Hydroculture Cultivation of Strawberries as Potential Reference Material for Microcystin Analysis: Approaches and Pitfalls
Abstract
1. Introduction
2. Results
2.1. Cultivation Approach and Harvest Measurements
2.2. Microcystin Congeners Quantification in Hoagland and Accumulation in Plant Material
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Standards
5.2. Cultivation and Treatment
5.3. Analytical Analysis
5.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATX | Anatoxin-a |
| CYN | Cylindrospermopsin |
| CRM | Certified reference material |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| MC | Microcystin congener |
| MS/MS | Tandem mass spectrometry |
| MU | Measurement uncertainty |
| RM | Reference material |
| SPE | Solid-phase extraction |
| STX | Saxitoxin |
| UHPLC | Ultra-High Performance Liquid Chromatography |
References
- Svirčev, Z.; Lalić, D.; Bojadzija Savic, G.; Tokodi, N.; Drobac Backovic, D.; Chen, L.; Meriluoto, J.; Codd, G. Global Geographical and Historical Overview of Cyanotoxin Distribution and Cyanobacterial Poisonings. Arch. Toxicol. 2019, 93, 2429–2481. [Google Scholar] [CrossRef] [PubMed]
- Visser, P.M.; Verspagen, J.M.H.; Sandrini, G.; Stal, L.J.; Matthijs, H.C.P.; Davis, T.W.; Paerl, H.W.; Huisman, J. How Rising CO2 and Global Warming May Stimulate Harmful Cyanobacterial Blooms. Harmful Algae 2016, 54, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Huisman, J. Blooms Like It Hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Taranu, Z.E.; Pick, F.R.; Creed, I.F.; Zastepa, A.; Watson, S.B. Meteorological and Nutrient Conditions Influence Microcystin Congeners in Freshwaters. Toxins 2019, 11, 620. [Google Scholar] [CrossRef]
- Svirčev, Z.; Drobac, D.; Tokodi, N.; Mijović, B.; Codd, G.A.; Meriluoto, J. Toxicology of Microcystins with Reference to Cases of Human Intoxications and Epidemiological Investigations of Exposures to Cyanobacteria and Cyanotoxins. Arch. Toxicol. 2017, 91, 621–650. [Google Scholar] [CrossRef]
- Drobac, D.; Tokodi, N.; Simeunović, J.; Baltić, V.; Stanić, D.; Svirčev, Z. Human Exposure to Cyanotoxins and Their Effects on Health. Arh. Hig. Rada Toksikol. 2013, 64, 119–130. [Google Scholar] [CrossRef]
- Testai, E.; Buratti, F.M.; Funari, E.; Manganelli, M.; Vichi, S.; Arnich, N.; Fessard, V.; Sialehaamoa, A. Review and Analysis of Occurrence, Exposure and Toxicity of Cyanobacteria Toxins in Food. EFSA Extern. Sci. Rep. 2016, 13, 998E. [Google Scholar] [CrossRef]
- World Health Organization. Cyanobacterial Toxins: Microcystins. Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Kanoshina, I.; Lips, U.; Leppänen, J.-M. The Influence of Weather Conditions (Temperature and Wind) on Cyanobacterial Bloom Development in the Gulf of Finland (Baltic Sea). Harmful Algae 2003, 2, 29–41. [Google Scholar] [CrossRef]
- Karlsson, K.; Sipiä, V.; Kankaanpää, H.; Meriluoto, J. Mass Spectrometric Detection of Nodularin and Desmethylnodularin in Mussels and Flounders. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003, 784, 243–253. [Google Scholar] [CrossRef]
- Mazur-Marzec, H.; Meriluoto, J.; Pliński, M.; Szafranek, J. Characterization of Nodularin Variants in Nodularia Spumigena from the Baltic Sea Using Liquid Chromatography/Mass Spectrometry/Mass Spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 2023–2032. [Google Scholar] [CrossRef]
- Ohtani, I.; Moore, R.E.; Runnegar, M.T.C. Cylindrospermopsin: A Potent Hepatotoxin from the Blue-Green Alga Cylindrospermopsis Raciborskii. J. Am. Chem. Soc. 1992, 114, 7941–7942. [Google Scholar] [CrossRef]
- Hawkins, P.R.; Chandrasena, N.R.; Jones, G.J.; Humpage, A.R.; Falconer, I.R. Isolation and Toxicity of Cylindrospermopsis Raciborskii from an Ornamental Lake. Toxicon 1997, 35, 341–346. [Google Scholar] [CrossRef]
- World Health Organization. Cyanobacterial Toxins: Saxitoxins. Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization. Cyanobacterial Toxins: Anatoxin-a and Analogues. Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization. Cyanobacterial Toxins: Cylindrospermopsins. Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Crush, J.R.; Briggs, L.R.; Sprosen, J.M.; Nichols, S.N. Effect of Irrigation with Lake Water Containing Microcystins on Microcystin Content and Growth of Ryegrass, Clover, Rape, and Lettuce. Environ. Toxicol. 2008, 23, 246–252. [Google Scholar] [CrossRef]
- Van Hassel, W.H.R.; Ahn, A.-C.; Huybrechts, B.; Masquelier, J.; Wilmotte, A.; Andjelkovic, M. LC-MS/MS Validation and Quantification of Cyanotoxins in Algal Food Supplements from the Belgium Market and Their Molecular Origins. Toxins 2022, 14, 513. [Google Scholar] [CrossRef]
- Gilroy, D.J.; Kauffman, K.W.; Hall, R.A.; Huang, X.; Chu, F.S. Assessing Potential Health Risks from Microcystin Toxins in Blue-Green Algae Dietary Supplements. Environ. Health Perspect. 2000, 108, 435–439. [Google Scholar] [CrossRef]
- Samdal, I.A.; Ballot, A.; Løvberg, K.E.; Miles, C.O. Multihapten Approach Leading to a Sensitive ELISA with Broad Cross-Reactivity to Microcystins and Nodularin. Environ. Sci. Technol. 2014, 48, 8035–8043. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Xie, P.; Guo, L.; Li, L.; Miyabara, Y.; Park, H.D. Organ Distribution and Bioaccumulation of Microcystins in Freshwater Fish at Different Trophic Levels from the Eutrophic Lake Chaohu, China. Environ. Toxicol. 2005, 20, 293–300. [Google Scholar] [CrossRef]
- Chen, J.; Xie, P. Tissue Distributions and Seasonal Dynamics of the Hepatotoxic Microcystins-LR and -RR in Two Freshwater Shrimps, Palaemon Modestus and Macrobrachium Nipponensis, from a Large Shallow, Eutrophic Lake of the Subtropical China. Toxicon 2005, 45, 615–625. [Google Scholar] [CrossRef]
- Codd, G.A.; Metcalf, J.S.; Beattie, K.A. Retention of Microcystis Aeruginosa and Microcystin by Salad Lettuce (Lactuca sativa) after Spray Irrigation with Water Containing Cyanobacteria. Toxicon Off. J. Int. Soc. Toxinology 1999, 37, 1181–1185. [Google Scholar] [CrossRef]
- Wijewickrama, M.M.; Manage, P.M. Accumulation of Microcystin-LR in Grains of Two Rice Varieties (Oryza sativa L.) and a Leafy Vegetable, Ipomoea Aquatica. Toxins 2019, 11, 432. [Google Scholar] [CrossRef]
- Xiang, L.; Li, Y.-W.; Wang, Z.-R.; Liu, B.-L.; Zhao, H.-M.; Li, H.; Cai, Q.-Y.; Mo, C.-H.; Li, Q.X. Bioaccumulation and Phytotoxicity and Human Health Risk from Microcystin-LR under Various Treatments: A Pot Study. Toxins 2020, 12, 523. [Google Scholar] [CrossRef]
- Trung, B.; Dao, T.-S.; Faassen, E.; Lürling, M. Cyanobacterial Blooms and Microcystins in Southern Vietnam. Toxins 2018, 10, 471. [Google Scholar] [CrossRef]
- Carlsson, P.; Rita, D. Sedimentation of Nodularia Spumigena and Distribution of Nodularin in the Food Web during Transport of a Cyanobacterial Bloom from the Baltic Sea to the Kattegat. Harmful Algae 2019, 86, 74–83. [Google Scholar] [CrossRef]
- Amzil, Z.; Derrien, A.; Terre Terrillon, A.; Duval, A.; Connes, C.; Marco-Miralles, F.; Nézan, E.; Mertens, K.N. Monitoring the Emergence of Algal Toxins in Shellfish: First Report on Detection of Brevetoxins in French Mediterranean Mussels. Mar. Drugs 2021, 19, 393. [Google Scholar] [CrossRef]
- Skafi, M.; Vo Duy, S.; Munoz, G.; Dinh, Q.T.; Simon, D.F.; Juneau, P.; Sauvé, S. Occurrence of Microcystins, Anabaenopeptins and Other Cyanotoxins in Fish from a Freshwater Wildlife Reserve Impacted by Harmful Cyanobacterial Blooms. Toxicon 2021, 194, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Klijnstra, M.D.; Faassen, E.J.; Gerssen, A. A Generic LC-HRMS Screening Method for Marine and Freshwater Phycotoxins in Fish, Shellfish, Water, and Supplements. Toxins 2021, 13, 823. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.R.; Xiong, A.; Deeds, J.R.; Stutts, W.L.; Samdal, I.A.; Løvberg, K.E.; Miles, C.O. Microcystin Toxins at Potentially Hazardous Levels in Algal Dietary Supplements Revealed by a Combination of Bioassay, Immunoassay, and Mass Spectrometric Methods. J. Agric. Food Chem. 2020, 68, 8016–8025. [Google Scholar] [CrossRef]
- Miller, A.; Russell, C. Food Crops Irrigated with Cyanobacteria-Contaminated Water: An Emerging Public Health Issue in Canada. Environ. Health Rev. 2017, 60, 58–63. [Google Scholar] [CrossRef]
- Gkelis, S.; Lanaras, T.; Sivonen, K. The Presence of Microcystins and Other Cyanobacterial Bioactive Peptides in Aquatic Fauna Collected from Greek Freshwaters. Aquat. Toxicol. 2006, 78, 32–41. [Google Scholar] [CrossRef]
- Van Hassel, W.H.R.; Tardy, E.; Cottyn, B.; Andjelkovic, M.; Decombel, A.; Van Wichelen, J.; Masquelier, J.; Rajkovic, A. Irrigation-Dependent Accumulation of Microcystin in Different Crops under Mid-Scale Greenhouse Conditions. J. Agric. Food Res. 2025, 20, 101753. [Google Scholar] [CrossRef]
- Mohamed, Z.A.; Al Shehri, A.M. Microcystins in Groundwater Wells and Their Accumulation in Vegetable Plants Irrigated with Contaminated Waters in Saudi Arabia. J. Hazard. Mater. 2009, 172, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Mugani, R.; El Khalloufi, F.; Kasada, M.; Redouane, E.M.; Haida, M.; Aba, R.P.; Essadki, Y.; Zerrifi, S.E.A.; Herter, S.-O.; Hejjaj, A.; et al. Monitoring of Toxic Cyanobacterial Blooms in Lalla Takerkoust Reservoir by Satellite Imagery and Microcystin Transfer to Surrounding Farms. Harmful Algae 2024, 135, 102631. [Google Scholar] [CrossRef]
- Cao, Q.; You, B.; Liu, W.; Zhu, B.; Xie, L.; Cheng, C. Effect of Different Irrigation Methods on the Toxicity and Bioavailability of Microcystin-LR to Lettuce and Carrot. Environ. Sci. Pollut. Res. 2023, 30, 104554–104562. [Google Scholar] [CrossRef] [PubMed]
- Levizou, E.; Papadimitriou, T.; Papavasileiou, E.; Papadimitriou, N.; Kormas, K.A. Root Vegetables Bioaccumulate Microcystins-LR in a Developmental Stage-Dependent Manner under Realistic Exposure Scenario: The Case of Carrot and Radish. Agric. Water Manag. 2020, 240, 106274. [Google Scholar] [CrossRef]
- Haida, M.; El Khalloufi, F.; Mugani, R.; Redouane, E.M.; Campos, A.; Vasconcelos, V.; Oudra, B. Effects of Irrigation with Microcystin-Containing Water on Growth, Physiology, and Antioxidant Defense in Strawberry Fragaria Vulgaris under Hydroponic Culture. Toxins 2022, 14, 198. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Han, F.X.; Wang, F.; Zhang, H.; Shi, Z. Accumulation and Phytotoxicity of Microcystin-LR in Rice (Oryza sativa). Ecotoxicol. Environ. Saf. 2012, 76, 193–199. [Google Scholar] [CrossRef]
- Machado, J.; Azevedo, J.; Freitas, M.; Pinto, E.; Almeida, A.; Vasconcelos, V.; Campos, A. Analysis of the Use of Microcystin-Contaminated Water in the Growth and Nutritional Quality of the Root-Vegetable, Daucus Carota. Environ. Sci. Pollut. Res. 2017, 24, 752–764. [Google Scholar] [CrossRef]
- Abdullahi, H.; Tanimu, Y.; Akinyemi, S.A.; do Carmo Bittencourt-Oliveira, M.; Chia, M.A. Assessment of Microcystins in Surface Water and Irrigated Vegetables in Kwaru Stream, Hayin Danmani, Kaduna-Nigeria. Environ. Sci. Pollut. Res. 2022, 29, 78303–78313. [Google Scholar] [CrossRef]
- Díez-Quijada Jiménez, L.; Guzmán-Guillén, R.; Cătunescu, G.M.; Campos, A.; Vasconcelos, V.; Jos, Á.; Cameán, A.M. A New Method for the Simultaneous Determination of Cyanotoxins (Microcystins and Cylindrospermopsin) in Mussels Using SPE-UPLC-MS/MS. Environ. Res. 2020, 185, 109284. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Jos, A.; Cameán, A.; Oliveira, F.; Barreiro, A.; Machado, J.; Azevedo, J.; Pinto, E.; Almeida, A.; Campos, A.; et al. Analysis of the Use of Cylindrospermopsin and/or Microcystin-Contaminated Water in the Growth, Mineral Content, and Contamination of Spinacia Oleracea and Lactuca sativa. Toxins 2019, 11, 624. [Google Scholar] [CrossRef]
- Prieto, A.I.; Guzmán-Guillén, R.; Díez-Quijada, L.; Campos, A.; Vasconcelos, V.; Jos, Á.; Cameán, A.M. Validation of a Method for Cylindrospermopsin Determination in Vegetables: Application to Real Samples Such as Lettuce (Lactuca sativa L.). Toxins 2018, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.D.; Waack, J.; Lewis, A.; Edwards, C.; Lawton, L. Development and Single-Laboratory Validation of a UHPLC-MS/MS Method for Quantitation of Microcystins and Nodularin in Natural Water, Cyanobacteria, Shellfish and Algal Supplement Tablet Powders. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1074–1075, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Van Hassel, W.H.R.; Masquelier, J.; Andjelkovic, M.; Rajkovic, A. Towards a Better Quantification of Cyanotoxins in Fruits and Vegetables: Validation and Application of an UHPLC-MS/MS-Based Method on Belgian Products. Separations 2022, 9, 319. [Google Scholar] [CrossRef]
- Roy-Lachapelle, A.; Solliec, M.; Sauvé, S. Determination of BMAA and Three Alkaloid Cyanotoxins in Lake Water Using Dansyl Chloride Derivatization and High-Resolution Mass Spectrometry. Anal. Bioanal. Chem. 2015, 407, 5487–5501. [Google Scholar] [CrossRef]
- Roy-Lachapelle, A.; Solliec, M.; Bouchard, M.F.; Sauvé, S. Detection of Cyanotoxins in Algae Dietary Supplements. Toxins 2017, 9, 76. [Google Scholar] [CrossRef]
- Roy-Lachapelle, A.; Duy, S.V.; Munoz, G.; Dinh, Q.T.; Bahl, E.; Simon, D.F.; Sauvé, S. Analysis of Multiclass Cyanotoxins (Microcystins, Anabaenopeptins, Cylindrospermopsin and Anatoxins) in Lake Waters Using on-Line SPE Liquid Chromatography High-Resolution Orbitrap Mass Spectrometry. Anal. Methods 2019, 11, 5289–5300. [Google Scholar] [CrossRef]
- Zervou, S.-K.; Christophoridis, C.; Kaloudis, T.; Triantis, T.M.; Hiskia, A. New SPE-LC-MS/MS Method for Simultaneous Determination of Multi-Class Cyanobacterial and Algal Toxins. J. Hazard. Mater. 2017, 323, 56–66. [Google Scholar] [CrossRef]
- Thomas, K.M.; Wright, E.J.; Beach, D.G.; McCarron, P. Multi–Class Cyanobacterial Toxin Analysis Using Hydrophilic Interaction Liquid Chromatography–Mass Spectrometry. J. Chromatogr. A 2024, 1738, 465483. [Google Scholar] [CrossRef]
- Al-Sammak, M.A.; Hoagland, K.D.; Snow, D.D.; Cassada, D. Methods for Simultaneous Detection of the Cyanotoxins BMAA, DABA, and Anatoxin- A in Environmental Samples. Toxicon 2013, 76, 316–325. [Google Scholar] [CrossRef]
- Van Camp, C.; Van Hassel, W.H.R.; Abdallah, M.F.; Masquelier, J. Simultaneous Detection and Quantification of Aflatoxin M1, Eight Microcystin Congeners and Nodularin in Dairy Milk by LC-MS/MS. Chemosensors 2023, 11, 511. [Google Scholar] [CrossRef]
- Van Camp, C.; Van Hassel, W.H.R.; Van Wichelen, J.; Masquelier, J. Development and Validation of a LC-MS Method for the Quantification of Microcystins in Fish for Their Monitoring in Environmental and Food Context. J. Agric. Food Res. 2024, 18, 101374. [Google Scholar] [CrossRef]
- ISO 17034:2016; General Requirements for the Competence of Reference Material Producers. Technical Committee ISO/CASCO; Technical Committee CEN/CLC/TC 1. ISO: Geneva, Switzerland, 2016.
- Sano, T.; Takagi, H.; Nishikawa, M.; Kaya, K. NIES Certified Reference Material for Microcystins, Hepatotoxic Cyclic Peptide Toxins from Cyanobacterial Blooms in Eutrophic Water Bodies. Anal. Bioanal. Chem. 2008, 391, 2005–2010. [Google Scholar] [CrossRef]
- Hollingdale, C.; Thomas, K.; Lewis, N.; Békri, K.; McCarron, P.; Quilliam, M.A. Feasibility Study on Production of a Matrix Reference Material for Cyanobacterial Toxins. Anal. Bioanal. Chem. 2015, 407, 5353–5363. [Google Scholar] [CrossRef]
- Turner, A.D.; Beach, D.G.; Foss, A.; Samdal, I.A.; Løvberg, K.L.E.; Waack, J.; Edwards, C.; Lawton, L.A.; Dean, K.J.; Maskrey, B.H.; et al. A Feasibility Study into the Production of a Mussel Matrix Reference Material for the Cyanobacterial Toxins Microcystins and Nodularins. Toxins 2023, 15, 27. [Google Scholar] [CrossRef]
- Sahoo, S.; Sahoo, D.; Sahoo, K.K. Optimization of an Efficient Hydroponic Cultivation Method for High Yield of Strawberry Plants. S. Afr. J. Bot. 2024, 167, 429–440. [Google Scholar] [CrossRef]
- Haida, M.; El khalloufi, F.; Tamegart, L.; Mugani, R.; Essadki, Y.; Redouane, E.M.; Azevedo, J.; Araújo, M.J.; Campos, A.; Vasconcelos, V.; et al. Tracing the Fate of Microcystins from Irrigation Water to Food Chains: Studies with Fragaria Vulgaris and Meriones Shawi. Toxicon 2023, 236, 107345. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, J.A.; Tettenhorst, D.R.; de la Cruz, A. Method 544, V1.0: Determination of Microcystins and Nodularin in Drinking Water by Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS); EPA/600/R-14/474; U. S. Environmental Protection Agency: Cincinnati, OH, USA, 2015.
- Kamp, L.; Church, J.L.; Carpino, J.; Faltin-Mara, E.; Rubio, F. The Effects of Water Sample Treatment, Preparation, and Storage Prior to Cyanotoxin Analysis for Cylindrospermopsin, Microcystin and Saxitoxin. Chem. Biol. Interact. 2016, 246, 45–51. [Google Scholar] [CrossRef]
- Seo, C.; Lee, J.W.; Jung, W.-K.; Lee, Y.-M.; Lee, S.; Lee, S.G. Examination of Microcystin Adsorption by the Type of Plastic Materials Used during the Procedure of Microcystin Analysis. Toxins 2022, 14, 625. [Google Scholar] [CrossRef]
- de Maagd, P.G.-J.; Hendriks, A.J.; Seinen, W.; Sijm, D.T.H.M. PH-Dependent Hydrophobicity of the Cyanobacteria Toxin Microcystin-LR. Water Res. 1999, 33, 677–680. [Google Scholar] [CrossRef]
- Zhou, S.; Yu, Y.; Sun, J.; Zhu, S.; Deng, J. Oxidation of Microcystin-LR by Copper (II) Coupled with Ascorbic Acid: Kinetic Modeling towards Generation of H2O2. Chem. Eng. J. 2018, 333, 443–450. [Google Scholar] [CrossRef]
- Rodríguez, E.; Majado, M.E.; Meriluoto, J.; Acero, J.L. Oxidation of Microcystins by Permanganate: Reaction Kinetics and Implications for Water Treatment. Water Res. 2007, 41, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants Without Soil; College of Agriculture, University of California: Berkeley, CA, USA, 1950. [Google Scholar]
- Corbel, S.; Mougin, C.; Nélieu, S.; Delarue, G.; Bouaïcha, N. Evaluation of the Transfer and the Accumulation of Microcystins in Tomato (Solanum Lycopersicum Cultivar MicroTom) Tissues Using a Cyanobacterial Extract Containing Microcystins and the Radiolabeled Microcystin-LR (14C-MC-LR). Sci. Total Environ. 2016, 541, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Drobac, D.; Tokodi, N.; Kiprovski, B.; Malenčić, D.; Važić, T.; Nybom, S.; Meriluoto, J.; Svirčev, Z. Microcystin Accumulation and Potential Effects on Antioxidant Capacity of Leaves and Fruits of Capsicum annuum. J. Toxicol. Environ. Health A 2017, 80, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Van Hassel, W.H.R.; Abdallah, M.F.; Gracia Guzman Velasquez, M.; Miles, C.O.; Samdal, I.A.; Masquelier, J.; Rajkovic, A. Experimental Accumulation and Depuration Kinetics and Natural Occurrence of Microcystin-LR in Basil (Ocimum basilicum L.). Environ. Pollut. 2024, 347, 123715. [Google Scholar] [CrossRef]
- Van Hassel, W.H.R.; Huybrechts, B.; Masquelier, J.; Wilmotte, A.; Andjelkovic, M. Development, Validation and Application of a Targeted LC-MS Method for Quantification of Microcystins and Nodularin: Towards a Better Characterization of Drinking Water. Water 2022, 14, 1195. [Google Scholar] [CrossRef]
- Xu, S.; Chen, M.; Feng, T.; Zhan, L.; Zhou, L.; Yu, G. Use Ggbreak to Effectively Utilize Plotting Space to Deal With Large Datasets and Outliers. Front. Genet. 2021, 12, 774846. [Google Scholar] [CrossRef]
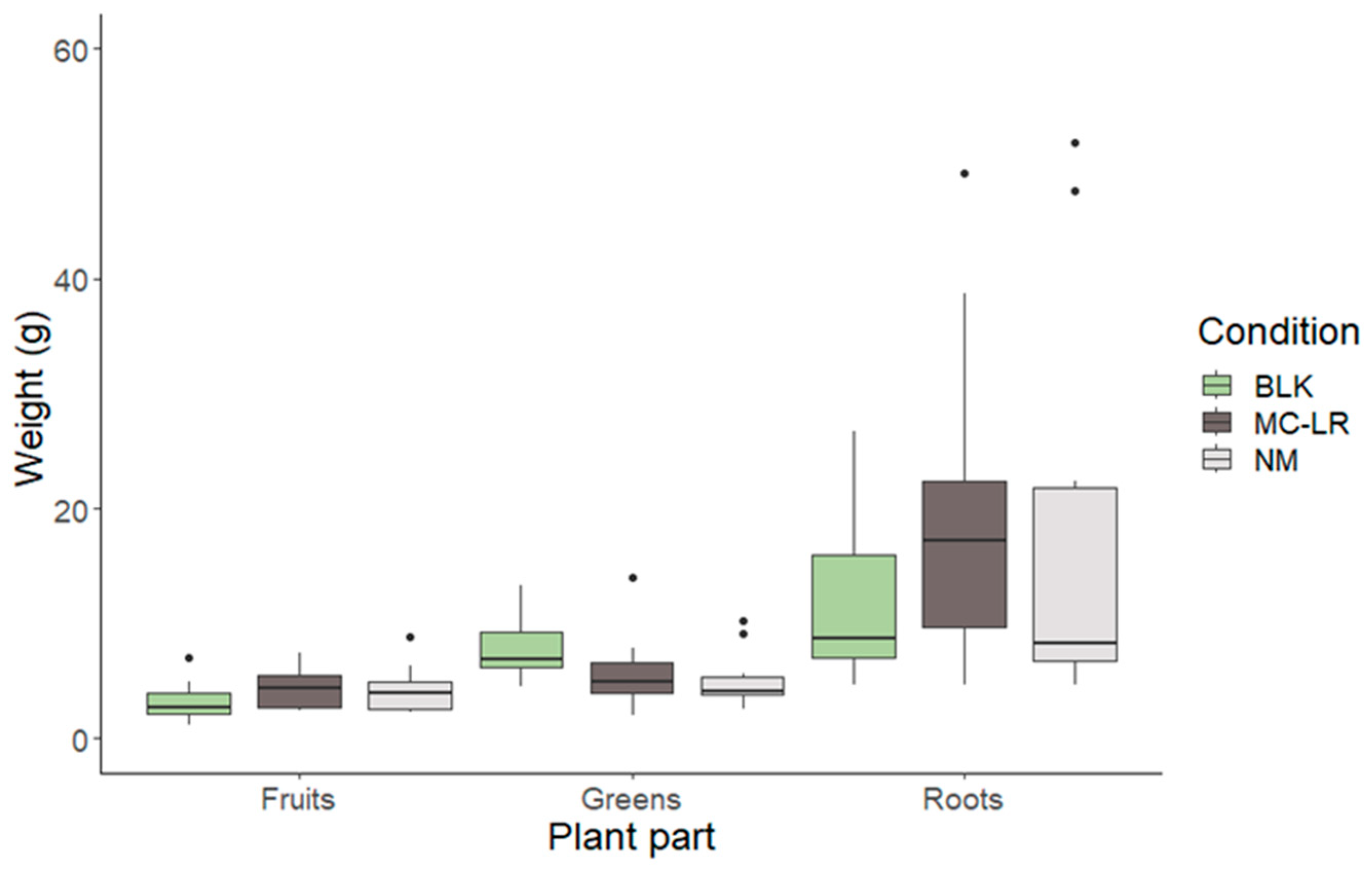


| Cultivation Method | Toxin Concentration in Solution | Concentration (ng g−1) | Reference |
|---|---|---|---|
| Liquid hydroculture | 100 µg L−1 MC-LR | 2.8 | current study |
| Liquid hydroculture | 100 µg L−1 MCs | 3.4 | current study |
| Substrate hydroponic | 20 µg L−1 MCs | 6.59 | [61] |
| Soil-based drip irrigation | 100 µg L−1 MC-LR | 3.4 | [34] |
| Soil-based spray irrigation | 100 µg L−1 MC-LR | 5.6 | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Hassel, W.H.R.; Guillaume, B.; Masquelier, J. Hydroculture Cultivation of Strawberries as Potential Reference Material for Microcystin Analysis: Approaches and Pitfalls. Toxins 2025, 17, 285. https://doi.org/10.3390/toxins17060285
Van Hassel WHR, Guillaume B, Masquelier J. Hydroculture Cultivation of Strawberries as Potential Reference Material for Microcystin Analysis: Approaches and Pitfalls. Toxins. 2025; 17(6):285. https://doi.org/10.3390/toxins17060285
Chicago/Turabian StyleVan Hassel, Wannes Hugo R., Benoît Guillaume, and Julien Masquelier. 2025. "Hydroculture Cultivation of Strawberries as Potential Reference Material for Microcystin Analysis: Approaches and Pitfalls" Toxins 17, no. 6: 285. https://doi.org/10.3390/toxins17060285
APA StyleVan Hassel, W. H. R., Guillaume, B., & Masquelier, J. (2025). Hydroculture Cultivation of Strawberries as Potential Reference Material for Microcystin Analysis: Approaches and Pitfalls. Toxins, 17(6), 285. https://doi.org/10.3390/toxins17060285





