Modelling the Bioaccumulation of Ciguatoxins in Parrotfish on the Great Barrier Reef Reveals Why Biomagnification Is Not a Property of Ciguatoxin Food Chains
Abstract
1. Introduction
2. Results and Discussion
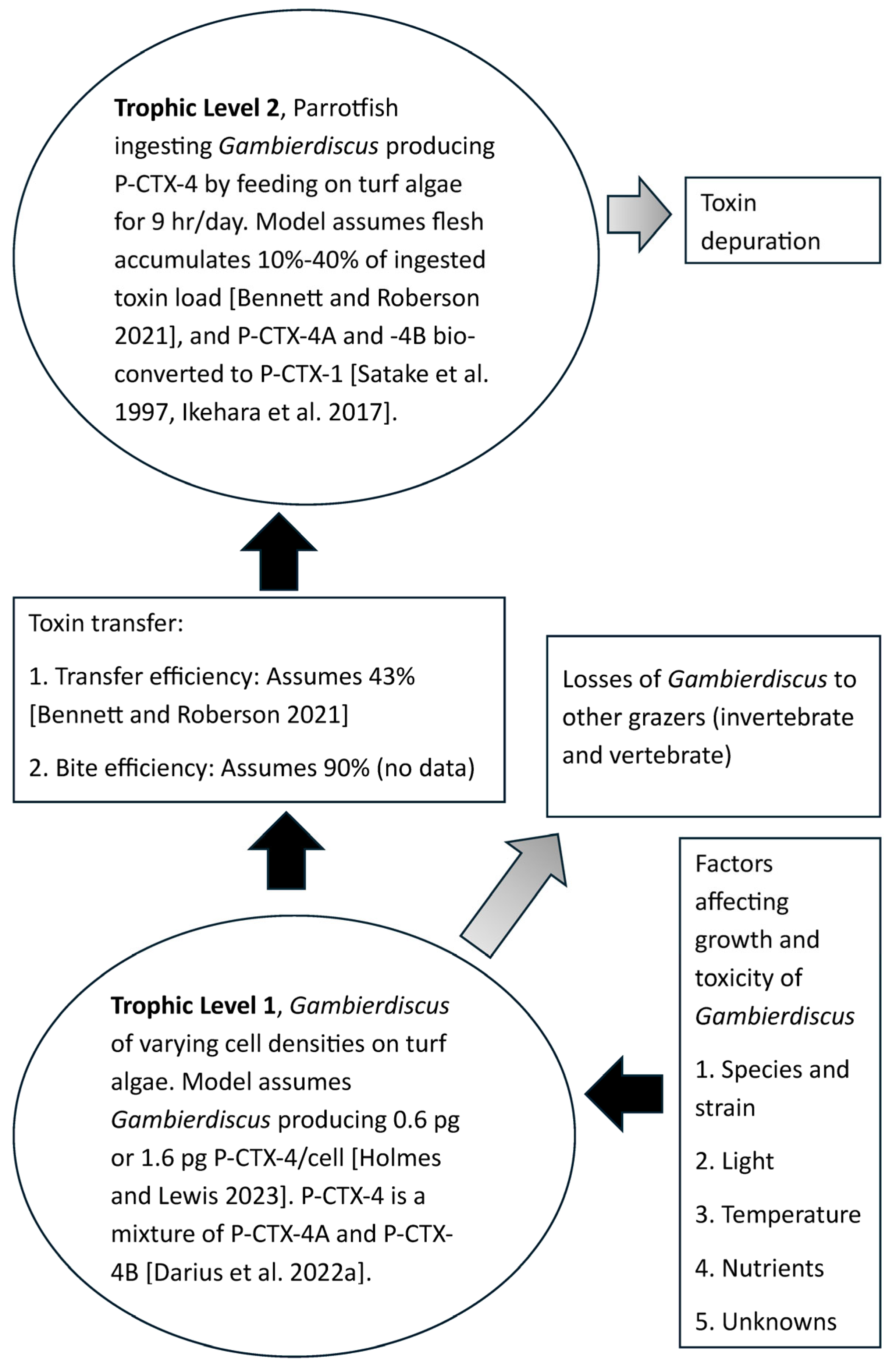
2.1. Construction of a Model for Production of Ciguatoxic Parrotfish on the Great Barrier Reef
2.2. Biomagnification Is Not a Property of the Ciguatoxin Food Chains
- Differences in relative toxicity between fishes of sequential trophic levels (e.g., herbivore prey and carnivorous predator);
- Differences in toxicity between different sized fish of the same species within the same trophic level;
- Differences in toxicity between different sized fish of different species, but within the same trophic level/guild.
2.2.1. Differences in Relative Toxicity Between Fishes of Sequential Trophic Levels (e.g., Herbivore Prey and Carnivorous Predator)
2.2.2. Differences in Toxicity Between Different-Sized Fish of the Same Species Within the Same Trophic Level
2.2.3. Differences in Toxicity Between Different-Sized Fish of Different Species, but Within the Same Trophic Level/Guild
2.3. Influence of Prey Size on Bioaccumulation of CTX into Predators
3. Summary and Conclusions
4. Material and Methods
4.1. Model for Accumulation of P-CTX into Parrotfish
4.2. Background for Model Interpretation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gillespie, N.C.; Lewis, R.J.; Pearn, J.H.; Bourke, A.T.C.; Holmes, M.J.; Bourke, J.B.; Shields, W.J. Ciguatera in Australia: Occurrence, clinical features, pathophysiology and management. Med. J. Aust. 1986, 145, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Lehane, L.; Lewis, R.J. Ciguatera: Recent advances but the risk remains. Int. J. Food Microbiol. 2000, 61, 91–125. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.P.; Brewer, T.D.; Johnstone, R.; Fleming, L.E.; Lewis, R.J. Ciguatera Fish Poisoning in the Pacific Islands (1998 to 2008). PLoS Negl. Trop. Dis. 2011, 5, e1416. [Google Scholar] [CrossRef] [PubMed]
- FAO; WHO. Report of the Expert Meeting on Ciguatera Poisoning: Rome, 19–23 November 2018; Food Safety and Quality No. 9; Rome, Italy. 2020. Available online: https://books.google.com.sg/books?hl=zh-TW&lr=&id=HhvtDwAAQBAJ&oi=fnd&pg=PR6&dq=10.4060/ca8817en&ots=UUfascmP-s&sig=UbOO36DHmBc8aCkBNQpXUxfuHdk#v=onepage&q=10.4060%2Fca8817en&f=false (accessed on 1 April 2025).
- Kretzschmar, A.L.; Larsson, M.E.; Hoppenrath, M.; Doblin, M.A.; Murray, S.A. Characterisation of Two Toxic Gambierdiscus spp. (Gonyaulacales, Dinophyceae) from the Great Barrier Reef (Australia): G. lewisii sp. nov. and G. holmesii sp. nov. Protist 2019, 170, 125699. [Google Scholar] [CrossRef]
- Li, Z.; Park, J.S.; Kang, N.S.; Chomérat, N.; Mertens, K.; Gu, H.; Lee, K.-W.; Kim, K.H.; Baek, S.H.; Shin, K.; et al. A new potentially toxic dinoflagellate Fukuyoa koreansis sp. nov. (Gonyaulacales, Dinophyceae) from Korean coastal waters: Morphology, phylogeny, and effects of temperature and salinity on growth. Harmful Algae 2021, 109, 102107. [Google Scholar] [CrossRef]
- Nguyen-Ngoc, L.; Larsen, J.; Doan-Nhu, H.; Nguyen, X.-V.; Chomérat, N.; Lundholm, N.; Phan-Tan, L.; Dao, H.V.; Nguyen, L.-N.; Nguyen, H.-H.; et al. Gambierdiscus (gonyaulacales, dinophyceae) diversity in Vietnamese waters with description of G. vietnamensis sp. nov. J. Phycol. 2023, 59, 496–517. [Google Scholar] [CrossRef]
- Murray, S.A.; Verma, A.; Hoppenrath, M.; Harwood, D.T.; Murray, J.S.; Smith, K.F.; Lewis, R.; Finch, S.C.; Islam, S.S.; Ashfaq, A.; et al. High ciguatoxin-producing Gambierdiscus clade (Gonyaulacales, Dinophyceae) as a source of toxins causing ciguatera poisoning. Sci. Total. Env. 2025, 994, 179990. [Google Scholar] [CrossRef]
- Holmes, M.J.; Venables, B.; Lewis, R.J. Critical review and conceptual and quantitative models for the transfer and depuration of ciguatoxins in fishes. Toxins 2021, 13, 515. [Google Scholar] [CrossRef]
- Satake, M.; Murata, M.; Yasumoto, T. The structure of CTX3C, a ciguatoxin congener isolated from Gambierdiscus toxicus. Tetrahedron Lett. 1993, 34, 1975–1978. [Google Scholar] [CrossRef]
- Mudge, E.M.; Miles, C.O.; Ivanova, L.; Uhlig, S.; James, K.S.; Erdner, D.L.; Fæste, C.K.; McCarron, P.; Robertson, A. Algal ciguatoxin identified as source of ciguatera poisoning in the Caribbean. Chemosphere 2023, 330, 138659. [Google Scholar] [CrossRef]
- Mudge, E.M.; Robertson, A.; Uhlig, S.; McCarron, P.; Miles, C.O. 3-epimers of 1 Caribbean ciguatoxins in fish and algae. Toxicon 2023, 237, 107536. [Google Scholar] [CrossRef]
- Murata, M.; Legrand, A.-M.; Ishibashi, Y.; Fukui, M.; Yasumoto, T. Structures and Configurations of Ciguatoxin from the Moray Eel Gymnothorax javanicus and Its Likely Precursor from the Dinoflagellate Gambierdiscus toxicus. J. Am. Chem. Soc. 1990, 112, 4380–4386. [Google Scholar] [CrossRef]
- Hamilton, B.; Hurbungs, M.; Vernoux, J.P.; Jones, A.; Lewis, R.J. Isolation and characterisation of Indian Ocean ciguatoxin. Toxicon 2002, 40, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, B.; Hurbungs, M.; Jones, A.; Lewis, R.J. Multiple ciguatoxins present in Indian Ocean reef fish. Toxicon 2002, 40, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J.; Holmes, M.J. Origin and transfer of toxins involved in ciguatera. Comp. Biochem. Physiol. 1993, 106, 615–628. [Google Scholar] [CrossRef]
- Satake, M.; Ishibashi, Y.; Legrand, A.-M.; Yasumoto, T. Isolation and Structure of Ciguatoxin-4A, a New Ciguatoxin Precursor, from Cultures of Dinoflagellate Gambierdiscus toxicus and Parrotfish Scarus gibbus. Biosci. Biotech. Biochem. 1997, 60, 2103–2105. [Google Scholar] [CrossRef]
- Ikehara, T.; Kuniyoshi, K.; Oshiro, N.; Yasumoto, T. Biooxidation of Ciguatoxins Leads to Species-Specific Toxin Profiles. Toxins 2017, 9, 205. [Google Scholar] [CrossRef]
- Yasumoto, T. The Chemistry and Biological Function of Natural Marine Toxins. Chem. Rec. 2001, 1, 228–242. [Google Scholar] [CrossRef]
- Holmes, M.J.; Lewis, R.J. Reviewing Evidence for Disturbance to Coral Reefs Increasing the Risk of Ciguatera. Toxins 2025, 17, 195. [Google Scholar] [CrossRef]
- Loeffler, C.R.; Spielmeyer, A.; Blaschke, V.; Bodi, D.; Kappenstein, O. Ciguatera poisoning in Europe: A traceback to Indian Ocean sourced snapper fish (Lutjanus bohar). Food Control 2023, 151, 109799. [Google Scholar] [CrossRef]
- Barreiro-Crespo, L.; Sanchez-Henao, A.; Gimeno-Monforte, S.; Reverté, J.; Campàs, M.; Tsumuraya, T.; Diogène, J.; Tunin-Ley, A.; Maillot, F.; Flores, C.; et al. Toxicity and toxin profile of La Réunion (Indian Ocean) fish containing CTX-like compounds. Harmful Algae 2025, 147, 102882. [Google Scholar] [CrossRef]
- Li, X.; Lew, K.; Leyau, Y.L.; Shen, P.; Chua, J.; Lin, K.J.; Wu, Y.; Chan, S.H. Application of high-resolution mass spectrometry for ciguatoxin detection in fish from the Asia–Pacific Region. Toxins 2025, 17, 100. [Google Scholar] [CrossRef]
- Watson, R.A.; Green, B.S.; Tracey, S.R.; Farmery, A.; Pitcher, T.J. Provenance of global seafood. Fish Fish. 2016, 17, 585–595. [Google Scholar] [CrossRef]
- Loeffler, C.R.; Spielmeyer, A.; Friedemann, M.; Kapp, K.; Schwank, U.; Kappenstein, O.; Bodi, D. Food safety risk in Germany from mislabeled imported fish: Ciguatera outbreak trace-back, toxin elucidation, and public health implications. Front. Mar. Sci. 2022, 9, 849857. [Google Scholar] [CrossRef]
- Lewis, R.J.; Sellin, R.J. Multiple ciguatoxins in the flesh of fish. Toxicon 1992, 30, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.E.; Lewis, R.J.; Taylor, J.M. Pacific ciguatoxin-1 associated with a large common-source outbreak of ciguatera in East Arnhem Land, Australia. Nat. Toxins 1997, 5, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, B.; Whittle, N.; Shaw, G.; Eaglesham, G.; Moore, M.M.; Lewis, R.J. Human fatality associated with Pacific ciguatoxin contaminated fish. Toxicon 2010, 56, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Kohli, G.S.; Haslauer, K.; Sarowar, C.; Kretzschmar, A.L.; Boulter, M.; Harwood, D.T.; Laczka, O.; Murray, S.A. Qualitative and quantitative assessment of the presence of ciguatoxin, P-CTX-1B, in Spanish Mackerel (Scomberomorus commerson) from waters in New South Wales (Australia). Toxicol. Rep. 2017, 4, 328–334. [Google Scholar] [CrossRef]
- Holmes, M.J.; Lewis, R.J. Origin of ciguateric fish: Quantitative modelling of the flow of ciguatoxin through a marine food chain. Toxins 2022, 14, 534. [Google Scholar] [CrossRef]
- Holmes, M.J.; Lewis, R.J. Model of the origin of a ciguatoxic grouper (Plectropomus leopardus). Toxins 2023, 15, 230. [Google Scholar] [CrossRef]
- USFDA Natural Toxins. Fish and Fishery Products Hazards and Control Guidance, 4th ed.; U.S. Department of Health and Human Services, Food and Drug Administration, Center for Food Safety and Applied Nutrition, Table A5–11: White Oak, MD, USA, 2021. Available online: https://www.fda.gov/media/80400/download (accessed on 14 December 2022).
- Parsons, M.L.; Richlen, M.L.; Smith, T.B.; Anderson, D.M.; Abram, A.L.; Erdner, D.L.; Robertson, A. CiguaMOD I: A conceptual model of ciguatoxin loading in the Greater Caribbean Region. Harmful Algae 2024, 131, 102561. [Google Scholar] [CrossRef] [PubMed]
- Cheal, A.; Emslie, M.; Miller, I.; Sweatman, H. The distribution of herbivorous fishes on the Great Barrier Reef. Mar. Biol. 2012, 159, 1143–1154. [Google Scholar] [CrossRef]
- Bellwood, D.R. Direct estimate of bioerosion by two parrotfish species, Chlorurus gibbus and C. sordidus, on the Great Barrier Reef, Australia. Mar. Biol. 1995, 121, 421–429. [Google Scholar] [CrossRef]
- Bonaldo, R.M.; Bellwood, D.R. Size-dependent variation in the functional role of the parrotfish Scarus rivulatus on the Great Barrier Reef, Australia. Mar. Ecol. Prog. Ser. 2008, 360, 237–244. [Google Scholar] [CrossRef]
- Welsh, J.Q.; Bellwood, D.R. How far do roving schools of herbivores rove? A case study using Scarus rivulatus. Coral Reefs 2012, 31, 991–1003. [Google Scholar] [CrossRef]
- Clements, K.D.; German, D.P.; Piche, J.; Tribollet, A.; Choat, J.H. Integrating ecological roles and trophic diversification on coral reefs: Multiple lines of evidence identify parrotfishes as microphages. Biol. J. Linn. Soc. 2017, 120, 729–751. [Google Scholar] [CrossRef]
- Adam, T.C.; Duran, A.; Fuchs, C.E.; Roycroft, M.V.; Rojas, M.C.; Ruttenberg, B.I.; Burkepile, D.E. Comparative analysis of foraging behavior and bite mechanics reveals complex functional diversity among Caribbean parrotfishes. Mar. Ecol. Prog. Ser. 2018, 597, 207–220. [Google Scholar] [CrossRef]
- Lange, I.D.; Perry, C.T.; Morgan, K.M.; Roche, R.; Benkwitt, C.; Graham, N.A.J. Site-Level Variation in Parrotfish Grazing and Bioerosion as a Function of Species-Specific Feeding Metrics. Diversity 2020, 12, 379. [Google Scholar] [CrossRef]
- Nicholson, G.M.; Clements, K.D. Resolving resource partitioning in parrotfishes (Scarini) using microhistology of feeding substrata. Coral Reefs 2020, 39, 1313–1327. [Google Scholar] [CrossRef]
- Nicholson, G.M.; Clements, K.D. Fine-scale analysis of substrata grazed by parrotfishes (Labridae:Scarini) on the outer-shelf of the Great Barrier Reef, Australia. Mar. Biol. 2023, 170, 121. [Google Scholar] [CrossRef]
- Houk, P.; Rhodes, K.; Cuetos-Bueno, J.; Lindfield, S.; Fread, V.; McIlwain, J.L. Commercial coral-reef fisheries across Micronesia: A need for improving management. Coral Reefs 2012, 31, 13–26. [Google Scholar] [CrossRef]
- Steneck, R.S.; Mumby, P.J.; MacDonald, C.; Rasher, D.B.; Stoyle, G. Attenuating effects of ecosystem management on coral reefs. Sci. Adv. 2018, 4, eaao5493. [Google Scholar] [CrossRef]
- Rassweiler, A.; Lauer, M.; Lester, S.E.; Holbrook, S.J.; Schmitt, R.J.; Moussa, R.M.; Munsterman, K.S.; Lenihan, H.S.; Brooks, A.J.; Wencélius, J.; et al. Perceptions and responses of Pacific Island fishers to changing coral reefs. Ambio 2020, 49, 130–143. [Google Scholar] [CrossRef]
- Taylor, B.M.; Duenas, A.E.K.; Lange, I.D. Decadal changes in parrotfish assemblages around reefs of Guam, Micronesia. Coral Reefs 2022, 41, 1693–1703. [Google Scholar] [CrossRef]
- Taylor, B.M.; Prince, J.; Mutz, S.; Pardee, C.; Wiley, J.; Robertson, D.R.; Choat, J.H. A widespread, consistent, and perplexing biphasic pattern in log catch-at-age data from a widely harvested family of tropical reef fishes. Fish Fish. 2024, 25, 910–917. [Google Scholar] [CrossRef]
- Cheal, A.J.; MacNeil, M.A.; Cripps, E.; Emslie, M.J.; Jonker, M.; Schaffelke, B.; Sweatman, H. Coral–macroalgal phase shifts or reef resilience: Links with diversity and functional roles of herbivorous fishes on the Great Barrier Reef. Coral Reefs 2010, 29, 1005–1015. [Google Scholar] [CrossRef]
- Webley, J.; McInnes, K.; Teixeira, D.; Lawson, A.; Quinn, R. Statewide Recreational Fishing Survey 2013–2014; Queensland Government Report: Brisbane, Australia, 2015; p. 127. Available online: https://era.dpi.qld.gov.au/id/eprint/6513/ (accessed on 1 April 2025).
- Jupiter, S.D.; Weeks, R.; Jenkins, A.P.; Egli, D.P.; Cakacaka, A. Effects of a single intensive harvest event on fish populations inside a customary marine closure. Coral Reefs 2012, 31, 321–334. [Google Scholar] [CrossRef]
- Teixeira, D.; Janes, R.; Webley, J. 2019/20 Statewide Recreational Fishing Survey Key Results; Project Report; Fisheries Queensland: Brisbane, Australia, 2021; p. 18. Available online: https://era.dpi.qld.gov.au/id/eprint/7879/ (accessed on 1 April 2025).
- QFish. Available online: http://qfish.fisheries.qld.gov.au/ (accessed on 24 April 2025).
- Perkins, J.C.; Zenger, K.R.; Liu, Y.; Strugnell, J.M. Ciguatera poisoning: A review of the ecology and detection methods for Gambierdiscus and Fukuyoa species. Harmful Algae 2024, 139, 102735. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.; Gregory, J. An outbreak of ciguatera fish poisoning in Victoria. Commun. Dis. Intell. 2000, 24, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Chungue, E.; Bagnis, R.; Fusetani, N.; Hashimoto, Y. Isolation of two toxins from a parrotfish Scarus gibbus. Toxicon 1977, 15, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, T.; Nakajima, I.; Chungue, E.; Bagnis, R. Toxins in the gut contents of parrotfish. Bull. Jap. Soc. Sci. Fish. 1977, 43, 69–74. [Google Scholar] [CrossRef]
- Nicholson, G.M.; Clements, K.D. Ecomorphological divergence and trophic resource partitioning in 15 syntopic Indo-Pacific parrotfishes (Labridae: Scarini). Biol. J. Linn. Soc. 2021, 132, 590–611. [Google Scholar] [CrossRef]
- Bray, D.J.; Fishes of Australia. Introduction to Australia’s Fishes. In Fishes of Australia; Bray, D.J., Gomon, M.F., Eds.; Museums Victoria and OzFishNet: Melbourne, Australia, 2018; Available online: https://fishesofaustralia.net.au/ (accessed on 28 April 2025).
- Marshell, A.; Mumby, P.J. The role of surgeonfish (Acanthuridae) in maintaining algal turf biomass on coral reefs. J. Exp. Mar. Biol. Ecol. 2015, 473, 152–160. [Google Scholar] [CrossRef]
- Robinson, J.P.W.; McDevitt-Irwin, J.M.; Dajka, C.-K.; Hadj-Hammou, J.; Howlett, S.; Graba-Landry, A.; Hoey, A.S.; Nash, K.L.; Wilson, S.K.; Graham, N.A.J. Habitat and fishing control grazing potential on coral reefs. Funct. Ecol. 2019, 34, 240–251. [Google Scholar] [CrossRef]
- Tebbett, S.B.; Bellwood, D.R. Algal turf productivity on coral reefs: A meta-analysis. Mar. Environ. Res. 2021, 168, 105311. [Google Scholar] [CrossRef] [PubMed]
- Darius, H.T.; Revel, T.; Viallon, J.; Sibat, M.; Cruchet, P.; Longo, S.; Hardison, D.R.; Holland, W.C.; Tester, P.A.; Litaker, R.W.; et al. Comparative study on the performance of three detection methods for the quantification of Pacific ciguatoxins in French Polynesian strains of Gambierdiscus polynesiensis. Mar. Drugs 2022, 20, 348. [Google Scholar] [CrossRef] [PubMed]
- Sibat, M.; Mai, T.; Chomérat, N.; Bilien, G.; Lhaute, K.; Hess, P.; Séchet, V.; Jauffraus, T. Gambierdiscus polynesiensis from New Caledonia (South West Pacific Ocean): Morpho-molecular characterization, toxin profile and response to light intensity. Harmful Algae 2025, 145, 102859. [Google Scholar] [CrossRef] [PubMed]
- Tester, P.A.; Litaker, R.W.; Soler-Onís, E.; Fernández-Zabala, J.; Berdalet, E. Using artificial substrates to quantify Gambierdiscus and other toxic benthic dinoflagellates for monitoring purposes. Harmful Algae 2022, 120, 102351. [Google Scholar] [CrossRef]
- Richlen, M.L.; Horn, K.; Uva, V.; Fachon, E.; Heidmann, S.L.; Smith, T.B.; Parsons, M.L.; Anderson, D.M. Gambierdiscus species diversity and community structure in St. Thomas, USVI and the Florida Keys, USA. Harmful Algae 2024, 131, 102562. [Google Scholar] [CrossRef]
- Ledreux, A.; Brand, H.; Chinain, M.; Dechraoui Bottein, M.-Y.; Ramsdell, J.S. Dynamics of ciguatoxins from Gambierdiscus polynesiensis in the benthic herbivore Mugil cephalus: Trophic transfer implications. Harmful Algae 2014, 39, 165–174. [Google Scholar] [CrossRef]
- Clausing, R.J.; Losen, B.; Oberhaensli, F.R.; Taiana Darius, H.; Sibat, M.; Hess, P.; Swarzenski, P.A.; Chinain, M.; Dechraoui Bottein, M.-Y. Experimental evidence of dietary ciguatoxin accumulation in an herbivorous coral reef fish. Aquat. Toxicol. 2018, 200, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Clausing, R.J.; Ben Gharbia, H.; Sdiri, K.; Sibat, M.; Ranada-Mestizo, M.L.; Lavenu, L.; Hess, P.; Chinain, M.; Bottein, M.-Y.D. Tissue Distribution and Metabolization of Ciguatoxins in an Herbivorous Fish following Experimental Dietary Exposure to Gambierdiscus polynesiensis. Mar. Drugs 2024, 22, 14. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mak, Y.L.; Chang, Y.-H.; Xiao, C.; Chen, Y.-M.; Shen, J.; Wang, Q.; Ruan, Y.; Lam, P.K.S. Uptake and Depuration Kinetics of Pacific Ciguatoxins in Orange-Spotted Grouper (Epinephelus coioides). Environ. Sci. Technol. 2020, 54, 4475–4483. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.T.; Robertson, A. Depuration Kinetics and Growth Dilution of Caribbean Ciguatoxin in the Omnivore Lagodon rhomboides: Implications for Trophic Transfer and Ciguatera Risk. Toxins 2021, 13, 774. [Google Scholar] [CrossRef]
- Randall, J.E. A review of ciguatera, a tropical fish poisoning, with a tentative explanation of its cause. Bull. Mar. Sci. 1958, 8, 236–267. [Google Scholar]
- Meyer, L.; Capper, A.; Carter, S.; Simpfendorfer, C. An investigation into ciguatoxin bioaccumulation in sharks. Toxicon 2016, 119, 234–243. [Google Scholar] [CrossRef]
- Dao, H.V.; Le, H.H.K.; Pham, K.X.; Phan, V.B.; Nguyen, A.P.; Doan, T.T.; Nguyen, X.-V.; Nguyen, N.-T.N.; Nguyen, X.-T.T.; Nguyen, T.N.; et al. Pacific Ciguatoxin-1 (P-CTX-1) in a Moray eel (Gymnothorax javanicus) Responsible for Ciguatera in Khanh Hoa Province, Viet Nam. Toxins 2025, 17, 186. [Google Scholar] [CrossRef]
- Drouillard, K.G. Biomagnification. In Encyclopedia of Ecology; Jørgensen, S.E., Fath, B.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 441–448. [Google Scholar]
- Gill, A.B. The dynamics of prey choice in fish: The importance of prey size and satiation. J. Fish Biol. 2003, 63, 105–116. [Google Scholar] [CrossRef]
- St John, J. Ontogenetic changes in the diet of the coral reef grouper Plectropomus leopardus (Serranidae): Patterns in taxa, size and habitat of prey. Mar. Ecol. Prog. Ser. 1999, 180, 233–246. [Google Scholar] [CrossRef]
- Mumby, P.J.; Dahlgren, C.P.; Harborne, A.R.; Kappel, C.V.; Micheli, F.; Brumbaugh, D.R.; Holmes, K.E.; Mendes, J.M.; Broad, K.; Sanchirico, J.N.; et al. Fishing, Trophic Cascades, and the Process of Grazing on Coral Reefs. Science 2006, 311, 98–101. [Google Scholar] [CrossRef]
- Garcia, T.D.; Strictar, L.; Fugi, R.; Vidotto-Magnoni, A.P. Does size matter? Exploring the influence of body size on predator–prey relationships, hunting mode and prey characteristics in Neotropical fishes. Ecol. Freshw. Fish. 2025, 34, e12803. [Google Scholar] [CrossRef]
- Magnelia, S.J.; Kohler, C.C.; Tindall, D.R. Acanthurids do not avoid consuming cultured toxic dinoflagellates yet do not become ciguatoxic. Trans. Am. Fish. Soc. 1992, 121, 737–745. [Google Scholar] [CrossRef]
- Nukina, M.; Koyanagi, L.M.; Scheur, P.J. Two interchangeable forms of ciguatoxin. Toxicon 1984, 22, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J.; Sellin, M.; Poli, M.A.; Norton, R.S.; Macleod, J.K.; Sheil, M.M. Purification and characterization of ciguatoxins from moray eel (Lycodontis javanicus, Muraenidae). Toxicon 1991, 29, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J.; Sellin, M.; Street, R.; Holmes, M.J.; Gillespie, N.C. Excretion of ciguatoxin from Moray eels (Muraenidae) of the central Pacific. In Proceedings of the Third International Conference on Ciguatera Fish Poisoning, La Parguera, Puerto Rico, 30 April–5 May 1990; Tosteson, T.R., Ed.; Polyscience Publications: Quebec City, QC, Canada, 1992; pp. 131–143. [Google Scholar]
- Mak, Y.L.; Wai, T.-C.; Murphy, M.B.; Chan, W.H.; Wu, J.J.; Lam, J.C.W.; Chan, L.L.; Lam, P.K.S. Pacific ciguatoxins in food web components of coral reef systems in the Republic of Kiribati. Environ. Sci. Technol. 2013, 47, 14070–14079. [Google Scholar] [CrossRef]
- Chan, W.H.; Mak, Y.L.; Wu, J.J.; Jin, L.; Sit, W.H.; Lam, J.C.W.; de Mitcheson, Y.S.; Chan, L.L.; Lam, P.K.S.; Murphy, M.B. Spatial distribution of ciguateric fish in the Republic of Kiribati. Chemosphere 2011, 84, 117–123. [Google Scholar] [CrossRef]
- Chan, T.Y.K. Regional Variations in the Risk and Severity of Ciguatera Caused by Eating Moray Eels. Toxins 2017, 9, 201. [Google Scholar] [CrossRef]
- Dao, H.V.; Le, H.H.K.; Le, T.T.T.; Pham, K.X.; Bui, M.Q.; Chan, L.L. Ciguatoxin in moray eels raising the risk for seafood safety in Viet Nam. Fish. Sci. 2022, 88, 821–830. [Google Scholar] [CrossRef]
- Helfman, G.S.; Clark, J.B. Rotational Feeding: Overcoming Gape-Limited Foraging in Anguillid Eels. Copeia 1986, 3, 679–685. [Google Scholar] [CrossRef]
- Darius, H.T.; Ponton, D.; Revel, T.; Cruchet, P.; Ung, A.; Tchou Fouca, M.; Chinain, M. Ciguatera risk assessment in two toxic sites of French Polynesia using the receptor-binding assay. Toxicon 2007, 50, 612–626. [Google Scholar] [CrossRef]
- Darius, H.T.; Paillon, C.; Mou-Tham, G.; Ung, A.; Cruchet, P.; Revel, T.; Viallon, J.; Vigliola, L.; Ponton, D.; Chinain, M. Evaluating Age and Growth Relationship to Ciguatoxicity in Five Coral Reef Fish Species from French Polynesia. Mar. Drugs 2022, 20, 251. [Google Scholar] [CrossRef]
- Bienfang, P.; DeFelice, S.; Laws, E.; Wallsgrove, N.; Caldwell, P. Ciguatoxicity in the main Hawaiian Islands: Spatial and temporal variability in the introduced reef carnivore Cephalopholis argus. J. Res. Environ. Sci. Toxicol. 2012, 1, 47–57. [Google Scholar]
- Caillaud, A.; Eixarch, H.; de la Iglesia, P.; Rodriguez, M.; Dominguez, L.; Andree, K.B.; Diogène, J. Towards the standardisation of the neuroblastoma (neuro-2a) cell-based assay for ciguatoxin-like toxicity detection in fish: Application to fish caught in the Canary Islands. Food Addit. Contam. 2012, 29, 1000–1010. [Google Scholar] [CrossRef]
- O’Toole, A.C.; Dechraoui Bottein, M.-Y.; Danylchuk, A.J.; Ramsdell, J.S.; Cooke, S.J. Linking ciguatera poisoning to spatial ecology of fish: A novel approach to examining the distribution of biotoxin levels in the great barracuda by combining non-lethal blood sampling and biotelemetry. Sci. Total Environ. 2012, 427–428, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Gaboriau, M.; Ponton, D.; Taiana Darius, H.; Chinain, M. Ciguatera fish toxicity in French Polynesia: Size does not always matter. Toxicon 2014, 84, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Soliño, L.; Widgy, S.; Pautonnier, A.; Turquet, J.; Loeffler, C.R.; Flores Quintana, H.A.; Diogène, J. Prevalence of ciguatoxins in lionfish (Pterois spp.) from Guadeloupe, Saint Martin, and Saint Barthélmy Islands (Caribbean). Toxicon 2015, 102, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Henao, A.; García-Álvarez, N.; Sergent, F.S.; Estévez, P.; Gago-Martínez, A.; Martín, F.; Ramos-Sosa, M.; Fernández, A.; Diogène, J.; Real, F. Presence of CTXs in moray eels and dusky groupers in the marine environment of the Canary Islands. Aquat. Toxicol. 2020, 221, 105427. [Google Scholar] [CrossRef]
- Zhu, J.; Lee, W.-H.; Wu, J.; Zhou, S.; Yip, K.-C.; Liu, X.; Kirata, T.; Chan, L.-L. The Occurrence, Distribution, and Toxicity of High-Risk Ciguatera Fish Species (Grouper and Snapper) in Kiritimati Island and Marakei Island of the Republic of Kiribati. Toxins 2022, 14, 208. [Google Scholar] [CrossRef]
- Chinain, M.; Howell, C.G.; Roué, M.; Ung, A.; Henry, K.; Revel, T.; Cruchet, P.; Viallon, J.; Darius, H.T. Ciguatera poisoning in French Polynesia: A review of the distribution and toxicity of Gambierdiscus spp., and related impacts on food web components and human health. Harmful Algae 2023, 129, 102525. [Google Scholar] [CrossRef]
- Clua, E.; Brena, P.F.; Lecasble, C.; Ghnassia, R.; Chauvet, C. Prevalence and proposal for cost-effective management of the ciguatera risk in the Noumea fish market, New Caledonia (South Pacific). Toxicon 2011, 58, 591–601. [Google Scholar] [CrossRef]
- Sydney Fish Market. Seafood Handling Guidelines, 5th ed.; Locked Bag 247; Sydney Fish Market Pty Ltd.: Pyrmont, Australia, 2015; pp. 35–36. [Google Scholar]
- Sanchez-Henao, J.A.; García-Álvarez, N.; Fernández, A.; Saavedra, P.; Silva Sergent, F.; Padilla, D.; Acosta-Hernández, B.; Martel Suárez, M.; Diogène, J.; Real, F. Predictive score and probability of CTX-like toxicity in fish samples from the official control of ciguatera in the Canary Islands. Sci. Total Environ. 2019, 673, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.R.; Churro, C.; Rodrigues, S.M.; Frazão, B.; Barbosa, M.; Godinho, L.; Soliño, L.; Timóteo, V.; Gouveia, N. A 15-Year Retrospective Review of Ciguatera in the Madeira Islands (North-East Atlantic, Portugal). Toxins 2023, 15, 630. [Google Scholar] [CrossRef] [PubMed]
- Gwinn, D.C.; Allen, M.S.; Johnston, F.D.; Brown, P.; Todd, C.R.; Arlinghaus, R. Rethinking length-based fisheries regulations: The value of protecting old and large fish with harvest slots. Fish Fish. 2013, 16, 259–281. [Google Scholar] [CrossRef]
- Amin, A.M.; El-Ganainy, A.A.; Sabrah, M.M. Length-Weight Relationships of Thirteen Species of Parrotfish (Family Scaridae) inhabiting the Egyptian coasts of the Red Sea. Egypt. J. Aquat. Biol. Fish. 2019, 23, 357–366. [Google Scholar] [CrossRef]
- Green, A.L.; Maypa, A.P.; Almany, G.R.; Rhodes, K.L.; Weeks, R.; Abesamis, R.A.; Gleason, M.G.; Mumby, P.J.; White, A.T. Larval dispersal and movement patterns of coral reef fishes, and implications for marine reserve network design. Biol. Rev. 2015, 90, 1215–1247. [Google Scholar] [CrossRef]
- Johnson, G.B.; Taylor, B.M.; Robbins, W.D.; Franklin, E.C.; Toonen, R.; Bowen, B.; Choat, J.H. Diversity and Structure of Parrotfish Assemblages across the Northern Great Barrier Reef. Diversity 2019, 11, 14. [Google Scholar] [CrossRef]
- Almany, G.R. Priority Effects in Coral Reef Fish Communities of the Great Barrier Reef. Ecology 2004, 85, 2872–2880. [Google Scholar] [CrossRef]
- Almany, G.R.; Webster, M.S. The predation gauntlet: Early post-settlement mortality in reef fishes. Coral Reefs 2006, 25, 19–22. [Google Scholar] [CrossRef]
- Chen, L.-S. Post-settlement diet shift of Chlorurus sordidus and Scarus schlegeli (Pisces: Scaridae). Zool. Stud. 2002, 41, 47–58. [Google Scholar]
- Kingsford, M.J. Spatial and temporal variation in predation on reef fishes by coral trout (Plectropomus leopardus, Serranidae). Coral Reefs 1992, 11, 193–198. [Google Scholar] [CrossRef]
- St John, J. Temporal variation in the diet of a coral reef piscivore (Pisces: Serranidae) was not seasonal. Coral Reefs 2001, 20, 163–170. [Google Scholar] [CrossRef]
- Frisch, A.J.; Cameron, D.S.; Pratchett, M.S.; Williamson, D.H.; Williams, A.J.; Reynolds, A.D.; Hoey, A.S.; Rizzari, J.R.; Evans, L.; Kerrigan, B.; et al. Key aspects of the biology, fisheries and management of Coral grouper. Rev. Fish Biol. Fish. 2016, 26, 303–325. [Google Scholar] [CrossRef]
- Craig, P.C.; Choat, J.H.; Axe, L.M.; Saucerman, S. Population biology and harvest of the coral reef surgeonfish Acanthurus lineatus in American Samoa. Fish. Bull. 1997, 95, 680–893. [Google Scholar]
- Doherty, P.J.; Dufour, V.; Galzin, R.; Hixon, M.A.; Meekan, M.G.; Planes, S. High Mortality during Settlement Is a Population Bottleneck for a Tropical Surgeonfish. Ecology 2004, 85, 2422–2428. [Google Scholar] [CrossRef]
- Trip, E.D.L.; Craig, P.; Green, A.; Choat, J.H. Recruitment dynamics and first year growth of the coral reef surgeonfish Ctenochaetus striatus, with implications for acanthurid growth models. Coral Reefs 2014, 33, 879–889. [Google Scholar] [CrossRef]
- Myers, R.F. Guam’s Small-Boat-based Fisheries. Mar. Fish. Rev. 1993, 55, 117–128. [Google Scholar]
- Ebisawa, A.; Ohta, I.; Uehara, M.; Nakamura, H.; Kanashiro, K.; Yasui, R. Life history variables, annual change in sex ratios with age, and total mortality observed on commercial catch on Pacific steephead parrotfish, Chlorurus microrhinos in waters off the Okinawa Island, southwestern Japan. Reg. Stud. Mar. Sci. 2016, 8, 65–76. [Google Scholar] [CrossRef]
- Cook, D.T.; Schmitt, R.J.; Holbrook, S.J.; Moeller, H.V. Modeling the effects of selectively fishing key functional groups of herbivores on coral resilience. Ecosphere 2024, 15, e4749. [Google Scholar] [CrossRef]
- FishBase. FishBase. World Wide Web Electronic Publication. Version (02/2025). Froese, R.; Pauly, D. (Eds.) 2025. Available online: www.fishbase.org (accessed on 28 April 2025).
- Kamikawa, K.T.; Cruz, E.; Essington, T.E.; Hospital, J.; Brodziak, J.K.T.; Branch, T.A. Length-weight relationships for 85 species from Guam. J. Appl. Ichthyol. 2015, 1171–1174. [Google Scholar] [CrossRef]
- Tester, P.A.; Kibler, S.R.; Holland, W.C.; Usup, G.; Vandersea, M.W.; Leaw, C.P.; Teen, L.P.; Larsen, J.; Mohammad-Noor, N.; Faust, M.A.; et al. Sampling harmful benthic dinoflagellates: Comparison of artificial and natural substrate methods. Harmful Algae 2014, 39, 8–25. [Google Scholar] [CrossRef]
- Yong, H.L.; Mustapa, N.I.; Lee, L.K.; Lim, Z.F.; Tan, T.H.; Usup, G.; Guc, H.; Litaker, R.W.; Tester, P.A.; Lim, P.T.; et al. Habitat complexity affects benthic harmful dinoflagellate assemblages in the fringing reef of Rawa Island, Malaysia. Harmful Algae 2018, 78, 56–68. [Google Scholar] [CrossRef]
- Fernández-Zabala, J.; Amorim, A.; Tuya, F.; Herrera, R.; Soler-Onís, E. Playing hide and seek: Distribution with depth of potentially harmful epibenthic dinoflagellates of Southern El Hierro Island, Canary Islands (NE Atlantic). Harmful Algae 2022, 117, 102271. [Google Scholar] [CrossRef]
- Argyle, P.A.; Rhodes, L.L.; Smith, K.F.; Harwood, D.T.; Halafihi, T.; Marsden, I.D. Diversity and distribution of benthic dinoflagellates in Tonga include the potentially harmful genera Gambierdiscus and Fukuyoa. Harmful Algae 2023, 130, 102524. [Google Scholar] [CrossRef]
- Kassim, N.S.; Lee, L.K.; Hii, K.S.; Azmi, N.F.M.; Baharudin, N.S.; Liu, M.; Gu, H.; Lim, P.T.; Leaw, C.P. Molecular diversity of benthic harmful dinoflagellates on a tropical reef: Comparing natural and artificial substrate sampling methods using DNA metabarcoding and morphological analysis. Harmful Algae 2025, 142, 102795. [Google Scholar] [CrossRef]
- Nakahara, H.; Sakami, I.T.; Chinain, M.; Ishida, Y. The role of macroalgae in epiphytism of the toxic dinoflagellate Gambierdiscus toxicus (Dinophyceae). Phycol. Res. 1996, 44, 113–117. [Google Scholar] [CrossRef]
- Fabricius, K.E.; Metzner, J. Scleractinian walls of mouths: Predation on coral larvae by corals. Coral Reefs 2004, 23, 245–248. [Google Scholar] [CrossRef]
- Zhu, J.; Li, J.; Wu, J.; Liu, X.; Lin, Y.; Deng, H.; Qin, X.; Wong, M.H.; Chan, L.L. The prevalence of marine lipophilic phycotoxins causes potential risks in a tropical small island developing State. Environ. Sci. Technol. 2024, 58, 9815–9827. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.L.; Richlen, M.L.; Smith, T.B.; Solow, A.R.; Anderson, D.M. Evaluation of 24-h screen deployments as a standardized platform to monitor Gambierdiscus populations in the Florida Keys and U.S. Virgin Islands. Harmful Algae 2021, 103, 101998. [Google Scholar] [CrossRef]
- Fernández-Zabala, J.; Tuya, F.; Amorim, A.; Soler-Onís, E. Benthic dinoflagellates: Testing the reliability of the artificial substrate method in the Macaronesian region. Harmful Algae 2019, 87, 101634. [Google Scholar] [CrossRef]
- Parsons, M.L.; Brandt, A.L.; Ellsworth, A.; Leynse, A.K.; Rains, L.K.; Anderson, D.M. Assessing the use of artificial substrates to monitor Gambierdiscus populations in the Florida Keys. Harmful Algae 2017, 68, 52–66. [Google Scholar] [CrossRef]
- Cruz-Rivera, E.; Villareal, T.A. Macroalgal palatability and the flux of ciguatera toxins through marine food webs. Harmful Algae 2006, 5, 497–525. [Google Scholar] [CrossRef]
- Lee, L.K.; Lim, Z.F.; Gu, H.; Chan, L.L.; Litaker, R.W.; Tester, P.A.; Leaw, C.P.; Lim, P.T. Effects of substratum and depth on benthic harmful dinoflagellate assemblages. Sci. Rep. 2020, 10, 11251. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Lin, X.; Liu, X.; Hii, K.S.; Li, H.; Li, Y.; Xu, X.; Xiao, J.; Mohamed, H.F.; Zheng, X.; et al. Morphology, phylogeny, and toxicity of three Gambierdiscus species from the South China Sea, including a coral-killing bloom of G. carpenteri in reef tanks. Mar. Environ. Res. 2025, 206, 107031. [Google Scholar] [CrossRef] [PubMed]
- Chinain, M.; Darius, H.T.; Ung, A.; Cruchet, P.; Wang, Z.; Ponton, D.; Laurent, D.; Pauillac, S. Growth and toxin production in the ciguatera-causing dinoflagellate Gambierdiscus polynesiensis (Dinophyceae) in culture. Toxicon 2010, 56, 739–750. [Google Scholar] [CrossRef] [PubMed]

| Days Feeding on Turf Algae to Accumulate 0.1 μg P-CTX-1 eq./kg in Flesh of Parrotfish | ||||
|---|---|---|---|---|
| Gambierdiscus Densities on Turf Algae (Cells/cm2) | 25 cm (Total Length) Scarus niger, Medium-Bodied Scraper | 25 cm (Total Length) Chlorurus strongylocephalus, Large-Bodied Excavator | ||
| 0.6 pg P-CTX-4/Gambierdiscus | 1.6 pg P-CTX-4/Gambierdiscus | 0.6 pg P-CTX-4/Gambierdiscus | 1.6 pg P-CTX-4/Gambierdiscus | |
| 1 | 144–575 | 54–216 | 286–1143 | 107–429 |
| 10 | 14–58 | 5–22 | 29–114 | 11–43 |
| 100 | 1–6 | <1–2 | 3–11 | 1–4 |
| Scarus niger | Scarus tricolor | Scarus frenatus | |||||
|---|---|---|---|---|---|---|---|
| Fish | Total Length (cm) | Weight (g) | Area Grazed (m2/y) | Weight (g) | Area Grazed (m2/y) | Weight (g) | Area Grazed (m2/y) |
| A | 20 | 211 | 27 | 208 | 21 | 226 | 47 |
| B | 40 | 1805 | 129 | 1724 | 65 | 1934 | 257 |
| Ratio (B/A) | 2.0 | 8.6 | 4.8 | 8.3 | 3.1 | 8.6 | 5.5 |
| Chlorurus spp. (Max Total Length 70–80 cm) | |||
|---|---|---|---|
| Fish | Total Length (cm) | Weight (g) Chlorurus spp.—C. microrhinos | Area Grazed (m2/y) C. strongylocephalus |
| A | 30 | 654–676 | 45 |
| B | 60 | 5266–5633 | 215 |
| Ratio (B/A) | 2.0 | 8.1–8.3 | 4.8 |
| Days Feeding on Turf Algae Supporting Gambierdiscus Producing 0.6 pg P-CTX-4/Cell to Produce 0.1 μg P-CTX-1 eq./kg in Flesh of Scarus niger Parrotfish | |||
|---|---|---|---|
| Gambierdiscus Densities on Turf Algae (cells/cm2) | 10 cm (Total Length) | 20 cm (Total Length) | 40 cm (Total Length) |
| 1 | 87–348 | 127–509 | 186–745 |
| 10 | 9–35 | 13–51 | 19–75 |
| 100 | <1–4 | 1–5 | 2–7 |
| Gambierdiscus Densities on Turf Algae (cells/cm2) | 40 cm (Total Length) Scarus niger, a Large Individual for this Scraper Species (Muscle Weight Assumed to be 42% of Total Weight = 436 g) | 60 cm (Total Length) Chlorurus strongylocephalus, a Large Individual for This Excavator Species (Muscle Weight Assumed to be 42% of Total Weight = 2366 g) | ||
|---|---|---|---|---|
| 0.6 pg P-CTX-4/Gambierdiscus | 1.6 pg P-CTX-4/Gambierdiscus | 0.6 pg P-CTX-4/Gambierdiscus | 1.6 pg P-CTX-4/Gambierdiscus | |
| 1 | 0.01–0.04 | 0.03–0.10 | <0.01–0.01 | 0.01–0.03 |
| 10 | 0.09–0.38 | 0.28–1.11 | 0.03–0.12 | 0.08–0.32 |
| Parrotfish (S. niger) as Model Prey for Predatory Grouper | |||
|---|---|---|---|
| Parrotfish | A (Small) | B (Large) | Ratio |
| Parrotfish total length (cm) | 5 | 10 | 2 (B:A) |
| Weight (g) | 3.4 | 23 | 6.8 (B:A) |
| Total load (ng) of P-CTX-1 eq. ingested by a single parrotfish feeding on 0.6 pg P-CTX-4/cell for 30 days at 10 Gambierdiscus/cm2 | 0.78 | 3.6 | 4.6 (B:A) |
| P-CTX-1 eq. load (ng) for equivalent weight of fish (23 g) = ~6.8 fish of 5 cm total length | 5.3 | 3.6 | 1.5 (A:B) |
| Variable | Model Values | Calculations, Assumptions, and Comments |
|---|---|---|
| Model target for P-CTX-1 concentration in flesh of parrotfish | 0.1 μg P-CTX-1/kg | 0.1 µg P-CTX-1/kg fish would likely cause mild poisoning in 2 out of 10 people [2] and is 10-fold more than the US FDA-recommended limit of 0.01 μg P-CTX-1 equivalents (eq.)/kg. |
| Flesh (fillet) recovery from parrotfish | 42% | Median value of a range of meat recoveries for fillets (40–49%) taken from internet fishing sites for 5 species of Scarus spp. |
| Flesh (fillet) CTX burden | Range calculated as between 10 and 40% of the CTX load ingested by parrotfish | Flesh estimated to accumulate between 10 and 40% of the toxin load of the fish based upon Caribbean pinfish [70]. Clausing et al. [68] recently reported a slightly higher relative proportion of CTX retained in the muscle of surgeonfish (45%). |
| Parrotfish CTX load (μg) | Calculated depending upon fish weight (Table 9) | Based upon a 43% transfer rate (Table 8, [70]) and considering the P-CTX-4 analogs ingested (P-CTX-4A and -4B) are bio-converted to P-CTX-1 by the parrotfish. The ingested toxins are treated as P-CTX-1 eq. |
| Daily grazing rates (m2/d) for parrotfish on turf algae | Calculated from annual grazing rates (m2/y) depending upon species and fish total length (TL, cm) | Annual grazing rates (m2/y) calculated using equations derived by Lange et al. [40]: S. niger = 0.0367(TL2.2), S. tricolor = 0.1836(TL1.591), S. frenatus = 0.0138(TL2.439), C. strongylopcephalus = 0.0209(TL2.256) |
| The time parrotfish spend grazing on turf algae each day | 9 h | Parrotfish are diurnal feeders that spend >90% of daylight hours feeding [35,36,37] and 9 h is consistent with the daily feeding times we used previously for surgeonfish on the Great Barrier Reef [31]. We have modified the daily feeding from 12 h used by Lange et al. [40] for parrotfish feeding close to the equator in the Maldives and Chagos Archipelago. However, feeding duration likely varies throughout the day, between seasons and with latitude |
| The efficacy of the parrotfish bite to remove and ingest Gambierdiscus from turf algae | 90% | This rate is an assumption as there are no data available but is unlikely to be 100%. As scraping and excavator parrotfish are targeting microorganisms for nutrition [38,41,42,57] we assume the efficiency to be high |
| Variable | Model Values | Calculations, Assumptions, and Comments |
|---|---|---|
| The transfer rate for CTX between trophic level 1 and 2 | 43% | Based upon an average net CTX assimilation of 43% in pinfish [70]; also see Holmes and Lewis [30,31]. This term accounts for CTX losses between trophic levels. This transfer efficiency is similar to that reported for CTX from G. polynesiensis into mullet (42%, [66]). The actual transfer rates for the modelled species are not known |
| P-CTX-4 concentrations produced by Gambierdiscus consumed by parrotfish. These concentrations are varied depending upon the scenario being explored | 0.6 pg or 1.6 pg P-CTX-4/cell | P-CTX-4 concentrations are assumed to be composed of a mix of P-CTX-4A and -4B; 0.6 pg P-CTX-4/cell is the maximum known concentration produced by a strain of G. polynesiensis from French Polynesia [62]; 1.6 pg/cell is a hypothetical concentration based upon mouse bioassay of Gambierdiscus strains isolated from Platypus Bay and the Great Barrier Reef, Australia [30,31] |
| Gambierdiscus densities on turf algae | 0.1, 1, 10, 100, 1000 cells/cm2 | Hypothetical (possible) cell densities of CTX-producing Gambierdiscus based upon ranges reported from 24 h benthic screen assays ([64] and references therein). We are not aware of any reports of cell densities ≥ 1000 cells/cm2; ~1 cell/cm2 is the median of a global range on screen assays [64] |
| Scraping Species | Common/Local Name | Maximum Total Length 1 (cm) | Weight (g)–Total Length (TL, cm) Relationships | Reference for Weight–Length Relationships |
|---|---|---|---|---|
| Scarus niger | Swarthy parrotfish | 40 | Weight = 0.0411∙TL2.7481 | [103] |
| S. tricolor | Tricolor parrotfish | 40 | FishBase calculator based upon geometric mean of 2 studies | [118] |
| S. frenatus | Sixband parrotfish | 47 | Weight = 0.0366∙TL2.8162 | [103] |
| Excavator species | ||||
| Chlorurus microrhinos | Steephead parrotfish | 80 | Weight = 0.0174∙TL3.07 | [119] |
| C. strongylocephalus | Steephead parrotfish | 70 | FishBase calculator based upon geometric mean of 5 studies | [118] |
| Model Parameters for Two-Trophic-Level Food Chain | Description or Relevance | How Well Does the Model Parameter Simulate Reality? |
|---|---|---|
| Density of Gambierdiscus on turf algae | Actual densities not known but model explores an exponential range from 0.1 cells/cm2 | Good, because model considers exponential range of possible densities. However, model does not account for finer-scale spatial or temporal factors that influence growth |
| Gambierdiscus species composition on turf algae | Model based upon monospecific composition of Gambierdiscus on turf algae eaten by parrotfish | Variable, as data suggests that sites can host multiple species [65]. Our model simulates worst-case scenarios (monospecific toxic blooms). Although this overestimates toxin production from mixed species assemblages on turf algae, it is useful to model toxic cell densities. The model could easily be adjusted for mixtures of species |
| Gambierdiscus CTX production | Highest known concentration [0.6 pg P-CTX-4/cell, 62] and a higher hypothetical concentration (1.6 pg P-CTX-4/cell) | Data-dependent. However, model does not account for environmental factors that influence toxin production. Model does not account for variation in toxicity of cells ingested by parrotfish |
| Grazing rates for parrotfish | Species-specific rates used from the literature | Data-dependent. Based upon published rates for area grazed/y [40]. Model does not account for seasonality affecting grazing rates or for schooling behaviour that can also affect grazing rates [37] |
| Parrotfish grazing (h/day) | Estimated average, consistent with previous modelling for the Great Barrier Reef [31] | Model adjusted from 12 h grazing/day for parrotfish near equator [40] to 9 h/day. Our model does not account for seasonality and latitude. Our model would underestimate ingested CTX if fish were grazing for up to 12 h/day |
| Grazing efficiency | Accounts for losses of material not ingested from the bite. Assumed 90% | No data, but as the fish are targeting microorganisms for nutrition, we assume the efficacy is high |
| Transfer efficiency of CTX between trophic levels | 43% | Data-dependent, 43% [70]. Ledreux et al. [66] reported 42%. But rates for the species modelled not known |
| Bioconversion of P-CTX-4 to P-CTX-1 in parrotfish | Assumed 1:1 bioconversion from P-CTX-4 to P-CTX-1 to accumulate in muscle (fillets). We do not know or assume where in the fish that the bioconversion occurs. Conversion rates have relevance for the toxicity of the fillets consumed by people | Rates of bioconversion for P-CTX-4A and P-CTX-4B not known. Assuming a 1:1 bioconversion (P-CTX-1 eq.) our model likely overestimates the toxicity of the fillets |
| Biotransfer of CTX between parrotfish tissues, from gut to muscle. Model estimates between 10 and 40% of ingested load accumulates in muscle [70] | Losses occur with each toxin transfer, and it takes time for CTX to accumulate into muscle (fillets) [67,68,69] | Time for transfer between parrotfish tissues not known and not incorporated in model. Our model is based on an immediate transfer which could overestimate the toxicity of the fillets. Clausing et al. [68] reported 45% of CTX retained by muscle of surgeonfish. On this basis, our use of 10–40% could slightly underestimate the toxicity of muscle (fillets) |
| CTX load accumulated in parrotfish muscle (fillet) | Based upon CTX load ingested after losses during transfer and grazing efficiency | Worst-case scenario. On-going, possibly simultaneous rates of accumulation, bioconversion, and depuration not incorporated in model |
| Depuration of CTX from parrotfish muscle | Depuration is time-dependent so becomes more important the longer the duration explored in the model scenarios | Not included over the ~1 month we mostly limit model interpretation. Our model likely produces worst-case scenarios that overestimate the toxicity of muscle (fillets) because P-CTX-1, -2, and -3 have been suggested to depurate from groupers with half-life of ~1 month [69]. Additionally, only 26% of the P-CTX3C-load ingested was retained by surgeonfish after 4 months of feeding on G. polynesiensis [68], which suggests a faster depuration rate than Li et al. [69] |
| Estimating the Number of Gambierdiscus to Produce a Flesh Concentration of 0.1 μg P-CTX-1 eq./kg in a 25 cm Parrotfish (S. niger) | ||
|---|---|---|
| Calculating | Result of Calculation | Source/Reference for Calculation |
| Parrotfish weight for 25 cm fish | 285 g | Table 9 |
| Muscle (flesh) weight for 25 cm fish | 120 g | Table 7 |
| P-CTX load to produce a concentration of 0.1 μg P-CTX-1 eq./kg in 120 g flesh | 1.2 × 10−8 g | |
| P-CTX load for the fish based upon flesh having 10% to 40% of toxin | 3.0 × 10−8 to 1.2 × 10−7 g | Table 7 |
| Number of Gambierdiscus producing 0.6 pg P-CTX-1 eq./cell to produce 3.0 × 10−8 to 1.2 × 10−7 g P-CTX-1 eq. | 5.0 × 104 to 2.0 × 105 cells | |
| Number of Gambierdiscus producing 0.6 pg P-CTX-1 eq./cell to produce 3.0 × 10−8 to 1.2 × 10−7 g P-CTX-1 eq. incorporating an assimilation efficiency of 43% across trophic levels | 1.2 × 105 to 4.7 × 105 cells | Table 8 |
| Estimating minimum number of days of feeding by S. niger to ingest 1.2 × 105 to 4.7 × 105 Gambierdiscus | ||
| Area of turf algae scraped in 1 day (9 h) by parrotfish | 897.2 cm2 | Table 7 |
| Number of Gambierdiscus ingested/day from turf algae with 10 Gambierdiscus/cm2 | 8972 cells | |
| Number of days to ingest 1.2 × 105 to 4.7 × 105 cells | 12.9 to 51.8 days | |
| Number of days to ingest 1.2 × 105 to 4.7 × 105 cells adjusted for a 90% ingestion efficiency | 14.4 to 57.5 days | Table 7 |
| Model output | 14 to 58 days (see Table 1) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holmes, M.J.; Lewis, R.J. Modelling the Bioaccumulation of Ciguatoxins in Parrotfish on the Great Barrier Reef Reveals Why Biomagnification Is Not a Property of Ciguatoxin Food Chains. Toxins 2025, 17, 380. https://doi.org/10.3390/toxins17080380
Holmes MJ, Lewis RJ. Modelling the Bioaccumulation of Ciguatoxins in Parrotfish on the Great Barrier Reef Reveals Why Biomagnification Is Not a Property of Ciguatoxin Food Chains. Toxins. 2025; 17(8):380. https://doi.org/10.3390/toxins17080380
Chicago/Turabian StyleHolmes, Michael J., and Richard J. Lewis. 2025. "Modelling the Bioaccumulation of Ciguatoxins in Parrotfish on the Great Barrier Reef Reveals Why Biomagnification Is Not a Property of Ciguatoxin Food Chains" Toxins 17, no. 8: 380. https://doi.org/10.3390/toxins17080380
APA StyleHolmes, M. J., & Lewis, R. J. (2025). Modelling the Bioaccumulation of Ciguatoxins in Parrotfish on the Great Barrier Reef Reveals Why Biomagnification Is Not a Property of Ciguatoxin Food Chains. Toxins, 17(8), 380. https://doi.org/10.3390/toxins17080380




