Dietary Supplementation of Zinc Oxide Quantum Dots Protective Against Clostridium perfringens Induced Negative Effects in Broilers
Abstract
1. Introduction
2. Results
2.1. In Vitro Antibacterial Activities of ZnO-QDs
2.2. Growth Performance
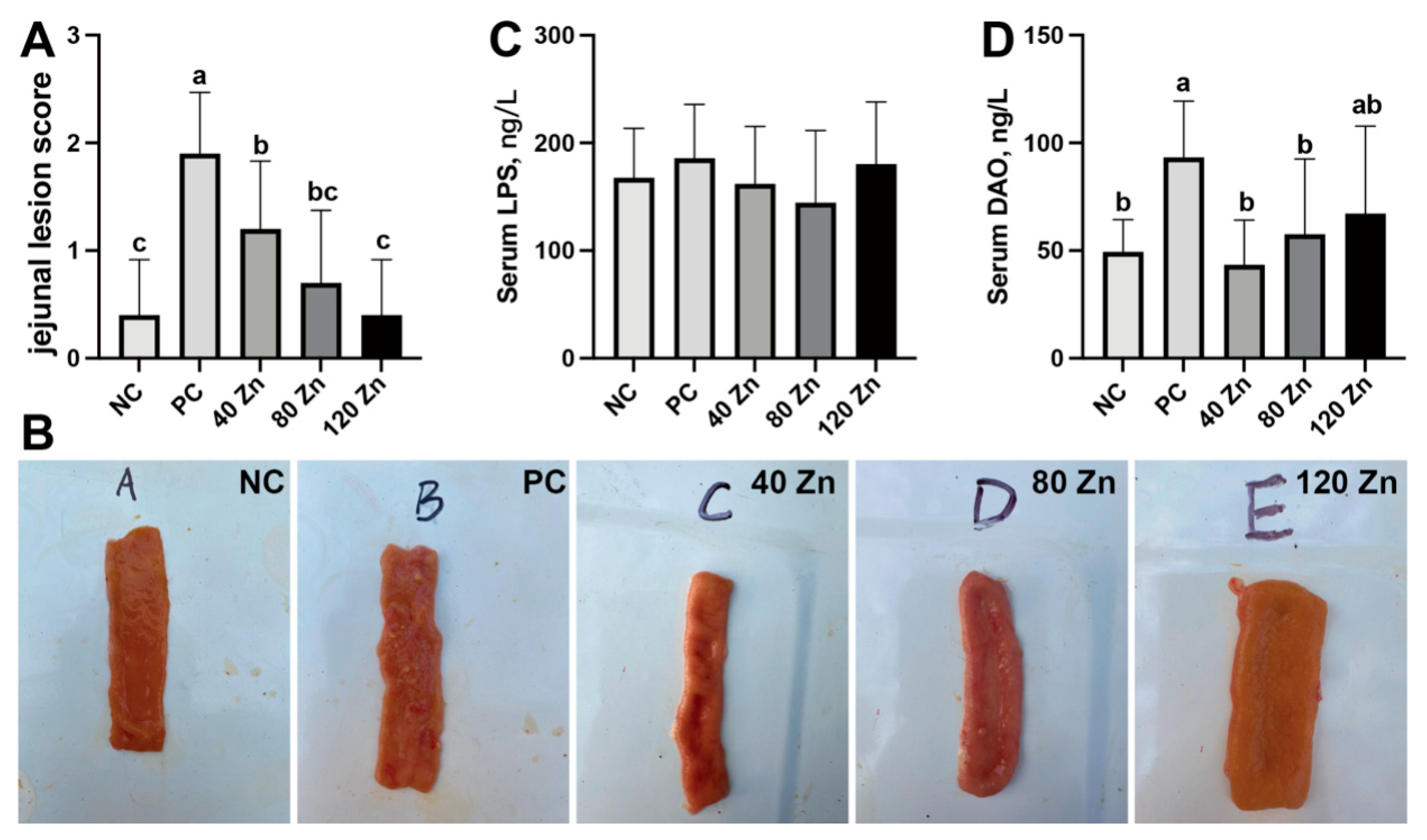
2.3. Intestinal Health and Serum Biochemistry
2.4. Jejunal Redox Status
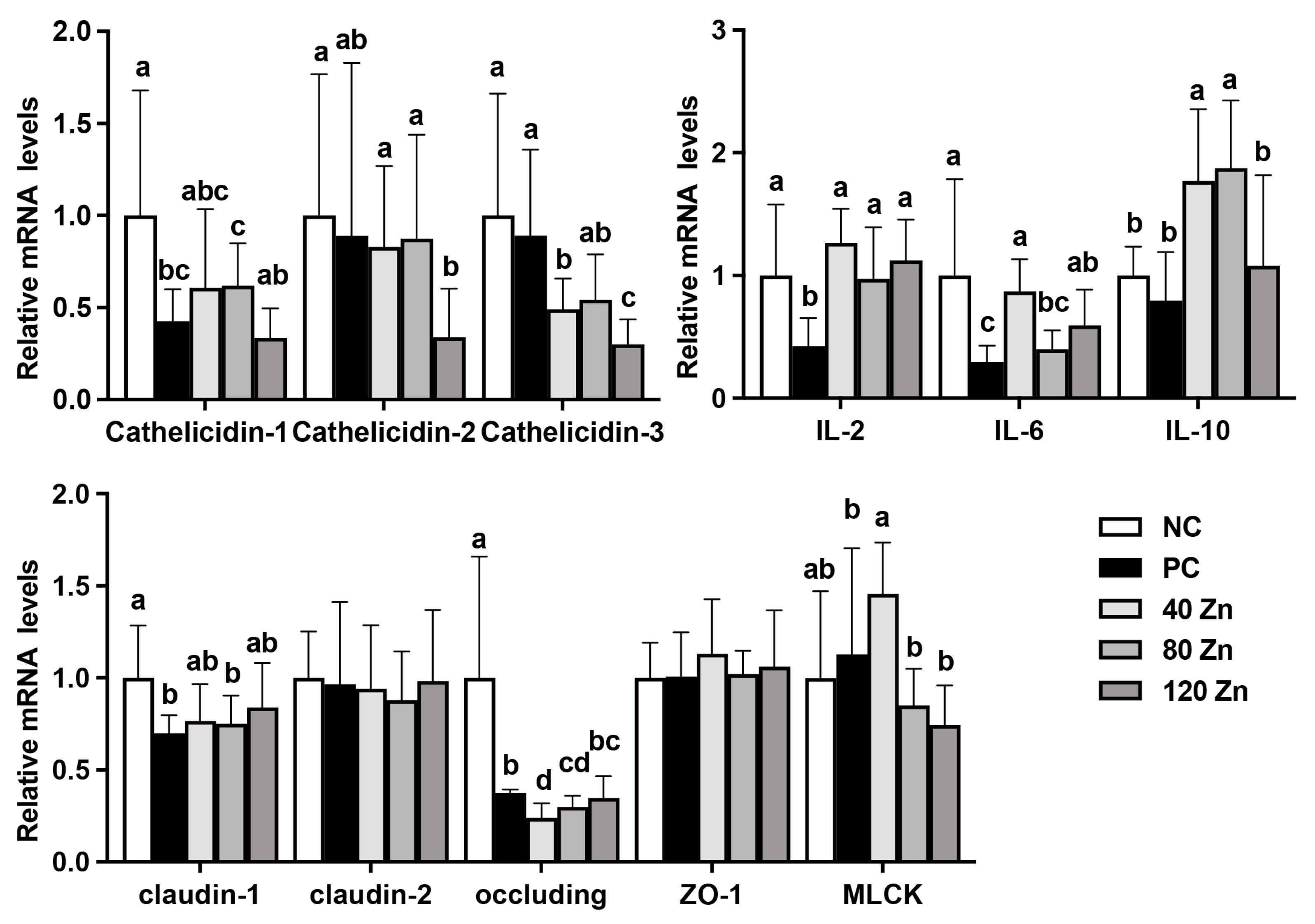
2.5. The mRNA Levels of Antimicrobial Peptide-, Cytokine-, and Tight Junction-Related Genes
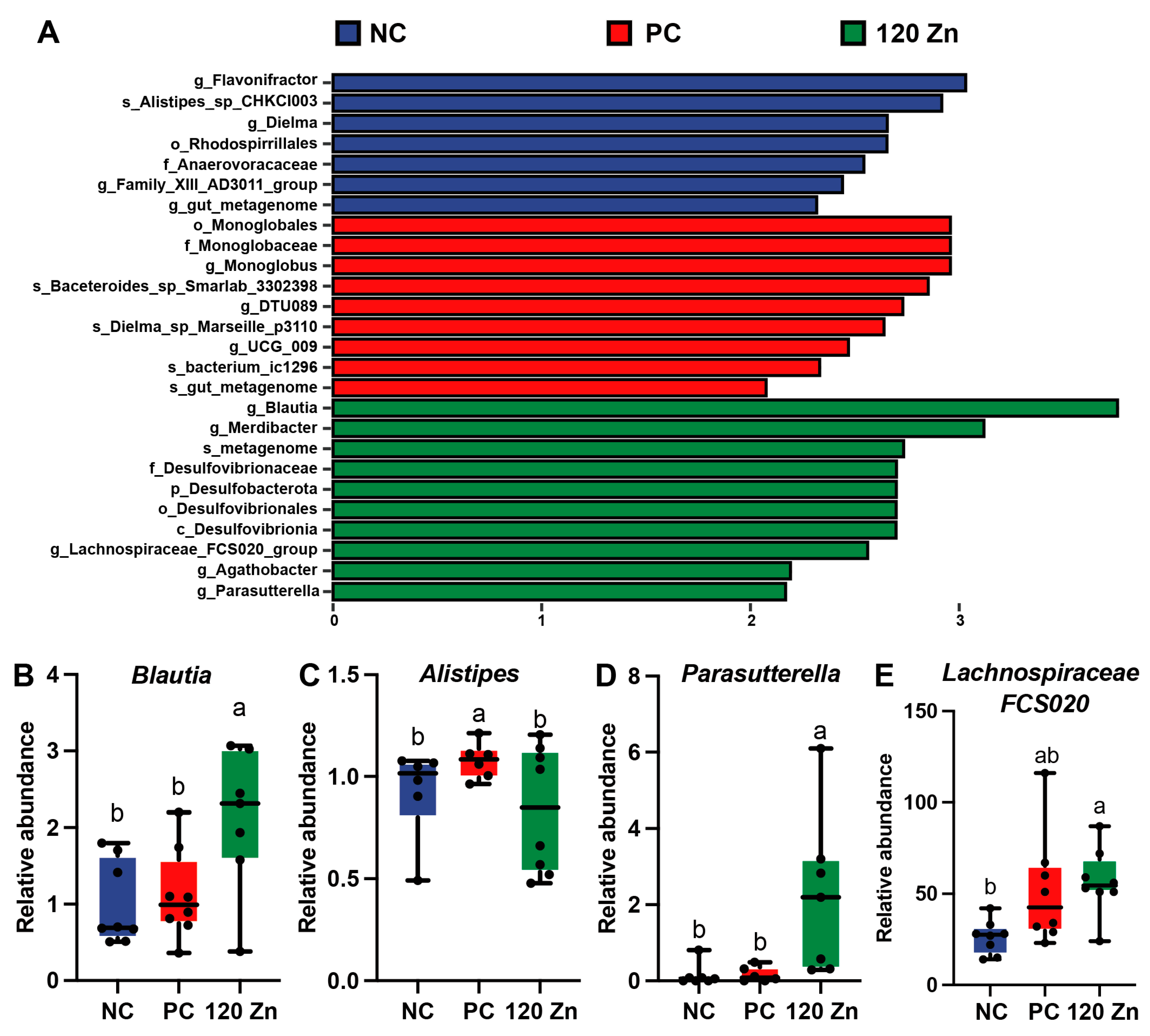
2.6. Gut Microbiota
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Experimental Materials
5.2. Antibacterial Activities In Vitro Experiment
5.3. Birds, Treatment, and Growth Performance
5.4. Intestinal Lesion Score
5.5. Serum Biochemistry and Jejunum Antioxidant Parameter Analysis
5.6. Real-Time Quantitative PCR
5.7. Gut Microbiota Analysis
5.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, K.W.; Lillehoj, H.S. Role of Clostridium perfringens Necrotic Enteritis B-like Toxin in Disease Pathogenesis. Vaccines 2021, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Caly, D.L.; D’Inca, R.; Auclair, E.; Drider, D. Alternatives to Antibiotics to Prevent Necrotic Enteritis in Broiler Chickens: A Microbiologist’s Perspective. Front. Microbiol. 2015, 6, 1336. [Google Scholar] [CrossRef] [PubMed]
- Broom, L.J. Necrotic enteritis; current knowledge and diet-related mitigation. World Poult. Sci. J. 2017, 73, 281–291. [Google Scholar] [CrossRef]
- Emami, N.K.; Calik, A.; White, M.B.; Young, M.; Dalloul, R.A. Necrotic Enteritis in Broiler Chickens: The Role of Tight Junctions and Mucosal Immune Responses in Alleviating the Effect of the Disease. Microorganisms 2019, 7, 231. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Adams, V.; Bannam, T.L.; Miyamoto, K.; Garcia, J.P.; Uzal, F.A.; Rood, J.I.; McClane, B.A. Toxin plasmids of Clostridium perfringens. Microbiol. Mol. Biol. Rev. 2013, 77, 208–233. [Google Scholar] [CrossRef]
- Kiu, R.; Hall, L.J. An update on the human and animal enteric pathogen Clostridium perfringens. Emerg Microbes Infect. 2018, 7, 141. [Google Scholar] [CrossRef]
- Moore, R.J. Necrotic enteritis and antibiotic-free production of broiler chickens: Challenges in testing and using alternative products. Anim. Nutr. 2024, 16, 288–298. [Google Scholar] [CrossRef]
- Hofacre, C.L.; Smith, J.A.; Mathis, G.F. An optimist’s view on limiting necrotic enteritis and maintaining broiler gut health and performance in today’s marketing, food safety, and regulatory climate. Poult. Sci. 2018, 97, 1929–1933. [Google Scholar] [CrossRef]
- Cao, K.X.; Deng, Z.C.; Li, S.J.; Yi, D.; He, X.; Yang, X.J.; Guo, Y.M.; Sun, L.H. Poultry Nutrition: Achievement, Challenge, and Strategy. J. Nutr. 2024, 154, 3554–3565. [Google Scholar] [CrossRef]
- Zhu, C.; Lv, H.; Chen, Z.; Wang, L.; Wu, X.; Chen, Z.; Zhang, W.; Liang, R.; Jiang, Z. Dietary Zinc Oxide Modulates Antioxidant Capacity, Small Intestine Development, and Jejunal Gene Expression in Weaned Piglets. Biol. Trace Elem. Res. 2017, 175, 331–338. [Google Scholar] [CrossRef]
- Grilli, E.; Tugnoli, B.; Vitari, F.; Domeneghini, C.; Morlacchini, M.; Piva, A.; Prandini, A. Low doses of microencapsulated zinc oxide improve performance and modulate the ileum architecture, inflammatory cytokines and tight junctions expression of weaned pigs. Animal 2015, 9, 1760–1768. [Google Scholar] [CrossRef]
- Radi, A.M.; Abdel Azeem, N.M.; El-Nahass, E.S. Comparative effects of zinc oxide and zinc oxide nanoparticle as feed additives on growth, feed choice test, tissue residues, and histopathological changes in broiler chickens. Environ. Sci. Pollut. Res. Int. 2021, 28, 5158–5167. [Google Scholar] [CrossRef] [PubMed]
- Ramiah, S.K.; Awad, E.A.; Mookiah, S.; Idrus, Z. Effects of zinc oxide nanoparticles on growth performance and concentrations of malondialdehyde, zinc in tissues, and corticosterone in broiler chickens under heat stress conditions. Poult. Sci. 2019, 98, 3828–3838. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Sasidharan, A.; Divya Rani, V.V.; Menon, D.; Nair, S.; Manzoor, K.; Raina, S. Role of size scale of ZnO nanoparticles and microparticles on toxicity toward bacteria and osteoblast cancer cells. J. Mater. Sci. Mater. Med. 2009, 20 (Suppl. S1), S235–S241. [Google Scholar] [CrossRef] [PubMed]
- Happy, A.; Soumya, M.; Venkat Kumar, S.; Rajeshkumar, S. Mechanistic study on antibacterial action of zinc oxide nanoparticles synthesized using green route. Chem. Biol. Interact. 2018, 286, 60–70. [Google Scholar] [CrossRef]
- Kumar Verma, A. ZnO quantum dots a novel nanomaterial for various applications: Recent advances and challenges. Indian J. Biochem. Biophys. 2022, 59, 1190–1198. [Google Scholar]
- Li, Y.; Xie, S.; Xu, D.; Shu, G.; Wang, X. Antibacterial activity of ZnO quantum dots and its protective effects of chicks infected withSalmonella pullorum. Nanotechnology 2021, 32, 505104. [Google Scholar] [CrossRef]
- Shi, L.; Ruan, M.L.; Zhang, B.B.; Gong, G.X.; Li, X.W.; Refaie, A.; Sun, L.-H.; Deng, Z.-C. Effects of Dietary Supplementation of Zinc Oxide Quantum Dots on Growth Performance and Gut Health in Broilers. Biol. Trace Elem. Res. 2024. [Google Scholar] [CrossRef]
- Du, F.; Niu, J.; Hong, Y.; Fang, X.; Geng, Z.; Liu, J.; Xu, F.; Liu, T.; Chen, Q.; Zhai, J.; et al. Microwave-Assisted Synthesized ZnO@APTES Quantum Dots Exhibits Potent Antibacterial Efficacy Against Methicillin-Resistant Staphylococcus aureus without Inducing Resistance. Int. J. Nanomed. 2025, 20, 523–540. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, R.F.; Sa-Correia, I.; Valvano, M.A. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol. Rev. 2016, 40, 480–493. [Google Scholar] [CrossRef]
- Mak, P.H.W.; Rehman, M.A.; Kiarie, E.G.; Topp, E.; Diarra, M.S. Production systems and important antimicrobial resistant-pathogenic bacteria in poultry: A review. J. Anim. Sci. Biotechnol. 2022, 13, 148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fan, X.; Zhong, Z.; Xu, G.; Shen, J. Association of plasma diamine oxidase and intestinal fatty acid-binding protein with severity of disease in patient with heat stroke. Am. J. Emerg. Med. 2015, 33, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, Q.; Ci, X.; Chen, S.; Xie, Z.; Li, H.; Zhang, H.; Chen, F.; Xie, Q. Evaluation of the efficacy of chlorogenic acid in reducing small intestine injury, oxidative stress, and inflammation in chickens challenged with Clostridium perfringens type A. Poult. Sci. 2020, 99, 6606–6618. [Google Scholar] [CrossRef]
- Liu, N.; Lin, L.; Wang, J.; Zhang, F.; Wang, J.P. Dietary cysteamine hydrochloride protects against oxidation, inflammation, and mucosal barrier disruption of broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. 2018, 96, 4339–4347. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, T.; Chen, Y.; Zhou, X.; Zhong, Y.; Liu, H.; Zhong, Z.; Hu, Y.; Liao, F.; Wang, X.; et al. Zinc Oxide Quantum Dots May Provide a Novel Potential Treatment for Antibiotic-Resistant Streptococcus agalactiae in Lama glama. Molecules 2023, 28, 5115. [Google Scholar] [CrossRef]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial host defence peptides: Functions and clinical potential. Nat. Rev. Drug Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef]
- Kosciuczuk, E.M.; Lisowski, P.; Jarczak, J.; Strzalkowska, N.; Jozwik, A.; Horbanczuk, J.; Krzyżewski, J.; Zwierzchowski, L.; Bagnicka, E. Cathelicidins: Family of antimicrobial peptides. A review. Mol. Biol. Rep. 2012, 39, 10957–10970. [Google Scholar] [CrossRef]
- Waititu, S.M.; Yitbarek, A.; Matini, E.; Echeverry, H.; Kiarie, E.; Rodriguez-Lecompte, J.C.; Nyachoti, C.M. Effect of supplementing direct-fed microbials on broiler performance, nutrient digestibilities, and immune responses. Poult. Sci. 2014, 93, 625–635. [Google Scholar] [CrossRef]
- Swaggerty, C.L.; Kogut, M.H.; Ferro, P.J.; Rothwell, L.; Pevzner, I.Y.; Kaiser, P. Differential cytokine mRNA expression in heterophils isolated from Salmonella-resistant and -susceptible chickens. Immunology 2004, 113, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Gadde, U.; Oh, S.T.; Lee, Y.S.; Davis, E.; Zimmerman, N.; Rehberger, T.; Lillehoj, H.S. The Effects of Direct-fed Microbial Supplementation, as an Alternative to Antibiotics, on Growth Performance, Intestinal Immune Status, and Epithelial Barrier Gene Expression in Broiler Chickens. Probiotics Antimicrob. Proteins 2017, 9, 397–405. [Google Scholar] [CrossRef]
- Fazal, F.; Gu, L.; Ihnatovych, I.; Han, Y.; Hu, W.; Antic, N.; Carreira, F.; Blomquist, J.F.; Hope, T.J.; Ucker, D.S. Inhibiting myosin light chain kinase induces apoptosis in vitro and in vivo. Mol. Cell. Biol. 2005, 25, 6259–6266. [Google Scholar] [CrossRef]
- Yadav, S.; Jha, R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Ani. Sci. Biotech. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Moschen, A.R.; Gerner, R.R.; Wang, J.; Klepsch, V.; Adolph, T.E.; Reider, S.J.; Hackl, H.; Pfister, A.; Schilling, J.; Moser, P.L.; et al. Lipocalin 2 Protects from Inflammation and Tumorigenesis Associated with Gut Microbiota Alterations. Cell Host Microbe 2016, 19, 455–469. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria with Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia-a new functional genus with potential probiotic properties? Gut Microbes 2021, 13, 1875796. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Park, J.; Kim, M. Gut microbiota-derived short-chain Fatty acids, T cells, and inflammation. Immune Netw. 2014, 14, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.F.; Huang, W.C.; Wu, C.W.; Huang, C.Y.; Yang, Y.C.S.H.; Tung, Y.T. Acute sleep deprivation exacerbates systemic inflammation and psychiatry disorders through gut microbiota dysbiosis and disruption of circadian rhythms. Microbiol. Res. 2023, 268, 127292. [Google Scholar] [CrossRef]
- Hu, R.; Wu, S.; Li, B.; Tan, J.; Yan, J.; Wang, Y.; Tang, Z.; Liu, M.; Fu, C.; Zhang, H.; et al. Dietary ferulic acid and vanillic acid on inflammation, gut barrier function and growth performance in lipopolysaccharide-challenged piglets. Anim. Nutr. 2022, 8, 144–152. [Google Scholar] [CrossRef]
- Du, E.; Gan, L.; Li, Z.; Wang, W.; Liu, D.; Guo, Y. In vitro antibacterial activity of thymol and carvacrol and their effects on broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2015, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Orou, S.F.C.; Hang, K.J.; Thien, M.T.; Ying, Y.L.; Diem, N.D.N.; Goh, B.H.; Pung, S.Y.; Pung, Y.F. Antibacterial activity by ZnO nanorods and ZnO nanodisks: A model used to illustrate “Nanotoxicity Threshold”. J. Ind. Eng. Chem. 2018, 62, 333–340. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, P.; Shi, X.; Tan, H. Inhibitory properties of Chinese Herbal Formula SanHuang decoction on biofilm formation by antibiotic-resistant Staphylococcal strains. Sci. Rep. 2021, 11, 7134. [Google Scholar] [CrossRef]
- Wu, Y.; Bai, J.; Zhong, K.; Huang, Y.; Gao, H. A dual antibacterial mechanism involved in membrane disruption and DNA binding of 2R,3R-dihydromyricetin from pine needles of Cedrus deodara against Staphylococcus aureus. Food Chem. 2017, 218, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Hu, D.; Cheng, E.W.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.C.; Wang, J.; Wang, J.; Yan, Y.Q.; Huang, Y.X.; Chen, C.Q.; Sun, L.; Liu, M. Tannic acid extracted from gallnut improves intestinal health with regulation of redox homeostasis and gut microbiota of weaned piglets. Anim. Res. One Health 2024, 2, 16–27. [Google Scholar] [CrossRef]
- Liu, D.; Guo, S.; Guo, Y. Xylanase supplementation to a wheat-based diet alleviated the intestinal mucosal barrier impairment of broiler chickens challenged by Clostridium perfringens. Avian Pathol. 2012, 41, 291–298. [Google Scholar] [CrossRef]
- Wang, S.Q.; Peng, Z.; Sun, H.; Han, Y.M.; Zhang, B.; Pineda, L.; Boerboom, G.; Sun, L.-H.; Liu, Y.; Deng, Z.-C. Evaluating the Impact of an Organic Trace Mineral mix on the Redox Homeostasis, Immunity, and Performance of Sows and their Offspring. Biol. Trace Elem. Res. 2024, 203, 1798–1807. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.W.; Sun, H.; Yan, Y.Q.; Xia, Z.Y.; Refaie, A.; Zhang, N.-Y.; Wang, S.; Tan, C.; Sun, L.-H. T-2 toxin-induced splenic injury by disrupting the gut microbiota-spleen axis via promoting IL-6/JAK/STAT1 signaling-mediated inflammation and apoptosis and its mitigation by elemental nano-selenium. Arch. Toxicol. 2025. [Google Scholar] [CrossRef]
- Yang, J.C.; Liu, M.; Huang, R.H.; Zhao, L.; Niu, Q.J.; Xu, Z.J.; Wei, J.-T.; Lei, X.G.; Sun, L.-H. Loss of SELENOW aggravates muscle loss with regulation of protein synthesis and the ubiquitin-proteasome system. Sci. Adv. 2024, 10, eadj4122. [Google Scholar] [CrossRef]
- Yan, Y.Q.; Liu, M.; Xu, Z.J.; Xu, Z.J.; Huang, Y.X.; Li, X.M.; Chen, C.-J.; Zuo, G.; Yang, J.-C.; Lei, X.G.; et al. Optimum Doses and Forms of Selenium Maintaining Reproductive Health via Regulating Homeostasis of Gut Microbiota and Testicular Redox, Inflammation, Cell Proliferation, and Apoptosis in Roosters. J. Nutr. 2024, 154, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahe, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Lyu, F.; Han, F.; Ge, C.; Mao, W.; Chen, L.; Hu, H.; Chen, G.; Lang, Q.; Fang, C. OmicStudio: A composable bioinformatics cloud platform with real-time feedback that can generate high-quality graphs for publication. Imeta 2023, 2, e85. [Google Scholar] [CrossRef]
- Cao, K.X.; Deng, Z.C.; Liu, M.; Huang, Y.X.; Yang, J.C.; Sun, L.H. Heat Stress Impairs Male Reproductive System with Potential Disruption of Retinol Metabolism and Microbial Balance in the Testis of Mice. J. Nutr. 2023, 153, 3373–3381. [Google Scholar] [CrossRef]
- Deng, J.; Peng, Z.; Xia, Z.; Mo, Y.; Guo, L.; Wei, J.; Sun, L.; Liu, M. Five glutathione S-transferase isozymes played crucial role in the detoxification of aflatoxin B1 in chicken liver. J. Anim. Sci. Biotechnol. 2025, 16, 54. [Google Scholar] [CrossRef]


| Strain | MIC (mg/mL) | |
|---|---|---|
| ZnO-QDs | ZnO | |
| E. coli | 0.413 | 3.800 |
| S. Pulorum | 0.052 | 0.059 |
| S. aureus | 0.026 | 0.238 |
| C. perfringens | 0.413 | 1.900 |
| NC | PC | 40 Zn | 80 Zn | 120 Zn | |
|---|---|---|---|---|---|
| Day 0 BW, g/bird | 45.05 ± 0.37 | 45.08 ± 0.52 | 45.12 ± 0.50 | 45.00 ± 0.31 | 44.86 ± 0.60 |
| Day 21 BW, g/bird | 740.91 ± 35.16 b | 717.18 ± 30.37 c | 757.95 ± 42.29 ab | 738.85 ± 30.79 bc | 775.70 ± 22.84 a |
| Day 28 BW, g/bird | 1256.07 ± 81.38 | 1210.32 ± 67.61 | 1205.90 ± 87.84 | 1262.47 ± 61.14 | 1252.01 ± 61.27 |
| 1 to 28 days | |||||
| ADG, g/d/bird | 43.25 ± 1.03 | 41.62 ± 0.85 | 41.46 ± 1.11 | 43.48 ± 0.77 | 43.11 ± 0.78 |
| ADFI, g/d/bird | 65.78 ± 1.23 | 66.23 ± 1.08 | 63.26 ± 1.36 | 66.51 ± 1.31 | 65.2 ± 1.55 |
| FCR, g/g | 1.52 ± 0.07 b,# | 1.59 ± 0.08 a,# | 1.53 ± 0.09 ab | 1.53 ± 0.11 ab | 1.52 ± 0.12 ab |
| NC | PC | 40 Zn | 80 Zn | 120 Zn | |
|---|---|---|---|---|---|
| CAT, U/mg protein | 0.76 ± 0.10 a | 0.57 ± 0.18 bc | 0.75 ± 0.18 ab | 0.45 ± 0.15 c | 0.61 ± 0.18 bc |
| SOD, U/mg protein | 78.86 ± 22.82 a | 46.75 ± 10.04 b | 60.24 ± 20.04 ab | 48.13 ± 8.26 b | 62.73 ± 26.89 ab |
| GPX, U/mg protein | 1.50 ± 0.58 b | 1.25 ± 0.69 b | 2.60 ± 1.18 a | 1.80 ± 0.78 ab | 2.42 ± 1.10 a |
| GR, U/g protein | 5.71 ± 2.68 | 3.64 ± 1.69 | 4.34 ± 2.06 | 5.92 ± 3.17 | 4.47 ± 1.75 |
| MDA, nmol/mg protein | 2.47 ± 0.94 ab | 3.07 ± 0.99 a | 2.15 ± 0.6 b | 1.79 ± 0.41 b | 2.27 ± 1.02 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, L.; Niu, Q.-J.; Xu, H.-H.; Huang, Y.-X.; Zhao, Y.-W.; Refaie, A.; Sun, L.-H.; Deng, Z.-C. Dietary Supplementation of Zinc Oxide Quantum Dots Protective Against Clostridium perfringens Induced Negative Effects in Broilers. Toxins 2025, 17, 272. https://doi.org/10.3390/toxins17060272
Shi L, Niu Q-J, Xu H-H, Huang Y-X, Zhao Y-W, Refaie A, Sun L-H, Deng Z-C. Dietary Supplementation of Zinc Oxide Quantum Dots Protective Against Clostridium perfringens Induced Negative Effects in Broilers. Toxins. 2025; 17(6):272. https://doi.org/10.3390/toxins17060272
Chicago/Turabian StyleShi, Lei, Qin-Jian Niu, Hao-Hua Xu, Yu-Xuan Huang, Yu-Wei Zhao, Alainaa Refaie, Lv-Hui Sun, and Zhang-Chao Deng. 2025. "Dietary Supplementation of Zinc Oxide Quantum Dots Protective Against Clostridium perfringens Induced Negative Effects in Broilers" Toxins 17, no. 6: 272. https://doi.org/10.3390/toxins17060272
APA StyleShi, L., Niu, Q.-J., Xu, H.-H., Huang, Y.-X., Zhao, Y.-W., Refaie, A., Sun, L.-H., & Deng, Z.-C. (2025). Dietary Supplementation of Zinc Oxide Quantum Dots Protective Against Clostridium perfringens Induced Negative Effects in Broilers. Toxins, 17(6), 272. https://doi.org/10.3390/toxins17060272





