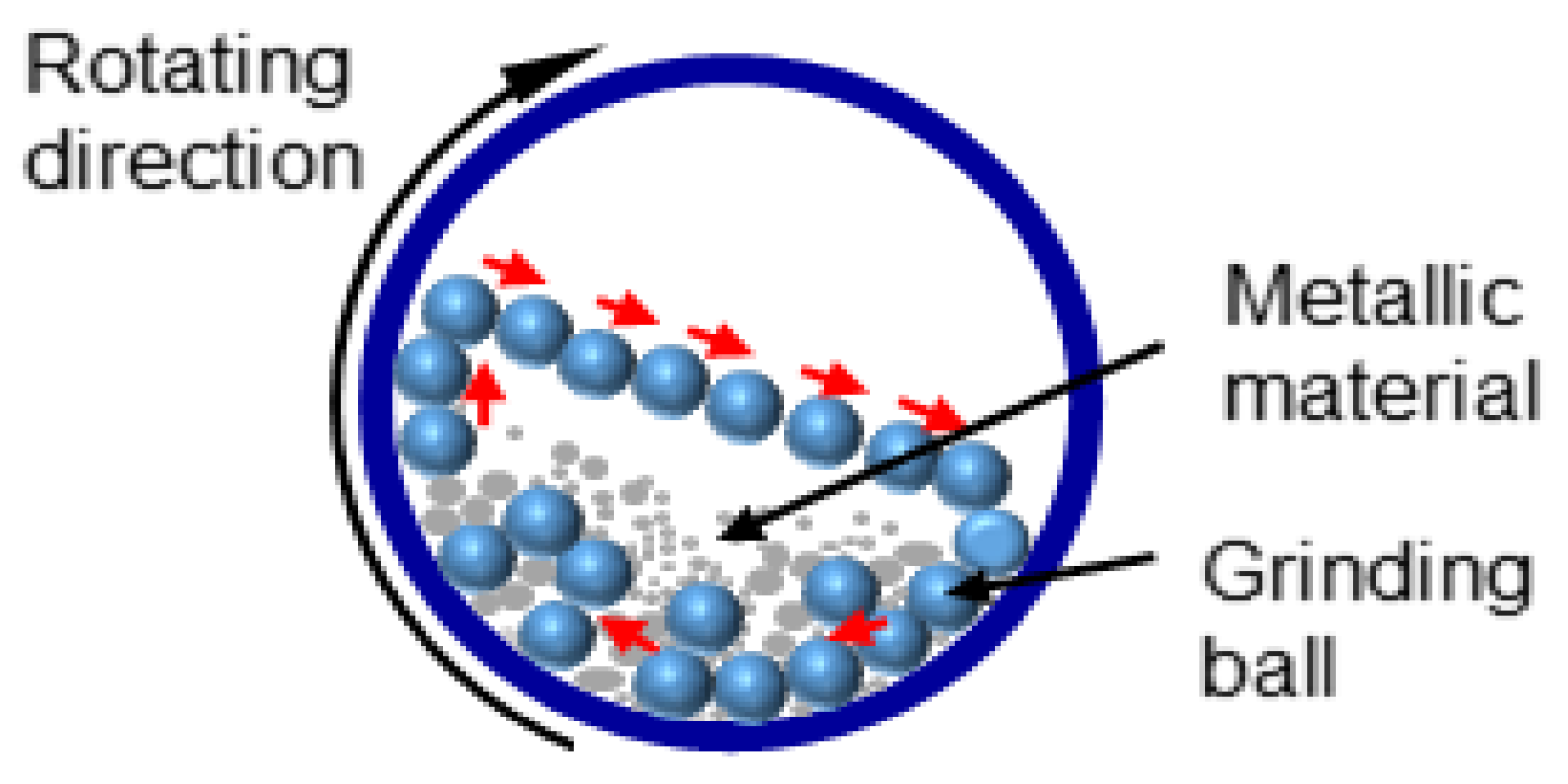
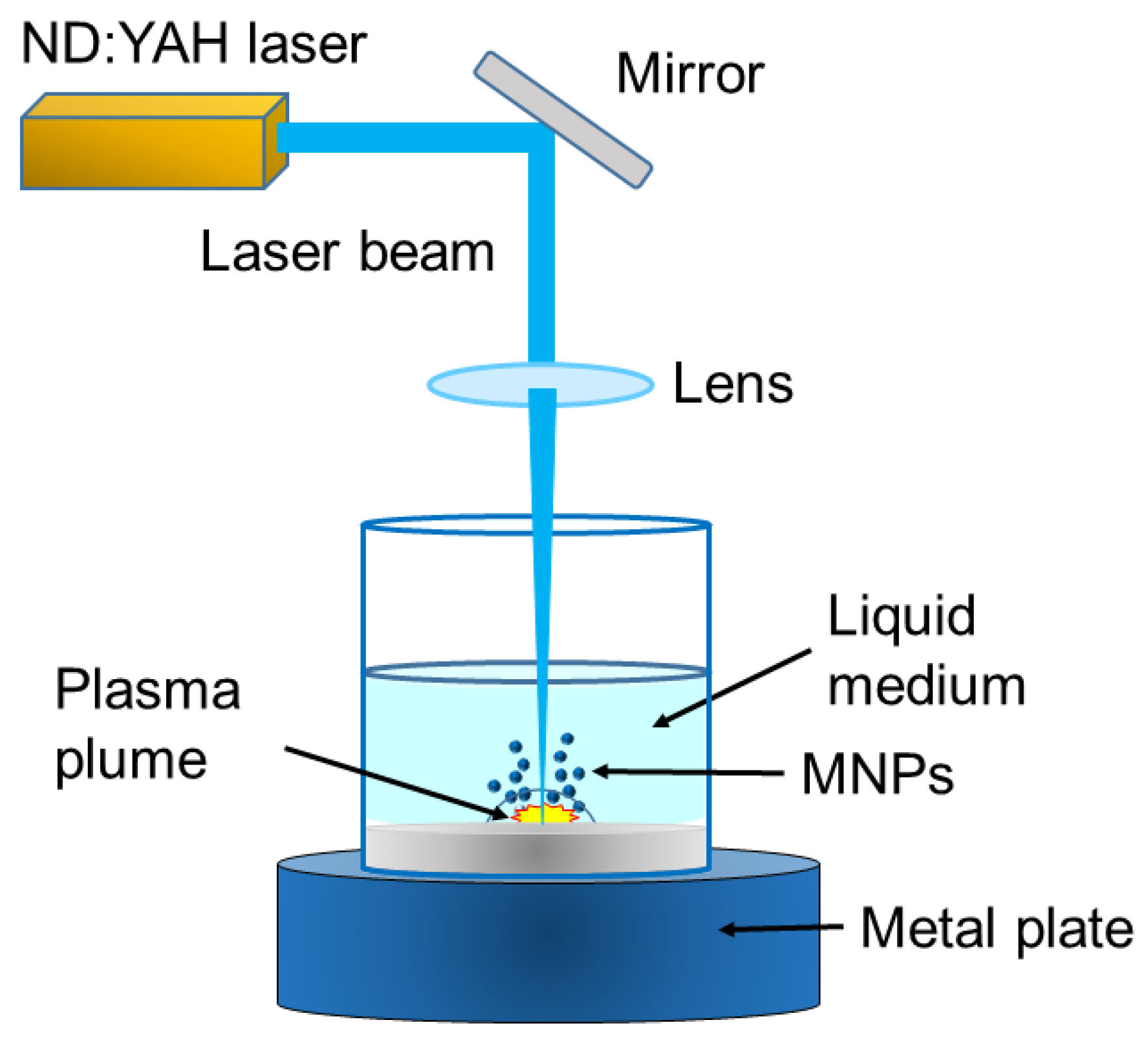
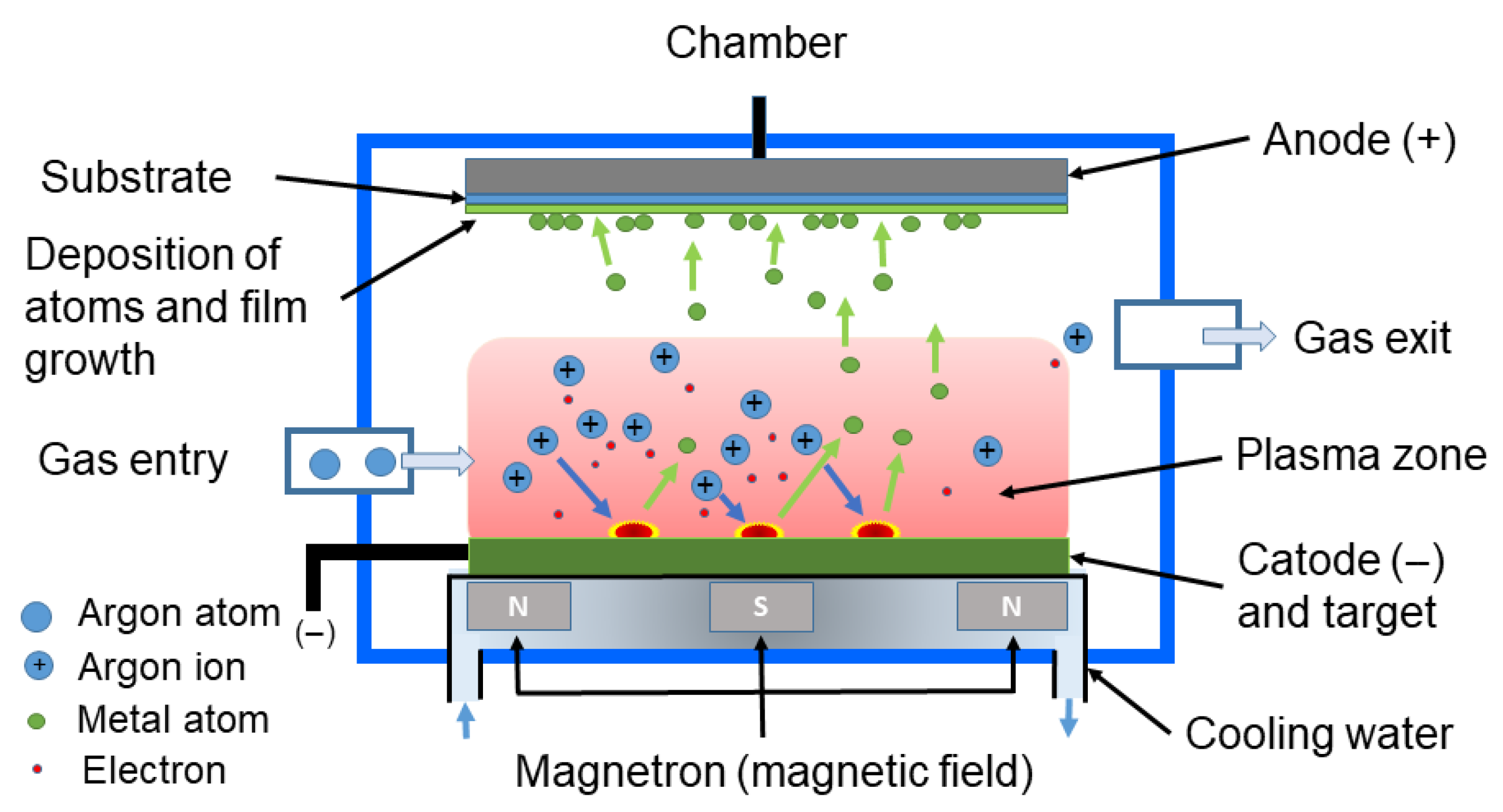
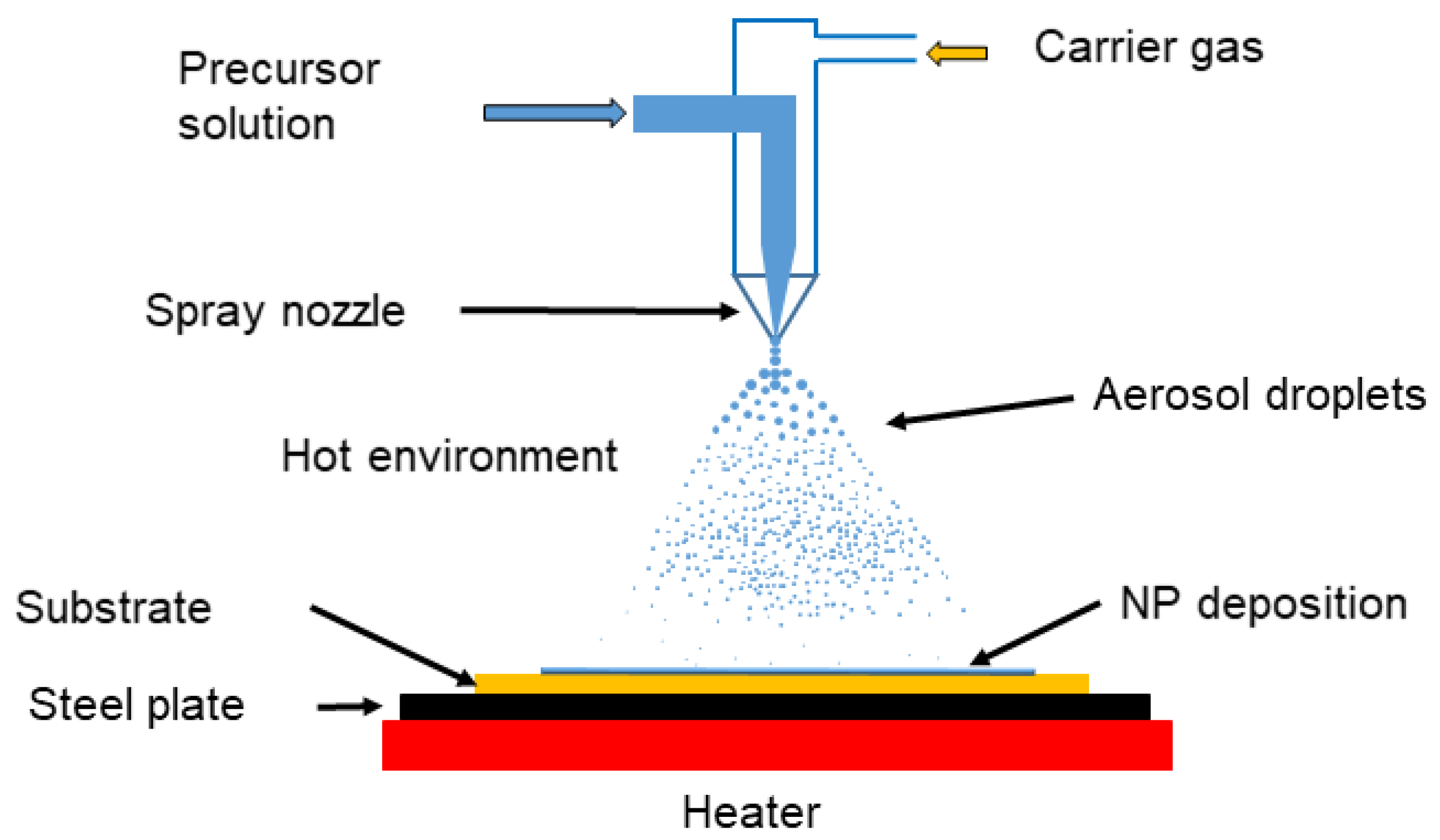


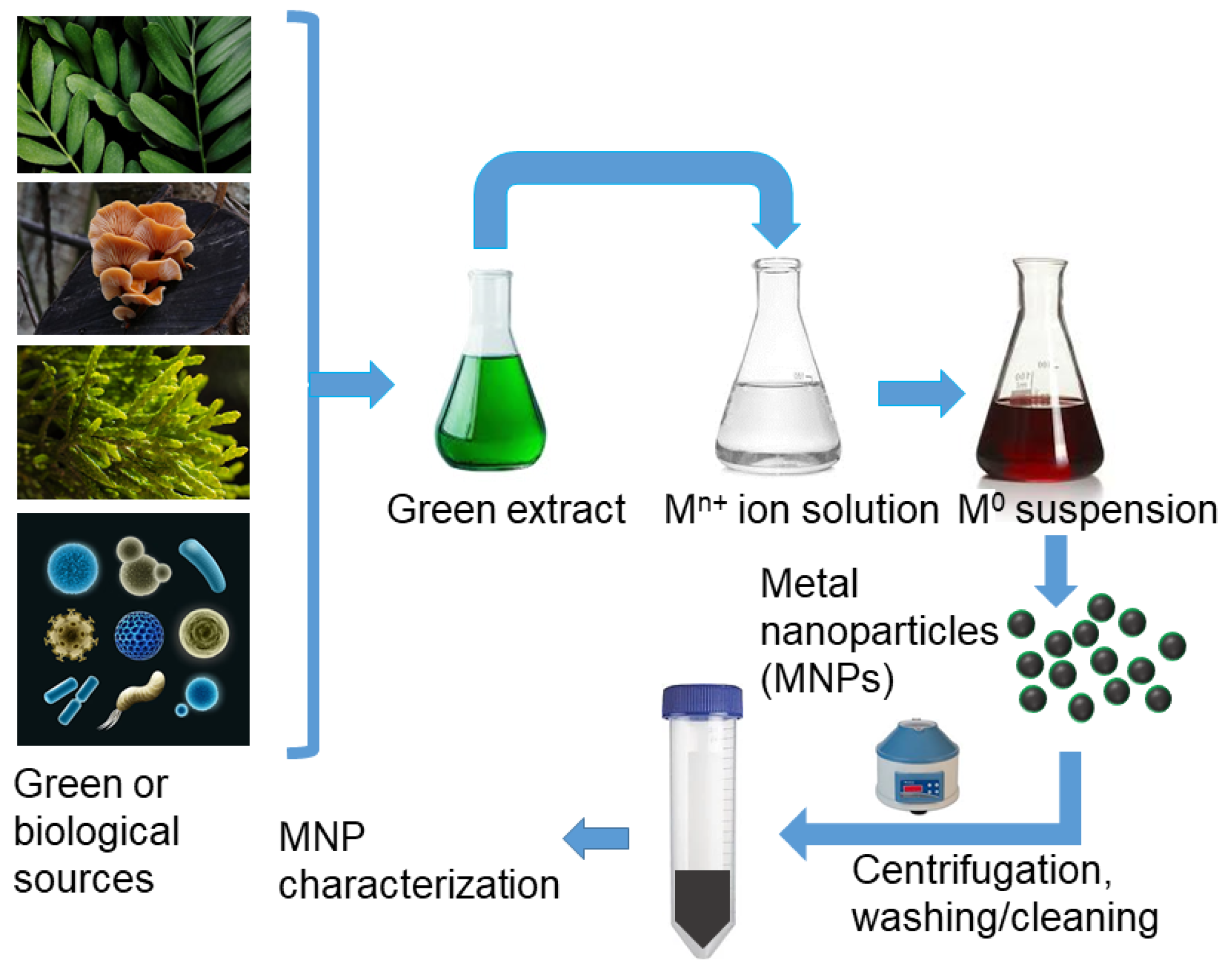

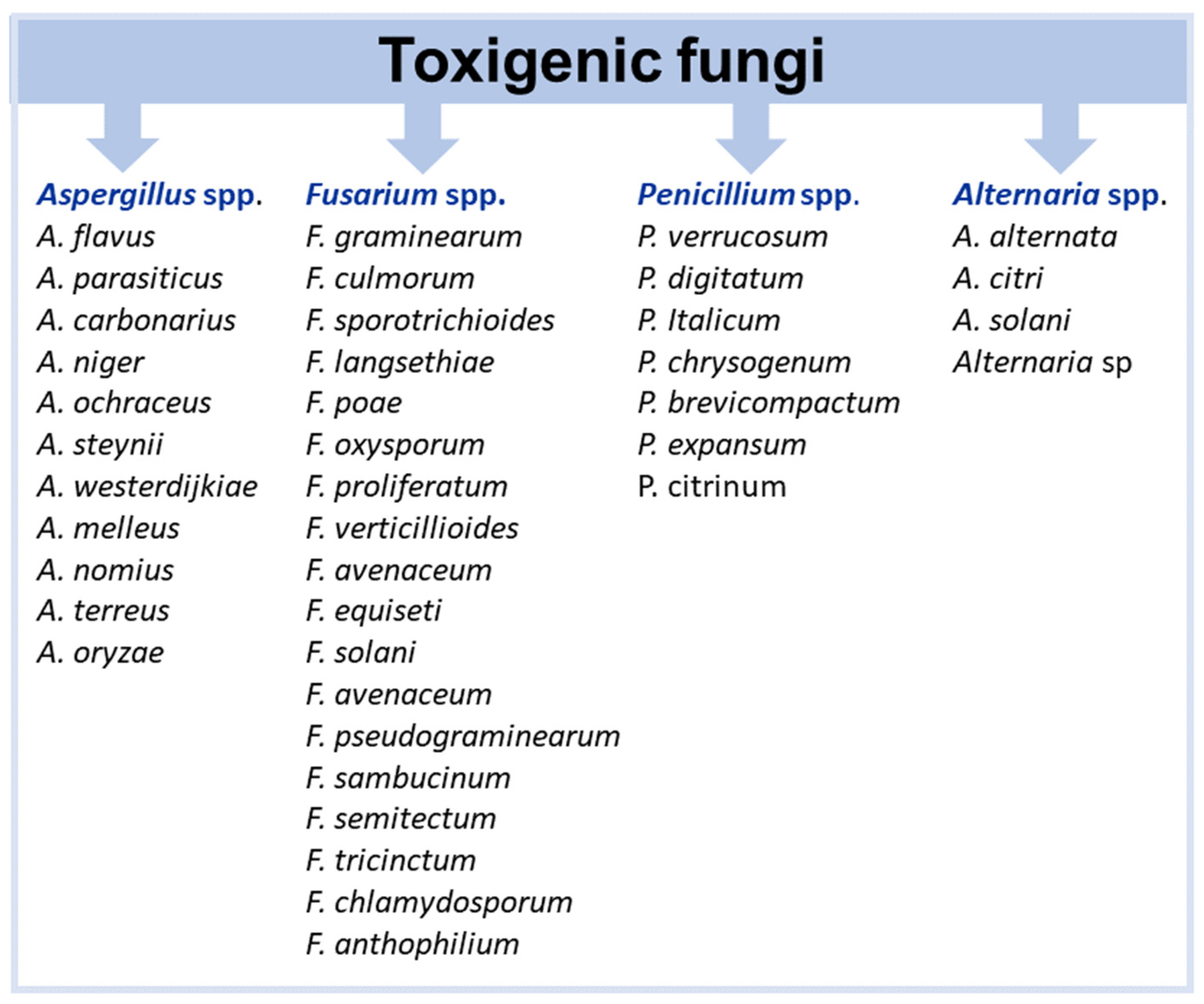

| Nanoparticle Properties | Antifungal Properties | |||||
|---|---|---|---|---|---|---|
| Synthesis Method | Size (nm) | Shape | Fungal species | Methodology | Growth reduction (%)/Treatment | Ref. |
| Chemical | 18 ± 4 246 ± 40 × 48 ± 6 786 ± 142 × 9330 ± 1500 | Spherical Platelet Elongated rod | F. oxysporum f. sp. lycopersici, F. solani | Medium: Potato Dextrose Agar (PDA). Inoculum: From a spore suspension (1 × 106 spores/mL). ZnONP concentration: 100, 250, 500, 750, and 1000 ppm. Incubation: 29 °C, 6 days. Fungal growth record: Colony diameter. | 0–55 ± 3.1%/100–1000 ppm, 0–65 ± 1.8%/100–1000 ppm, 0–31 ± 2.1%/100–1000 ppm, Depending on the NP size and shape | [365] |
| Chemical | 30 | Spherical | F. graminearum, P. citrinum, A. flavus | Medium: PDA. Inoculum: Agar plugs (6 mm) from a fungal culture. ZnONP concentration: 10, 25, 50, and 100 mM. Incubation: 25 °C, 8 days. Fungal growth record: Colony diameter and mycelium weight. | ∼50%/100 mM | [366] |
| Chemical | 70 ± 15 | Spherical | P. expansum | Medium: PDA. Inoculum: Agar plugs (14 mm) from a fungal culture. ZnONP concentration: 3, 6, and 12 mM. Incubation: 25 °C, 12 days. Fungal growth record: Colony diameter. | 91%/12 mM | [367] |
| Chemical | 47.2 | Irregular | A. alternata, F. verticillioides | Medium: Malt Extract Agar (MEA). Inoculum: From a spore suspension (1 × 106 spores/mL). ZnONP concentration: 2–5000 ppm. Incubation: 25 °C, 12 days. Fungal growth record: Diameter inhibition zone. | 22.73–36.28 mm/2–5000 ppm, 23.77–34.77 mm/2–5000 ppm, For each species, respectively | [368] |
| ChemicaL | ~30 ± 10 | A. alternata, F. oxysporum | Medium: Suspension of spores + NPs. Inoculum: From a spore suspension (—). ZnONP concentration: —. Incubation: 24 ± 2 °C, 24 h. Fungal growth record: Spore germination. | 78.00–42.61%/— | [369] | |
| Chemical | 20 | Spherical | A. flavus, A. ochraceus, A. niger | Medium: Yeast Extract Sucrose (YES). Inoculum: From a spore suspension (5 × 106 spores/mL). ZnONP concentration: (0, 2, 4, 6, 8, and 10 ppm. Incubation: 22–25 ± 2 °C, 20 days. Fungal growth record. Mycelial damage. | 100%/8 ppm, 100%/10 ppm, 100%/10 ppm, For each species, respectively | [370] |
| Biological (Trichoderma harzianum) | 8–23 | Spherical, rod, and hexagonal | Fusarium sp. | Medium: PDA. Inoculum: Disks 5 mm + inoculum (—). ZnONP concentration: 20, 40, and 100 ppm. Incubation: 35 °C, 5–7 days. Fungal growth record: Colony diameter. | 100%/≥20 ppm | [371] |
| Biological (Cinnamomum camphora L. leaf) | 13.92–21.13 | Spherical | A. alternata | Medium: PDA and Potato Dextrose Broth (PDB). Inoculum: Agar plugs (6 mm) from a fungal culture. ZnONP concentration: 10, 20, 40, 80, and 160 ppm. Incubation: 25 °C, 8 days. Fungal growth record: Colony diameter. | 21.59–68.50%/20–160 ppm | [372] |
| Biological (Aeromonas hydrophila) | 57.72 | Spherical, oval | A. flavus, A. niger | Medium: Mueller Hinton Agar (MHA). Inoculum: From a spore suspension (—) (well 8 mm). ZnONP concentration: 5, 10, 15, 20, and 25 ppm. Incubation: 37 °C, 24 h. Fungal growth record: Diameter inhibition zone and MIC (according to CLSI). | 19 ± 1.0 mm/25 ppm, <19 ± 1.0 mm/25 ppm, For each species, respectively 100% (MIC)/2.9 ± 0.01 ppm, 100% (MIC)/2.0 ± 0.04 ppm, For each species, respectively | [373] |
| Biological (Parthenium hysterophorus L. leaf) | 27 ± 5 84 ± 2 | Spherical, Hexagonal | A. niger, A. flavus, F. oxysporum, F. culmorum | Medium: PDA. Inoculum: From a spore suspension (—). ZnONP concentration: 25 ppm (wells 5 mm). Incubation: Room temperature, 48 h. Fungal growth record: Diameter inhibition zone. | 24–14 mm/50 ppm (size 27 ± 5 nm), 21–12 mm/50 ppm (size 84 ± 2 nm), Depending on the species | [374] |
| Biological (Nyctanthes arbor-tristis flower) | 12–32 | Spherical | A. alternata, A. niger, F. oxysporum, P. expansum | Medium: Liquid medium (—). Inoculum: From a spore suspension (1 × 105 spores/mL). ZnONP concentration: 16–256 ppm. Incubation: 28 °C, 3 days. Fungal growth record: MIC. | 100%/64 ppm, 100%/16 ppm, 100%/64 ppm, 100%/128 ppm, For each species, respectively | [375] |
| Biological (Penicillium chrysogenum) | 9.0–35.0 | Hexagonal | F. solani, F. oxysporum, A. terreus | Medium: PDA. Inoculum: From a spore suspension (—). ZnONP concentration: 10,000 ppm (discs 7 mm). Incubation: 30 °C, 5 days. Fungal growth record: Diameter inhibition zone. | 12.33 ± 0.88/10,000 ppm, 11.83 ± 1.36/10,000 ppm, 14 ± 0.5 mm/10,000 ppm, For each species, respectively | [341] |
| Biological (Daedalea Mushroom) | 18.53 | Irregular | A. niger | Medium: PDA. Inoculum: Agar plugs (1.4 cm) from a fungal culture. ZnONP concentration: 100, 250, 750 ppm. Incubation: 26 °C, 6 days. Fungal growth record: Colony diameter. | 22%/100 ppm, 88%/250 ppm | [376] |
| Biological (Chlorella vulgaris) | 35 | Hexagonal | A. flavus | Medium: MHA. Inoculum: From a spore suspension (2–5 × 105 spores/mL). ZnONP concentration: 10,000 ppm (disks). Incubation: 35 °C, 2 days. Fungal growth record: Diameter inhibition. | 13–14 mm/10,000 ppm | [377] |
| Biological (Tinospora cordifolia) | 32 | Hexagonal | F. oxysporum | Medium: PDA. Inoculum: From spore suspension (—). ZnONP concentration: 100 ppm (wells 6 mm). Incubation: 25 °C, 5–7 days. Fungal growth record: Diameter inhibition zone. | 47–51 mm/100 ppm | [378] |
| Biological (Serratia nematodiphila) | 15–30 (23) | Hexagonal | Alternaria sp. | Medium: PDA. Inoculum: Agar plugs (4 mm) from a fungal culture. ZnONP concentration: 50, 100, 150, 200, and 250 ppm. Incubation: (—), 5 days. Fungal growth record: Colony diameter (cd) and spore viability (sv). | 21.67–85.93% (cd)/50–250 ppm, 18.18–92.22% (sv)/50–250 ppm | [379] |
| Biological (Sargassum vulgare) | 50–150 | Spherical | A. niger, A. flavus | Medium: Sabouraud Dextrose Agar (SDA). Inoculum: From a spore suspension (1 × 106 spores/mL). ZnONP concentration: 1, 5, 10, 30, and 50 ppm (wells). Incubation: 22 °C, 3 days. Fungal growth record: Diameter inhibition zone, MIC, and MFC. | 11–32 mm/1–50 ppm, 9–27 mm/1–50 ppm, For each species, respectively MIC and MFC/10 and 20 ppm, MIC and MFC/40 and 50 ppm, For each species, respectively | [380] |
| Biological (Lemon peels extract) | 16.8 | Rounded, Elongated, Spherical | A. citri | Medium: PDA. Inoculum: From a spore suspension (—). ZnONP concentration: 10–100 ppm. Incubation: 28 °C, 5 days. Fungal growth record: Diameter inhibition zone. | 21.5–51.5 mm/10–100 ppm | [345] |
| Biological (Melia azedarach leaf) | 30–40 | Hexagonal | F. oxysporum | Medium: Czapek-Dox Broth (CZB) and Czapek-Dox agar (CZA). Inoculum: From a spore suspension (1 × 106 spores/mL). ZnONP concentration: 250 ppm. Incubation: 25 ± 2 °C, 3 days. Fungal growth record: MIC and MFC. | MIC/93.33 ppm. MFC/208.3 ppm | [381] |
| Biological (Pithecellobium dulce peel) | 11.5 ± 2 | Spherical | A. flavus, A. niger | Medium: PDB. Inoculum: From a spore suspension (—). ZnONP concentration: 500 and 1000 ppm. Incubation: 37 °C, 3 days. Fungal growth record: Fungal biomass. | 37.81–63.57%/500–1000 ppm. 40.21–43.04%/500–1000 ppm. For each species, respectively | [382] |
| Biological (Saussurea lappa plant root) | 26 ± 1 | Hexagonal | A. niger, A. flavus, F. oxysporum | Medium: —. Inoculum: —. ZnONP concentration: 50, 100, 170 ppm. Incubation: —. Fungal growth record: Diameter inhibition zone. | 2.0–2.7 mm/50–170 ppm 1.7–2.7 mm/50–170 ppm 1.7–2.1 mm/50–170 ppm For each species, respectively | [383] |
| Commercial | <50 | Spherical | P. expansum, A. alternata | Medium: PDA. Inoculum: From a spore suspension (1 × 105 spores/mL). ZnONP concentration: 3, 6, 12, and 15 mM. Incubation: 25 °C, 30–112 h. Fungal growth record: Colony diameter. | 50%/5.08 mM. 50%/5.49 mM. For each species, respectively | [384] |
| Commercial | 20 | Spherical | F. oxysporum, A. solani | Medium: PDA. Inoculum: Agar plugs (5 mm) from a fungal culture. ZnONP concentration: 100, 250, 500, 700, and 1000 ppm. Incubation: 25 ± 2 °C, 7–11 days. Fungal growth record: Colony diameter. | 0.0–91.13%/100–1000 ppm, 29.67–98.69%/100–1000 ppm, For each species, respectively | [347] |
| Commercial | <100 | — | A. alternata (10 isolates) | Medium: PDA. Inoculum: Agar plug (5 mm) from a fungal culture. ZnONP concentration: 10, 25, 50, 100, 250, 500, and 1000 ppm. Incubation: 25 °C, 70% RH, 4 days. Fungal growth record: Colony diameter. Medium: Tomato fruit Inoculum: Agar plugs (5 mm) from a fungal culture. ZnONP concentration: 1000 ppm. Incubation: 25 °C, 70% RH, 4 days. Fungal growth record: Lesion diameter. | 50%/250–388 ppm (mean 303 ppm) 14.24–32.50%/1000 ppm | [385] |
| Commercial | 70 ± 15 | — | F. oxysporum, P. expansum | Medium: PDA. Inoculum: Agar plugs (1 cm) from a fungal culture. ZnONP concentration: 0, 2, 4, 6, 8, and 12 ppm. Incubation: 25 °C, 12 days. Fungal growth record: Colony diameter. | 19.3–77.5%/2–12 ppm, 25.3–100%/2–12 ppm, For each species, respectively | [386] |
| Commercial | 170–430 | — | F. oxysporum | Medium: PDA. Inoculum: Agar plugs (0.5 mm) from a fungal culture. ZnONP concentration: 10−3 M and 5 × 10−3 M. Incubation: 25 °C (—). Fungal growth record: Colony diameter. | 51.7%/5 × 10−3 M photoactivated | [387] |
| Nanoparticle Properties | Anti-Mycotoxin Properties | |||||
|---|---|---|---|---|---|---|
| Synthesis Method | Size (nm) | Shape | Fungal Species | Methodology | Mycotoxin/Reduction (%)/Treatment | Ref |
| Chemical | 30 | Spherical | F. graminearum (DON), A. flavus (AFB1), P. citrinum (CIT) | Medium: PDA. Inoculum: Agar plugs (6 mm) from a fungal culture. Concentration of ZnONPs: 100 mM. Incubation: 25–30 °C, moisture 80–90%, 20 days. Deoxynivalenol (DON), aflatoxin B1 (AFB1), and citrinin (CIT) analysis: TLC. | DON/100%/100 mM AFB1/∼60%/100 mM CIT/∼5%/100 mM | [366] |
| Chemical | 20 | Spherical | A. flavus (Aflatoxins AFs), A. ochraceus (OTA), A. niger (FB2) | Medium: Yeast Extract Sucrose (YES). Inoculum: From a spore suspension (5 × 106 spores/mL). Concentration of ZnONPs: 0, 2, 4, 6, 8, and 10 ppm. Incubation: 22–25 ± 2 °C, 20 days. Aflatoxins (AFs), OTA and FB2 analysis: HPLC. | AFs/100%/8 ppm, OTA/100%/10 ppm, FB2/100%/10 ppm | [370] |
| Commercial | 70 ± 15 | — | F. oxysporum, P. expansum | Medium: PDA. Inoculum: Agar plugs (1 cm) from a fungal culture. ZnONP concentration: 0, 2, 4, 6, 8, and 12 ppm. Incubation: 25 °C, 12 days. Fusaric acid (FA) and patulin (PAT) analysis: HPLC. | FA/10.26–99.5%/2–12 ppm, PAT/13.3–92.26%/2–12 ppm | [386] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateo, E.M.; Mateo, F.; Tarazona, A.; Jiménez, M. Engineered Metal Nanoparticles: A Possible Small Solution to Big Problems Associated with Toxigenic Fungi and Mycotoxins. Toxins 2025, 17, 378. https://doi.org/10.3390/toxins17080378
Mateo EM, Mateo F, Tarazona A, Jiménez M. Engineered Metal Nanoparticles: A Possible Small Solution to Big Problems Associated with Toxigenic Fungi and Mycotoxins. Toxins. 2025; 17(8):378. https://doi.org/10.3390/toxins17080378
Chicago/Turabian StyleMateo, Eva María, Fernando Mateo, Andrea Tarazona, and Misericordia Jiménez. 2025. "Engineered Metal Nanoparticles: A Possible Small Solution to Big Problems Associated with Toxigenic Fungi and Mycotoxins" Toxins 17, no. 8: 378. https://doi.org/10.3390/toxins17080378
APA StyleMateo, E. M., Mateo, F., Tarazona, A., & Jiménez, M. (2025). Engineered Metal Nanoparticles: A Possible Small Solution to Big Problems Associated with Toxigenic Fungi and Mycotoxins. Toxins, 17(8), 378. https://doi.org/10.3390/toxins17080378






