Growth Dynamics and Toxin Production of Pseudo-nitzschia Species Isolated from the Central Adriatic Sea
Abstract
1. Introduction
2. Results
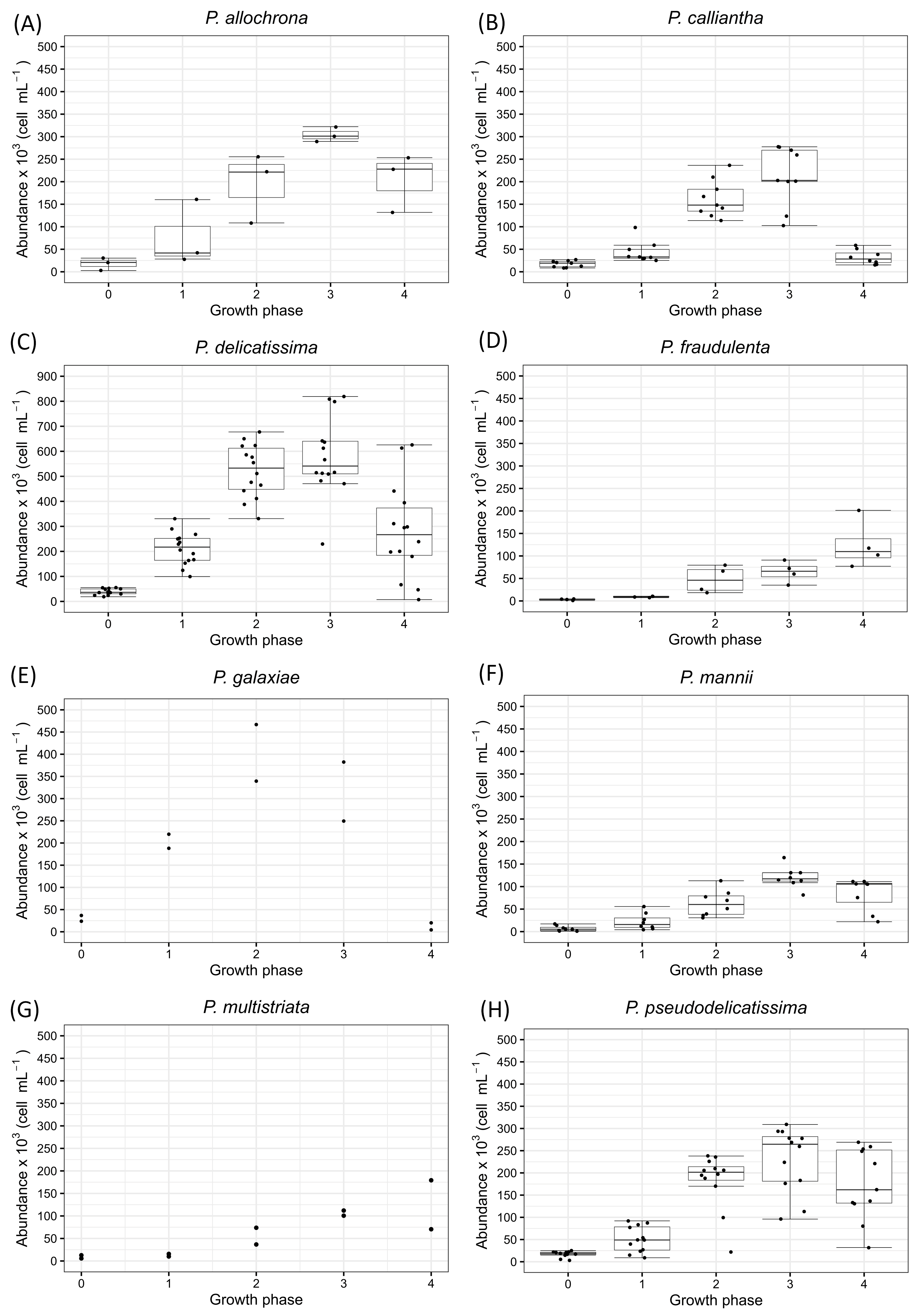
2.1. Pseudo-nitzschia allochrona
2.2. Pseudo-nitzschia calliantha
2.3. Pseudo-nitzschia delicatissima
2.4. Pseudo-nitzschia fraudulenta
2.5. Pseudo-nitzschia galaxiae
2.6. Pseudo-nitzschia mannii
2.7. Pseudo-nitzschia mutistriata
2.8. Pseudo-nitzschia pseudodelicatissima
3. Discussion
3.1. Abundance and Growth Rate
3.2. Toxicity
4. Conclusions
5. Materials and Methods
5.1. Cell Cultures
5.2. Experimental Setup
5.3. Cell Abundance and Growth Rate Determination
5.4. Toxin Analysis
5.5. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lelong, A.; Hégaret, H.; Soudant, P.; Bates, S.S. Pseudo-nitzschia (Bacillariophyceae) species, domoic acid and amnesic shellfish poisoning: Revisiting Previous Paradigms. Phycologia 2012, 51, 168–216. [Google Scholar] [CrossRef]
- Dong, H.C.; Lundholm, N.; Teng, S.T.; Li, A.; Wang, C.; Hu, Y.; Li, Y. Occurrence of Pseudo-nitzschia species and associated domoic acid production along the Guangdong Coast, South China Sea. Harmful Algae 2020, 98, 101899. [Google Scholar] [CrossRef]
- Lundholm, N.; Bernard, C.; Churro, C.; Escalera, L.; Hoppenrath, M.; Iwataki, M.; Larsen, J.; Mertens, K.; Moestrup, Ø.; Murray, S.; et al. (Eds.) (2009 Onwards). IOC-UNESCO Taxonomic Reference List of Harmful Micro Algae. Available online: https://www.marinespecies.org/hab (accessed on 2 March 2025).
- Lundholm, N.; Christensen, A.L.; Olesen, A.K.J.; Beszteri, B.; Eggers, S.L.; Krock, B.; Altenburger, A. Diversity, toxicity, and distribution of potentially toxic diatoms in Antarctic waters––with description of Pseudo-nitzschia meridionalis sp. nov. and P. glacialis sp. nov. Harmful Algae 2024, 139, 102724. [Google Scholar] [CrossRef]
- von Dassow, P.; Mikhno, M.; Percopo, I.; Orellana, V.R.; Aguilera, V.; Álvarez, G.; Araya, M.; Cornejo-Guzmán, S.; Llona, T.; Mardones, J.I.; et al. Diversity and toxicity of the planktonic diatom genus Pseudo-nitzschia from coastal and offshore waters of the Southeast Pacific, including Pseudo-nitzschia dampieri sp. nov. Harmful Algae 2023, 130, 102520. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, University of Galway. 2025. Available online: https://www.algaebase.org (accessed on 2 March 2025).
- Teitelbaum, J.S.; Zatorre, R.J.; Carpenter, S.; Gendron, D.; Evans, A.C.; Gjedde, A.; Cashman, N.R. Neurologic sequelae of domoic acid intoxication due to the ingestion of contaminated mussels. N. Engl. J. Med. 1990, 322, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Trainer, V.L.; Bates, S.S.; Lundholm, N.; Thessen, A.E.; Cochlan, W.P.; Adams, N.G.; Trick, C.G. Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae 2012, 14, 271–300. [Google Scholar] [CrossRef]
- Lefebvre Kathi, A.; Dovel Shonna, L.; Silver Mary, W. Tissue distribution and neurotoxic effects of domoic acid in a prominent vector species, the northern anchovy Engraulis mordax. Mar. Biol. 2001, 138, 693–700. [Google Scholar] [CrossRef]
- Wright, J.L.C.; Boyd, R.K.; de Freitas, A.S.W.; Falk, M.; Foxall, R.A.; Jamieson, W.D.; Laycock, M.V.; McCulloch, A.W.; McInnes, A.G.; Odense, P.; et al. Identification of domoic acid, a neuroexcitatory amino acid, in toxic mussels from Eastern Prince Edward Island. Can. J. Chem. 1989, 67, 481–490. [Google Scholar] [CrossRef]
- Perl, T.M.; Bédard, L.; Kosatsky, T.; Hockin, J.C.; Todd, E.C.D.; Remis, R.S. An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. N. Engl. J. Med. 1990, 322, 1775–1780. [Google Scholar] [CrossRef]
- Todd, E.C.D. Domoic Acid and Amnesic Shellfish Poisoning—A Review. J. Food Prot. 1993, 56, 69–83. [Google Scholar] [CrossRef]
- Bates, S.S.; Garrison, D.L.; Horner, R.A. Bloom dynamics and physiology of domoic acid producing Pseudo-nitzschia species. In Physiological Ecology of Harmful Algal Blooms; Springer: Heidelberg, Germany, 1998; pp. 267–292. [Google Scholar]
- Ciminiello, P.; Dell’Aversano, C.; Fattorusso, E.; Forino, M.; Magno, G.S.; Tartaglione, L.; Quilliam, M.A.; Tubaro, A.; Poletti, R. Hydrophilic Interaction Liquid Chromatography/Mass Spectrometry for Determination of Domoic Acid in Adriatic Shellfish. Rapid Commun. Mass Spectrom. 2005, 19, 2030–2038. [Google Scholar] [CrossRef] [PubMed]
- European Parliament and Council. Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin. Off. J. Eur. Union 2004, L139, 55–205. [Google Scholar]
- Ujević, I.; Ninčević-Gladan, Ž.; Roje, R.; Skejić, S.; Arapov, J.; Marasović, I. Domoic acid-a new toxin in the Croatian Adriatic shellfish toxin profile. Molecules 2010, 15, 6835–6849. [Google Scholar] [CrossRef]
- Ljubešić, Z.; Bosak, S.; Viličić, D.; Borojević, K.K.; Marić, D.; Godrijan, J.; Ujević, I.; Peharec, P.; Dakovac, T. Ecology and taxonomy of potentially toxic Pseudo-nitzschia species in Lim Bay (North-Eastern Adriatic Sea). Harmful Algae 2011, 10, 713–722. [Google Scholar] [CrossRef]
- Marić, D.; Ljubešić, Z.; Godrijan, J.; Viličić, D.; Ujević, I.; Precali, R. Blooms of the potentially toxic diatom Pseudo-nitzschia calliantha Lundholm, Moestrup & Hasle in coastal waters of the Northern Adriatic Sea (Croatia). Estuar. Coast. Shelf Sci. 2011, 92, 323–331. [Google Scholar] [CrossRef]
- Kvrgić, K.; Lešić, T.; Džafić, N.; Pleadin, J. Occurrence and seasonal monitoring of domoic acid in three shellfish species from the Northern Adriatic Sea. Toxins 2022, 14, 33. [Google Scholar] [CrossRef]
- Ujević, I.; Roje-Busatto, R.; Ezgeta-Balić, D. Comparison of amnesic, paralytic and lipophilic toxins profiles in cockle (Acanthocardia tuberculata) and smooth clam (Callista chione) from the Central Adriatic Sea (Croatia). Toxicon 2019, 159, 32–37. [Google Scholar] [CrossRef]
- Arapov, J.; Skejić, S.; Bužančić, M.; Bakrač, A.; Vidjak, O.; Bojanić, N.; Ujević, I.; Gladan, Ž.N. Taxonomical diversity of Pseudo-nitzschia from the Central Adriatic Sea. Phycol. Res. 2017, 65, 280–290. [Google Scholar] [CrossRef]
- Arapov, J.; Ujević, I.; Pfannkuchen, D.M.; Godrijan, J.; Bakrač, A.; Gladan, Ž.N.; Marasovic, I. Domoic acid in phytoplankton net samples and shellfish from the Krka river estuary in the Central Adriatic Sea. Mediterr. Mar. Sci. 2016, 17, 340–350. [Google Scholar] [CrossRef]
- Bosak, S.; Burić, Z.; Djakovac, T.; Viličić, D. Seasonal distribution of plankton diatoms in Lim Bay, Northeastern Adriatic Sea. Acta Bot. Croat. 2009, 68, 351–365. [Google Scholar]
- Arapov, J.; Bužančić, M.; Penna, A.; Casabianca, S.; Capellacci, S.; Andreoni, F.; Skejić, S.; Bakrač, A.; Straka, M.; Mandić, J.; et al. High proliferation of Pseudo-nitzschia cf. arenysensis in the Adriatic Sea: Ecological and morphological characterisation. Mediterr. Mar. Sci. 2020, 21, 759–774. [Google Scholar] [CrossRef]
- Skejić, S.; Milić Roje, B.; Matić, F.; Arapov, J.; Francé, J.; Bužančić, M.; Bakrač, A.; Straka, M.; Ninčević Gladan, Ž. Phytoplankton assemblage over a 14-year period in the Adriatic Sea: Patterns and trends. Biology 2024, 13, 493. [Google Scholar] [CrossRef] [PubMed]
- Turk Dermastia, T.; Cerino, F.; Stanković, D.; Francé, J.; Ramšak, A.; Žnidarič Tušek, M.; Beran, A.; Natali, V.; Cabrini, M.; Mozetič, P. Ecological time series and integrative taxonomy unveil seasonality and diversity of the toxic diatom Pseudo-nitzschia h. Peragallo in the Northern Adriatic Sea. Harmful Algae 2020, 93, 101773. [Google Scholar] [CrossRef]
- Giulietti, S.; Romagnoli, T.; Siracusa, M.; Bacchiocchi, S.; Totti, C.; Accoroni, S. Integrative Taxonomy of the Pseudo-nitzschia (Bacillariophyceae) populations in the nw Adriatic Sea, with a focus on a novel cryptic species in the P. delicatissima species complex. Phycologia 2021, 60, 247–264. [Google Scholar] [CrossRef]
- Arapov, J.; Tomašević, T.; Bonačić, T.; Pejković, M.; Bužančić, M.; Bušelić, I.; Lepen Pleić, I.; Casabianca, S.; Penna, A.; Skejić, S.; et al. A new insight into the taxonomy of Pseudo-nitzschia genus from the Adriatic Sea: Description of P. brasiliana, P. galaxiae, P. hasleana, and P. linea. J. Mar. Sci. Eng. 2023, 11, 1370. [Google Scholar] [CrossRef]
- Bonačić, T.; Arapov, J.; Bušelić, I.; Lepen Pleić, I.; Milić Roje, B.; Tomašević, T.; Bužančić, M.; Mladinić, M.; Casabianca, S.; Penna, A.; et al. Advancing the taxonomy of the diatom Pseudo-nitzschia through an integrative study conducted in the Central and Southeastern Adriatic Sea. Plants 2025, 14, 245. [Google Scholar] [CrossRef]
- Turk, T.; Franc, J.; Accoroni, S.; Totti, C.; Cerino, F.; Immacolata, M.; Bernardi, F.; Finotto, S.; Godrijan, J.; Drakulović, D. Comprehensive insights into Pseudo-nitzschia research in the Adriatic Sea: Diverse perspectives and emerging discoveries. Estuar. Coast. Shelf Sci. 2025, 319, 109283. [Google Scholar] [CrossRef]
- Penna, A.; Casabianca, S.; Perini, F.; Bastianini, M.; Riccardi, E.; Pigozzi, S.; Scardi, M. Toxic Pseudo-nitzschia spp. in the Northwestern Adriatic Sea: Characterization of species composition by genetic and molecular quantitative analyses. J. Plankton Res. 2013, 35, 352–366. [Google Scholar] [CrossRef]
- Tanković, M.S.; Baričević, A.; Gerić, M.; Domijan, A.-M.; Pfannkuchen, D.M.; Kužat, N.; Ujević, I.; Kuralić, M.; Rožman, M.; Matković, K.; et al. Characterisation and Toxicological activity of three different Pseudo-nitzschia species from the Northern Adriatic Sea (Croatia). Environ. Res. 2022, 214, 114108. [Google Scholar] [CrossRef]
- Turk Dermastia, T.; Dall’Ara, S.; Dolenc, J.; Mozetič, P. Toxicity of the diatom genus Pseudo-nitzschia (bacillariophyceae): Insights from toxicity tests and genetic screening in the Northern Adriatic Sea. Toxins 2022, 14, 60. [Google Scholar] [CrossRef]
- Arapov, J.; Ujević, I.; Straka, M.; Skejić, S.; Bužančić, M.; Bakrač, A.; Ninčević Gladan, Ž. First evidence of domoic acid production in Pseudo-nitzschia calliantha cultures from the Central Adriatic Sea. Acta Adriat. 2020, 61, 135–144. [Google Scholar] [CrossRef]
- Fehling, J.; Davidson, K.; Bates, S.S. Growth dynamics of non-toxic Pseudo-nitzschia delicatissima and toxic P. seriata (Bacillariophyceae) under simulated spring and summer photoperiods. Harmful Algae 2005, 4, 763–769. [Google Scholar] [CrossRef]
- Cerino, F.; Orsini, L.; Sarno, D.; Dell’Aversano, C.; Tartaglione, L.; Zingone, A. The alternation of different morphotypes in the seasonal cycle of the toxic diatom Pseudo-nitzschia galaxiae. Harmful Algae 2005, 4, 33–48. [Google Scholar] [CrossRef]
- Moschandreou, K.K.; Nikolaidis, G. The genus Pseudo-nitzschia (Bacillariophyceae) in Greek Coastal waters. Bot. Mar. 2010, 53, 159–172. [Google Scholar] [CrossRef]
- Thessen, A.E.; Bowers, H.A.; Stoecker, D.K. Intra- and interspecies differences in growth and toxicity of Pseudo-nitzschia while using different nitrogen sources. Harmful Algae 2009, 8, 792–810. [Google Scholar] [CrossRef]
- Lapworth, C.J.; Hallegraeff, G.M.; Ajani, P.A. Identification of domoic-acid producing Pseudo-nitzschia species in Australian waters. In Harmful Algal Blooms 2000; Hallegraeff, G.M., Blackburn, S.I., Bolch, C.J., Lewis, R.J., Eds.; Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 2001; pp. 38–41. [Google Scholar]
- Moschandreou, K.K.; Papaefthimiou, D.; Katikou, P.; Kalopesa, E.; Panou, A.; Nikolaidis, G. Morphology, phylogeny and toxin analysis of Pseudo-nitzschia pseudodelicatissima (Bacillariophyceae) isolated from the Thermaikos Gulf, Greece. Phycologia 2010, 49, 260–273. [Google Scholar] [CrossRef]
- Lundholm, N.; Moestrup, Ø.; Hoef-emden, K. A Study of the Pseudo-nitzschia pseudodelicatissima/cuspidata complex (Bacillariophyceae): What is P. pseudodelicatissima? J. Psychol. 2003, 39, 797–813. [Google Scholar] [CrossRef]
- Orsini, L.; Sarno, D.; Procaccini, G.; Poletti, R.; Dahlmann, J.; Montresor, M. Toxic Pseudo-nitzschia multistriata (Bacillariophyceae) from the Gulf of Naples: Morphology, toxin analysis and phylogenetic relationships with other Pseudo-nitzschia species. Eur. J. Phycol. 2002, 37, 247–257. [Google Scholar] [CrossRef]
- Bates, S.S.; Hubbard, K.A.; Lundholm, N.; Montresor, M.; Leaw, C.P. Pseudo-nitzschia, Nitzschia, and domoic acid: New research since 2011. Harmful Algae 2018, 79, 3–43. [Google Scholar] [CrossRef]
- Percopo, I.; Ruggiero, M.V.; Sarno, D.; Longobardi, L.; Rossi, R.; Piredda, R.; Zingone, A. Phenological segregation suggests speciation by time in the planktonic diatom Pseudo-nitzschia allochrona sp. nov. Ecol. Evol. 2022, 12, e9155. [Google Scholar] [CrossRef]
- Amato, A.; Montresor, M. Morphology, phylogeny, and sexual cycle of Pseudo-nitzschia mannii sp. nov. (Bacillariophyceae): A pseudo-cryptic species within the P. pseudodelicatissima complex. Phycologia 2008, 47, 487–497. [Google Scholar] [CrossRef]
- Quijano-Scheggia, S.; Garcés, E.; Andree, K.B.; De la Iglesia, P.; Diogène, J.; Fortuño, J.M.; Camp, J. Pseudo-nitzschia species on the Catalan coast: Characterisation and contribution to the current knowledge of the distribution of this genus in the Mediterranean Sea. Sci. Mar. 2010, 74, 395–410. [Google Scholar] [CrossRef]
- Hlaili, A.S.; Khalifa, I.S.; Bouchouicha-Smida, D.; Garali, S.M.; Ksouri, J.; Chalghaf, M.; Bates, S.S.; Lundholm, N.; Kooistra, W.H.C.F.; de la Iglesia, P. Toxic and potentially toxic diatom blooms in Tunisian (SW Mediterranean) waters: Review of ten years of investigations. Adv. Environ. Res. 2016, 48, 51–69. [Google Scholar]
- Sahraoui, I.; Bates, S.S.; Bouchouicha, D.; Mabrouk, H.H. Hlaili Toxicity of Pseudo-nitzschia populations from Bizerte lagoon, Tunisia, southwest Mediterranean, and first report of domoic acid production by P. brasiliana. Diatom Res. 2011, 26, 293–303. [Google Scholar] [CrossRef]
- Fernandes, L.F.; Hubbard, K.A.; Richlen, M.L.; Smith, J.; Bates, S.S.; Ehrman, J.; Léger, C.; Mafra, L.L.; Kulis, D.; Quilliam, M.; et al. Diversity and toxicity of the diatom Pseudo-nitzschia Peragallo in the Gulf of Maine, Northwestern Atlantic Ocean. Deep. Res. Part II Top. Stud. Oceanogr. 2014, 103, 139–162. [Google Scholar] [CrossRef]
- Tatters, A.O.; Fu, F.-X.; Hutchins, D.A. High CO2 and silicate limitation synergistically increase the toxicity of Pseudo-nitzschia fraudulenta. PLoS ONE 2012, 7, 32116. [Google Scholar] [CrossRef]
- Lema, K.A.; Latimier, M.; Nézan, É.; Fauchot, J.; Le Gac, M. Inter and intra-specific growth and domoic acid production in relation to nutrient ratios and concentrations in Pseudo-nitzschia: Phosphate an important factor. Harmful Algae 2017, 64, 11–19. [Google Scholar] [CrossRef]
- Moschandreou, K.K.; Baxevanis, A.D.; Katikou, P.; Papaefthimiou, D.; Nikolaidis, G.; Abatzopoulos, T.J. Inter- and Intra-Specific Diversity of Pseudo-Nitzschia (Bacillariophyceae) in the Northeastern Mediterranean. Eur. J. Phycol. 2012, 47, 321–339. [Google Scholar] [CrossRef]
- Pistocchi, R.; Guerrini, F.; Pezzolesi, L.; Riccardi, M.; Vanucci, S.; Ciminiello, P.; Dell’Aversano, C.; Forino, M.; Fattorusso, E.; Tartaglione, L.; et al. Toxin levels and profiles in microalgae from the North-Western Adriatic Sea—15 Years of studies on cultured species. Mar. Drugs 2012, 10, 140–162. [Google Scholar] [CrossRef]
- Lundholm, N.; Krock, B.; John, U.; Skov, J.; Cheng, J.; Pančić, M.; Wohlrab, S.; Rigby, K.; Nielsen, T.G.; Selander, E.; et al. Induction of domoic acid production in diatoms—Types of grazers and diatoms are important. Harmful Algae 2018, 79, 64–73. [Google Scholar] [CrossRef]
- Fehling, J.; Davidson, K.; Bolch, C.; Tett, P. Seasonally of Pseudo-nitzschia spp. (Bacillariophyceae) In Western Scottish waters. Mar. Ecol. Prog. Ser. 2006, 323, 91–105. [Google Scholar] [CrossRef]
- Pan, Y.; Subba Rao, D.V.; Mann, K.H. Acclimation to low light intensity in photosynthesis and growth of Pseudo-nitzschia multiseries Hasle, a neurotoxigenic diatom. J. Plankton Res. 1996, 18, 1427–1438. [Google Scholar] [CrossRef]
- Bates, S.S.; Bird, C.J.; de Freitas, A.S.W.; Foxall, R.; Gilgan, M.; Hanic, L.A.; Johnson, G.R.; McCulloch, A.W.; Odense, P.; Pocklington; et al. Pennate diatom Nitzschia pungens is the primary source of domoic acid, a toxin in shellfish from eastern Prince Edward Island, Canada. Can. J. Fish Aquat. Sci. 1989, 46, 1203–1215. [Google Scholar] [CrossRef]
- Mafra, L.L.; Bricelj, V.M.; Fennel, K. Domoic acid uptake and elimination kinetics in oysters and mussels in relation to body size and anatomical distribution of toxin. Aquat. Toxicol. 2010, 100, 17–29. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Plenum Publishing Corp.: New York, NY, USA, 1975; pp. 29–60. [Google Scholar] [CrossRef]
- D’Alelio, D.; Amato, A.; Luedeking, A.; Montresor, M. Sexual and vegetative phases in the planktonic diatom Pseudo-nitzschia multistriata. Harmful Algae 2009, 8, 225–232. [Google Scholar] [CrossRef]
- Wood, A.; Everroad, R.C.; Wingard, L.M. Measuring growth rates in microalgal cultures. In Algal Culturing Techniques; Elsevier Academic Press: Burlington, MA, USA, 2005; pp. 269–285. [Google Scholar]
- Guillard, R.R.; Sieracki, M.S. Counting cells in cultures with the light microscope. In Algal Culturing Techniques; Elsevier Academic Press: Burlington, MA, USA, 2005; pp. 239–252. [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
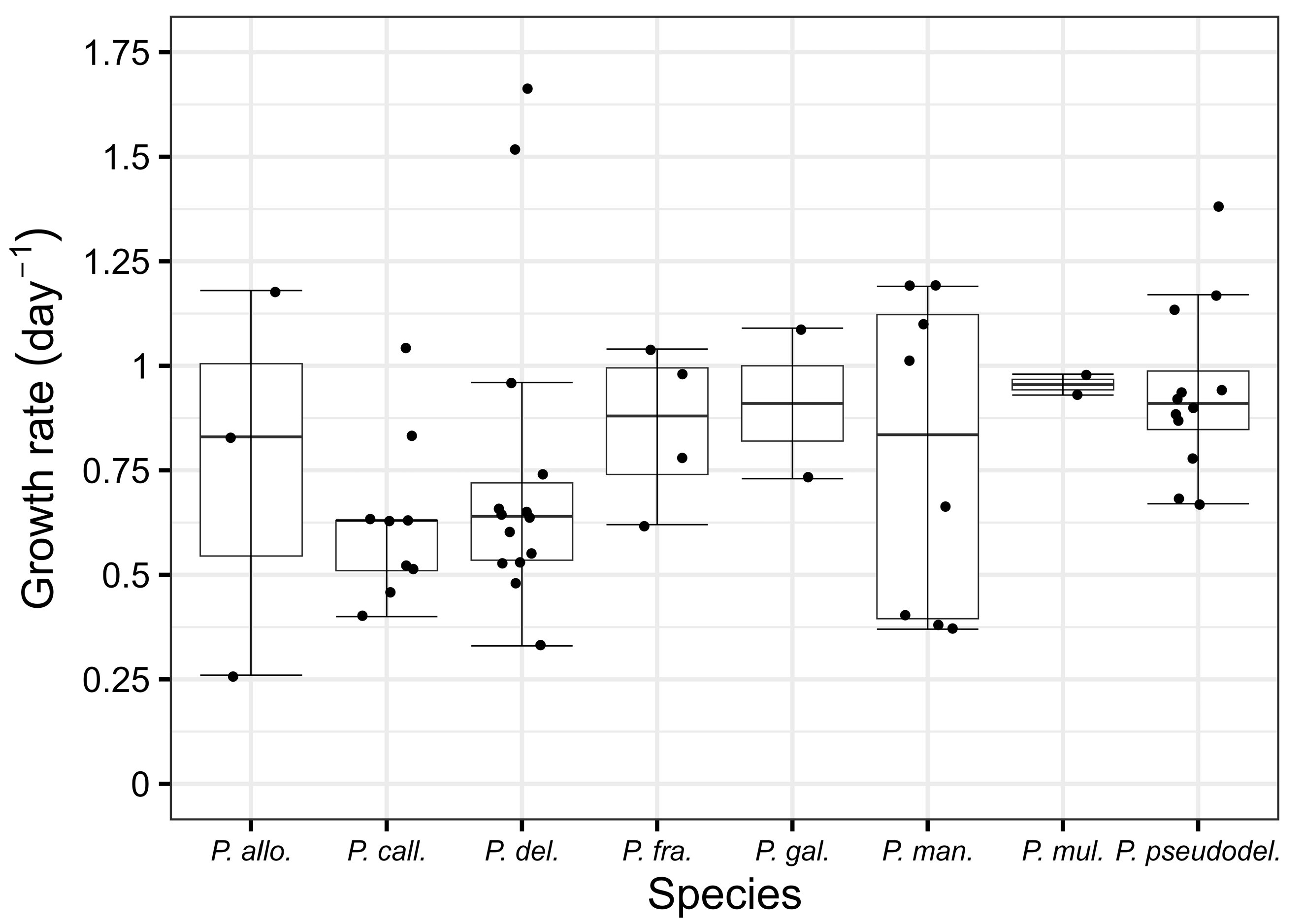


| Species | Strain | pDA in Cell Culture (ng/mL) | Growth Rate µ (day−1) | Maximum Abundance × 103 (cell mL−1) |
|---|---|---|---|---|
| P. allochrona | S222al | ND | 0.26 | 322.20 |
| S223al | ND | 0.83 | 301.20 | |
| S280al | ND | 1.18 | 289.40 | |
| P. calliantha | M074ca | ND | 0.63 | 201.20 |
| V061ca | ND | 0.83 | 276.68 | |
| V065ca | ND | 0.51 | 124.44 | |
| V068ca | ND | 0.63 | 277.60 | |
| V070ca | ND | 1.04 | 259.40 | |
| V071ca | ND | 0.46 | 270.00 | |
| V072ca | ND | 0.40 | 202.80 | |
| V077ca | ND | 0.52 | 200.60 | |
| V079ca | ND | 0.63 | 113.75 | |
| P. delicatissima | K057de | ND | 0.53 | 512.22 |
| K058de | ND | 0.65 | 789.60 | |
| K129de | ND | 0.64 | 636.60 | |
| K134de | ND | 0.66 | 515.80 | |
| M075de | ND | 0.74 | 482.00 | |
| M085de | ND | 0.96 | 411.20 | |
| M088de | ND | 0.48 | 509.20 | |
| M144de | ND | 0.33 | 566.40 | |
| M461de | ND | 1.66 | 641.60 | |
| M462de | ND | 1.52 | 514.40 | |
| V040de | ND | 0.64 | 808.60 | |
| V041de | ND | 0.55 | 819.00 | |
| V042de | ND | 0.60 | 612.40 | |
| V043de | ND | 0.53 | 677.40 | |
| P. fraudulenta | K450fr | ND | 0.98 | 201.30 |
| K455fr | ND | 0.78 | 117.20 | |
| K456fr | ND | 0.62 | 77.10 | |
| K458fr | ND | 1.04 | 102.20 | |
| P. galaxiae | K136ga | ND | 1.09 | 466.80 |
| M232ga | ND | 0.73 | 382.40 | |
| P. mannii | K231ma | ND | 0.66 | 108.70 |
| K237ma | ND | 0.37 | 130.80 | |
| M233ma | ND | 0.38 | 114.70 | |
| M236ma | ND | 1.01 | 105.30 | |
| M239ma | ND | 1.19 | 164.35 | |
| M240ma | ND | 1.19 | 130.80 | |
| M241ma | ND | 0.40 | 113.20 | |
| V229ma | ND | 1.10 | 119.64 | |
| P. multistriata | S290mu | ND | 0.93 | 179.20 |
| S442mu | ND | 0.98 | 100.60 | |
| P. pseudodelicatissima | K328ps | 1.78–2.10 | 1.38 | 113.10 |
| K336ps | 5.42–6.32 | 1.17 | 309.20 | |
| K339ps | 0.96–5.66 | 0.87 | 259.80 | |
| K340ps | 4.72 | 0.90 | 268.60 | |
| K349ps | 4.78 | 0.94 | 294.40 | |
| K350ps | ND | 0.68 | 96.27 | |
| K351ps | 5.44 | 0.92 | 278.00 | |
| K352ps | 0.96–6.26 | 0.67 | 293.40 | |
| K356ps | 0.22–0.93 | 0.78 | 236.40 | |
| K357ps | 1.00–4.01 | 0.88 | 187.96 | |
| K358ps | 0.68–3.93 | 0.94 | 277.80 | |
| K359ps | 0.28–0.68 | 1.13 | 237.60 |
| Strain | Growth Phase | Mean Abundance × 103 (cell mL−1) | Mean DA Concentration in Culture (ng mL−1) | Mean DA Concentration (pg cell−1) |
|---|---|---|---|---|
| K328ps | 3 | 89.20 | 1.78 | 0.0128 |
| 4 | 105.44 | 2.10 | 0.0097 | |
| K336ps | 4 | 316.33 | 6.32 | 0.0014 |
| 5 | 271.83 | 5.42 | 0.0027 | |
| K339ps | 2 | 48.51 | 0.96 | 0.0163 |
| 3 | 209.55 | 4.18 | 0.0095 | |
| 4 | 282.60 | 5.66 | 0.0070 | |
| 5 | 202.16 | 4.04 | 0.0026 | |
| K340ps | 5 | 236.03 | 4.72 | 0.0034 |
| K349ps | 5 | 238.62 | 4.78 | 0.0116 |
| K351ps | 5 | 272.54 | 5.44 | 0.0021 |
| K352ps | 2 | 48.79 | 0.96 | 0.0086 |
| 3 | 281.37 | 5.62 | 0.0044 | |
| 4 | 313.56 | 6.26 | 0.0044 | |
| 5 | 223.43 | 4.46 | 0.0052 | |
| K356ps | 3 | 233.23 | 0.78 | 0.0035 |
| 4 | 222.42 | 0.22 | 0.0010 | |
| 5 | 154.35 | 0.93 | 0.0061 | |
| K357ps | 2 | 89.83 | 1.00 | 0.0112 |
| 3 | 187.17 | 3.89 | 0.0208 | |
| 4 | 165.07 | 4.01 | 0.0241 | |
| 5 | 140.90 | 3.53 | 0.0250 | |
| K358ps | 2 | 69.42 | 1.09 | 0.0160 |
| 3 | 196.40 | 3.93 | 0.0200 | |
| 4 | 286.95 | 2.98 | 0.0104 | |
| 5 | 92.45 | 0.68 | 0.0007 | |
| K359ps | 2 | 79.61 | 0.28 | 0.0036 |
| 3 | 247.47 | 0.54 | 0.0022 | |
| 4 | 224.36 | 0.34 | 0.0015 |
| Time (min) | A (%) | B (%) |
|---|---|---|
| 0 | 90 | 10 |
| 4 | 20 | 80 |
| 6 | 20 | 80 |
| 6.5 | 90 | 10 |
| 10.5 | 90 | 10 |
| 11 | 90 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomašević, T.; Arapov, J.; Ujević, I.; Bonačić, T.; Bužančić, M.; Bulić, A.; Skejić, S.; Roje-Busatto, R.; Ninčević Gladan, Ž. Growth Dynamics and Toxin Production of Pseudo-nitzschia Species Isolated from the Central Adriatic Sea. Toxins 2025, 17, 307. https://doi.org/10.3390/toxins17060307
Tomašević T, Arapov J, Ujević I, Bonačić T, Bužančić M, Bulić A, Skejić S, Roje-Busatto R, Ninčević Gladan Ž. Growth Dynamics and Toxin Production of Pseudo-nitzschia Species Isolated from the Central Adriatic Sea. Toxins. 2025; 17(6):307. https://doi.org/10.3390/toxins17060307
Chicago/Turabian StyleTomašević, Tina, Jasna Arapov, Ivana Ujević, Tina Bonačić, Mia Bužančić, Antonija Bulić, Sanda Skejić, Romana Roje-Busatto, and Živana Ninčević Gladan. 2025. "Growth Dynamics and Toxin Production of Pseudo-nitzschia Species Isolated from the Central Adriatic Sea" Toxins 17, no. 6: 307. https://doi.org/10.3390/toxins17060307
APA StyleTomašević, T., Arapov, J., Ujević, I., Bonačić, T., Bužančić, M., Bulić, A., Skejić, S., Roje-Busatto, R., & Ninčević Gladan, Ž. (2025). Growth Dynamics and Toxin Production of Pseudo-nitzschia Species Isolated from the Central Adriatic Sea. Toxins, 17(6), 307. https://doi.org/10.3390/toxins17060307





